ABSTRACT
Pituitary adenomas constitute one of the most common intracranial tumors. MicroRNAs play an important role in development and progression of pituitary adenomas. In this study, we showed that miR-219a-2-3p was significantly down-regulated in pituitary adenomas cells. Overexpression of miR-219a-2-3p suppressed the proliferation and promoted apoptosis of pituitary adenomas cells. After bioinformatics analysis, we found that MDM2 was one of the downstream targets of miR-219a-2-3p. Further researches showed that miR-219a-2-3p could reduce the protein level of MDM2 by binding to the 3ʹ-UTR of MDM2 and promoted p53 expression. Then, we overexpressed both miR-219a-2-3p and MDM2 in the same group and found that it could counteract the effect of overexpressing miR-219a-2-3p alone on proliferation and apoptosis of pituitary adenoma cells. Taken together, these results suggested that miR-219a-2-3p regulated the proliferation and apoptosis by targeting MDM2/p53 in pituitary adenomas. Therefore, miR-219a-2-3p may serve as a novel marker and therapeutic target for pituitary adenomas.
Graphical abstract
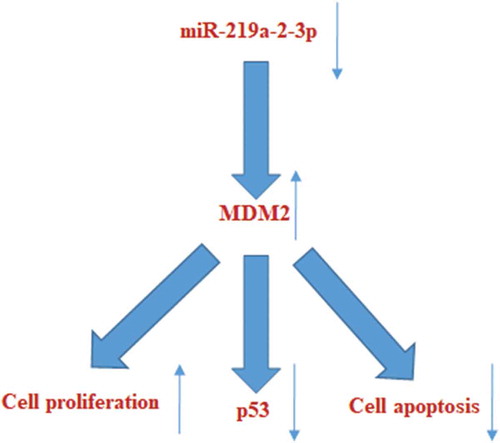
Overexpression of miR-219a-2-3p could inhibit proliferation, promote apoptosis and p53 expression in pituitary adenomas cells by suppressing MDM2 expression.
Pituitary adenoma is a common intracranial tumor, and its pathogenic rate is only second to glioma and meningioma, accounting for about 22.5% [Citation1]. There are many types of pituitary adenomas depending on the hormone secretion. 42.70% of Chinese patients did not secrete hormones, while the other patients secreted a variety of hormones, such as follicle-stimulating hormone, prolactin, growth hormone and adrenocorticotropin [Citation2]. The dysregulation of hormone secretion is just an important factor in the malignant process of pituitary adenoma. However, due to the lack of early diagnosis and therapeutic strategies for recurrent cases, the survival rate of the malignant patients is not optimistic. The various types of pituitary adenomas lead to various pathological mechanisms. Therefore, elucidating the molecular pathogenesis of pituitary adenoma and identifying reliable biomarkers for diagnosis and treatment are still the key points in this field.
MicroRNAs(miRNAs), composed of 18-25nt, usually regulates the expression of the target gene by binding to the 3ʹ-untranslated regions (3ʹ -UTRs), so as to perform its function. In the past decades, numerous reports have been published on the association between miRNAs and tumors, among which studies on the role of miRNAs in regulating the occurrence and development of pituitary adenoma are also common [Citation3,Citation4].
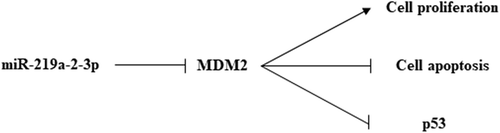
Mir-219a is a miRNA that has been studied in recent years, mainly concentrated in miR-219a-5p. In breast cancer [Citation5], ovarian cancer [Citation6,Citation7] and non-small cell lung cancer [Citation8], miR-219a-5p has been proved to be involved in the proliferation, metastasis or apoptosis of cancer cells as a tumor suppressor. However, few studies have reported on miR-219a-2-3p. But, when using next-generation sequencing to detect miRNAs expression in different types of pituitary adenoma (nonfunctioning pituitary adenomas, NFPAs, growth hormone-secreting pituitary adenomas, GHPAs and prolactin-secreting pituitary adenomas, PRLPAs) and normal tissues, Zongze He et al. found that miR-219a-2-3p had low expression in all three types of pituitary adenoma tissues and it was the lowest miRNA in the overall [Citation9]. On the basis of the above studies, since the specific molecular mechanism of miR-219a-2-3p in pituitary tumor has not been studied, in this study, we firstly detected if miR-219a-2-3p could influence proliferation and apoptosis of pituitary adenoma cells, then the target genes of miR-219a-2-3p were predicted by bioinformatics, recognized mouse double minute 2 (MDM2) as the possible target gene of miR-219a-2-3p, which is an equally important factor but also have not been in-depth study in pituitary adenoma. The negative regulatory relationship between MDM2-p53 has been demonstrated in cancers [Citation10]. In pituitary adenoma, Suliman M et al. found that MDM2 was a downstream gene of p53, indicating MDM2/p53 may be a functional factor in pituitary adenoma [Citation11]. So, p53, as a tumor-suppressor, was also investigated in our study. Finally, we verified the molecular mechanism of miR-219a-2-3p to manage pituitary adenoma cells through regulating the MDM2/p53.
Materials and methods
Cell lines
Mouse pituitary adenomas cell lines AtT-20, GT1.1 and pituitary cell MPC were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). MPC cell and GT1.1 cell were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Life Technologies, Gaithersburg, MD, USA) supplemented with 10% horse serum (Gibco, Life Technologies, Gaithersburg, MD, USA) and 2.5% fetal bovine serum (FBS, Gibco, Life Technologies, Gaithersburg, MD, USA), AtT-20 cell was cultured in F-12K medium (Gibco, Life Technologies, Gaithersburg, MD, USA) supplemented with 10% horse serum (Gibco, Life Technologies, Gaithersburg, MD, USA) and 2.5% FBS (Gibco, Life Technologies, Gaithersburg, MD, USA). All of them were incubated at 37°C in a humidified atmosphere with 5% CO2.
RT-qPCR
The miRNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA) was used to extract miRNAs from cell lines according to the manufacturer’s instructions. The reverse transcription of miRNAs used the One Step PrimeScript miRNA cDNA Synthesis Kit (Takara, Dalian, China). Quantitative real-time PCR(RT-qPCR) was performed on the ABI7500 Real-time PCR system (Applied Biosystems, Foster City, CA, USA) using the SYBR® PremixEx Taq II Kit (Takara, Dalian, China). U6 was used as the internal control. 2− Δ Δ CT was applied to calculate the relative expression quantity. The primers of miR-219a-2-3p and U6 were listed as follows: miR-219a-2-3p F: 5-ACACTCCAGCTGGGAGAATTGTGGCTGGAC-3; miR-219a-2-3p R: 5-CTCAACTGGTGTCGTGGA-3; U6 F: 5-CTCGCTTCGGCAGCACA-3; U6 R: 5- AACGCTTCACGAATTTGCGT-3.
Mimics, pcDNA3.1-MDM2 and their negative controls transfection
MiR-219a-2-3p mimics, pcDNA3.1-MDM2 and their negative controls were purchased from Ribobio Company. Each of them (50nM) was transfected into cells using Lipofectamine RNAiMAX transfection reagent (Life Technologies). After 48 h, cells in each group were digested by trypsin and collected for further experiments.
Cell proliferation assay
Cells from different groups (2000 cells/well, 3 replicates per group) in the 96-well plates were cultured for 24, 48 and 72 h, respectively. Cell proliferation was determined by MTS Cell Proliferation Assay Kit (Abcam, Cambridge, MA, England). Added 20 µL/well MTS Reagent into each well and incubated for 4 h at 37°C in standard culture conditions. Shook the plate briefly on a shaker and measured the absorbance of cells using a plate reader at OD = 490 nm.
Cell apoptosis assay
Cell culture Medium for each group was transferred to 15 mL conical tubes and placed on ice. The cells in the culture plate were lightly moistened with 2 mL PBS, and removed the solution. Added 0.5 mL 0.25% trypsin and incubated until cells were completely detached from the culture wall. The cells were gently suspended in the 1× binding buffer. 0.5 mL of the cell suspension was transferred from the cell culture plate (5 × 105 cells) to a clean centrifuge tube. Added 1.25 µL ANNEXIN V-FITC (BD Biosciences, Franklin Lakes, NJ, USA) and incubated for 15 min at room temperature (18-24°C) in dark, then mixed with 10 µL PI for 5 min. Samples were then diluted with 400 µL binding buffer and analyzed by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
3′-UTR luciferase plasmid construction and dual-luciferase reporter gene assay
We used 2 miRNA databases, TargetScan (http://www.targetscan.org/Release7.1) and microRNA.org (http://www.microrna.org/microrna/home.do), to predict the target genes of miR-219a-2-3p. The wide-type (WT) or mutant 3ʹ-UTR of MDM2 was cloned into psi-CHECK2 vector by double digestion to construct the double luciferase reporter vectors. Each constructed psi-CHECK2 vector (30 ng) was co-transfected with miR-219a-2-3p mimics, inhibitor or negative control (50nM). After 48 h, luciferase activity (F) and renilla luciferase activity (R) were measured with a Dual-Luciferase Assay kit (Promega). R/F was used to represent the change of fluorescence activity in each group, indicating the binding ability between miR-219a-2-3p and 3ʹ-UTR of MDM2.
Western blot analysis
We harvested the cells of all groups, then lysed in a buffer containing protease inhibitor called RIPA (Beyotime, Shanghai, China) for 30 min at 4°C with gently shaking every 10 min. The cracked mixture was centrifuged on 12,000rpm for 10 min at 4°C. Proteins concentration was determined using the bicinchoninic acid protein assay kit (BCA1‐1KT, Sigma‐Aldrich Chemical Company, st. Louis, MO, USA). The total cell proteins (30μg/lane) were separated by electrophoresis in sodium dodecyl sulfate polyacrylamide(SDS-PAGE) gel, after that, separated proteins were transferred to polyvinylidene fluoride (PVDF) membrane. Then, used TBST with 5% skim milk powder to block the membranes for 2 h at room temperature. Antigen-antibody crosslinking reaction was carried on by incubating the membranes with anti-MDM2, anti-p53 or anti-GAPDH (Abcam, Cambridge, MA, England) overnight. The secondary antibody(Invitrogen) which conjugated with horseradish peroxidase (HRP) was used to cascade amplify signals and enhanced chemical luminescence reagent (PerkinElmer Life Sciences, MA, USA) was used to visualize bands. Density scanning was performed with Image J software.
Statistical analysis
All experimental data were analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). All the final results were presented as mean ± standard deviation. Statistical analysis carried by the Student’s t-test or one-way ANOVA determined the differences between two groups. P < 0.05 was considered as statistical significance.
Results
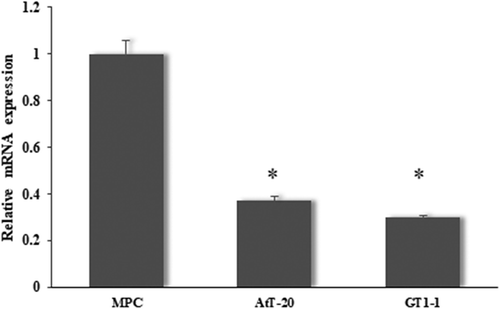
MiR-219a-2-3p was down-regulated in pituitary adenomas cell lines
In order to determine whether miR-219a-2-3p differentially expressed in pituitary adenomas cell lines, pituitary cell MPC, pituitary adenomas cancer cells GT1.1 and AtT-20 were analyzed by the RT-qPCR. The result showed that the expression of miR-219a-2-3p was significantly decreased in both pituitary adenomas cells in comparison with MPC cell ().
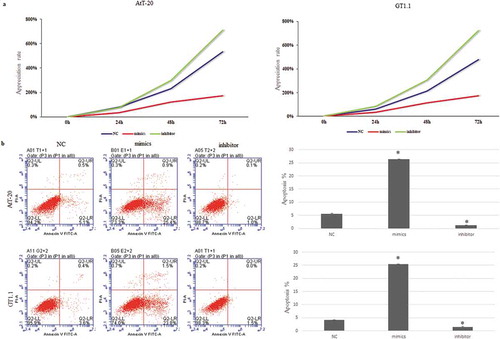
MiR-219a-2-3p suppressed the proliferation and promoted apoptosis of pituitary adenomas cells
To evaluate the function of miR-219a-2-3p in pituitary adenomas cells, miR-219a-2-3p was overexpressed by transfecting miR-219a-2-3p mimics into both AtT-20 and GT1.1 cells and inhibition of miR-219a-2-3p was achieved by transfecting miR-219a-2-3p inhibitor into both cells. MTS was used to further investigate the proliferation and flow cytometry was used to detect apoptosis of pituitary adenomas cells. Overexpression of miR-219a-2-3p significantly decreased the proliferation ()) and promoted apoptosis as compared to the negative control ()) (p < 0.05). These results suggested that miR-219a-2-3p could obviously suppress the vitality of AtT-20 and GT1.1 cells.
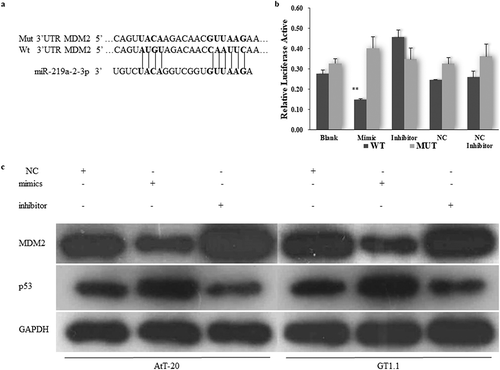
MDM2 is the direct target gene of miR-219a-2-3p
To clarify the molecular mechanisms of miR-219a-2-3p on the pituitary adenomas cells function, the downstream target genes of miR-219a-2-3p were predicted by the TargetScan and microRNA.org databases. We recognized MDM2 as a target gene of miR-219a-2-3p, which has a binding site at the 3′-UTR ()). Then, luciferase reporter assay was performed to detect whether miR-219a-2-3p directly binds to MDM2. WT/mutant psi-CHECK2-MDM2-3′UTR was co-transfected with miR-219a-2-3p mimics, inhibitor or negative control respectively. Results showed that the fluorescence activity of WT psi-CHECK2-MDM2-3′UTR but not the mutant was significantly decreased by miR-219a-2-3p mimics ()). These results indicated MDM2 was the direct target of miR-219a-2-3p.
MDM2 was inversely correlated with the expression of miR-219a-2-3p and p53 was positively correlated with miR-219a-2-3p
To detect the effects of miR-219a-2-3p on MDM2 expression in pituitary adenomas cells, the protein levels of MDM2 and p53 were detected by western blot assay using GAPDH as an internal reference, basing on the overexpression or inhibition of miR-219a-2-3p. The results showed that overexpression of miR-219a-2-3p significantly decreased the protein level of MDM2 and promoted the expression of p53 as compared to the negative control, the miR-219a-2-3p inhibition group did the opposite ()). These results suggested that miR-219a-2-3p negatively regulated the MDM2 and positively affected p53 expression in pituitary adenomas cells.
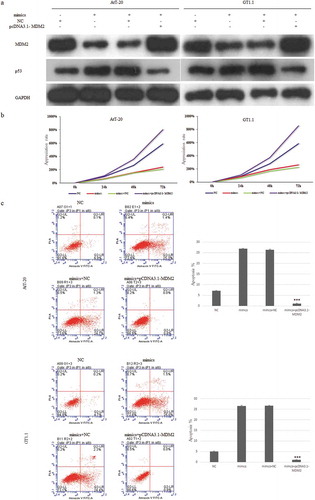
Restoration of the expression of MDM2 can attenuate the effect of miR-219a-2-3p on proliferation, apoptosis and p53 expression in pituitary adenomas cells
In order to further verify the involvement of MDM2 in the proliferation and apoptosis of pituitary adenoma cells regulated by miR-219a-2-3p, we co-transfected miR-219a-2-3p mimics and pcDNA3.1-MDM2 or its NC control into pituitary adenomas cells. Western blot was used to detect the protein level of MDM2 and p53 using GAPDH as internal parameters. MTS assay was used to detect the cell proliferation of each group, flow cytometry was used to detect apoptosis. The results showed that after co-transfecting miR-219a-2-3p mimics and pcDNA3.1- MDM2, the expression of MDM2 and p53 were reverted ()), the proliferation was enhanced ()) and its apoptosis was decreased comparing with the mimics group ()). These results suggested that the MDM2 participated in the regulation of proliferation and apoptosis caused by miR-219a-2-3p in pituitary adenomas cells, and the tumor-suppressor p53 maybe was an important factor in the process. The schematic representation of possible mechanisms is shown in .
Discussion
Numerous studies have been conducted on miRNAs and different types of pituitary adenoma. MiRNAs play an important role both in the diagnosis and prognostic of pituitary adenoma. For diagnosis, miR-26a played an important role in cell cycle regulation in ACTH-secreting pituitary adenomas [Citation12], and may be as a biomarker for diagnosis of invasive/noninvasive pituitary tumors [Citation13]. Some miRNAs, like miR-329, miR-300, miR-381 and miR-655, which could target PTTG1 were downregulated in pituitary adenoma and inhibited pituitary tumors cell tumorigenesis [Citation14]. MiR-34a [Citation15] and miR-107 [Citation16] inhibited the cell proliferation of pituitary adenomas. The expressions of miR-20a, miR-106b and miR-17-5p in NFPA metastatic tumor tissues were higher than those in primary tumor tissues suggesting these miRNAs had potential as diagnostic biomarkers for pituitary malignancy [Citation17]. For prognostic, miR-450b-5p, miR-424, miR-503, miR-542-3p, miR-629, and miR-214 were correlated with tumor size in NFPA [Citation18]. High expression of miR-106b~25 (miR-25-3p, miR-93-3p, miR-93-5p and miR106b-5p) was associated with unfavorable surgical outcome, distinguishes recurrent and progressive tumors in corticotroph pituitary adenomas [Citation19].
In our study, to elucidate the effect of miR-219a-2-3p in pituitary adenoma cells, we detected the expression of miR-219a-2-3p in pituitary adenoma cells and normal cell. The expression of miR-219a-2-3p showed a downward trend in pituitary adenoma cells. Then we focused on the functions of miR-219a-2-3p in pituitary adenoma cells, overexpression of miR-219a-2-3p significantly reduced the proliferation and promoted apoptosis of pituitary adenoma cells. These results revealed that miR-219a-2-3p plays a tumor suppressive role in pituitary adenoma.
It is well known that the function of miRNA depends on the regulation of the expression of downstream target genes. In this study, MDM2 was predicted to be the target gene of miR-219a-2-3p by bioinformatics database. MDM2 gene expression have been found in various tumors [Citation20], and MDM2 amplification is closely related to tumor growth and metastasis [Citation21–Citation23]. Further, Yao X et al. found that MDM2 almost 100% expressed in regrowth and recurrence pituitary tumor tissue, and far higher than the primary tumor tissues through histological test for 35 cases of NFPA patients, indicating MDM2 may be a biomarker and potential drug target for NFPA treatment [Citation24]. In pituitary adenoma, Suliman M et al. found that MDM2 expressed in the cytoplasm of tumors as a downstream gene of p53, indicating MDM2 and p53 may be functional factors in pituitary adenoma [Citation11].
Our study showed that miR-219a-2-3p bound the 3ʹ-UTR of MDM2, overexpression of miR-219a-2-3p decreased the protein levels of MDM2 and promoted p53 expression. Then, we restored the expression of MDM2 in the pituitary adenoma cells which had overexpressed miR-219a-2-3p, the expression of MDM2 and p53 was reverted and the effect of miR-219a-2-3p on the proliferation and apoptosis was attenuated.
Although the expression and role of miRNAs have been widely studied in pituitary adenomas, we still lack the information of miRNAs in tumor systemic circulation, which also poses an obstacle to the diagnostic potential of miRNAs. Because some pituitary adenomas are hormone-secreting tumors, the role of miRNAs may be less important since hormone detection can well monitor tumor growth and function. However, for nonfunctional pituitary adenomas, miRNAs have potential to be a blood biomarker, which facilitates post-operative diagnosis and patient follow-up.
In conclusion, synthesizing the functions of miR-219a-2-3p in the pituitary adenoma cells and the role of its downstream target gene MDM2 in cancer progression, our findings provide a new perspective for tumor research on pituitary adenoma. Down-regulated miR-219a-2-3p was found in pituitary adenoma and was related to the proliferation and apoptosis of pituitary adenoma. Excessive miR-219a-2-3p induces inhibition of the growth of cancer cells by regulating MDM2 and p53. This study always indicated that miR-219a-2-3p/MDM2/p53 axis maybe is a new direction for pituitary adenoma research. Targeting miR-219a-2-3p may be a potential therapeutic strategy for pituitary adenoma.
Undoubtedly, more experiments need to deeply verify the mechanism. In particular, the detailed roles of MDM2/p53 and its downstream pathway in the regulation mechanism of miR-219a-2-3p in pituitary adenoma are the focus of the next study. For example, whether the MDM2-p53 negative feedback loop, which existed in many diseases, also participate in the regulation of miR-219a-2-3p in pituitary adenoma, that is exactly what we’re going to research next.
Authors’ contributions
Yibiao Wang designed the experiment and Hao Peng supervised the experimental performance. All authors participated in the experiments, data analysis. Hao Peng finished the data interpretation and Yibiao Wang wrote the paper. All authors read and approved this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
Additional information
Funding
References
- Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613–619. PubMed PMID: 15374075
- Zhu X, Wang Y, Zhao X, et al. Incidence of pituitary apoplexy and its risk factors in Chinese people: a database study of patients with pituitary adenoma. PloS One. 2015;10(9):e0139088. PubMed PMID: 26407083
- Feng Y, Mao ZG, Wang X, et al. MicroRNAs and target genes in pituitary adenomas. Hormone Metab Res. 2018;50(3):179–192. PubMed PMID: 29523005
- Yang Q, Li X. Molecular network basis of invasive pituitary adenoma: a review. Front Endocrinol (Lausanne). 2019;10(undefined):7. PubMed PMID: 30733705
- Gao C, Xiao G, Piersigilli A, et al. Context-dependent roles of MDMX (MDM4) and MDM2 in breast cancer proliferation and circulating tumor cells. BCR. 2019;21(1):5. PubMed PMID: 30642351
- Li L, Zhang R, Li SJ. Long noncoding RNA SNHG14 promotes ovarian cancer cell proliferation and metastasis via sponging miR-219a-5p. Eur Rev Med Pharmacol Sci. 2019;23(10):4136–4142. PubMed PMID: 31173283
- Wang L, Yu M, Zhao S. lncRNA MEG3 modified epithelial-mesenchymal transition of ovarian cancer cells by sponging miR-219a-5p and regulating EGFR. J Cell Biochem. 2019;undefined(undefined):undefined. PubMed PMID: 31161607
- Rao C, Miao X, Zhao G, et al. MiR-219a-5p enhances cisplatin sensitivity of human non-small cell lung cancer by targeting FGF9. Biomed Pharmacother. 2019;114(undefined):108662. PubMed PMID: 30999114
- He Z, Chen L, Hu X, et al. Next-generation sequencing of microRNAs reveals a unique expression pattern in different types of pituitary adenomas. Endocr J. 2019 Aug 29;66(8):709–722. PubMed PMID: 31061247.
- Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24(17):2899–2908. PubMed PMID: 15838523
- Suliman M, Royds J, Cullen D, et al. Mdm2 and the p53 pathway in human pituitary adenomas. Clin Endocrinol (Oxf). 2001;54(3):317–325. PubMed PMID: 11298083
- Gentilin E, Tagliati F, Filieri C, et al. miR-26a plays an important role in cell cycle regulation in ACTH-secreting pituitary adenomas by modulating protein kinase Cδ. Endocrinology. 2013;154(5):1690–1700. PubMed PMID: 23525216
- Yu C, Li J, Sun F, et al. Expression and clinical significance of miR-26a and Pleomorphic Adenoma Gene 1 (PLAG1) in invasive pituitary adenoma. Med Sci Monit. 2016;22:(undefined):5101–5108. PubMed PMID: 28012286
- Liang HQ, Wang RJ, Diao CF, et al. The PTTG1-targeting miRNAs miR-329, miR-300, miR-381, and miR-655 inhibit pituitary tumor cell tumorigenesis and are involved in a p53/PTTG1 regulation feedback loop. Oncotarget. 2015;6(30):29413–29427. PubMed PMID: 26320179
- Yang Z, Zhang T, Wang Q, et al. Overexpression of microRNA-34a attenuates proliferation and induces apoptosis in pituitary adenoma cells via SOX7. Mol Ther Oncolytics. 2018;10(undefined):40–47. PubMed PMID: 30109259
- Trivellin G, Butz H, Delhove J, et al. MicroRNA miR-107 is overexpressed in pituitary adenomas and inhibits the expression of aryl hydrocarbon receptor-interacting protein in vitro. Am J Physiol Endocrinol Metab. 2012;303(6):E708–19. PubMed PMID: 22811466
- Wei Z, Zhou C, Liu M, et al. MicroRNA involvement in a metastatic non-functioning pituitary carcinoma. Pituitary. 2015;18(5):710–721. PubMed PMID: 25862551
- Butz H, Likó I, Czirják S, et al. MicroRNA profile indicates downregulation of the TGFβ pathway in sporadic non-functioning pituitary adenomas. Pituitary. 2011;14(2):112–124. PubMed PMID: 21063788
- Garbicz F, Mehlich D, Rak B, et al. Increased expression of the microRNA 106b~25 cluster and its host gene MCM7 in corticotroph pituitary adenomas is associated with tumor invasion and Crooke’s cell morphology. Pituitary. 2017;20(4):450–463. PubMed PMID: 28432562
- Momand J, Jung D, Wilczynski S, et al. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26(15):3453–3459. PubMed PMID: 9671804
- Wu D, Niu X, Tao J, et al. MicroRNA-379-5p plays a tumor-suppressive role in human bladder cancer growth and metastasis by directly targeting MDM2. Oncol Rep. 2017;37(6):3502–3508. PubMed PMID: 28498468
- Turbin DA, Cheang MC, Bajdik CD, et al. MDM2 protein expression is a negative prognostic marker in breast carcinoma. Mod Pathol. 2006;19(1):69–74. PubMed PMID: 16258514
- Patterson DM, Gao D, Trahan DN, et al. Effect of MDM2 and vascular endothelial growth factor inhibition on tumor angiogenesis and metastasis in neuroblastoma. Angiogenesis. 2011;14(3):255–266. PubMed PMID: 21484514
- Yao X, Gao H, Li C, et al. Analysis of Ki67, HMGA1, MDM2, and RB expression in nonfunctioning pituitary adenomas. J Neurooncol. 2017;132(2):199–206. PubMed PMID: 28255749