ABSTRACT
Oxidized low-density lipoprotein (ox-LDL) leads to atherosclerosis via lectin-like oxidized lipoprotein receptor-1 (LOX-1), one of the major receptor for ox-LDL. Inhibition of the binding of ox-LDL to LOX-1 decreases the proinflammatory and atherosclerotic events. The aim of the present study was to investigate whether protamine, a polybasic nuclear protein, interferes the binding of ox-LDL to LOX-1. Using sandwich ELISA with newly generated antibody, we measured the blocking effect of protamine on the binding of ox-LDL to LOX-1. Protamine dose-dependently inhibited the binding of ox-LDL to LOX-1. DiI-labeled ox-LDL uptake assay in two types of cultured human endothelial cells was performed with fluorescence microplate reader. Activation of extracellular-signal-regulated kinase (ERK)1/2 by ox-LDL was analyzed by immunoblotting. We found that protamine suppressed uptake of ox-LDL in endothelial cells and inhibited ERK1/2 activation by ox-LDL. These results suggest that protamine may possess anti-atherogenic potential by inhibiting ox-LDL binding to LOX-1 through electrostatic interactions.
Graphical abstract
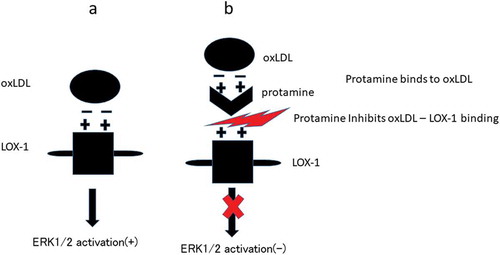
Protamine inhibits the binding of ox-LDL to several scavenger receptors.
Ischemic heart disease and stroke are global clinical issues. Atherosclerosis is closely associated with these diseases. Oxidized low-density lipoprotein (ox-LDL) is involved in atherosclerotic process by various mechanisms, including endothelial cell dysfunction, macrophage foam cell formation, and vascular smooth muscle cell migration and proliferation [Citation1–Citation3]. The action of ox-LDL on endothelial cells is mediated by several scavenger receptors including SR-A, CD36 and lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) [Citation4]. Among these scavenger receptors, LOX-1 is expressed in several pro-inflammatory situations and appears to play a crucial role in endothelial dysfunction [Citation5–Citation7]. Therefore, LOX-1 could be a potential target of the therapy for patients with atherosclerosis.
LOX-1 was initially identified in vascular endothelial cells as a receptor for ox-LDL [Citation8]. In endothelial cells LOX-1 is more highly expressed than other scavenger receptors when endothelial cells are treated with ox-LDL [Citation9]. Among the four domains comprising LOX-1, lectin-like domain at the C-terminus (CTLD, C-type lectin-like domain) is essential for the binding to ox-LDL [Citation10,Citation11]. A linear arrangement of electropositive residues has been reported to cross the dimer surface of CTLD, called basic spine [Citation12,Citation13]. When LDL is oxidized, the surface becomes negatively charged [Citation14,Citation15]. Therefore, inhibition of the binding of LOX-1 to ox-LDL could be accomplished by blocking electrostatic interaction between the basic spine in LOX-1 and ox-LDL. Protamine, derived from human sperm, is a protein of molecular weight 6674 [Citation16]. The 50 residue sequence of human sperm protamine is: ARYRC CRSQS RSRYY RQRQR SRRRR RRSCQ TRRRA MRCCR PRYRP RCRRH.
As protamine is a protein which is rich in the basic amino acid arginine it is assumed to bind to ox-LDL. To date, few studies have investigated inhibiting the electrostatic interaction between LOX-1 and ox-LDL. Protamine is used to neutralize the anti-coagulant effect of heparin [Citation16]. Protamine can be administered at the end of several surgical treatments such as carotid endarterectomy to reverse the anticoagulant effects of heparin and to limit the risk for postoperative bleeding [Citation17]. As anaphylactic reactions can be caused by injection of protamine during cardiac surgery [Citation18], we need to be careful in the case of clinical application.
Oxidation of LDL stimulates mitogen-activated protein kinase (MAPK) in several cells [Citation19–Citation21]. Extracellular-signal-regulated kinase (ERK) 1/2 is significantly activated by ox-LDL compared with native LDL. Several studies have described the activation of ERK1/2 by ox-LDL via LOX-1 [Citation21–Citation23]. ERK1/2 activation by ox-LDL is prevented by some inhibitors [Citation23,Citation24].
In the present study, we investigated the effect of protamine on the binding of ox-LDL to LOX-1 in a cell free system and its effect on the uptake of ox-LDL in two types of human endothelial cells. We also examined whether protamine blocks the activation of ERK1/2 by ox-LDL.
Materials and methods
Antibodies
A human monoclonal anti-human apoB antibody was generated from the HuCAL Platinum collection of human antibody genes [Citation25]. This antibody recognizes human oxidized LDL (data not shown). Anti-human IgG HRP (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) was used as a secondary antibody.
Preparation of oxidized LDL
Human LDL was purchased from Lee Biosolutions (Maryland Heights, MO). LDL (0.5 mg protein/mL) was incubated with CuSO4 (10 μmol/L) at 37°C. Ox-LDL was dialyzed with 1mM EDTA in phosphate-buffered saline (PBS). The degree of oxidation was assessed by measuring the mobility of the protein on an agarose gel (Helena Laboratories).
Preparation of recombinant LOX-1
CTLD, the extracellular ligand-binding domain of LOX-1, was cloned into the pET-22b bacterial expression vector containing a hexahistidine tag [Citation26]. This LOX-1 construct was a kind gift from Dr. Sachiko Machida (NFRI NARO, Japan). A His-tagged CTLD protein was prepared using a modified method described in a previous report [Citation27]. Briefly, a bacterial lysate was sonicated and centrifuged. The inclusion body pellets were solubilized in buffer containing guanidine hydrochloride. The solubilized inclusion body was purified using a His-tag affinity column (Nacalai Tesque, Kyoto, Japan). The purified protein was dialyzed stepwise for refolding.
Sandwich ELISA for ox-LDL binding to the scavenger receptor
A sandwich ELISA was carried out using a modified method described in a previous report [Citation28]. Each recombinant protein (0.6 μg/well), including LOX-1, CD36 (RayBiotech, Inc., Norcross, GA) and CD204 (SR-A) (LD Biopharma,Inc., San Diego, CA), was immobilized on 96-well plates (Greiner Bio-One) through incubation overnight at 4°C. In case of immobilizing protamine (Wako Pure Chemical Industries, Ltd., Osaka, Japan), the protein was added to the plate at various concentrations overnight at 4°C. All the subsequent steps were performed at room temperature. After washing with PBS, the plates were incubated with blocking buffer (10 mM HEPES (pH 7.0), 2 mM EDTA, 3% bovine serum albumin (BSA)) for 30 min. After blocking, ox-LDL was added, along with NaCl or amino acids or protamine for 1.5 h. Arginine and lysine were neutralized to pH 7.0 using H2SO4. After three washes with PBS, the plates were incubated for 1 h with an anti-human apoB monoclonal antibody. After six washes with PBS, the plates were incubated with the peroxidase-conjugated anti-human IgG for 1 h. The plates were washed with PBS, and a substrate solution (TMB solution, Bio-Rad) was added to the plate. A colorimetric assay was performed using a microplate reader (Berthold Technologies, Bad Wildbad, Germany) at 450 nm.
Surface plasmon resonance assay
The surface plasmon resonance assay was performed with a Biacore X100 apparatus (GE Healthcare). Protamine was immobilized on a CM5 sensor chip (GE Healthcare). The interaction between immobilized protamine and ox-LDL was examined at 25°C by a single-cycle kinetics analysis program, with running buffer (15 mM HEPES (pH 7.5), 1.5 mM EDTA, 0.15 M NaCl). The molecular weight of the ox-LDL particle was assumed to be 2.5 MDa. We calculated the binding kinetics by using a bivalent analyte model with BIAevaluation software (GE Healthcare).
Cell culture
Human Coronary Artery Endothelial Cells (HCAECs) and Human Aortic Endothelial Cells (HAOECs) were purchased from Toyobo (Osaka, Japan). The cells were grown in endothelial cell growth medium. These cell lines were maintained at 37°C in a humidified 5% CO2 incubator.
Cell viability assay
HCAECs and HAOECs were seeded at a density of 3,000 cells/well (HCAECs) or 5,000 cells/well (HAOECs) in 96-well plate and incubated in endothelial cell growth medium for more than 20 h at 37°C. Endothelial cells were treated with protamine at various concentrations for 24 h. Cell viability was determined using a Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). The color intensity was measured in a microplate reader (Thermo Electron Corporation, Vantaa, Finland) at 450 nm.
Measurement of uptake of DiI-labeled ox-LDL in endothelial cells
Endothelial cells were seeded in 96-well plate at 3,000 cells/well (HCAECs) or 5,000 cells/well (HAOECs) and incubated in endothelial cell growth medium for more than 20 h at 37°C. After treatment with protamine for 1.5 h, the cells were incubated with 1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ-tetramethyllindocarbocyanine (DiI)-ox-LDL (6 μg/mL) (Alfa Aesar) and protamine for 20 h. The cells were washed with PBS twice. To measure the amounts of DiI-oxLDL uptake in the cells, 0.1% sodium dodecyl sulfate (SDS) solution with 0.1 M NaCl was added to each well, and the plates were incubated at room temperature. Quantitative fluorescence analysis was performed using a fluorescence microplate reader (SH-9000, Corona Electric Co., Ltd. Ibaraki, Japan) with excitation and emission wavelengths at 520 nm and 574 nm, respectively.
Western blotting for detection of phospho-ERK1/2
Endothelial cells were seeded in 35 mm dishes and incubated in endothelial cell growth medium for more than 20 h at 37°C. After the cells were treated with protamine for 1.5 h, ox-LDL for HCAECs (40 μg/mL) or for HAOECs (50 μg/mL) were added and incubated for 10 min. The cells were washed twice with PBS and lysed in a radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS and 1% Nonidet P-40) with a protease inhibitor and phosphatase inhibitor. The proteins in the cell lysates were separated using SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 1% BSA and incubated with a rabbit anti-phospho-ERK1/2 antibody or anti-total ERK1/2 antibody (Cell Signaling Technology Japan, K.K., Tokyo) overnight at 4°C. After 3 washes with T-PBS, the membranes were incubated with peroxidase-conjugated anti-rabbit IgG antibody (New England Biolabs, Ipswich, MA) for 1 h at room temperature. The protein bands were visualized using Immobilon Western (Millipore, Corporation, Billerica, MA).
Statistical analysis
Data are presented as means ± standard deviation. Statistical analysis was performed by the Tukey-Kramer test (statcel 4, OMS publishing Inc., Tokorozawa, Japan). The differences were considered statistically significant at P < 0.05.
Results
Inhibition of the binding ox-LDL to LOX-1 by acidic and basic amino acid
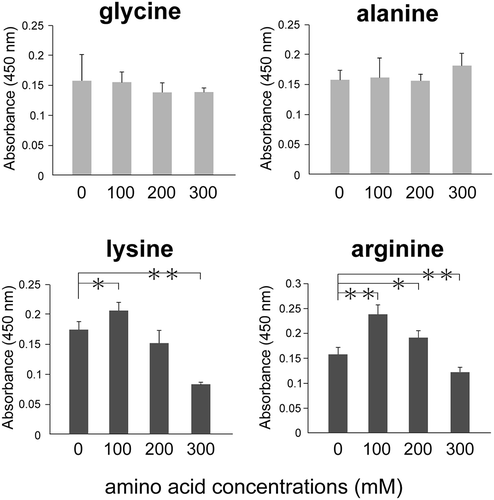
The positive charge of the cluster in LOX-1 is thought to be responsible for the binding to ox-LDL, which is negatively charged. Since substances with an electric charge could interfere with these electrostatic interactions, our first approach was to examine whether charged reagents can inhibit the binding of ox-LDL to LOX-1. To investigate whether NaCl inhibits the binding ox-LDL to LOX-1, we performed a sandwich ELISA using ox-LDL as a ligand for LOX-1. NaCl blocked the binding of ox-LDL to LOX-1, dose-dependently (). Moreover, to examine if amino acids could inhibit the binding of ox-LDL to LOX-1, we tested several amino acids which have electrically charged side chains. Neutral amino acids showed comparable reactivity with the control, while both basic amino acid showed increased reactivity at 100 mM and dose-dependently inhibitory effect at more than 100 mM () . These results suggest that amino acids which have electrically charged side chains could more effectively inhibit the binding of ox-LDL to LOX-1 than neutral amino acids at more than 300 mM.
Figure 1. NaCl inhibits the binding of ox-LDL to LOX-1.
Ox-LDL was incubated along with NaCl at the indicated concentration in a recombinant LOX-1-immobilized ELISA system. A colorimetric assay was performed and values are expressed as mean absorbance at 450 nm. Error bars represent the SD for five samples. **P < 0.01.
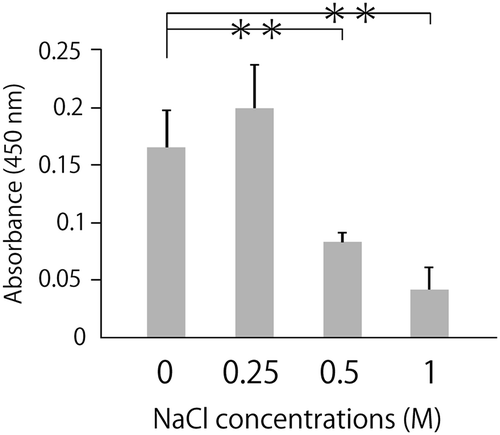
Figure 2. Charged amino acids inhibit the binding of ox-LDL to LOX-1.
Ox-LDL was incubated with amino acids and analyzed using a recombinant LOX-1-immobilized ELISA system. A colorimetric assay was performed and values are expressed as mean absorbance at 450 nm. The X axis shows amino acid concentrations. Error bars represent the SD for five samples. **P < 0.01, *P < 0.05.
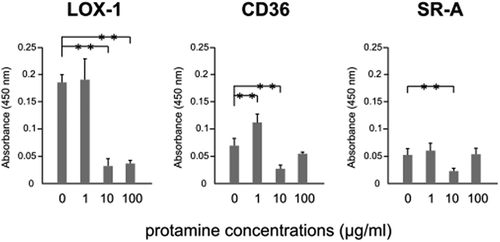
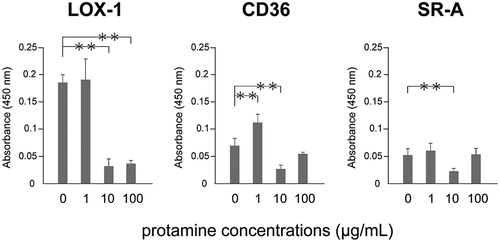
Protamine inhibits the binding of ox-LDL to the scavenger receptor
Several scavenger receptors including LOX-1, CD36 and SR-A are expressed on the surface of endothelial cells. Because protamine contains many arginines, we next examined whether protamine interferes with the binding of ox-LDL to the scavenger receptor using a sandwich ELISA. Protamine decreased the binding of ox-LDL to CD36 or SR-A at 10 μg/mL, while the binding of ox-LDL to LOX-1 was markedly inhibited by protamine at more than 10 μg/mL (). These results suggest that protamine could inhibit the binding of ox-LDL to the scavenger receptor in vitro.
Figure 3. Protamine inhibits the binding of ox-LDL to the receptor.
Ox-LDL was incubated with protamine at the indicated concentration in a recombinant receptor protein-immobilized ELISA system. A colorimetric assay was performed and values are expressed as mean absorbance at 450 nm. Error bars represent the SD for five samples. **P < 0.01.
To clarify the mechanism by which protamine inhibits the binding of ox-LDL to LOX-1, we confirmed the binding of ox-LDL to protamine using surface plasmon resonance (). The protamine-immobilized sensor chip was used in this study. The KD value for the interaction of protamine with ox-LDL was estimated to be 6.3 × 10−3 M. These results suggest that protamine can bind to ox-LDL.
Protamine inhibits the uptake of ox-LDL in human endothelial cells
We examined whether protamine could interfere with the uptake of ox-LDL into two types of human endothelial cells. First, we ascertained the cytotoxicity of protamine in the cells. HCAECs and HAOECs were treated with various concentrations of protamine for 24 h. shows that protamine did not produce detectable cytotoxicity as compared with the control up to 100 μg/mL for HCAECs or 50 μg/mL for HAOECs. To examine the effect of protamine on the uptake of ox-LDL in HCAECs and HAOECs, DiI-ox-LDL was added to the cells with protamine and incubated for 20 h. Protamine inhibited the uptake of DiI-ox-LDL in both endothelial cell types ().
Figure 5. Protamine inhibits the uptake of ox-LDL to endothelial cells.
(a) Effect of protamine on the cell viability of HCAECs and HAOECs. HCAECs and HAOECs were treated with protamine at the indicated concentration for 24h. A cell counting kit was used and values are expressed as absorbance at 450 nm. Error bars represent the SD for eight samples. (b) Protamine inhibits the uptake of ox-LDL in HCAECs and HAOECs. DiI-ox-LDL uptake in HCAECs or HAOECs was measured in the presence of various concentrations of protamine. Values are expressed as fluorescence intensity in arbitrary units. Error bars represent the SD for six samples. **P < 0.01, *P < 0.05. (c) Inhibitory effect of protamine on the activation of ERK1/2 induced by ox-LDL in HCAECs or HAOECs. Endothelial cells were preincubated with protamine and were treated with or without ox-LDL for 10 min. The expression of protein levels of phosphor ERK1/2 and total ERK1/2 was quantitated using Image J software. The ratio (phosphor ERK1/2/total ERK1/2) is shown.
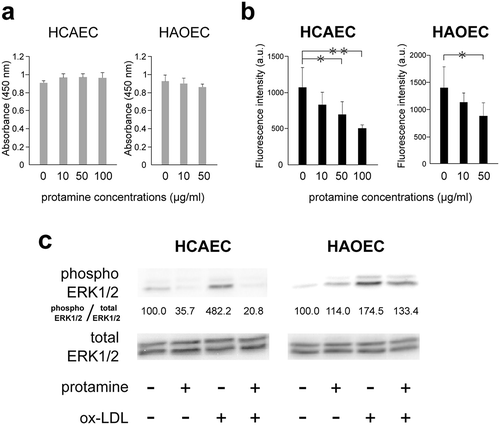
We examined the effect of ox-LDL on ERK1/2 activity which is involved in progression of atherosclerosis. ERK1/2 is rapidly activated by ox-LDL [Citation16,Citation18]. Therefore, we analyzed the ERK1/2 activity at 10 min after treatment. shows that protamine inhibited the activation of ERK1/2 by ox-LDL in both types of endothelial cells. These results suggest that protamine inhibits the uptake of ox-LDL and the activation of ERK1/2 in HCAECs and HAOECs.
In summary, as shown in , our data demonstrate for the first time that protamine inhibits the binding of ox-LDL to LOX-1 through protamine-oxLDL binding. Moreover, cell-based assays indicate that protamine interferes with the uptake of ox-LDL and reduces ERK1/2 activation by ox-LDL in endothelial cells.
Discussion
Our study suggests that protamine inhibits the electrostatic interactions between ox-LDL and LOX-1. The CTLD in LOX-1 forms homodimer which provides a linear arrangement of arginine residues [Citation12,Citation13], whereas ox-LDL has a negative charge because of oxidization of its components. In addition, mutations of some residues present in the basic spine in the CTLD impair binding to ox-LDL [Citation13]. In the present study, we showed that protamine, which contains many arginines, also inhibited the binding of ox-LDL to the scavenger receptor and the uptake of ox-LDL in vascular endothelial cells. Ox-LDL-binding of CD36 or SR-A is thought to be mediated through electrostatic interactions [Citation29–Citation31]. Accordingly, these electrostatic interactions are required for the binding ox-LDL with LOX-1.
In this study, we showed that NaCl or basic amino acids inhibited the binding of ox-LDL to LOX-1 at several hundreds of mM, but protamine inhibited the binding at less than several μM. These results indicate that protamine has an inhibitory concentration that is about 100,000 times smaller compared with that of NaCl or basic amino acids. This effect may be caused by the continuous arginine sequence of protamine and multi-site binding to ox-LDL. In contrast, NaCl or amino acids distribute uniformly in aqueous solutions and may demonstrate instable interaction to ox-LDL or LOX-1.
There are two targets to block the binding of ox-LDL to LOX-1. One is LOX-1, and the other is ox-LDL. Although several receptors for ox-LDL have been reported, a single inhibitor which interferes with the binding of ox-LDL to all its receptors has not been found. Inhibition of the binding of ox-LDL to each receptor requires unique inhibitors to bind to each receptor. In the present study, we showed that protamine bound ox-LDL and protamine inhibited the binding of ox-LDL to the several scavenger receptors. These results suggest that protamine blocked the binding of ox-LDL to the scavenger receptor through the interaction with ox-LDL rather than the scavenger receptor owing to many basic amino acids of protamine.
The positive charge of lactoferrin or developmental endothelial locus-1 (Del-1) is thought to be important for binding to ox-LDL, which is negatively charged [Citation32,Citation33]. Therefore, these proteins protect cells from the actions of ox-LDL. In addition, the heparin-bound fraction of human lipoprotein-deficient serum inhibits the interaction with ox-LDL [Citation34]. These results indicate that substances with a positive charge interfere with the binding of ox-LDL to the LOX-1 receptor. These evidences suggest that the positively charged amino acids of these proteins are critical for their interaction with ox-LDL. Therefore, reagent which contains several positively charged amino acids could be effective to inhibit the binding of ox-LDL to the scavenger receptor.
The hydrophobic tunnel residing within the CTLD dimer has been reported as important for ox-LDL recognition and binding [Citation35]. Several compounds, including oxidized phospholipids, have been reported as inhibiting the binding of ox-LDL to LOX-1 [Citation35–Citation37]. These compounds were computationally predicted to bind to the hydrophobic tunnel. Although we did not take this hydrophobic tunnel into consideration in the current study, the binding of ox-LDL to LOX-1 might be more effectively inhibited by the combination of protamine and these compounds.
We showed that the inhibitory effect of protamine on uptake of ox-LDL and on the activation of ERK1/2. The activation of intracellular signaling pathways including ERK1/2 could be regulated due to inhibition of uptake of ox-LDL. These pathways are involved in progression of atherosclerosis. Therefore, protamine might be useful for anti-atherogenic therapy. As protamine is a natural product and is already used clinically, it could be applied clinically. But, further study is required to confirm that protamine is a promising inhibitor for the binding of ox-LDL to its receptors clinically.
Author Contributions
Conceptualization: Yukitoshi Takemura, Shunichiro Kubota.
Formal analysis: Yukitoshi Takemura.
Funding acquisition: Shunichiro Kubota.
Investigation: Yukitoshi Takemura, Masaki Okamoto, Makoto Hasegawa.
Project administration: Shunichiro Kubota.
Supervision: Yukitoshi Takemura, Shunichiro Kubota.
Writing the original draft: Yukitoshi Takemura.
Writing the review and editing: Yukitoshi Takemura, Kenichi Hatanaka, Shunichiro Kubota.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Steinberg D. The LDL modification hypothesis of atherogenesis: an update. J Lipid Res. 2008;50:S376–S381.
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325.
- Di Pietro N, Formoso G, Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vascul Pharmacol. 2016;84:1–7.
- Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711.
- Pirillo A, Norata GD, Catapano AL. LOX-1, oxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786.
- Lubrano V, Balzan S. Roles of LOX-1 in microvascular dysfunction. Microvasc Res. 2016;105:132–140.
- Chistiakov DA, Orekhov AN, Bobryshev YV. LOX-1-mediated effects on vascular cells in atherosclerosis. Cell Physiol Biochem. 2016;38:1851–1859.
- Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77.
- Khaidakov M, Mitra S, Wang X, et al. Large impact of low concentration oxidized LDL on angiogenic potential of human endothelial cells: a microarray study. PLoS One. 2012;7:e47421.
- Chen M, Narumiya S, Masaki T, et al. Conserved C-terminal residues within the lectin-like domain of LOX-1 are essential for oxidized low-density-lipoprotein binding. Biochem J. 2001;355:289–296.
- Chen MY, Inoue K, Narumiya S, et al. Requirements of basic amino acid residues within the lectin- like domain of LOX-1 for the binding of oxidized low-density lipoprotein. FEBS Lett. 2001;499:215–219.
- Park H, Adsit FG, Boyington JC. The 1.4 angstrom crystal structure of the human oxidized low density lipoprotein receptor lox-1. J Biol Chem. 2005;280:13593–13599.
- Ohki I, Ishigaki T, Oyama T, et al. Crystal structure of human lectin-like, oxidized low-density lipoprotein receptor 1 ligand binding domain and its ligand recognition mode to OxLDL. Structure. 2005;13:905–917.
- Steinbrecher UP. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987;262:3603–3608.
- Zhang H, Yang Y, Steinbrecher UP. Structural requirements for the binding of modified proteins to the scavenger receptor of macrophages. J Biol Chem. 1993;268:5535–5542.
- Levi M, Eerenberg E, Kamphuisen PW. Bleeding risk and reversal strategies for old and new anticoagulants and antiplatelet agents. J Thromb Haemost. 2011;9:1705–1712.
- Baracchini C, Ballotta E. The benefit of heparin reversal with protamine during carotid endarterectomy. JAMA Surg. 2016;151:255–256.
- Nybo M, Madsen JS. Serious anaphylactic reactions due to protamine sulfate: a systematic literature review. Basic Clin Pharmacol Toxicol. 2008;103:192–196.
- Kusuhara M, Chait A, Cader A, et al. Oxidized LDL stimulates mitogen-activated protein kinases in smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 1997;17:141–148.
- Apostolov EO, Basnakian AG, Yin X, et al. Modified LDLs induce proliferation-mediated death of human vascular endothelial cells through MAPK pathway. Am J Physiol Heart Circ Physiol. 2007;292:H1836–H1846.
- Tanigawa H, Miura S, Zhang B, et al. Low-density lipoprotein oxidized to various degrees activates ERK1/2 through Lox-1. Atherosclerosis. 2006;188:245–250.
- Biocca S, Falconi M, Filesi I, et al. Functional analysis and molecular dynamics simulation of LOX-1 K167N polymorphism reveal alteration of receptor activity. PLoS One. 2009;4:e4648.
- Zhang Z, Zhang M, Li Y, et al. Simvastatin inhibits the additive activation of ERK1/2 and proliferation of rat vascular smooth muscle cells induced by combined mechanical stress and oxLDL through LOX-1 pathway. Cell Signal. 2013;25:332–340.
- Tsai KL, Chang YL, Huang PH, et al. Ginkgo biloba extract inhibits oxidized low-density lipoprotein (oxLDL)-induced matrix metalloproteinase activation by the modulation of the lectin-like oxLDL receptor 1-regulated signaling pathway in human umbilical vein endothelial cells. J Vasc Surg. 2016;63:204–215.e1.
- Shojima K, Sato A, Hanaki H, et al. Wnt5a promotes cancer cell invasion and proliferation by receptor-mediated endocytosis-dependent and -independent mechanisms, respectively. Sci Rep. 2015;5:8042.
- Kumano-Kuramochi M, Shimozu Y, Wakita C, et al. Identification of 4-hydroxy-2-nonenal-histidine adducts that serve as ligands for human lectin-like oxidized LDL receptor-1. Biochem J. 2012;442:171–180.
- Vohra RS, Murphy JE, Walker JH, et al. Functional refolding of a recombinant C-type lectin-like domain containing intramolecular disulfide bonds. Protein Expr Purif. 2007;52:415–421.
- Sato Y, Nishimichi N, Nakano A, et al. Determination of LOX-1-ligand activity in mouse plasma with a chicken monoclonal antibody for ApoB. Atherosclerosis. 2008;200:303–309.
- Kar NS, Ashraf MZ, Valiyaveettil M, et al. Mapping and characterization of the binding site for specific oxidized phospholipids and oxidized low density lipoprotein of scavenger receptor CD36. J Biol Chem. 2008;283:8765–8771.
- Doi T, Higashino KI, Kurihara Y, et al. Charged collagen structure mediates the recognition of negatively charged macromolecules by macrophage scavenger receptors. J Biol Chem. 1993;268:2126–2133.
- Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR-A) multifunctional? — the mouse ’ s tale. J Clin Investig. 2001;108:649–654.
- Kajikawa M, Ohta T, Takase M, et al. Lactoferrin inhibits cholesterol accumulation in macrophages mediated by acetylated or oxidized low-density lipoproteins. Biochim Biophys Acta. 1994;1213:82–90.
- Kakino A, Fujita Y, Nakano A, et al. Developmental endothelial locus-1 (Del-1) inhibits oxidized low-density lipoprotein activity by direct binding, and its overexpression attenuates atherogenesis in mice. Circ J. 2016;80:2541–2549.
- Suginohara Y, Miyazaki A, Hakamata H, et al. The heparin-bound fraction of human lipoprotein-deficient serum inhibits endocytic uptake of oxidized low density lipoprotein by macrophages. Atherosclerosis. 1996;120:167–179.
- Francone OL, Tu M, Royer LJ, et al. The hydrophobic tunnel present in LOX-1 is essential for oxidized LDL recognition and binding. J Lipid Res. 2009;50:546–555.
- Falconi M, Ciccone S, D’Arrigo P, et al. Design of a novel LOX-1 receptor antagonist mimicking the natural substrate. Biochem Biophys Res Commun. 2013;438:340–345.
- Thakkar S, Wang X, Khaidakov M, et al. Structure-based design targeted at LOX-1, a receptor for oxidized low-density lipoprotein. Sci Rep. 2015;5:16740.