ABSTRACT
Oxytocin is produced by neurons in the paraventricular nucleus (PVN) and the supraoptic nucleus in the hypothalamus. Various ion channels are considered to regulate the excitability of oxytocin neurons and its secretion. A-type currents of voltage-gated potassium channels (Kv channels), generated by Kv4.2/4.3 channels, are known to be involved in the regulation of neuron excitability. However, it is unclear whether the Kv4.2/4.3 channels participate in the regulation of excitability in PVN oxytocin neurons. Here, we investigated the contribution of the Kv4.2/4.3 channels to PVN oxytocin neuron excitability. By using transgenic rat brain slices with the oxytocin-monomeric red fluorescent protein 1 fusion transgene, we examined the excitability of oxytocin neurons by electrophysiological technique. In some oxytocin neurons, the application of Kv4.2/4.3 channel blocker increased firing frequency and membrane potential with extended action potential half-width. Our present study indicates the contribution of Kv4.2/4.3 channels to PVN oxytocin neuron excitability regulation.
Abbreviation: PVN, paraventricular nucleus; Oxt-mRFP1, Oxt-monometric red fluorescent protein 1; PaTx-1, Phrixotoxin-1; TEA, Tetraethylammonium Chloride; TTX, tetrodotoxin; aCSF, artificial cerebrospinal fluid;PBS, phosphate buffered saline 3v, third ventricle.
GRAPHICAL ABSTRACT
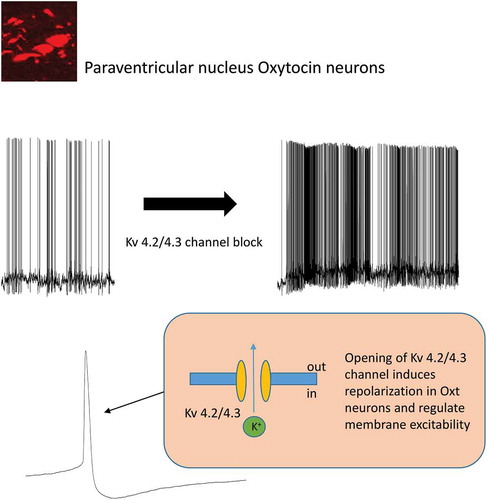
The opening of Kv 4.3/4.2 channels may induce repolarization of PVN oxytosin neurons and regulate membrane excitability.
Since the discovery of oxytocin in 1906, its effects on peripheral organs, such as uterine contractile activity during labor and the promotion of milk ejection, have been intensively studied [Citation1]. However, recent studies have reported additional contribution of oxytocin to physiological functions in the central nervous system, such as promoting trust, as well as therapeutic effects for social anxiety and autism [Citation2,Citation3]. Previously, we reported the contribution of oxytocin in the central nervous system to food intake and body weight regulation [Citation4–Citation6]. Moreover, oxytocin has also recently been reported to be a therapeutic target for diseases such as obesity and metabolic syndrome [Citation7,Citation8]. Therefore, elucidation of the mechanism that regulates oxytocin neuron excitability is necessary in order to understand the physiological functions of oxytocin.
Oxytocin is a peptide consisting of nine amino acids, and it is produced by neurons in the paraventricular nucleus (PVN) and the supraoptic nucleus of the hypothalamus[Citation1]. PVN neurons produce oxytocin either in magnocellular neurons or parvocellular neurons. In the PVN region, most of the magnocellular neurons are considered to secrete oxytocin to bloodstreams through the pituitary, whereas oxytocin produced in the parvocellular neurons is considered to exert its actions through neuronal projections throughout the brain [Citation1].
Similar to other neurotransmitters, oxytocin is considered to be secreted by the intracellular Ca2+ influx following membrane depolarization, which ultimately induces the exocytosis of oxytocin-containing vesicles [Citation9]. Therefore, various ion channels that control membrane potential are considered to participate in regulating the excitability of oxytocin neurons and oxytocin secretion. However, detail regulatory mechanisms of excitability and secretion remain unclear.
voltage gated potassium channel (Kv channels) are ion channels that contribute to the regulation of neuron excitability. In excitable cells such as neurons, pancreatic beta cells and cardiac muscle cells, Kv channels are known to regulate resting membrane potential and repolarization after membrane depolarization [Citation10].
A previous study reported the presence of A-type currents in oxytocin neurons of the hypothalamus [Citation11]. A-type currents are mainly generated by Kv4.1, Kv4.2 and Kv4.3 channels [Citation12]. Especially, Kv4.2 and Kv4.3 channels mRNA are known to be expressed in almost all regions of the mammalian brain that include PVN oxytocin neurons [Citation12,Citation13]. Also, it has been reported that the inhibition of Kv channels enhances oxytocin secretion from neurohypophysial neurons [Citation14,Citation15].
Therefore, Kv4.2 and Kv4.3 channels are candidates for the regulation of PVN oxytocin neuron excitability. However, to date, the contribution of these channels to PVN oxytocin neuron excitability remains unclear. In the present study, we focused on elucidating the contribution of Kv4.2 and Kv4.3 channels to the excitability of PVN oxytocin neurons. Using the brain slice patch-clamp technique, we compared the responsiveness of PVN oxytocin neurons to a Kv4.2 and Kv4.3 channel blocker with factors such as firing frequency, membrane potential and action potential half-width, and observed the co-expression of oxytocin and Kv4.3 channels in the PVN region by immunohistochemistry.
Materials and methods
Animal
Male Wistar rats (200–300 g), purchased from SLC (Shizuoka, Japan), and male and female transgenic rats bearing an Oxt-monometric red fluorescent protein 1 (Oxt-mRFP1) fusion transgene (200–650 g) [Citation16], kindly provided by Professor Yoichi Ueta, were used in the present study. The animals were housed in a 12 h light/dark cycle (0700–1900) with conventional food (CE-2; CLEA, Tokyo, Japan) and water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of Fukushima Medical University.
Drugs
Phrixotoxin-1 (PaTx-1), a Kv4.2 and Kv4.3 channel blocker, was obtained from Alomone Labs (Jerusalem, Israel). Tetraethylammonium Chloride (TEA) and tetrodotoxin (TTX) were obtained from FUJIFILM Wako (Osaka, Japan). All reagents (PaTx-1: 100 nM, TEA: 30 mM, TTX: 300 nM) were dissolved in artificial cerebrospinal fluid (aCSF). The PaTx-1 concentration used in this study (100 nM) was based on a previous report [Citation17].
Electrophysiology
Under anesthesia, the Oxt-mRFP1 rats were transcardially perfused with an ice-cold solution containing (in mM) 230 sucrose, 2 KCl, 1 KH2PO4, 0.5 CaCl2, 1 MgCl2, 26 NaHCO3, and 10 D-glucose, and whole brains were isolated. Coronal brain slices (250 μm) were prepared in an ice-cold solution using a vibrating microtome (Campden Instruments, Leics., UK). The slices were recovered at room temperature for 1 h or more in aCSF containing (in mM) 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, 21.4 NaHCO3, and 10 D-glucose, with a gas mixture of 95% O2 and 5% CO2. The brain slices were transferred to a recording chamber and perfused continuously at 2–4 mL/min with gassed aCSF. Whole-cell recordings were performed using an EPC-800 patch clamp amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) filtering at 1 kHz. The data were digitized with an analog-to-digital converter (Molecular Devices, CA, USA) and stored on a computer using Clampex 10.5 software (Molecular Devices). Patch electrodes (4–8 MΩ) were filled with an internal solution containing (in mM) 120 K-gluconate, 10 KCl, 10 HEPES, 5 EGTA, 0.3 CaCl2, 1 MgCl2, 2 Mg-ATP, and 1 Na-GTP at pH 7.3 adjusted with KOH. Whole-cell patch recordings were performed in a current-clamp mode with zero holding current. The electrophysiological experiments were performed at room temperature (22–25ºC), and drugs were applied to each brain slice via bath perfusion.
Membrane potentials of firing neurons were determined from slow time-scale recordings with a clean baseline as described previously [Citation18]. Firing frequency was measured and compared in 1 min before and after the application of PaTx-1. Action potential half-width was defined as the duration of the action potential halfway between the resting membrane potential and the peak potential. Action potential half-width was measured and compared in 1 min before and after the application of PaTx-1. Currents of neurons were measured with voltage-clamp recording. Once whole-cell configuration is acquired, all records in a voltage-clamp mode were initiated after 3 min stabilization period. The effects of each drug on the A-type currents were investigated more than 3 min after applying each drug. All data were analyzed using clampfit software 10.5 (Molecular Devices).
Measurement of oxytocin release from PVN slices
The PVN area located at −0.9 to −2.0 mm from the bregma was dissected, from which three 400 μm thick slices ware prepared using a vibrating microtome in a buffer composed of (in mM) 229 mannitol, 3 KCl, 26 NaHCO3, 1 H3PO4, 0.5 CaCl2, 7MgCl2, 7 glucose, and 1 kynurenate at pH 7.4 with 95% O2 and 5% CO2 mixed gas. Six slices from two Wistar rats composed one sample. The prepared PVN slices were incubated at 37ºC for 1 h in aCSF composed of (in mM) 126 NaCl, 3 KCl, 26 NaHCO3, 1 H3PO4, 2 CaCl2, and 1 MgSO4 at pH 7.4 with 95% O2 and 5% CO2 mixed gas with or without PaTx-1 (100 nM). Oxytocin in the supernatant was determined using an oxytocin ELISA kit (Enzo Life Science)
Immunohistochemistry
The Wistar rats were transcardially perfused with 4% paraformaldehyde containing 0.2% picric acid, and their brains were removed. Brain sections were then cut at a thickness of 40 μm using a freezing microtome. Sections at 160 μm intervals between −0.9 and −2.0 mm from the bregma (PVN containing sections) were washed with 0.01 M phosphate buffered saline (PBS: pH 7.4) and incubated with a blocking solution (2% bovine serum albumin, 5% normal equine serum, 0.3% Triton-X) for 1 h. After blocking, the sections were incubated with mouse anti-oxytocin antibody (1:1000, Millipore MAB5296, CA) and rabbit anti-Kv4.3 antibody (1:500, Alomone Labs) overnight at 4ºC. The sections were then rinsed with PBS, and incubated with DyLight 594-labeled goat anti-mouse IgG (1:400, Life Technologies, CA, USA) and Alexa Fluor 488-labeled anti-rabbit IgG (1:400, Life Technologies). After rinsing with PBS, the sections were then covered with a mounting medium (Dako, CA, USA). Fluorescence images were acquired by using a confocal-laser scanning microscope (Fluoview FV10i; Olympus, Tokyo, Japan). The numbers of oxytocin-positive, Kv4.3 channel-positive and double-positive neurons in each section were counted and averaged from four to five sections in each rat brain. These data were collected individually from three rats.
In order to examine the specificity of the antibody for the Kv4.3 channels, an absorption test was performed. Rabbit anti-Kv4.3 antibody was pre-incubated with Kv4.3 peptide (control antigen, Alomone Labs) overnight (Molecular ratio, antibody: Kv4.3 peptide = 1:20). The staining procedure was the same as that described above. The absorption test revealed that immunoreactivity was completely abolished from all sections, which were incubated with a pre-absorbed antibody with Kv4.3.
Statistical analysis
The statistical significance of firing frequency, membrane potential and action potential half-width between before and after drug applications in the electrophysiological investigation was assessed using a paired t-test. Oxytocin release experiment was assessed using a student’s t-test. All data were expressed as mean ± SEM.
Results
The excitability of some PVN oxytocin neurons is regulated by Kv4.2 and Kv4.3 channels
We first investigated the contribution of Kv4.2 and Kv4.3 channels to the excitability of PVN oxytocin neurons using a Kv4.2 and Kv4.3 channel blocker PaTx-1. Oxt-mRFP1 rats were used in order to identify PVN oxytocin neurons for electrophysiological studies. Because Kv4.2 and Kv4.3 channels are known to contribute to the repolarization of the action potential, PVN oxytocin neurons with slight firing before drug application were investigated in the current study.
) shows a representative membrane potential of the neuron that reacted to PaTx-1. We performed further analysis to investigate the characteristics of PaTx-1 on PVN oxytocin neurons. The ratemeter recording indicates the increase of number of spikes after PaTx-1 application with gradual time-dependent reduction ()). This result indicates that PVN oxytocin neurons showed a possible frequency adaptation to PaTx-1. The interspike interval histogram of less than 1.0 sec of the representative membrane potential shows that PaTx-1 shifted the peak of the histogram to the left compared to the control, which indicates that interspike interval was shortened by the application of PaTx-1 ()). Therefore, when analyzing the effects of PaTx-1, we used the data for 1 min after PaTx-1 application in order to eliminate the effect of the frequency adaptation in current-clamp mode experiments.
Figure 1. The effects of PaTx-1 (100 nM), a selective Kv4.2 and Kv4.3 channel blocker, on firing frequency and membrane potential of PVN oxytocin neurons.
(a) A representative recording of membrane potential from the neuron reacted to PaTx-1. The black bar indicates the duration of PaTx-1 application. (b) A ratemeter for the effect of PaTx-1. The black bar indicates the PaTx-1 application. (c) An interspike interval histograms for control (white bar) and under PaTx-1 (black). (d, e) Changes in firing frequency (d) and membrane potential (e) of all investigated neurons (n = 19) before and after PaTx-1 application. (f) A Scatter plot of the change in firing frequency and membrane potential in all recorded neurons (n = 19). The dotted line indicates 1 Hz. (g, i) The effects of PaTx-1 on firing frequency and membrane potential of PVN oxytocin neurons in the activated group (n = 6, paired t-test, **p < 0.01, ***p < 0.001). (h, j) The effects of PaTx-1 on firing frequency and membrane potential of PVN oxytocin neurons in the non-activated group (n = 13, paired t-test). Data are presented as means ± SEM. PaTx-1: phrixotoxin-1, PVN: paraventricular nucleus.
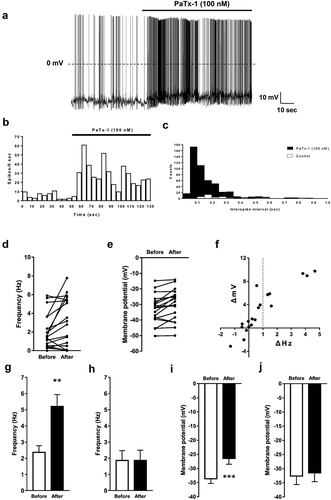
) show the change of firing frequency ()) and membrane potential ()) of all investigated neurons (n = 19) before and after PaTx-1 application, respectively. These results indicate that there were cells with and without changes in firing frequency and membrane potential upon applying PaTx-1. The scatter diagram of each neuron shows the change in membrane potential and firing frequency before and after the application of PaTx-1 ()). These results indicate the tendency that oxytocin neurons are more likely to react to PaTx-1 when they are more depolarized with action potential firings.
Because of the obvious difference in the reaction of PVN oxytocin neurons to PaTx-1, we analyzed the neurons that responded to PaTx-1 separately from those which did not respond. Since it is reported that oxytocin secretion from oxytocin neurons depended on stimulus frequency [Citation14], we mainly focused on the change of firing frequency. Thus, we classified oxytocin neurons that showed an increase of firing frequency of more than 1 Hz as “activated” and all others as “non-activated” (). This criterion is based on a report showing that a non-selective Kv channel blocker 4-AP, which can also inhibit Kv4.2 and Kv4.3 channels, increased a firing frequency of PVN oxytocin neurons by 1 Hz [Citation19]. We then compared the effects of PaTx-1 (). Approximately 31.58% (n = 6/19) of neurons were in the activated group and 68.42% (n = 13/19) were in the non-activated group. Firing frequency and membrane potential were significantly increased by the application of PaTx-1 in the activated group (); n = 6). However, there was no significant difference in the non-activated group (); n = 13). Therefore, it is suggested that in the activated group the excitability of PVN oxytocin neurons was increased by the inhibition of Kv4.2 and Kv4.3 channels.
The involvement of Kv4.2 and Kv4.3 channels in the action potential half-width of PVN oxytocin neurons
The change of action potential half-width in neurons is one of the indexes of the influence of Kv4.2 and Kv4.3 channels on the A-type currents and repolarization of the action potential [Citation20]. Therefore, we compared the change of action potential duration by measuring action potential half-width before and after the application of PaTx-1 in both the activated and non-activated groups.
) show a representative action potential before and after PaTx-1 application in the activated and non-activated groups, respectively. The action potential half-width in the activated group was significantly extended by the PaTx-1 application (); n = 6). However, no significant difference was observed in the non-activated group (); n = 9).
Figure 2. The effects of PaTx-1 (100 nM), a selective Kv4.2 and Kv4.3 channel blocker, on action potential half-width in PVN oxytocin neurons.
(a, b) Representative membrane potential recordings from PVN oxytocin neurons before (left) and after (right) PaTx-1application in the activated (a) and non-activated (b) groups. Black bars indicate the expanded time scale area for the enlarged action potential shown in the inset. The bar in the inset shows the half-width of action potential used for the analysis. (c) The effects of PaTx-1 on half-width of action potential of PVN oxytocin neurons in the activated group (n = 6, paired t-test, **p < 0.01). (d) The effects of PaTx-1 on the action potential half-width of PVN oxytocin neurons in the non-activated group (n = 9, paired t-test). Data are presented as means ± SEM. PaTx-1: phrixotoxin-1, PVN: paraventricular nucleus.
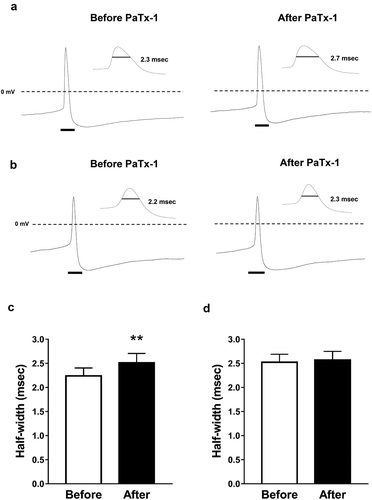
Since PaTx-1 extended the half-width of the action potential in PVN oxytocin neurons, Kv4.2 and Kv4.3 channels may be involved in the action potential repolarization dependently on A-type currents of PVN oxytocin neurons in the activated group.
The existence of A-type currents in PVN oxytocin neurons
Since it is suggested that the generation of A-type currents, mainly regulated by Kv4.2 and Kv4.3 channels, were involved in action potential repolarization of PVN oxytocin neurons in the activated group, we measured the A-type currents in PVN oxytocin neurons.
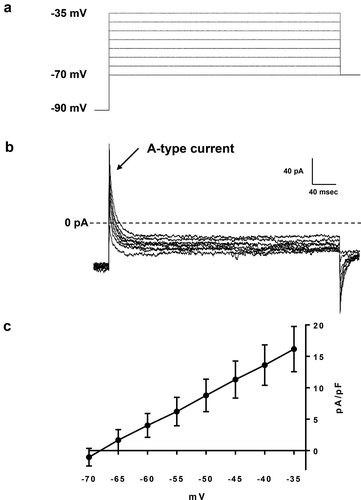
For the measurement of A-type currents, the cells were held at −90 mV and the currents were evoked by 400 ms voltage depolarization to voltage values between −70 mV to −35 mV in 5 mV increments under presence 30 mM TEA ()). ) shows a representative A-type current of a PVN oxytocin neuron. The character of A-type currents in PVN oxytocin neurons was shown in the current-voltage relationship curve (); n = 8). These results demonstrated that A-type currents are indeed positive in PVN oxytocin neurons. As shown in the supplementary figure, PaTx-1 inhibited A-type currents in a PVN oxytocin neuron. Therefore, it is suggested that the A-type current that is regulated by Kv4.2 and Kv4.3 channels can be existed in PVN oxytocin neurons and also can be inhibited by application of PaTx-1.
Figure 3. Measurements of A-type currents of PVN oxytocin neurons.
(a) A voltage protocol for measurement of A-type currents of PVN oxytocin neurons. Recorded cells were held at −90 mV and the currents were evoked by 400 ms voltage depolarization to voltage values between −70 to −35 mV in 5 mV increments under presence 30 mM TEA (b) A representative A-type current of PVN oxytocin neurons. (c) A current-voltage relationship curve of A-type currents of PVN oxytocin neurons (n = 8).
The contribution of Kv4.2 and Kv4.3 channels to oxytocin release from PVN neurons
Oxytocin release was reported to be regulated by stimulus frequency [Citation14]. In the present results (), the firing frequency of some PVN oxytocin neurons was increased by PaTx-1. These results suggest that the inhibition of Kv4.2 and Kv4.3 channels by PaTx-1 may increase the oxytocin release from PVN neurons. Therefore, we investigated whether the PaTx-1 application can increase oxytocin release from brain slices including the PVN region by ex vivo batch incubation experiment. However, no significant increase of oxytocin release was shown upon PaTx-1 application (); n = 4 each).
Figure 4. Measurements of secreted oxytocin from brain slices including the PVN region and expression of oxytocin and Kv4.3 in the PVN region.
(a) The effect of PaTx-1 (100 nM) on oxytocin release from brain slices including the PVN region (n = 4 each, student’s t-test). (b–d) Confocal images of double immunofluorescence for oxytocin (red: b), Kv4.3 (green: c) and merged images (d). Scale bars = 50 μm. Enlarged image of the dotted square is shown in the lower right corners of b–d. The arrowheads in each image indicate the co-localized neurons, oxytocin and Kv4.3. Scale bars = 10 μm. PaTx-1: phrixotoxin-1, PVN: paraventricular nucleus, 3v: third ventricle.
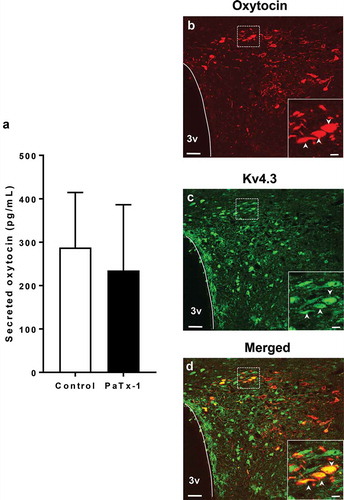
Based on the results () and oxytocin release experiment, it is possible to consider that Kv4.2 or Kv4.3 channels are expressed only in part of PVN oxytocin neurons. Referring to the previous report that Kv4.3 channel mRNA is mainly expressed in PVN oxytocin neurons [Citation13], we investigated the co-localization of Kv4.3 channels and oxytocin in PVN neurons by using immunohistochemistry. Oxytocin and the Kv4.3 channels were apparently co-expressed in the same neurons in PVN ()). The Kv4.3 channels were found to be expressed in 21.47 ± 4.22% of oxytocin neurons investigated (n = 3 rats).
Discussion
We investigated the effects of a selective Kv4.2 and Kv4.3 channel blocker PaTx-1 on excitability regulation of oxytocin neurons for the first time. We found that the excitability of some PVN oxytocin neurons was regulated by repolarization dependently on A-type currents of Kv4.2 and K.4.3 channels. However, oxytocin release from PVN oxytocin neurons was not increased by the inhibition of Kv4.2 and Kv4.3 channels using its blocker PaTx-1.
Kv channels are generally known to regulate membrane potential in neurons and other excitable cells. In pancreatic beta-cells, high glucose levels induce membrane depolarization and action potential firing via inhibition of the KATP channels, which ultimately leads to insulin secretion [Citation21]. This rise in beta-cell membrane potential induces the activation of Kv2.1 channels in the beta-cell membrane, which leads to membrane repolarization and acts as a brake for insulin secretion [Citation22]. Knockout or inhibition of the Kv2.1 channels is reported to increase insulin secretion [Citation23,Citation24]. Therefore, Kv channel activation regulates membrane potential and cell excitability by inducing membrane repolarization. Kv4.2 and Kv4.3 channels are also known to be important for the regulation of neuron excitability by generating A-type currents [Citation25]. It is reported in the past that the half-width of action potential of PVN neurons was extended by application of a Kv channel blocker 4-AP [Citation19]. Our present data showed that a Kv4.2 and Kv4.3 channel blocker PaTx-1 can also extend the half-width of action potential in PVN oxytocin neurons. Our present study also suggests that the A-type currents of Kv4.2 and Kv4.3 channels may regulate the excitability of some PVN oxytocin neurons by affecting the duration from depolarization to repolarization.
In the current study, we showed that the excitability in some PVN oxytocin neurons is regulated by Kv4.2 and Kv4.3 channels, and that Kv4.3 channels and oxytocin are partly co-expressed in the PVN region. We also found that not all oxytocin neurons in PVN react to the Kv4.2 and Kv4.3 channel blocker PaTx-1. The percentage of oxytocin neurons, which reacted to PaTx-1 (31.58%), was approximately corresponding to the percentage of Kv4.3-expressing PVN oxytocin neurons (21.47%). A previous report also showed that Kv4.2 or Kv4.3 mRNA is expressed in some PVN oxytocin neurons [Citation13]. Our electrophysiological and immunohistochemistrical results can support the previous report. On the other hand, the role of neurons with Kv4.2 and Kv4.3 channels but negative with oxytocin was not investigated in this study. A previous report shows that PVN neurons with Kv4.2 or Kv4.3 channels own mRNA of vasopressin, corticotropin-releasing hormone or thyrotropin-releasing hormone [Citation13]. Therefore, it is suggested that Kv4.2 and Kv4.3 channels in oxytocin negative neurons may regulate the excitability of the neurons including these peptides. Further study is required to elucidate the function of Kv4.2 and Kv4.3 channels in these neurons.
Our results show that the duration of repolarization (half-width of action potential) was extended and the firing frequency was increased by PaTx-1 in the activated group. These are consistent with a report by Carrasquillo et al. showing that the knockout of Kv4.2 or Kv4.3 channels in cortical pyramidal neurons induced the extending action potential half-width and the increased firing frequency caused by current stimuli [Citation20]. Also, the reduction of A-type current density is reported to increase firing frequency in neurons [Citation26]. Therefore, it can be considered that the increasing of membrane potential and firing frequency of the oxytocin neurons in the activated group was caused by inhibiting repolarization dependent on A-type currents induced by the blockade of the Kv4.2 and Kv4.3 channels. In other words, Kv4.2 and Kv4.3 channels may have an ability to return action potential to resting potential in PVN oxytocin neurons.
Because the oxytocin secretion is related to the increase of firing frequency [Citation27], it may be possible that oxytocin secretion from the neuronal terminal is increased following a membrane potential depolarization. We expected that the Kv4.2 and Kv4.3 channels may be involved in the oxytocin secretion dependent on a rise of firing frequency by Kv4.2 and Kv4.3 channels blocking in PVN oxytocin neurons of the activated group. However, oxytocin release was not increased by the PaTx-1 application to brain slices including the PVN region. It is reported that the electrically evoked oxytocin release was further increased by the 4-AP application on pituitary stalk [Citation15]. Based on this report and our result, we consider that this may be because inhibition of Kv4.2 and Kv4.3 channels are only involved in maintaining the excitability of the oxytocin neurons after its activation is triggered.
In general, various ion channels expressed in a neuron are interacting with each other. Therefore, blockade of Kv4.2 and Kv4.3 channels which leads to inhibition of A-type currents, also may affect other ion channels. It is known that inhibition of A-type currents could induce an increased Ca2+intracellular influx per spike [Citation28,Citation29], and activate various Ca2+-dependent mechanisms such as Ca2+-dependent K+ channels [Citation30]. Furthermore, in PVN neurons, it is reported that Ca2+blocker application can lower the effect of a Kv channel blocker 4-AP [Citation19], indicating the close interaction between Kv channels and Ca2+channels. Similar to these studies, properties of various ion channels could be affected by the blockade of Kv4.2 and Kv4.3 channels in this study. Investigation on effects of inhibiting A-type currents on various ion channels including Ca2+channels is required in the future.
In the present study, we investigated the possible contribution of the Kv4.2 and Kv4.3 channels to the excitability and oxytocin release of PVN oxytocin neurons. We found that the inhibition of these channels further activates PVN oxytocin neurons by suppressing the repolarization process of the action potential. This suggests that the Kv4.2 and Kv4.3 channels are involved in the regulation of PVN oxytocin neuron excitability. However, no increase of oxytocin release from PVN oxytocin neurons was found in this study. Therefore, we speculate that the inhibition of Kv4.2 and Kv4.3 contributes to making oxytocin neuron more feasible to the activated state by preventing repolarization once activated by another triggering factor and has less contribution to trigger the activation. Our findings may provide implications for understanding the physiological regulatory mechanisms of the excitability of PVN oxytocin neurons and oxytocin secretion. So far, there is no report showing Kv4.2 and Kv4.3 channels of PVN oxytocin neurons being increased in some particular physiological condition. However, our present study suggests the possibility that inhibition of Kv4.2 and Kv4.3 channels make PVN oxytocin neurons more prone to activated state once it is activated. Because oxytocin is involved in various physiological functions, and is also known as a potential therapeutic target for diseases such as autism, obesity and metabolic syndrome, Kv4.2 and Kv4.3 channel inhibitors may contribute to supporting therapeutic strategies in these diseases. The present data provide important insights to understand the mechanism of oxytocin neuron excitability, which may contribute to its clinical effect.
Author contributions
Kenju Shimomura contributed to the overall supervision of this study. Kenju Shimomura and Yuko Maejima designed the study protocols. Yoichi Ueta provided transgenic animals and important suggestions for the study. Kenju Shimomura, Yuko Maejima and Ryota Imai conducted experiments and collected data with supported by Shoko Yokota and Shoichiro Horita. Ryota Imai and Yuko Maejima performed the statistical analysis. Ryota Imai drafted the initial manuscript. Kenji Shimomura and Yuko Maejima critically reviewed and provided the manuscript for important intellectual information. All authors read and approved the final manuscript.
Supplementary_figure_final.pptx
Download MS Power Point (153.4 KB)Acknowledgments
The authors are grateful to editors and reviewers for their helpful comments on earlier versions of the manuscript.
Disclosure statement
Ryota Imai is employees of Tsumura & Co.
Additional information
Funding
References
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001 Apr;81:629–683.
- Kosfeld M, Heinrichs M, Zak PJ, et al. Oxytocin increases trust in humans. Nature. 2005 Jun;435:673–676.
- Jones C, Barrera I, Brothers S, et al. Oxytocin and social functioning. Dialogues Clin Neurosci. 2017 Jun;19:193–201.
- Maejima Y, Sakuma K, Santoso P, et al. Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS Lett. 2014 Nov;588:4404–4412.
- Maejima Y, Rita RS, Santoso P, et al. Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limited brain areas. Neuroendocrinology. 2015 Jan;101:35–44.
- Maejima Y, Iwasaki Y, Yamahara Y, et al. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY). 2011 Dec;3:1169–1177.
- Lawson EA, Marengi DA, Desanti RL, et al. Schoenfeld DA and Tolley CJ. Oxytocin reduces caloric intake in men. Obesity (Silver Spring). 2015 May;23:950–956.
- Zhang H, Wu C, Chen Q, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One. 2013 May;8:e61477.
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006 Feb;7:126–136.
- Rudy B, Maffie J, Amarillo Y, et al. Voltage gated potassium channels: structure and function of Kv1 to Kv9 Subfamilies. In: Editor Larry R, editor. Squire editor. encyclopedia of neuroscience. Massachusetts: Academic Press; 2009. p. 397–425.
- Fisher TE, Voisin DL, Bourque CW. Density of transient K+ current influences excitability in acutely isolated vasopressin and oxytocin neurones of rat hypothalamus. J Physiol. 1998 Sep;511:423–432.
- Serôdio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998 Feb;79:1081–1091.
- Lee SK, Lee S, Shin SY, et al. Single cell analysis of voltage-gated potassium channels that determines neuronal types of rat hypothalamic paraventricular nucleus neurons. Neuroscience. 2012 Mar;205:49–62.
- Bondy CA, Gainer H, Russell JT. Effects of stimulus frequency and potassium channel blockade on the secretion of vasopressin and oxytocin from the neurohypophysis. Neuroendocrinology. 1987 Sep;46:258–267.
- Racké K, Altes U, Baur AM, et al. Differential effects of potassium channel blockers on neurohypophysial release of oxytocin and vasopressin. Evidence for frequency-dependent interaction with the endogenous opioid inhibition of oxytocin release. Naunyn Schmiedebergs Arch Pharmacol. 1988 Nov;338:560–566.
- Katoh A, Fujihara H, Ohbuchi T, et al. Highly visible expression of an oxytocin-monomeric red fluorescent protein 1 fusion gene in the hypothalamus and posterior pituitary of transgenic rats. Endocrinology. 2011 Jul;152:2768–2774.
- Diochot S, Drici M-D, Moinier D, et al. Effects of phrixotoxins on the Kv4 family of potassium channels and implications for the role of Ito1 in cardiac electrogenesis. Br J Pharmacol. 1999 Jan;126:251–263.
- Maejima Y, Takahashi S, Takasu K, et al. Orexin action on oxytocin neurons in the paraventricular nucleus of the hypothalamus. Neuroreport. 2017 Apr;28:360–366.
- Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol. 2007 Aug;582:1219–1238.
- Carrasquillo Y, Burkhalter A, Nerbonne JM. A-type K+ channels encoded by Kv4.2, Kv4.3 and Kv1.4 differentially regulate intrinsic excitability of cortical pyramidal neurons. J Physiol. 2012 Aug;590:3877–3890.
- Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005 Aug;115:2047–2058.
- MacDonald PE, Wheeler MB. Voltage-dependent K(+) channels in pancreatic beta cells: role, regulation and potential as therapeutic targets. Diabetologia. 2003 Aug;46:1046–1062.
- Rita RS, Dezaki K, Kurashina T, et al. Partial blockade of Kv2.1 channel potentiates GLP-1’s insulinotropic effects in islets and reduces its dose required for improving glucose tolerance in type 2 diabetic male mice. Endocrinology. 2015 Jan;156:114–123.
- Maejima Y, Horita S, Kobayashi D, et al. Nesfatin-1 inhibits voltage gated K+ channels in pancreatic beta cells. Peptides. 2017 Sep;95:10–15.
- Jerng HH, Pfaffinger PJ. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004 Dec;27:343–369.
- Liss B, Franz O, Sewing S, et al. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and KChip3.1 transcription. Embo J. 2001 Oct;20:5715–5724.
- Brimble MJ, Dyball REJ, Forsling ML. Oxytocin release following osmotic activation of oxytocin neurones in the paraventricular and supraoptic nuclei. J Physiol. 1978 May;278:69–78.
- Hoffman DA, Magee JC, Colbert CM, et al. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997 Jun;387:869–875.
- Chen C. beta-Amyloid increases dendritic Ca2+ influx by inhibiting the A-type K+ current in hippocampal CA1 pyramidal neurons. Biochem Biophys Res Commun. 2005 Dec;338:1913–1919.
- Sah P, Davies P. Calcium-activated potassium currents in mammalian neurons. Clin Exp Pharmacol Physiol. 2000 Sep;27:657–663.
