ABSTRACT
Cells organize themselves to maintain proper shape, structure, and size during growth and division for their cellular functions. However, how these cellular organizations coordinate with the cell cycle is not well understood. This review focuses on cell morphogenesis and size of the membrane-bound nucleus in the fission yeast Schizosaccharomyces pombe. Growth polarity, an important factor for cell morphogenesis, in rod-shaped fission yeast is restricted to the cell tips and dynamically changes depending on the cell cycle stage. Furthermore, nuclear size in fission yeast is proportional to the cell size, resulting in a constant ratio between nuclear volume and cellular volume (N/C ratio). This review summarizes the signaling pathway(s) involved in growth polarity control and key factors involved in N/C ratio control and provides their roles in coordination between cell organization and the cell cycle.
Graphical abstract
Coordination of cellular organization with the cell cycle in fission yeast. Two signaling pathways for cell polarity control and two factors for nuclear size control.
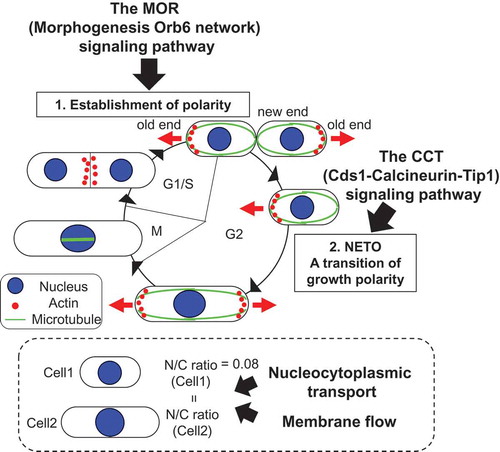
How cellular organizations which are important for cellular functions coordinate with the cell cycle is poorly understood. The fission yeast Schizosaccharomyces pombe is a simple unicellular organism [Citation1]. Its regular rod shape and genetic tractability make it a useful model system for investigating control mechanisms of a cellular organization such as cell morphogenesis and structures of membrane-bound organelles. This review focuses on cell morphogenesis and membrane-bound nuclear size in fission yeast and summarizes the signaling pathway(s) involved in the regulation of cell morphogenesis depending on the cell cycle stages ()) and key factors involved in control of nuclear size, which is proportional to cell size during the cell cycle ()) [Citation2].
Figure 1. Cell polarity of fission yeast is coordinated with the cell cycle (a) and its nuclear size is proportional to cell size (b), resulting in constant ratio between nuclear and cellular volume (N/C ratio) (c).
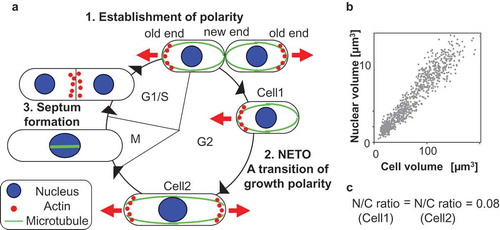
Fission yeast cells grow by extension at their cell ends and divide in their center to generate daughter cells of equal size with the same structure of membrane-bound organelles. The growth polarity dynamically changes at three stages of the cell cycle [Citation3,Citation4]: the initiation of monopolar growth following cell division, the transition of growth polarity called NETO (new end take off) [Citation5], and septum formation. After division, newly born cells grow in a monopolar manner only from the old end, which existed in the previous cell cycle. Upon NETO at a certain point in G2 phase, they initiate growth at the new end and grow in a bipolar manner ()). This growth polarity is maintained until entering mitosis. During monopolar and bipolar growth, actin is localized at the growing cell ends ()). Upon mitosis, growth ceases and actin at both cell ends disappears and is translocated to the cell middle where the septum formation occurs during cytokinesis ()). After cytokinesis, monopolar growth is redirected to the old cell end. Therefore, localization of actin is correlated with sites of polarized cell growth.
Role of the MOR (morphogenesis Orb6 network) signaling pathway in coordinating cell polarity control with the cell cycle
Essential components of the MOR
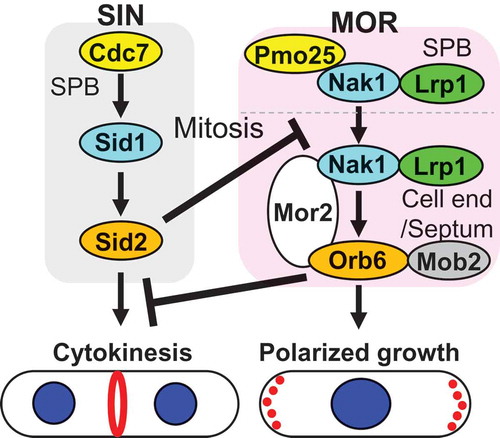
The MOR (Morphogenesis Orb6 network) signaling pathway in fission yeast is essential for the establishment of cell polarity following cytokinesis and the control of actin-based polarized growth during interphase. The MOR is analogous to the budding yeast regulation of Ace2 activity and morphogenesis (RAM) pathway [Citation6,Citation7] and the metazoan Hippo pathway [Citation8]. The MOR is composed of conserved proteins including Pmo25 (the MO25 family protein) [Citation9–Citation13], Nak1 (a Germinal Center kinase) [Citation9,Citation14,Citation15] and Nak1 regulator Lrp1 (Leucine-rich repeat protein) [Citation16,Citation17], all of which act upstream of Mor2 (Drosophila Furry/Fry-like protein) [Citation9,Citation18,Citation19], Orb6 (the NDR kinase) [Citation9,Citation20–Citation23], and Orb6 regulator Mob2 [Citation23] (, ). All of the MOR genes are essential for cell viability and their temperature-sensitive mutants at the restrictive temperature exhibit reduction in the kinase activity of Orb6, the most downstream kinase of the MOR, and completely lose cell polarity resulting in round cells [Citation9,Citation16,Citation24]. Orb6 kinase activity oscillates during the cell cycle: it is activated in early interphase following cytokinesis, maintains the high activity during interphase, and is inactivated upon mitosis [Citation9,Citation24]. This oscillation correlates with polarized growth: Orb6 kinase activity increases after cytokinesis, when cell polarity is established and is maintained following interphase, and the activity decreases upon mitosis, when polarized growth ceases.
Table 1. Components of the MOR and the SIN.
Role of the MOR components in Orb6 kinase activity
All the components of the MOR are essential for Orb6 kinase activity, which is required for actin localization at growing cell ends and polarized growth [Citation9–Citation23]. Orb6 localizes at growing cell ends and the division site and the localization is dependent on its interacting protein Mor2 [Citation9,Citation18]. Mor2 localization is similar to that of Orb6 and the localizations of Mor2 and Orb6 are interdependent [Citation9,Citation18]. On the other hand, Pmo25, Nak1, and Lrp1, upstream components of the MOR, localize to the SPBs and the division site [Citation9,Citation14–Citation17] (). Pmo25, the most upstream component of the MOR, interacts with Nak1 and is required for Nak1 kinase activity which is constant during the cell cycle [Citation9,Citation15]. Nak1 interacts with Lrp1 [Citation16,Citation17] and Mor2 [Citation9] and the Nak1–Mor2 interaction is required for Orb6 kinase activity [Citation24]. Fusion of Nak1 to Orb6 completely rescues loss of Orb6 kinase activity and cell polarity in mor2 mutant cells at the restrictive temperature, suggesting that Mor2 functions as a scaffold protein to promote activation of Orb6 [Citation24]. The fusion does not rescue a mutant of pmo25, consistent with the functional hierarchy between Pmo25 and Nak1 [Citation9] ().
What is a role of Pmo25 in the MOR? The SPB localization of Pmo25 and the kinase activities of Nak1 and Orb6 in interphase are under the control of the Cdc7 and Sid1 kinases in the septation initiation network (SIN) [Citation9], which is analogous to the budding yeast mitotic exit network (MEN) [Citation25] () and essential for actomyosin ring formation, ring constriction, and septum formation in the middle of the cell [Citation25]. This suggests that Pmo25 plays a connecting role between the SIN and the MOR. Indeed, Pmo25 physically and functionally interacts with Sid1 kinase in the SIN and Nak1 kinase in the MOR [Citation9,Citation26]. Further analysis is necessary to understand how Pmo25 connects between the SIN and the MOR.
Cross talk between the SIN and MOR pathways
The SIN is activated during mitosis to promote actomyosin ring assembly and cytokinesis [Citation25]. Insufficient activation of the SIN causes improper assembly of the actomyosin ring and failure of cytokinesis, generating multinucleated cells, whereas ectopic activation of the SIN promotes the formation of multiple septa at any point in the cell cycle [Citation25]. The SIN is therefore tightly controlled during the cell cycle. Interestingly, the ectopic activation of the SIN inhibits actin polarization toward growing cell ends through inactivation of the most downstream MOR component, Orb6 [Citation24,Citation25]. The mechanism of Orb6 kinase inhibition by activation of the SIN is that the SIN effector kinase Sid2 phosphorylates Nak1 kinase, and this phosphorylation blocks the interaction of Nak1 with the scaffold protein Mor2 [Citation17,Citation24]. Why does the SIN inhibit the MOR? Loss of SIN inhibition of the MOR causes defects in cytokinesis [Citation24], suggesting that the MOR-dependent polarized growth interferes with the cytokinetic apparatus (). Furthermore, loss of MOR activity allows SIN mutants to promote actomyosin ring constriction and complete cytokinesis [Citation24]. These studies reveal that cross talk between the SIN and the MOR is important for the completion of cytokinesis and coordination of actin reorganization during the cell cycle.
Downstream targets of the MOR
To understand the mechanisms of how the MOR controls cell morphogenesis and its coordination with the cell cycle, it is necessary to identify downstream targets of the MOR. One approach to search for these targets is to identify substrates of Orb6 kinase. One of the substrates is RNA-binding protein Sts5 [Citation27]. Phosphorylation of Sts5 by Orb6 promotes Sts5 interaction with 14-3-3 protein Rad24 and then this interaction inhibits recruitment of Sts5 into RNA granules and degradation of Sts5-bound mRNAs which include factors for polarized cell growth [Citation27]. Perturbation of this control by Orb6 influences cell morphology and the pattern of polarized growth depending on nutritional conditions [Citation27]. In addition to Sts5, Gef1, a guanine exchange factor (GEF) for Cdc42 GTPase, a key regulator of cell polarity, is a substrate of Orb6 kinase [Citation28]. Phosphorylation of Gef1 promotes Gef1 association with Rad24 to restrict Cdc42 activation only at the growing cell ends [Citation28]. A recent phosphoproteomics study [Citation29] also reveals multiple Orb6 kinase targets including Sec3 and Sec5, components of the exocyst complex which affect polarized growth during interphase. Identification of additional Orb6 substrates and functional analysis of them are necessary to elucidate mechanisms of the MOR-dependent cell polarity control and its coordination with the cell cycle.
The CCT (Cds1-calcineurin-Tip1) signaling pathway links the completion of DNA replication and the transition of growth polarity (NETO)
NETO, essential components of the CCT pathway and the role in NETO
How nuclear cell-cycle events are coordinated with cell polarization is poorly understood. At a certain point in the G2 phase of the fission yeast cell cycle, the growth pattern undergoes a transition from monopolar to bipolar growth, referred to as NETO [Citation5]. For initiation of NETO, cells need to execute two requirements: completion of DNA replication and attainment of a certain cell size [Citation5]. This indicates that there are signaling pathways monitoring the two requirements and controlling the timing of NETO [Citation5]. Although a number of mutants defective in NETO have been isolated and their roles in NETO characterized [Citation30,Citation31], the signaling pathways controlling NETO remain elusive.
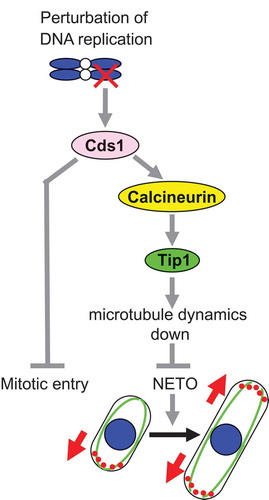
The CCT (Cds1-Calcineurin-Tip1) pathway in fission yeast is one of the signaling pathways and is crucial for a connection between the DNA replication checkpoint and NETO [Citation32]. The CCT pathway is composed of conserved proteins which are the checkpoint kinase Cds1 (CHK2 homologue) [Citation33,Citation34], a Ca2+/calmodulin-regulated type 2B protein phosphatase calcineurin [Citation35,Citation36], and a microtubule-plus-end tracking protein Tip1 (CLIP170 homologue) [Citation37,Citation38] (). When DNA replication is perturbed, activated Cds1 kinase delays both mitotic entry and the initiation of NETO [Citation32]. The Cds1 phosphorylates calcineurin, which subsequently dephosphorylates Tip1 serine-82 [Citation32]. Tip1 dephosphorylation is essential for the NETO delay that is accomplished by both a reduction in microtubule dynamics and accumulation of Tea1 (microtubule-associated cell polarity protein) [Citation39–Citation41] at the non-growing cell end [Citation32] (). Furthermore, calcineurin and Tip1, which function mainly in NETO delay, partially contribute to the maintenance of cell viability under the checkpoint activation [Citation32].
Downstream targets of Cds1 and calcineurin for the NETO delay
Search for kinases required for NETO using a haploid gene deletion library of non-essential protein kinases [Citation42] reveals that more than 30 kinases have a putative positive or a negative role in NETO and their relevant signaling pathways might be involved in the regulation of NETO [Citation43]. Further study of the kinases shows that Cki3 (a casein kinase 1γ homologue) is required not only for the NETO delay but also for maintenance of cell viability upon perturbed DNA replication [Citation44]. Cki3 functions downstream of Cds1 and calcineurin, and membrane localization of Cki3 regulated by palmitoyltransferase complex Erf2-Erf4 is essential for Cki3 kinase activity and NETO delay when the DNA replication checkpoint is activated [Citation44]. In addition to Cki3, three molecules (Trp1322/Ca2+ channel, Vcx1/antiporter, and Neo1/P-type ATPase transporter protein family) have been identified as regulators of NETO delay [Citation45]. Trp1322 and Vcx1, but not Neo1, act downstream of Cds1 and upstream of calcineurin and consistent with the hierarchy. Trp1322 and Vcx1 are required for activation of calcineurin upon DNA replication is perturbed [Citation45].
Nuclear size control: coordinating nuclear size with cell size
Membrane-bound organelles are maintained at an appropriate size during cell growth and division. The sizes of membrane-bound organelles are important for cell organization and function. However, little is known about the underlying mechanisms. The nucleus is an excellent model to study overall organelle growth and organelle size during cell growth as it is a simple shape and generally present in single copy within a cell. It has been reported that nuclear size correlates with cell size across a wide range of cell types and species [Citation46]. Budding and fission yeast cells maintain a nuclear size proportional to cell size, resulting in a constant ratio of nuclear volume to cellular volume (N/C ratio) [Citation2,Citation47]. In fission yeast, the N/C ratio is approximately 0.08 and remains constant throughout the cell cycle as cells grow [Citation2]. There is no clear increase in the ratio during or after DNA replication, indicating that DNA content is not a determinant of nuclear size. This is also confirmed by changing ploidy. Neither increases in ploidy nor a 16-fold increase in DNA content influence the N/C ratio [Citation2]. Furthermore, nuclear growth does not depend on DNA content in HeLa cells [Citation48].
What determines nuclear size? A study of multi-nucleated fission yeast cells reveals that the volume of each nucleus is proportional to that of its surrounding cytoplasm; closely located inner nuclei surrounded by less cytoplasm grow slower than the outer nuclei that are surrounded by a larger cytoplasmic volume [Citation2]. Effects of cytoplasm on nuclear size are observed in nuclear transplantation experiments of metazoan. When the nucleus of an erythrocyte cell is injected into the cytoplasm of a larger HeLa cell, the injected nucleus increases in size [Citation49,Citation50]. In vitro study of Xenopus egg extracts also demonstrates that the available space surrounding a nucleus determines the nuclear growth rate and the nuclear size [Citation51]. These observations suggest that available cytoplasmic space surrounding the nucleus or cytoplasmic factors influence nuclear size in eukaryotic cells.
The fission yeast is genetically tractable and a useful system to study nuclear size control in vivo and a gene deletion collection is available for systematic genomic screens [Citation52]. A genetic screen for fission yeast non-essential gene deletion mutants altering N/C ratio has been conducted and mutants with high N/C ratios were identified [Citation53]. The screen indicates that bulk nucleocytoplasmic transport and regulation of lipid biosynthesis are factors in nuclear size control [Citation53].
It has been reported that perturbed nucleocytoplasmic transport induced by prolonged inhibition of nuclear export of proteins by treatment with leptomycin B, an inhibitor of the exportin Crm1 [Citation54], causes nuclear size increase in both fission yeast [Citation2] and mammalian cells [Citation55]. Two genes encoding protein components of the same complex involved in mRNA export from the nucleus were identified in the fission yeast N/C ratio screen [Citation53]. A third component of this complex, Rae1, is an essential gene and its temperature-sensitive mutant rae1-167 exhibits an N/C ratio increase of approximately 50% compared to that of wild type (0.08) when the cells are incubated at restrictive temperature for 4 h. A significant increase of N/C ratio is detectable within 1 h of the temperature shift and this increase is caused by approximately 2-fold increased nuclear growth rate [Citation53]. What stimulates nuclear growth in rae1-167 mutant cells? In most cells of rae1-167 mutant, poly(A)+RNA accumulates in the nucleus within 15 min of a temperature shift to the restrictive temperature [Citation53,Citation56]. In addition to the rapid accumulation of poly(A)+RNA, within 30 min of shift to the restrictive temperature, rae1-167 cells exhibit significant nuclear protein accumulation compared to that of wild type cells in which distribution of proteins is uniform [Citation53]. Inhibition of transcription or protein synthesis can suppress the high N/C ratio observed in rae1-167 mutant cells following temperature shift [Citation53], indicating that continued RNA and protein synthesis are required for the N/C ratio increase. Mass spectrometry and microarray analyses reveal that a large number of proteins and mRNAs are accumulated in rae1-167 nuclei at the restrictive temperature for 1 h. The nuclear-accumulated proteins are normally located in the nucleus or shuttling between the nucleus and cytoplasm [Citation53]. So, it is more likely that bulk accumulation of general proteins and mRNAs following perturbation of nucleocytoplasmic transport changes the N/C ratio.
Mechanisms of nuclear size scaling have been identified in a range of systems. A study of Xenopus egg extracts of two different species implicates that the nucleocytoplasmic transport factors, importin α and Ntf2, and the import cargo lamin B3 in the determination of nuclear size [Citation57]. Altering lamin concentrations in Xenopus egg extracts and mammalian cells influences their nuclear size [Citation58]. Furthermore, a study of Xenopus egg extracts and human cells demonstrates that cytoplasmic levels of importin α regulate the scaling of both spindle and nuclear size which are coordinated with cell size [Citation59]. A study of mammalian cells also shows the density of the nuclear pore complex (NPC) and nuclear import capacity affect the nuclear import of lamin and nuclear size scale [Citation60].
Yeasts do not have nuclear lamin proteins but display nuclear size control, suggesting that there are other key players associated with the nuclear membrane that have not been identified. A screen for fission yeast essential gene deletion mutants has been carried out and identified mutants with high and low N/C ratios [Citation61]. The screen implicates LINC complexes in nuclear size control [Citation61]. LINC complexes are conserved protein complexes that connect nuclear membrane-associated chromatin to the cytoskeleton [Citation62]. In fission yeast, the KASH domain containing integral outer nuclear membrane protein Kms2 and the SUN domain containing integral inner nuclear membrane protein Sad1 form the complexes [Citation63]. The screen identified kms2 and sad1 mutants as altered N/C ratio mutants and both mutants exhibit enlarged N/C ratio, suggesting that connection of chromatin to the cytoskeleton by LINC complexes may have an important role on nuclear size control [Citation61]. Other conserved integral inner nuclear membrane protein Lem2 is identified as a barrier to membrane flow between the nucleus and other parts of the cellular membrane structures [Citation64]. Deletion of Lem2 increases membrane flow in to and out of the nucleus in response to changes in lipid synthesis and nucleocytoplasmic transport, resulting in nuclear size alteration [Citation64]. The endoplasmic reticulum protein Lnp1 can functionally compensate for lack of Lem2, indicating that Lnp1 acts as a barrier to membrane flow at the ER [Citation64]. These results demonstrate that this system regulating membrane flow is part of the mechanism that maintains nuclear size proportional to overall cellular membrane content and thus to cell size. This system could be used in the eukaryotic subcellular membrane network.
Acknowledgments
I would like to thank Dr. Dai Hirata (Asahi Shuzo Sake Brewing Co.), Takashi Toda (Hiroshima University), Sir Paul Nurse (The Crick Institute) for continuous support and helpful discussion; Dr. Tokichi Miyakawa (Hiroshima University) and Dr. Masaki Mizunuma (Hiroshima University) for their advice with encouragement; all the collaborators, all current and past students who participated in the projects I described here.
Disclosure statement
No potential conflict of interest was reported by the author.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
Additional information
Funding
References
- Hayles J, Nurse P. A journey into space. Nat Rev Mol Cell Biol. 2001;2:647–656.
- Neumann FR, Nurse P. Nuclear size control in fission yeast. J Cell Biol. 2007;179:593–600.
- Mitchison JM. Physiological and cytological methods for Schizosaccharomyces pombe. In: Prescott DM, editor. Methods in cell physiology. New York, NY: Academic Press. 1970. p. 131–165.
- Marks J, Hagan I, Hymas JS. Growth polarity and cytokinesis in fission yeast: the role of the cytoskeleton. J Cell Sci. 1986;5:229–241.
- Mitchison JM, Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985;75:357–376.
- Nelson B, Kurischoko C, Horecka J, et al. RAM: a conserved signaling network that regulates Ace2p transcriptional activity and polarized morphogenesis. Mol Biol Cell. 2003;14:3782–3803.
- Weiss EL. Mitotic exit and separation of mother and daughter cells. Genetics. 2012;192:1165–1202.
- Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015;25:499–513.
- Kanai M, Kume K, Miyahara K, et al. Fission yeast MO25 protein is localized at SPB and septum and is essential for cell morphogenesis. EMBO J. 2005;24:3012–3025.
- Mendoza M, Redemann S, Brunner D. The fission yeast MO25 protein functions in polar growth and cell separation. Eur J Cell Biol. 2005;84:915–926.
- Miyamoto H, Matsushiro A, Nozaki M. Molecular cloning of a novel mRNA sequence expressed in cleavage stage mouse embryo. Mol Reprod Dev. 1993;34:1–7.
- Nozaki M, Onishi Y, Togashi S, et al. Molecular characterization of the Drosophila Mo25 gene, which is conserved among Drosophila, mouse and yeast. DNA Cell Biol. 1996;15:505–509.
- Karos M, Fischer R. Molecular characterization of HymA, an evolutionarily highly conserved and highly expressed protein of Aspergillus nidulans. Mol Gen Genet. 1999;260:510–521.
- Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230.
- Leonhard K, Nurse P. Ste20/GCK kinase Nak1/Orb3 polarizes the actin cytoskeleton in fission yeast during the cell cycle. J Cell Sci. 2005;118:1033–1044.
- Kume K, Kubota S, Koyano T, et al. Fission yeast leucine-rich repeat protein Lrp1 is essential for cell morphogenesis as a component of the morphogenesis Orb6 network (MOR). Biosci Biotechnol Biochem. 2013;77:1086–1091.
- Gupta S, Mana-Capelli S, McLean JR, et al. Identification of SIN pathway targets reveals mechanisms of crosstalk between NDR kinase pathways. Curr Biol. 2013;23:333–338.
- Hirata D, Kishimoto N, Suda M, et al. Fission yeast Mor2/Cps12, a protein similar to Drosophila Furry, is essential for cell morphogenesis and its mutation induces Wee1-dependent G2 delay. EMBO J. 2002;21:4863–4874.
- Cong J, Geng W, He B, et al. The furry gene of Drosophila is important for maintaining the integrity of cellular extensions during morphogenesis. Development. 2001;128:2793–2802.
- Verde F, Wiley DJ, Nurse P. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc Natl Acad Sci USA. 1998;95:7526–7531.
- Hergovich A, Stegert MR, Schimiz D, et al. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006;7:253–264.
- Tamaskovic R, Bichsel SJ, Hemmings BA. NDR family of AGC kinases - essential regulators of the cell cycle and morphogenesis. FEBS Lett. 2003;546:73–80.
- Hou MC, Wiley DJ, Verde F, et al. Mob2p interacts with the protein kinase Orb6p to promote coordination of cell polarity with cell cycle progression. J Cell Sci. 2003;116:125–135.
- Ray S, Kume K, Gupta S, et al. The mitosis-to-interphase transition is coordinated by cross talk between the SIN and MOR pathways in Schizosaccharomyces pombe. J Cell Biol. 2010;190:793–804.
- Hotz M, Barral Y. The mitotic exit network: new turns on old pathways. Trends Cell Biol. 2014;24:145–152.
- Kume K, Goshima T, Miyahara K, et al. A method for Pmo25-associated kinase assay in fission yeast: the activity is dependent on two GC kinases Nak1 and Sid1. Biosci Biotechnol Biochem. 2007;71:615–617.
- Nunez I, Rodriguez PM, Wiley DJ, et al. Spatial control of translation repression and polarized growth by conserved NDR kinase Orb6 and RNA-binding protein Sts5. eLife. 2016;5:e14216.
- Das M, Nunez I, Rodriguez M, et al. Phosphorylation-dependent inhibition of Cdc42 GEF Gef1 by 14-3-3 protein Rad24 spatially regulates Cdc42 GTPase activity and oscillatory dynamics during cell morphogenesis. Mol Biol Cell. 2015;26:3520–3532.
- Tay YD, Leda M, Spanos C, et al. Fission yeast NDR/LATS kinase Orb6 regulates exocytosis via phosphorylation of the exocyst complex. Cell Rep. 2019;26:1654–1667.
- Martin SG, Chang F. New end take off: regulating cell polarity during the fission yeast cell cycle. Cell Cycle. 2005;4:1046–1049.
- Huisman SM, Brunner D. Cell polarity in fission yeast: a matter of confining, positioning, and switching growth zones. Semin Cell Dev Biol. 2011;22:799–805.
- Kume K, Koyano T, Kanai M, et al. Calcineurin ensures a link between the DNA replication checkpoint and microtubule-dependent polarized growth. Nat Cell Biol. 2011;13:234–242.
- Rhind N, Russell P. Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J Cell Sci. 2000;113:3889–3896.
- Lindsay HD, Griffiths DJF, Edwards RJ, et al. S-phase specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998;12(3):382–395.
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370.
- Yoshida T, Toda T, Yanagida M. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J Cell Sci. 1994;107:1725–1735.
- Brunner D, Nurse P. CLIP170-like tip1p spatially organizes microtubular dynamics in fission yeast. Cell. 2000;102:695–704.
- Busch K, Brunner D. The microtubule plus end-tracking proteins mal3p and tip1p cooperate for cell-end targeting of interphase microtubules. Curr Biol. 2004;14:548–559.
- Mata J, Nurse P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell. 1997;89:939–949.
- Martin SG, McDonald WH, Yates JR, et al. Tea4p links microtubule plus ends with the formin For3p in the establishment of cell polarity. Dev Cell. 2005;8:479–491.
- Tatebe H, Shimada K, Uzawa S, et al. Wsh3/Tea4 is a novel cell-end factor essential for bipolar distribution of Tea1 and protects cell polarity under environmental stress in S. pombe. Curr Biol. 2005;15:1006–1015.
- Bimbo A, Jia Y, Poh SL, et al. Systematic deletion analysis of fission yeast protein kinases. Eukaryot Cell. 2005;4:799–813.
- Koyano T, Kume K, Konishi M, et al. Search for kinases related to transition of growth polarity in fission yeast. Biosci Biotechnol Biochem. 2010;74:1129–1133.
- Koyano T, Konishi M, Martin SG, et al. Casein kinase 1γ ensures monopolar growth polarity under incomplete DNA replication downstream of Cds1 and calcineurin in fission yeast. Mol Cell Biol. 2015;35:1533–1542.
- Kume K, Hashimoto T, Suzuki M, et al. Identification of three signaling molecules required for calcineurin-dependent monopolar growth induced by the DNA replication checkpoint in fission yeast. Biochem Biophys Res Commun. 2017;491:883–889.
- Cooklin EG. Cell size and nuclear size. J Exp Zool. 1912;12:1–98.
- Jorgensen P, Edgington NP, Schneider BL, et al. The size of the nucleus increases as yeast cells grow. Mol Biol Cell. 2007;18:3523–3532.
- Maeshima K, Iino H, Hihara S, et al. Nuclear size, nuclear pore number and cell cycle. Nucleus. 2011;2:113–118.
- Harris H. The reactivation of the red cell nucleus. J Cell Sci. 1967;2:23–32.
- Gurdon JB. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976;36:523–540.
- Hara Y, Merten CA. Dynein-based accumulation of membranes regulates nuclear expansion in Xenopus laevis egg extracts. Dev Cell. 2015;33:562–575.
- Kim DU, Hayles J, Kim D, et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol. 2010;28:617–623.
- Kume K, Cantwell H, Neumann FR, et al. A systematic genomic screen implicates nucleocytoplasmic transport and membrane growth in nuclear size control. PLoS Genet. 2017;13:e1006767.
- Kudo N, Matsumori N, Taoka H, et al. Leptomycine B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–9117.
- Ganguly A, Bhattachrjee C, Bhave M, et al. Perturbation of nucleocytoplasmic transport affects size of nucleus and nucleolus in human cells. FEBS Lett. 2016;590:631–643.
- Brown JA, Bharathi A, Ghosh A, et al. A mutation in the Schizosaccharomyces pombe rae1 gene causes defects in poly(A)+RNA export and in the cytoskeleton. J Biol Chem. 1995;270:7411–7419.
- Levy DL, Heald R. Nuclear size is regulated by importin alpha and Ntf2 in Xenopus. Cell. 2010;143:288–298.
- Jevtic P, Edens LJ, Li X, et al. Concentration-dependent effects of nuclear lamins on nuclear size in Xenopus and mammalian cells. J Biol Chem. 2015;290:27557–27571.
- Brownlee C, Heald R. Importin α partitioning to the plasma membrane regulates intracellular scaling. Cell. 2019;176:805–815.
- Jevtic P, Schibler AC, Wesley CC, et al. The nucleoporin ELYS regulates nuclear size by controlling NPC number and nuclear import capacity. EMBO Rep. 2019;20:pii: e47283.
- Cantwell H, Nurse P. A systematic genetic screen identifies essential factors involved in nuclear size control. PLoS Genet. 2019;15:e1007929.
- Rothballer A, Schwartz TU, Kutay U. LINCing complex functions at the nuclear envelope: what the molecular architecture of the LINC complex can reveal about its function. Nucleus. 2013;4:29–36.
- King MC, Drivas TG, Blobel G. A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell. 2008;134:427–438.
- Kume K, Cantwell H, Burrell A, et al. Nuclear membrane protein Lem2 regulates nuclear size through membrane flow. Nat Commun. 2019;10:1871.