Abstract
Gastric cancer is a common malignancy with high mortality. Long noncoding RNA (lncRNA) zinc finger antisense (ZFAS)1 is upregulated in gastric cancer specimens compared with the para-carcinoma tissues. The silencing of ZFAS1 inhibited the growth, proliferation, cell cycle progress, migration, invasion and epithelial-mesenchymal transition (EMT), and enhanced the sensitivity to cis-platinum or paclitaxel in SGC7901 cells, as evidenced by the expression changes of proliferating cell nuclear antigen, Cyclin D1, Cyclin E, Cyclin B1, E-cadherin, N-cadherin, vimentin, matrix metalloproteinase (MMP)-2 and MMP-14. The ZFAS1 also activated the Wnt/β-catenin signaling. Subsequently, the ZFAS1 knockdown-induced the inhibition of migration, invasion, EMT and resistance to chemotherapeutic reagens was reversed by the overexpression of β-catenin. In summary, the silencing of ZFAS1 inhibited the growth, proliferation, cell cycle progress, migration, invasion, EMT and chemotherapeutic tolerance by blocking the Wnt/β-catenin signaling in gastric cancer cells.
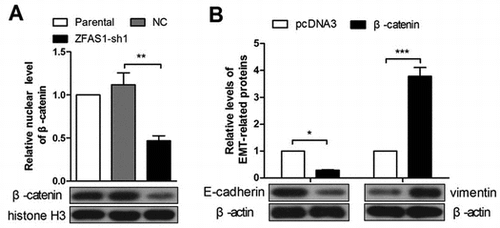
ZFAS1 knockdown decreases the nuclear level of β-catenin (A). Ectopic expression of β-catenin enhances EMT in ZFAS1-silenced SGC7901 cells (B).
Gastric cancer is the fifth most common malignancy and the third leading cause of cancer-related death in the world [Citation1]. Gastric cancer ranked the second leading cause of cancer death in China [Citation2]. Despite a declining morbidity, gastric cancer causes over 723,000 deaths one year [Citation3]. The high mortality and poor prognosis of gastric cancer are associated with late stage diagnosis and limited efficacy of therapies. Due to the lack of validated screening programs and the absence of significant symptoms at early stages, most gastric cancer cases are diagnosed at an advanced stage [Citation4,5]. Currently, some routine markers for clinical diagnosis of gastric cancer, such as carcinoembrionyc antigen (CEA), CA19.9 and CA50, are not enough sensitive and specific [Citation6–8]. Therefore, it is urgent to seek novel biomarkers for early diagnosis of gastric cancer.
Long noncoding RNA (lncRNA) is a class of RNA molecules longer than 200 nucleotides and without protein coding ability [Citation9]. Since the first lncRNA, H19, was reported in 1990 [Citation10], lncRNA has attracted an increasing attention. Once considered as “transcriptional noise”, lncRNA has been demonstrated with significant functions in human disease, including gastric cancer [Citation11,12]. LINC00152 overexpression accelerates the cell cycle process of gastric cancer cells [Citation13]. MALAT-1 promotes migration, invasion and epithelial-mesenchymal transition in gastric cancer cells [Citation14]. LncRNA PVT-1 is up-regulated in gastric cancer tissues from cisplatin-resistance patients and cisplatin-resistant gastric cancer cell lines, SGC7901/DDP and BGC823/DDP. The resistance of these two cell lines is attenuated by silence of PVT-1, and augmented by PVT-1 overexpression [Citation15]. LncRNA zinc finger antisense (ZFAS)1 was first identified in 2011, at that time, it was demonstrated downregulated in human breast cancer, and seen with an anti-tumor effect [Citation16]. However, recent studies reveal that ZFAS1 is highly expressed in hepatocellular carcinoma and colorectal cancer, promotes the metastasis of hepatocellular carcinoma, and enhances the tumorigenicity of colorectal cancer cells by involving the cell cycle [Citation17,18].
Recently, it has been reported that ZFAS1 expression is increased in gastric cancer tissue and cell lines, and play a cancer-promoting role in gastric cancer [Citation5,19]. However, the underlying mechanism has not been elucidated completely. In this study, we investigated the mechanism that ZFAS1 affects the proliferation, migration, invasion, epithelial-mesenchymal transition (EMT) and chemotherapeutic resistance in gastric cancer cells.
Material and methods
Clinical specimens
The clinical gastric cancer specimens and paired para-carcinoma tissues were collected in the First Affiliated Hospital of Jilin University from February 2017 to May 2017. The patients were diagnosed with gastric cancer based on the histopathological detection, and did not suffer any treatments before surgery. The clinical tissue collection and experiment procedures were complied with Declaration of Helsinki (2013), and the protocols were approved by the Ethics Committee of Jilin University.
Real-time PCR
The total RNA was isolated with a Total RNA Rapid Extraction Kit (BioTeke, Beijing, China) according to the manufacturer’s protocols. After concentration measurement, the RNA was reversely transcribed into cDNA with Super M-MLV reverse transcriptase (BioTeke), in presence of Oligo(dT) and random primers. All instruments in this section were pre-treated with Surface RNase Erasol (TIANDZ, Beijing, China), and all reagents were RNase-free.
The cDNA was used for real-time PCR to detect the levels of lncRNA ZFAS1 or β-catenin mRNA. The procedure was set as follows: the first cycle including 94 °C for 10 min, 94 °C for 10 s, 60°for 20 s, 72 °C for 30 s, followed with 40 cycles of 70 °C for 2 min 30 s, 40 °C for 5 min 30 s, melting from 60 °C to 94 °C for 1 s every 1 °C, and 25 °C for 1 min. The data were analyzed with 2-ΔΔCt method. The sequence information of real-time PCR primers was shown in Table .
Table 1. Sequence information of real-time PCR primers used in this study.
Plasmid construction
The two knockdown sequences of lncRNA ZFAS1, ZFAS1-shRNA-1 and ZFAS1-shRNA-2, were inserted into pRNA-H1.1 vector with BamHⅠ + HindⅢ sites. A random sequence with an equal length was synthesized as the negative control (NC). After screening with G418 (Invitrogen, Carlsbad, CA, USA), the stably ZFAS1-silenced SGC7901 cells were obtained. The sequences of sense, antisense and target sequence of each shRNA were shown in Table .
Table 2. Information of lncRNA ZFAS1 knockdown sequences.
For ZFAS1 overexpression, a DNA sequence containing ZFAS1 unit cloned from human gastric cancer cell genomic DNA was inserted into pcDNA3.1 vector at HindⅢ and EcoRⅠ sites. The sequence information of the amplification primers was shown: ZFAS1-sense: 5’-TTCAAGCTTCGGGGGCCCAGGGTGGAGA-3’, ZFAS1-antisense: 5’-CCGGAATTCTTTTGATTTAAAAGATTTTAT-3’. The pcDNA3.1-ZFAS1 plasmid was used for transient transfection in SGC7901 cells with the pcDNA3.1 empty vector as the control.
The β-catenin overexpression plasmid (pcDNA3-S33Y Beta-catenin) was purchased from Addgene (Cambrige, MA, USA), and pcDNA3 vector served as the control.
Cell culture, transfection and monoclonal cell line screening
Gastric cancer cell line SGC7901 was purchased from CHI scientific Inc. (MA, USA), and cultured with RPMI-1640 medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) at 37 °C in 5% CO2. The SGC-7901 cells were seeded in 6-well plates at a density of 1 × 105 per well. Twenty-four hours later, the SGC7901 cells was transfected with the plasmid containing ZFAS1-shRNA-1, ZFAS1-shRNA-2 or negative control (NC) in serum-free medium in the presence of Lipofectamine 2000 reagent (Invitrogen). Twenty-four hours later, G418 reagent (700 μg/mL) (Invitrogen) was used to treat the cells, and the medium was renewed every 2 days. After one week, individual cell colonies could be seen. The cells were continued to culture in RPMI-1640 medium without G418 reagent for about two weeks, and the monoclonal cell lines were obtained.
Cell counting kit (CCK)-8 assay
The CCK-8 assay was performed to detect cell viability. The SGC7901 cells were seeded in 96-well plates at a density of 3 × 103 per well. After cells were culturing 0, 24, 48, 72 and 96 h, CCK-8 reagent was used to treat cells with 10 μL per well for 1 h, and the optical density of solution was detected with a microplate reader at 450 nm.
Western blot
The cellular protein or nuclear protein was extracted from SGC7901 cells with RIPA lysis buffer (Beyotime, Haimen, Jiangsu, China) or a Nuclear Protein Extraction Kit (Wanleibio, Shenyang, Liaoning, China) according to the manufacturer’s protocols. After denaturation with boiling, the protein was separated with SDS-PAGE, and transferred onto PVDF membrane (Millipore, Boston, MA, USA). After blocking with 5% skim milk (YILI, Hohhot, Inner Mongolia, China) for 1 h, the membrane was incubated with one of the following antibodies at 4 °C overnight: rabbit anti-proliferating cell nuclear antigen (PCNA) (1:500, Sangon, Shanghai, China), rabbit anti-Cyclin D1 (1:400, Boster, Wuhan, Hubei, China), rabbit anti-Cyclin E (1:500, Bioss, Beijing, China), rabbit anti-Cyclin B1 (1:400, Boster), rabbit anti-matrix metalloproteinase (MMP)-2 (1:400, Boster), rabbit anti-MMP-14 (1:400, Boster), rabbit anti-N-cadherin (1:400, Boster), rabbit anti-vimentin (1:400, Boster), rabbit anti-E-cadherin (1:400, Proteintech, Wuhan, Hubei, China), rabbit anti-naked cuticle homolog 2 (NKD2) (1:300, Proteintech), rabbit anti-β-catenin (1:500, Proteintech), glycogen synthase kinase 3 beta (GSK3β) (1:1000; Proteintech), p-GSK3β (Ser9) (1:500; Proteintech), mouse anti-β-actin (1:500, Bioss), mouse anti-histone H3 (1:500, Bioss). After rinsing with TBST, the PVDF membrane was incubated with corresponding secondary antibody at 37 °C for 45 min, followed with a signal exposure with ECL reagent (Beyotime). β-actin served as the internal control for total cellular proteins, and histone H3 served as the internal control for nuclear proteins.
Flow cytometry
The SGC7901 cells were seeded in 6-well plates, and cultured to a confluence of 90%. The cells were collected, washed with PBS, and centrifuged at 309 g for 5 min. The supernatant was removed, and the cells were fixed with 70% ethanol at 4 °C for 2 h. After centrifugation at 309 g for 5 min, the cells were collected, treated with Apoptosis Detection Kit (Beyotime) according to the manufacturer’s protocol, and detected with a flow cytometer (BD, Franklin Lakers, NJ, USA).
Transwell assay
Transwell assay was performed to detect the migration and invasion of SGC7901 cells using transwell chambers with polycarbonate membrane (Corning, NY, USA). For invasion assay, the membrane was coated with Matrigel (BD) beforehand. The 200 μL cell suspension containing 1 × 104 cells was placed in the upper chamber to culture in seum-free medium. Eight hundred μL medium containing 30% FBS was added in the lower chamber to attract the cells. After culturing for 24 h, the polycarbonate membrane were washed with PBS, fixed with 4% paraformaldehyde (Sinopharm, Beijing, China), and stained with 0.5% crystal violet (Amresco, Solon, OH, USA) for 5 min. After washing with water, the cell numbers on the reverse surface of the membrane were counted under an inverted microscope at 200 × magnification.
The migration assay was performed as previous described only without Matrigel. The cell number in the upper chambers was adjusted to 5 × 103 per well.
Immunofluorescence assay
The SGC7901 cells were seeded on the glass slides in 24-well plates to culture to a confluence of 70–80%. The cells were fixed with 4% paraformaldehyde for 15 min, washed with PBS for 5 min thrice, and permeated with 0.1% triton X-100 (Amresco) for 30 min. Then the cells were blocking with goat serum (ZSGB-BIO, Beijing, China) for 15 min, and incubated with rabbit antibody against β-catenin (1:100; Proteintech) at 4 °C overnight. After washing with PBS, the cells were incubated with goat anti-rabbit Cy3-conjugated IgG (1:200; Beyotime) at room temperature for 60 min. After staining with DAPI (Biosharp, Hefei, Anhui, China) for several minutes, the cells were mounted with antifading mounting medium (Solarbio, Beijing, China), and photographed with a fluorescence microscope (Olympus, Tokyo, Japan) at 400x magnification.
Statistical analysis
The data in this study were presented as mean ± SD, in three individual experiments. The data from clinical sample were analyzed with paired t test, and the data from cell experiments were analyzed with one-way or two-way ANOVA test with post hoc Bonferroni’s multiple comparions. The data were analyzed with GraphPad Prism 5.01 software. A p value which <0.05 was seen as statistically significant. (*p < 0.05, **p < 0.01, ***p < 0.001, ns, no significance)
Results
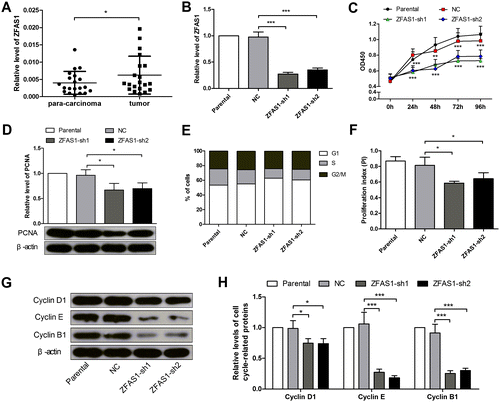
LncRNA ZFAS1 was upregulated in gastric cancer specimens
Twenty pairs of fresh gastric cancer specimens and the corresponding para-carcinoma tissues were collected, and the expression level of lncRNA ZFAS1 was detected by real-time PCR. The data showed that the ZFAS1 was increased 1.55-fold in the gastric cancer specimens, compared with the para-carcinoma tissues (Figure (A)).
Figure 1. Silencing of lncRNA ZFAS1 inhibited the proliferation and cell cycle process in gastric cancer cells. (A) The expression level of ZFAS1 in gastric cancer specimens and paired para-carcinoma tissues was detected by real-time PCR. (B) The expression level of ZFAS1 in SGC7901 cells with or without ZFAS1 knockdown. (C) The viability of SGC7901 cells was detected by CCK-8 assay (the data were compared with NC group). (D) The expression level of PCNA in SGC7901 cells was detected by western blot. (E) The percentage of SGC7901 cells in each phase. (F) The PI of SGC7901 cells (PI=(S + G2/M)/G1). (G, H) The expression levels of Cyclin D1, Cyclin E and Cyclin B1 in SGC7901 cells were detected by western blot. (*p < 0.05, **p < 0.01, ***p < 0.001, ns, no significance; ZFAS1, zinc finger antisense 1; PCNA, proliferating cell nuclear antigen; PI, proliferation index).
Silencing of ZFAS1 inhibited the proliferation and cell cycle process of gastric cancer cells
As the ZFAS1 was increased in the gastric cancer tissues, SGC7901 cells were transfected with ZFAS1 shRNA sequences, and stably silenced cells were screened. The real-time PCR results showed that the expression level of ZFAS1 was decreased by 72% and 64% in the ZFAS1-silenced cell lines, respectively (Figure (B)). Then the CCK-8 assay results showed that the silencing of ZFAS1 reduced the viability of SGC7901 cells significantly (Figure (C)). PCNA, a marker of proliferating cells, was decreased by 30% and 27% in the ZFAS1-silenced cells (Figure (D)). The flow cytometry assay result revealed that the silencing of ZFAS1 inhibited the cell cycle G1/S transition, and suppressed the proliferation index (PI) by 27% and 20% (Figure (E), (F)) (PI=(S + G2/M)/G1). The western blot results showed that several cell cycle-related proteins, Cyclin D1, Cyclin E, and Cyclin B1, were decreased significantly in the ZFAS1-silenced SGC7901 cells (Figure (G), (H)). The above results demonstrated that the silencing of ZFAS1 inhibited the proliferation and cell cycle transition in gastric cancer cells.
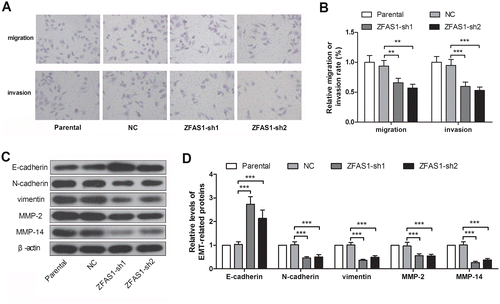
Knockdown of ZFAS1 suppressed the migration, invasion and epithelial-mesenchymal transition (EMT) of gastric cancer cells
Transwell assay was used to detect the migration and invasion of SGC7901 cells. The results showed that the ZFAS1 knockdown decreased the migration and invasion rate by 40 and 44%, respectively, in the SGC7901 cells (Figure (A), (B)). Then several EMT-related proteins we measured. As shown in the Figure , the epithelial marker, E-cadherin, was increased 2.65- and 2.07-fold after silencing of ZFAS1. However, the mesenchymal markers, N-cadherin and vimentin, were decreased by 55 and 64%, respectively, in the ZFAS1-silenced SGC7901 cells (Figure (C), (D)). In addition, the expression of MMP-2 and MMP-14, which play key roles in the cell invasion, were demonstrated with a decreased of 44% or 74%, in ZFAS1-silenced SGC7901 cells (Figure (C), (D)). The data in this section demonstrated that the knockdown of ZFAS1 suppressed the migration, invasion and EMT in gastric cancer cells.
Figure 2. Knockdown of ZFAS1 suppressed the migration, invasion and EMT in gastric cancer cells. (A) The transwell assay images of SGC7901 cells with or without ZFAS1 knockdown. (B) The migration or invasion rate of SGC7901 cells. (C-D) The expression levels of E-cadherin, N-cadherin, vimentin, MMP-2 and MMP-14 in SGC7901 cells were detected by western blot. (*p < 0.05, **p < 0.01, ***p < 0.001, ns, no significance; ZFAS1, zinc finger antisense 1; EMT, epithelial-mesenchymal transition; MMP, matrix metalloproteinase).
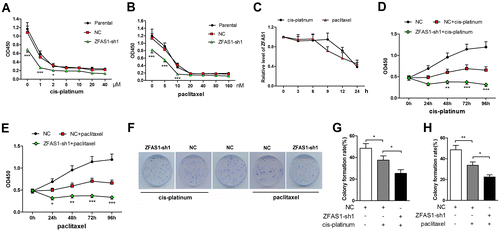
Silencing of ZFAS1 augmented the sensitivity to cis-platinum or paclitaxel in gastric cancer cancer cells
As the chemotherapeutic resistance is a pivotal aspect of cancer cell malignancies, we investigated the effect of ZFAS1 on the sensitivity of SGC7901 cells to cis-platinum or paclitaxel treatment. The SGC7901 cells were treated with cis-platinum or paclitaxel of grading concentrations, and the viability was detected with CCK-8 assay (Figure (A), (B)). Based on the CCK-8 assay data, 0.106 μM and 4.394 nM were determined as the half maximal inhibitory concentration (IC50) of cis-platinum and paclitaxel, and used to treat SGC7901 cells in the subsequent experiments. The ZFAS1 expression level was decreased with the treatment of cis-platinum (0.106 μM) or paclitaxel (4.394 nM) in a time-dependent manner (Figure (C)). The CK-8 assay data showed that the viability of ZFAS1-silenced SGC7901 cells was decreased more significantly than NC cells after treatment of cis-platinum or paclitaxel (Figure (D), (E)), suggesting that ZFAS1 knockdown promoted the cytotoxicity of cis-platinum or paclitaxel on SGC7901 cells. The colony formation assay results showed that the inhibition effect of cis-platinum or paclitaxel treatment on the proliferation of SGC7901 cells was augmented by ZFAS1 silencing (Figure (F)–(H)).
Figure 3. Knockdown of ZFAS1 augmented the drug-induced proliferation suppression in gastric cancer cells. (A-B) The viability of SGC7901 cells treated with grading concentrations of cis-platinum (A) or paclitaxel (B) was detected by CCK-8 assay. (C) The expression level of ZFAS1 in SGC7901 cells treated with cis-pltinum (0.106 μM) or paclitaxel (4.394 μM) was detected by real-time PCR. (D-E) The viability of SGC7901 cells treated with cis-pltinum (0.106 μM) (D) or paclitaxel (4.394 μM) (E) or/and ZFAS1 knockdown was detected with CCK-8 assay (the data were compared with NC + cis-platinum or NC + paclitaxel group). (F) The colony formation images of SGC7901 cells treated with cis-platinum or paclitaxel. (G-H) The colony formation rate of SGC7901 cells with or without cis-platinum (G) or paclitaxel (H) treatment. (*p < 0.05, **p < 0.01, ***p < 0.001, ns, no significance; ZFAS1, zinc finger antisense 1).
Silencing of ZFAS1-induced inhibition of malignancies was reversed by β-catenin
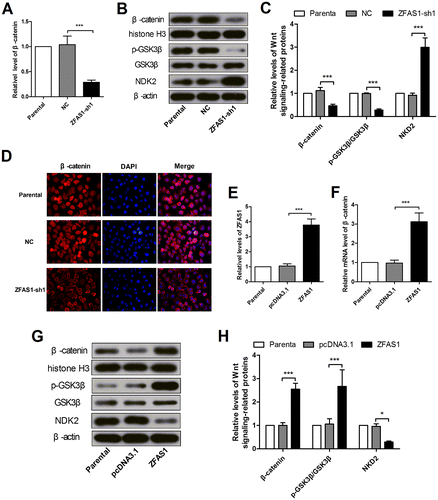
As Wnt signaling plays a key role in cell proliferation and migration, we detected Wnt signaling-related proteins, GSK3β and β-catenin, and a negative regulator of Wnt signaling, NKD2. The real-time PCR, immunofluorescence and immunoblotting results showed that the mRNA level, cellular and nuclear protein levels of β-catenin were all decreased after silencing of ZFAS1 (Figure (A)–(D)). The western blot results revealed that the GSK3β phosphorylation (Ser9) level was declined and the cellular NKD2 level was increased (Figure (B), (C)). These results suggested the inactivation of Wnt signaling. To confirm the effect of ZFAS1 on Wnt signaling, ZFAS1 was overexpressed in SGC7901 cells, and verified by real-time PCR (Figure (E)). The western blot results showed that the nuclear β-catenin level and the GSK3β phosphorylation level were incrased, and the cellular NKD2 level was decreased after ZFAS1 enhanced expression (Figure (G), (H)), which suggested the activation of Wnt signaling. The real-time PCR results also demonstrated the elevation of β-catenin at the transcriptional level (Figure (F)).
Figure 4. Wnt canonical signaling was modulated by ZFAS1 expression changes. (A) The mRNA level of β-catenin in SGC7901 cells with or without ZFAS1 knockdown was detected by real-time PCR. (B-C) The nuclear β-catenin level, cellular p-GSK3β, GSK3β and NKD2 levels in SGC7901 cells with or without ZFAS1 knockdown were detected by western blot. (D) The expression level of β-catenin in SGC7901 cells was detected with immunofluorescence assay. (E) The ZFAS1 level in SGC7901 cells after ZFAS1 overexpression was detected by real-time PCR. (F) The mRNA level of β-catenin in SGC7901 cells after ZFAS1 overexpression. (G-H) The nuclear β-catenin level, cellular p-GSK3β, GSK3β and NKD2 levels in SGC7901 cells with or without ZFAS1 overexpresion were detected by western blot. (*p < 0.05, **p < 0.01, ***p < 0.001, ns, no significance; ZFAS1, zinc finger antisense 1; NKD2, naked cuticle homolog 2; GSK3β, glycogen synthase kinase 3 beta).
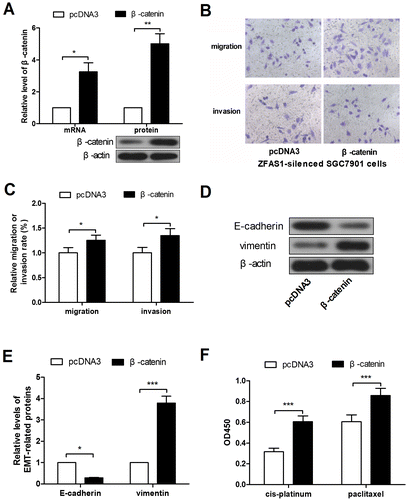
To investigate the role of Wnt signaling in gastric cancer cells, β-catenin overexpression plasmid was constructed, and verified by real-time PCR and western blot (Figure (A)). Then the ZFAS1-silenced SGC7901 cells were transfected with β-catenin overexpression plasmid. Transwell assay results revealed that the forced expression of β-catenin promoted the migration and invasion in ZFAS1-silenced SGC7901 cells (Figure (B), (C)). The western blot results showed that the E-cadherin expression level was decreased and the vimentin expression level was increased after overexpression of β-catenin (Figure (D), (E)), suggesting the enhanced EMT. Finally, CCK-8 assay results showed that the forced expression of β-catenin alleviated the cytotoxicity of cis-platinum and paclitaxel in ZFAS1-silenced SGC7901 cells (Figure (F)).
Figure 5. ZFAS1 silencing-induced malignancy suppression was reversed by β-catenin ectopic overexpression. (A) The levels of β-catenin mRNA and protein in SGC7901 cells after β-catenin overexpression. (B-C) The transwell assay was performed to detect the migration and invasion in ZFAS1-silenced SGC7901 cells after ectopic expression of β-catenin. (D-E) The expression level of E-cadherin and vimentin in ZFAS1-silenced SGC7901 cells after β-catenin overexpression. (F) The viability of ZFAS1-silenced SGC7901 cells with cis-platinum or paclitaxel treatment as well as forced expression of β-catenin was detected by CCK-8 assay. (*p < 0.05, **p < 0.01, ***p < 0.001, ns, no significance; ZFAS1, zinc finger antisense 1).
Discussion
LncRNA ZFAS1 was first identified as a tumor suppressor in human breast cancer [Citation16], but reported with pro-tumor functions in hepatocellular carcinoma and colorectal cancer [Citation17,18]. Recently, it has been reported that ZFAS1 is highly expressed in gastric cancer sepcimens and cell lines, and plays a pro-tumor role. In our study, ZFAS1 was upregulated in gastric cancer specimens comparing with the para-carcinoma tissues, and ZFAS1 knockdown inhibited the proliferation, migarition, invasion and EMT in SGC7901 cells, similar to the reports from Nie et al. and Pan et al. [Citation5,19] In addition, we also found that the silencing of ZFAS1 enhanced the cytotoxity of cis-platinum or paclitaxel on gastric cancer cells, inhibited the resistance to chemotherapeutic reagents. Notably, we observed the decrease of β-catenin and p-GSK3β levels, and the increase of cellular NKD2 in ZFAS1-silenced SGC7901 cells, which suggested the inactivation of canonical Wnt signaling.
Wnt signaling is an important extracellular signaling, involving in cell growth, differentiation, individual development, and diseases. WNTs (the products of WNT genes) can bind to various receptors and trigger different downstream signaling cascades including canonical Wnt/β-catenin signaling pathway, non-canonical Wnt/planar cell polarity, and Wnt/Ca2+ [Citation20]. Among the various signalings, canonical Wnt/β-catenin is highly evolutionary conserved, and β-catenin is a core mediator of canonical Wnt signaling. In absence of Wnt ligands, β-catenin is recruited into a destruction complex that contains GSK3β, adenomatous polyposis coli (APC), casein kinase (CK) 1α and Axin. Then β-catenin is phosphorylated by CK1α and GSK3β, followed by ubiquitylation and proteasomal degradation. Thus, β-catenin is kept at a low level [Citation21]. When Wnt ligand binds to the Frizzled receptors, the Frizzled receptors phosphorylated low-density lipoprotein receptor-related proteins 5/6 (LRP5/6) via interacting with Dishevelled proteins, and the phosphorylated LRP5/6 provides a binding site for GSK3β and Axin, then the destruction complex will be disassembled [Citation22,23]. At this time, β-catenin is accumulated in the cytoplasm, translocates into the nucleus, binds to the T-cell factor (TCF)/lymphoid enhancement factor (LEF), and activates the transcription of downstream genes, such as Jun, c-Myc and Cyclin D1 [Citation24]. NKD2 has been reported to negatively regulate Wnt canonical signaling activity by inhibiting Dishevelled [Citation22].
In our study, the reduced nuclear β-catenin and p-GSK3β levels, and the elevated cellular NKD2 level suggested the Wnt canonical signaling disturbance in the ZFAS1-silenced gastric cancer cells, and the opposite results of β-catenin, p-GSK3β and NKD2 revealed the activation of Wnt canonical signaling. Therefore, we speculated that the ZFAS1 knockdown inhibited the malignancies by blocking the Wnt canonical signaling in gastric cancer cells. To confirm the speculation, β-catenin was overexpressed to simulate the activation of Wnt canonical signaling in the ZFAS1-silenced SGC7901 cells. The data showed that the silencing of ZFAS1-induced the decrease of migration, invasion, EMT and chemotherapeutic tolerance, was reversed by the forced expression of β-catenin.
The canonical Wnt singaling plays a crucial role in regulating proliferation, stem cell maintenance and homeostasis in normal gastric mucosa [Citation25–27]. However, the dysregulation of the Wnt signaling has been known associated with gastric carcinogenesis, wherein β-catenin plays a crucial role. Investigations on gastric cancer specimens revealed that 20–30% gastric cancers presented nuclear β-catenin accumulation [Citation28,29]. The high expression of Wnt family members were detected in gastric cancer [Citation30]. In addition, the inhibition of Wnt/β-catenin suppresses the EMT in gastric cancer cells [Citation31].
It has been reported that ZFAS1 simultaneously recruits transcriptional factors, enhancer of zeste homolog 2 (EZH2) and LSD1, to bind to kruppel like factor 2 (KLF2) and NKD2 promoter regions, and inhibits their transcription. The ZFAS1 knockdown could enhance the expression of KLF2 and NKD2 in gastric cancer cells [Citation19]. NKD2 is a mammalian ortholog of drosophila naked cuticle, having frequent loss of heterozygosity in colorectal and gastric cancer [Citation32,33]. Since NKD2 is also an inhibitor of Wnt/β-catenin signaling, we speculated that the ZFAS1 knockdown suppressed the Wnt/β-catenin signaling by promoting the NKD2 expression, and then inhibited the malignancies in gastric cancer cells.
In summary, our data demonstrated that theZFAS1 is upregulated in gastric cancer specimens, and the silencing of ZFAS1 inhibited the proliferation, cell cycle progress, migration, invasion, EMT and resistance to chemotherapeutic reagents by blocking the canonical Wnt/β-catenin signaling in gastric cancer cells. These findings suggested that ZFAS1 may be considered as a diagnosis marker or a clinical therapy of gastric cancer.
Author contribution
Fan Zhang and Hong Xu conceived the ideas, coordinated the overall project, and revised the manuscript. Weiran Xu and Liang He participated in the design of the study, conducted most of the experiments, analyzed the results, and drafted the manuscript. Ying Li and Yan Tan provided help for the collection of clinical specimens. All the authors reviewed the results and approved the final version of the manuscript.
Disclosure statement
The authors declare that there is no conflict of interest.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
- Richman DM, Tirumani SH, Hornick JL, et al. Beyond gastric adenocarcinoma: multimodality assessment of common and uncommon gastric neoplasms. Abdom Radiol (NY). 2017;42(1):124–140.
- Riquelme I, Ili C, Roa JC, et al. Long non-coding RNAs in gastric cancer: mechanisms and potential applications. Oncotarget. 2016.
- Pan L, Liang W, Fu M, et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143(6):991–1004.
- Lee HS, Cho SB, Lee HE, et al. Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res. 2007;13(14):4154–4163.
- Nadauld LD, Ford JM. Molecular profiling of gastric cancer: toward personalized cancer medicine. J Clin Oncol. 2013;31(7):838–839.
- Yin Y, Li J, Chen S, et al. MicroRNAs as diagnostic biomarkers in gastric cancer. Int J Mol Sci. 2012;13(10):12544–12555.
- Li T, Mo X, Fu L, et al. Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget. 2016;7(8):8601–8612.
- Brannan CI, Dees EC, Ingram RS, et al. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10(1):28–36.
- Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641.
- Zhang M, Du X. Noncoding RNAs in gastric cancer: research progress and prospects. World J Gastroenterol. 2016;22(29):6610–6618.
- Chen WM, Huang MD, Sun DP, et al. Long intergenic non-coding RNA 00152 promotes tumor cell cycle progression by binding to EZH2 and repressing p15 and p21 in gastric cancer. Oncotarget. 2016;7(9):9773–9787.
- Chen D, Liu L, Wang K, et al. The role of MALAT-1 in the invasion and metastasis of gastric cancer. Scand J Gastroenterol. 2017;52(6–7):790–796.
- Zhang XW, Bu P, Liu L, et al. Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem Biophys Res Commun. 2015;462(3):227–232.
- Askarian-Amiri ME, Crawford J, French JD, et al. SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA. 2011;17(5):878–891.
- Li T, Xie J, Shen C, et al. Amplification of long noncoding RNA ZFAS1 promotes metastasis in hepatocellular carcinoma. Cancer Res. 2015;75(15):3181–3191.
- Thorenoor N, Faltejskova-Vychytilova P, Hombach S, et al. Long non-coding RNA ZFAS1 interacts with CDK1 and is involved in p53-dependent cell cycle control and apoptosis in colorectal cancer. Oncotarget. 2016;7(1):622–637.
- Nie F, Yu X, Huang M, et al. Long noncoding RNA ZFAS1 promotes gastric cancer cells proliferation by epigenetically repressing KLF2 and NKD2 expression. Oncotarget. 2017;8(24):38227–38238.
- Fujimura N. WNT/beta-Catenin Signaling in Vertebrate Eye Development. Front Cell Dev Biol. 2016;4(138).
- Pai SG, Carneiro BA, Mota JM, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):101.
- Zeng X, Huang H, Tamai K, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135(2):367–375.
- Tamai K, Zeng X, Liu C, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13(1):149–156.
- Shang S, Hua F, Hu ZW. The regulation of beta-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017;8(20):33972–33989.
- Sato N, Meijer L, Skaltsounis L, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10(1):55–63.
- Byun T, Karimi M, Marsh JL, et al. Expression of secreted Wnt antagonists in gastrointestinal tissues: potential role in stem cell homeostasis. J Clin Pathol. 2005;58(5):515–519.
- Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25–36.
- Woo DK, Kim HS, Lee HS, et al. Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer. 2001;95(2):108–113.
- Clements WM, Wang J, Sarnaik A, et al. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62(12):3503–3506.
- Chiurillo MA. Role of the Wnt/beta-catenin pathway in gastric cancer: an in-depth literature review. World J Exp Med. 2015;5(2):84–102.
- Song Y, Li ZX, Liu X, et al. The Wnt/beta-catenin and PI3K/Akt signaling pathways promote EMT in gastric cancer by epigenetic regulation via H3 lysine 27 acetylation. Tumour Biol. 2017;39(7):1010428317712617.
- Lu Y, Yu Y, Zhu Z, et al. Identification of a new target region by loss of heterozygosity at 5p15.33 in sporadic gastric carcinomas: genotype and phenotype related. Cancer Lett. 2005;224(2):329–337.
- Xu SF, Peng ZH, Li DP, et al. Refinement of heterozygosity loss on chromosome 5p15 in sporadic colorectal cancer. World J Gastroenterol. 2003;9(8):1713–1718.
