ABSTRACT
Juvenile idiopathic arthritis (JIA) is the most common cause of uveitis in children. While symptoms are usually mild, persistent eye inflammation could lead to severe complications and impaired vision. It is essential that JIA patients at risk are diagnosed with uveitis early, receive adequate treatment, and avoid developing complications, such as cataract, glaucoma, and amblyopia. The purpose of this mini-review is to summarize the screening strategies and clinical management for JIA-associated uveitis (JIA-U) as well as the current state of molecular markers linked to this condition. Because glaucoma is one of the most common causes of visual loss in JIA-U, special focus will be put on this serious complication. We conclude by describing the current evidence regarding the long-standing question of whether chronic anterior uveitis without arthritis may be the same disease entity as JIA-U.
Epidemiology
Uveitis primarily affects adults, with children accounting for approximately 5–10%.Citation1 Most pediatric cases (69–95%) in developed countries are non-infectious, and often occur in conjunction with other systemic inflammatory conditions. About half of all cases (41–47%) are associated with Juvenile Idiopathic Arthritis (JIA), the most prevalent disease in children with uveitis.Citation1–3
A meta-analysis from 2019 reported an approximate incidence of uveitis in JIA (JIA-U) of 13%, varying from 19% in Northern European countries to 5% in Southeast Asia.Citation4 There is uncertainty about whether geographical variation in uveitis prevalence among JIA cases is caused by genetic factors, environmental factors, or differences in uveitis screening practices.Citation5 Uveitis prevalence also varies considerably between disease categories of JIA, which is mostly absent in patients with systemic arthritis and rheumatoid factor-positive polyarthritis, but may affect half of the cases with oligoarthritis in some cohort studies.Citation6 The incidence of uveitis is higher in female JIA patients, which is not an independent predictor variable, but rather related to a positive antinuclear antibodies (ANA) status and onset of oligoarthritis at young age.Citation6–8 JIA-U development is most likely in the first 4 years after arthritis onset, but cases have been reported even after 10 years.Citation6,Citation9 About 10% of children are first diagnosed with uveitis, with JIA developing later.Citation5,Citation9,Citation10
Clinical presentation
The most common form of JIA-U is chronic non-granulomatous anterior uveitis, which is often asymptomatic in the early stages of the disease. Uveitis usually occurs bilaterally, involving both eyes simultaneously or starting unilaterally and rapidly followed by inflammation in the other eye within a few months. Patients can suffer from severe inflammation in both eyes or have a different severity in each eye. During active uveitis, a mild to moderate degree of fine anterior chamber cells are present, with or without non-granulomatous keratic precipitates or fine endothelial inflammatory deposits. A less typical manifestation of active JIA-U is the presence of granulomatous keratic precipitates and posterior segment involvement.Citation11
In contrast, acute anterior uveitis is characterized by significant symptoms, including a painful red eye and moderate to marked anterior chamber cells, and tends to occur in individuals with enthesitis-related arthritis who test positive for HLA-B27 (±15% of JIA-U cases).Citation5,Citation10
Screening
Regular ophthalmologic screening of JIA patients is essential for detecting and treating chronic anterior uveitis at an early stage, thereby preventing vision-threatening complications including cataracts, synechiae, and glaucoma.Citation9,Citation10,Citation12 This screening involves a combination of age-appropriate visual acuity testing, measurement of intraocular pressure and slit-lamp examination by the ophthalmologist, and should commence shortly after onset of arthritis.Citation5,Citation10,Citation12 Following a 2007 nation-wide study, the German Uveitis in Childhood Study Group proposed a set of screening frequencies for JIA-U based on the current International League of Associations for Rheumatology’s categories of JIA, ANA status, age at JIA onset, and JIA disease duration.Citation9 This study also identified patients at a relatively low, intermediate, and high risk for JIA-U, with a recommended screening interval of 3 months for the high-risk group and less frequent screening intervals for the other risk groups (). Although these recommendations are widely adopted, some have suggested adhering to shorter intervals for the first 6 months (every 2 months) following arthritis onset in patients with JIA who are most likely to develop uveitis (i.e., highest risk group),Citation13 in line with previous guidelines, including the 2006 British Society for Pediatric and Adolescent Rheumatology (BSPAR)/Royal College of Ophthalmology screening guidelinesCitation5,Citation10,Citation12 (but not the 2019 American College of Rheumatology/Arthritis Foundation screening guidelines.Citation14 The BSPAR guidelines also recommend bimonthly screening for the duration of 6 months after tapering or discontinuing immunosuppressive drugs for treating arthritis, such as methotrexate. Also, patients should be advised to monitor their unilateral vision regularly after discharge from screening, and when to seek medical attention.Citation5,Citation10,Citation12 In the near future, screening frequencies might be guided by more personalized prediction models that include molecular data such as inflammatory and genetic biomarkers, including erythrocyte sedimentation rate, S100A12, and the YST-amino acid motif in the HLA-DRB1 gene.Citation8,Citation15
Table 1. Summary of guidelines for JIA-U screening.
Genes and biomarkers
JIA is both a multifactorial and polygenetic disease, however, pathophysiology is still poorly understood.Citation16 A variety of immune cell subtypes may play a role in the underlying mechanisms of JIA-U. While a comprehensive understanding of the changes in immune cells in JIA-U is lacking, previous studies have shown that patients show activated peripheral monocyte populations and altered frequencies of distinct functional T cell subsets.Citation17,Citation18 Accumulating evidence suggests that B cells play an important role in JIA-U. It is consistent with clinical findings that ANAs are a classical risk factor for JIA-U, as antibodies are produced by plasma cells (differentiated B cells).Citation15 In addition, small studies have revealed increased abundance of CD20+ B cells in eye tissues of cases with JIA-U.Citation19,Citation20 Although rituximab, a monoclonal antibody targeting CD20 on B cells, is reported to be effective in small case series in treating refractory JIA-uveitis, it is not commonly used as a treatment modality.Citation21 According to RNA-sequencing analysis of circulating B cells in JIA-U, memory B cell-associated gene circuits are involved and may contribute to the disease process.Citation22 In addition, transcriptomic and proteomic analysis of iris tissue in JIA-U patients revealed increased expression for immunoglobulin genes and B cell-associated proteins.Citation23 Several case series presented histological and immunohistochemical reports of enucleated eyes and iridectomy specimens from patients with JIA-U at different stages of disease. These revealed an initial predominance of plasma cells (terminally differentiated B-cells) and significant numbers of CD20+ B cells.Citation15
The genetic predisposition for JIA-U involves genes that are implicated in immune pathways.Citation15 The amino acid motif (YST) at position 10–12 in the HLA-DRB1 gene, encoding the beta subunit of HLA-DR, was found to be the primary association for uveitis susceptibility in girls in a genome-wide association study of >500 JIA cases with over 4-year clinical follow-up.Citation24 The YST motif also appears in alleles previously associated with JIA-U.Citation24–26 This genetic marker for uveitis demonstrates sexual dimorphism that is conceptually consistent with the differences in clinical presentation between males and females; there is an apparent female predisposition to JIA-U, and the disease course in males is typically more complicated. According to the GWAS study, the YST motif was associated with uveitis in an unusually high proportion of girls. This makes it tempting to speculate that female patients without the YST motif have a very low risk for uveitis. For confirmation that absence of YST is predictive of low risk in girls, a prospective study with an ophthalmologic follow-up of at least 4 years is needed.
The YST-motif can be detected by polymerase chain reaction (PCR) using sequence-specific oligonucleotide probes. Additionally, TaqMan® SNP genotyping can be used, which uses the 5’ nuclease activity of Taq polymerase to generate a fluorescent signal for single nucleotide polymorphisms (i.e., position 11 in HLA-DRB1), since the YST-motif shows strong linkage disequilibrium and commercial assays are available. However, next-generation sequencing is increasingly used for HLA typing, and this method will type the variable exon sequences that encode the amino terminal domains that contain the YST-motif in great detail and allows also the detection of potentially relevant rare alleles that influence the function of this motif in the HLA-DR molecule.
In patients with JIA-U, serum autoantibodies directed against nuclear factors, such as histones, and chromatin are more common.Citation27,Citation28 These autoantibodies have also been shown to bind ocular tissues (including iris/ciliary tissues) and may not be exclusively targeted against nuclear antigens.Citation29 Other factors often found elevated in the blood of patients are acute phase proteins and innate stress mediators.Citation30,Citation31 In oligo- and polyarticular JIA-patients, elevated erythrocyte sedimentation rate is predictive for the risk of uveitis.Citation32 In addition, an increased level of the proinflammatory S100A12 (≥250 ng/ml) at JIA-onset is associated with an increased risk for uveitis in JIA.Citation8
Treatment
Managing JIA-U requires long-term treatment and a multidisciplinary approach, including collaboration between an ophthalmologist and pediatric immunologist. To prevent vision-threatening complications, the primary goal of treatment is to achieve stable remission as soon as possible. The recommended treatment target is to achieve zero cells in the anterior chamber in both eyes (SUN Criteria, anterior chamber cell grade equal to 0).Citation33,Citation34 A variety of treatments have been explored over the years to control inflammation and preserve visual function. The goal of therapy should be to minimize the risk of complications caused both by inflammation itself and by topical corticosteroid use, which can have iatrogenic effects.
For JIA-U, topical corticosteroids have historically been the mainstay of treatment, but today they are only used as an initial treatment. The reason is that prolonged and excessive use of topical corticosteroids may lead to severe adverse effects, including glaucoma, cataracts, and increased intraocular pressure.Citation12 As a rescue medication, short-term high dose systemic corticosteroids can be used. Note that peribulbar corticosteroid injections cause severe complications, including cataract and glaucoma development, and are not a standard treatment for these patients. Consequently, corticosteroids are not recommended for maintenance therapy in JIA-U, and the treatment transition in recent decades has been toward corticosteroid-sparing agents, which have proven to be valuable tools in lowering complications in retrospective studies.Citation5,Citation35 Methotrexate (MTX) is a corticosteroid-sparing agent (also known as conventional disease-modifying antirheumatic drugs (cDMARDs)) and is taken orally or subcutaneously, usually at a dose of 10–15 mg/m2/week.Citation36–38 Folic or folinic acid supplementation is recommended to prevent MTX side effects.Citation39 MTX has demonstrated efficacy in controlling ocular inflammation and reducing the need for corticosteroids in JIA-U. Based on a systematic review, improvement in disease severity was seen in 70–75% of patients on MTX.Citation40 Other cDMARDs can be used if MTX side effects present as gastrointestinal complaints (or less frequently elevated liver enzymes, hair loss, mouth sores, or rash).Citation41 In theory, MTX can be substituted with mycophenolate or azathioprine in these cases, but their efficacy varies more strongly.Citation42,Citation43
A “step-up approach” may be necessary when there is insufficient response to DMARDs or when uveitis severity is significant, particularly in young children with a complicated disease course. This step-up involves starting humanized monoclonal antibodies that inhibit cytokine signaling pathways, including inhibitors of tumor necrosis factor alpha (TNF-alpha) and interleukin-6 (IL-6).Citation44 According to positive results and safety profile from two international JIA-U trials (the SYCAMORE and the ADJUVITE trial), the anti-TNF-alpha agent adalimumab is considered to be the first choice biological agent for moderate-to-severe JIA-U.Citation45,Citation46 Infliximab is the second most frequently used anti-TNF-alpha agent and may also be used to switch in cases that do not respond to adalimumab therapy. Collectively, the switch toward an alternative anti-TNF-alpha agent in these cases may improve disease management for about three-quarters of patients.Citation47,Citation48 Testing for anti-adalimumab antibodies should be considered when JIA-U relapses under adalimumab therapy.Citation49 The use of low dose MTX in combination of an anti-TNF-alpha agent may reduce the development of antibodies.Citation50 Recurrences of uveitis may also be caused by decreased efficacy or decreased adherence due to the use of biosimilars. Side effects are more frequently described with the use of biosimilars, with pain at injection administration being the most common complaint.Citation51 Humanized monoclonal antibodies directed against the IL-6 receptor (tocilizumab) have been shown to be effective in JIA-U cases where anti-TNF-alpha therapy failed due to anti-drug antibodies.Citation14,Citation52,Citation53 However, despite all the current treatment options, it is still challenging to achieve stable remission without corticosteroids in some children. For these cases, novel therapeutic approaches remain an unmet need. Inhibitors of Janus kinase (JAK), anti-CD20 B cell therapy, and anti-CTLA4 (abatacept)Citation44 are some promising approaches, but the current data is limited for JIA-U. As of now, adalimumab has the most favorable risk profile among biologics for pediatric uveitis, with an adverse event rate of 10.60 per patient year (most common are infections and infestations) and a low serious adverse event rate (0.29 per patient year).Citation54
Complications
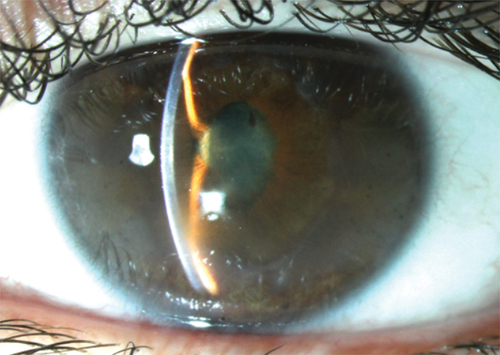
Uveitis in children with JIA has a high risk for development of complications because the asymptomatic nature of this type of uveitis makes it prone to diagnostic delay and undertreatmentCitation5,Citation55,Citation56 (). Consequently, diagnosis relies heavily on intensive screening of JIA cases. Severe complications are present at first presentation in 20–45% of JIA-U patients.Citation57 The most frequent vision-threatening complication in JIA-U is cataract.Citation57–59 The development of cataract might be related to prolonged treatment with topical corticosteroids.Citation60 Other frequent complications are band keratopathy (16–32%), glaucoma (8–19%), and posterior synechiae (25–29%). Less frequent complications are ocular hypotony (3–9%), cystoid macular edema (3–6%), optic disc swelling (3–4%), and epiretinal membrane formation (0–4%).Citation57–59 By adulthood, approximately 30% of eyes become visually impaired,Citation61 with secondary glaucoma as the most common cause.Citation2
In the past years, different studies have identified several factors that are associated with a poor prognosis, such as uveitis developing prior to arthritis in approximately 10–14% of patients, due to late detection.Citation9,Citation62 Similarly, Woreta et al. reported that active intraocular inflammation at presentation is a risk factor for at least one ocular complication during the initial visit.Citation63 In several studies, male patients were found to be associated with a complicated courseCitation64–66 and an increased risk for cataract surgery, development of cystoid macula edema and papillitis.Citation66 Male sex has also shown to be an independent risk factor for poor visual prognosis in patients with JIA-U compared to girls.Citation66,Citation67 Also, a short interval between arthritis and uveitis diagnosis is reported as a risk factor for uveitis in children with JIA.Citation56,Citation65,Citation68,Citation69 In a recent multicenter, prospective cohort study in patients with JIA-U, 39% had one or more ocular complications attending the 18-year ophthalmology visit. Children with a shorter time interval between diagnosis of arthritis and uveitis had a higher risk for developing complications.Citation56 In addition, uveitis at a younger age tend to have a greater risk for severe complications.Citation67,Citation70 In the study of Holland et al., age 3 years or younger at baseline was associated with the development of complications.Citation67 Topical corticosteroid treatment in young children might lead to earlier development of cataract and glaucoma.Citation10,Citation60 Lastly, severity of uveitis and complications at initial visit are prognostic factors for both later complications and vision loss.Citation9,Citation59,Citation63,Citation67,Citation71 Baseline cell grade ≥1+ cells have a high relative risk for development of new complications.Citation67 The presence of ≥1+ anterior chamber flare at the initial visit was associated with 20/50 or worse and 20/200 or worse vision.Citation67 Repeated laser flare photometry measurements may be of additional value. For example, a decrease in flare values by ≥50% of the initial value, 1 month after treatment intensification, is an early-stage prognostic factor for positive outcome.Citation72,Citation73 In addition, the objective measurements may also be useful for study purposes. For example, the previously mentioned ADJUVITE study used the anterior chamber flare values by the laser flare photometry as definition of active uveitis and as primary outcome.Citation45
Early start with immunomodulatory therapy seems to be protective for the development of complications.Citation35,Citation71 The incidence of complications has decreased during the recent years, probably because of the improvement of the systemic treatment for JIA and JIA-U.Citation35,Citation58 The start with MTX <1 year after onset of JIA-U postponed the development of cataract requiring surgery.Citation74 The development of novel immunomodulatory treatments and early start, in combination with the standardized uveitis screening, might improve visual outcomes.
As mentioned earlier, cataract is the most common complication that causes reversible vision loss. All surgeries in JIA-U are challenging because of the young age and underlying inflammation. The uveitis must be completely controlled for >2 months with systemic immunomodulatory therapy,Citation75–77 in order to lower the risk of recurrence of uveitis, anterior capsule phimosis and cystoid macular oedema after cataract surgery. Before considering intraocular lens placement, risk factors such as age, refractory nature of JIA-U, presence of other complications and condition of the other eye need to be taken into account to optimize the long-term visual outcome.Citation78–80
Secondary glaucoma
Secondary glaucoma is one of the most serious and potentially blinding complications in pediatric uveitis.Citation2,Citation81 Based on a retrospective analysis in a tertiary care referral center, secondary glaucoma developed in 26% of the children with uveitis,Citation81 with glaucoma developing most frequently in non-infectious uveitis (20% in JIA-U and 28% in idiopathic anterior uveitis).Citation81,Citation82
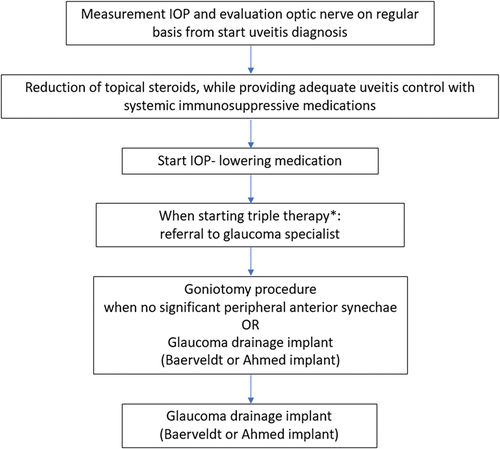
Uveitic glaucoma develops as a result of increased resistance to the aqueous humor outflow by several mechanisms, including mechanical obstruction due to entrapment of inflammatory cells and debris in the trabecular meshwork, swelling of the meshwork itself, and secondary scarring or collapse of the trabecular meshwork and/or Schlemm’s canal. Also, treatment with corticosteroids may increase outflow resistance by modifying the trabecular meshwork.Citation83 The proportion of eyes with elevated intraocular pressure (IOP) and glaucoma increases during the first 5 years after the uveitis diagnosis. After 5 years, the incidence of glaucoma remained stable.Citation81 Regular IOP evaluation for a longer period of time is mandatory for early detection of an IOP increase, taking into account the risk profile for developing uveitic glaucoma.Citation68 In JIA-U, ANA positivity, anterior segment complications (cataract, band keratopathy, and/or posterior synechiae) at diagnosis, IOP > 21 mmHg during the first uveitis remission and a higher amount of topical corticosteroids use are associated with an increased risk for glaucoma surgery. In addition, the necessity of using three types of IOP-lowering medication indicates a high risk for glaucoma surgery, as in a retrospective study the majority (68%) of eyes had glaucoma surgery within 1 year of starting a third type of medication.Citation84
With the introduction of Icare rebound tonometry, the IOP can be measured in young children during the normal clinical exam.Citation85 Additional evaluation is difficult in children, whereas visual field tests do not provide reliable responses in younger children. An optical coherence tomography (OCT) could evaluate the retinal nerve fiber layer and/or the macular ganglion cell layer; however, no normative database for children is available. Moreover, aspects such as optic disc swelling due to papillitis, epiretinal membrane, and dense vitreous opacities may distort the results, making OCT scans difficult to interpret. Repeatedly performing OCT scans per patient could provide useful information on changes in the optic disc over time.
The first step in the case of ocular hypertension is pharmacologically lowering the IOP and minimizing topical corticosteroids (). In 30–61% of the children with uveitic glaucoma, a surgical intervention is mandatory.Citation86–88 Based on recent research, the advice is to start with a goniotomy as a first intervention,Citation89,Citation90 because of the relative simplicity of the procedure, its high success rate and low risk profile. When peripheral anterior synechiae are present, especially in the area to be treated, or failure develops, it is advisable to use a glaucoma drainage implant (GDI) (most well-known devices are Baerveldt GDI and Ahmed GDI) (). GDI’s adequately reduce the IOP, but their complication profile is a relative disadvantage, with severe IOP fluctuations, bleb and tube problems in the long term.Citation91,Citation92 The use of a trabeculectomy or cyclodiode laser is less advisable in JIA-U, due to a relatively high amount of re-interventions needed after the primary surgery.Citation93,Citation94
Idiopathic chronic anterior uveitis and JIA-U: The same disease?
Idiopathic chronic anterior uveitis (iCAU) is a subgroup of pediatric uveitis patients that shows similarities with JIA-U. ICAU accounts for approximately 30–40% of the children with anterior uveitis and cannot be distinguished clinically from JIA-U, based on ophthalmological characteristics.Citation67,Citation95,Citation96 In contrast with JIA-U, no routine ophthalmologic screening for uveitis occurs in iCAU patients, resulting in more vision-threatening ocular complications compared to JIA-U because of late detection. However, long-term visual outcome is similar between the two groups when adequately treated with immunomodulatory therapy.Citation82 In particular, ANA-seropositive iCAU shows similarities with JIA-U with regard to uveitis characteristics, clinical course, and response to systemic treatment.Citation97 Therefore, it is not surprising that recent therapy guidelines and the Childhood Arthritis and Rheumatology Research Alliance recommend that JIA-U and iCAU should be treated in a similar manner.Citation98–101
Furthermore, the finding of shared genetic risk alleles for both JIA-U and iCAU (HLA-DQB1 × 04:02 and HLA-DRB1 × 08:01), leads to the hypothesis that iCAU is identical to JIA-U without arthritis.Citation26 While iCAU could be the first manifestation of cases that later develop JIA, only a minority of the patients with an onset of anterior uveitis will develop arthritis at a later stage.Citation26,Citation66,Citation97 However, the development of arthritis might be suppressed due to the common use of immunomodulatory therapy in JIA. Haasnoot et al. revealed that iCAU could not be distinguished from JIA-U by comparison of multi-cytokine profiles in aqueous humor,Citation102 which was consistent with the observation of previous studies.Citation19,Citation30,Citation103 Since the collection of aqueous humor is invasive and not preferable in children, Angeles-Han et al. studied proteomics in tears.Citation104 They discovered 29 differentially expressed proteins in a small cohort of children with JIA-U and iCAU that were involved in the extracellular exosomes. These proteins may provide clues to intrinsic differences between JIA-U and iCAU, despite their similar clinical phenotypes and shared genetic alleles. Investigation of serum of pediatric uveitis patients suggests, however, common immune activity, regulatory processes, and a common adaptive disease mechanism for both disease entities.Citation17,Citation30
Discussion and conclusion
Over the past decade, new insights have been gained into the pathogenesis, risk factors, and treatment strategies of JIA-U. In particular, adalimumab treatment in addition to MTX has resulted in better visual prognosis with fewer structural complications.Citation35,Citation105 Due to the risks for cataract and glaucoma, topical corticosteroids are no longer recommended for the maintenance treatment of JIA-U. The use of other biologicals, such as tocilizumab, has also been proven effective in cases refractory to anti-TNFα therapy. Several studies have shown that inflammation control is directly related to visual outcome.Citation71 Despite this, there are still patients who are refractory to biological therapy, or who developed antibodies against it.
Despite progress regarding the immunopathogenesis of JIA, many questions remain unanswered. A major question is whether biomarkers can predict patient response to treatment. Through the use of these biomarkers, early remission could be achieved with fewer side effects from multiple treatment strategies and biomarkers may aid in the prediction of uveitis in JIA patients. Predicting more accurately which patients are not at risk would reduce the need for prolonged and frequent screenings by eye specialists. A reduction in hospital visits and burden on families would result from this.
Although progress has been made regarding JIA-U treatment and pathogenesis in the last decade, complications remain prevalent, with secondary glaucoma being the most common cause of visual loss, despite intensive immunomodulatory therapy. Thus, prolonged inflammation and topical corticosteroid therapy should be avoided as much as possible to prevent structural damage. To estimate the risk of complications during follow-up, the risk profile of the patient outlined in our review can be considered, and measurements by laser flare photometry might be valuable.Citation73 It is important to treat these patients with a multidisciplinary team of ophthalmologists and pediatric rheumatologists.
Acknowledgments
This review is a summary of largely previously published work. The manuscript, have never been presented or published elsewhere.
Disclosure statement
J.H. de Boer participates in a trial from Roche, not related to JIA associated uveitis. The other authors report there are no competing interests to declare.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in English primary and referral centers. Am J Ophthalmol. 2003;135(5):676–680. doi:10.1016/S0002-9394(02)02148-7.
- de Boer J, Wulffraat N, Rothova Br AJ. Visual loss in uveitis of childhood. Br J Ophthalmol. 2003;87(7):879–884. doi:10.1136/bjo.87.7.879.
- Hettinga YM, de Groot-Mijnes JDF, Rothova A, de Boer JH. Infectious involvement in a tertiary center pediatric uveitis cohort. Br J Ophthalmol. 2015;99(1):103–107. doi:10.1136/bjophthalmol-2014-305367.
- Hayworth JL, Turk MA, Nevskaya T, Pope JE. The frequency of uveitis in patients with juvenile inflammatory rheumatic diseases. Joint Bone Spine. 2019;86(6):685–690. doi:10.1016/j.jbspin.2019.06.001.
- Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Clin Immunol. 2020;211:108322. doi:10.1016/j.clim.2019.108322.
- van Straalen JW, Giancane G, Amazrhar Y, et al. A clinical prediction model for estimating the risk of developing uveitis in patients with juvenile idiopathic arthritis. Rheumatology. 2021;60(6):2896–2905. doi:10.1093/rheumatology/keaa733.
- van Straalen JW, van Stigt Thans M, Wulffraat NM, de Roock S, Swart JF. A diagnostic prediction model for separating juvenile idiopathic arthritis and chronic musculoskeletal pain syndrome. J Pediatr. 2022;251:164–171.e6. doi:10.1016/j.jpeds.2022.04.029.
- Tappeiner C, Klotsche J, Sengler C, et al. Risk factors and biomarkers for the occurrence of uveitis in juvenile idiopathic arthritis. Arthritis Rheumatol. 2018;70(10):1685–1694. doi:10.1002/art.40544.
- Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology. 2007;46(6):1015–1019. doi:10.1093/rheumatology/kem053.
- Sen ES, Dick AD, Ramanan AV. Uveitis associated with juvenile idiopathic arthritis. Nat Rev Rheumatol. 2015;11(6):338–348. doi:10.1038/nrrheum.2015.20.
- Vitale AT, Graham E, de Boer JH. Juvenile idiopathic arthritis-associated uveitis: clinical features and complications, risk factors for severe course, and visual outcome. Ocul Immunol Inflamm. 2013;21(6):478–485. doi:10.3109/09273948.2013.815785.
- Clarke SLN, Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol. 2016;14(1):27. doi:10.1186/s12969-016-0088-2.
- Chia A, Lee V, Graham EM, Edelsten C. Factors related to severe uveitis at diagnosis in children with juvenile idiopathic arthritis in a screening program. Am J Ophthalmol. 2003;135(6):757–762. doi:10.1016/S0002-9394(03)00225-3.
- Angeles‐Han ST, Ringold S, Beukelman T, et al. 2019 American college of rheumatology/arthritis foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis–associated uveitis. Arthritis Care Res (Hoboken). 2019;71(6):703–716. doi:10.1002/acr.23871.
- Haasnoot A-MJW, Kuiper JJW, de Boer JH. Predicting uveitis in juvenile idiopathic arthritis: from biomarkers to clinical practice. Expert Rev Clin Immunol. 2019;15(6):657–666. doi:10.1080/1744666X.2019.1593139.
- Vastert SJ, Bhat P, Goldstein DA. Pathophysiology of JIA-associated uveitis. Ocul Immunol Inflamm. 2014;22(5):414–423. doi:10.3109/09273948.2014.926937.
- Walscheid K, Neekamp L, Heiligenhaus A, et al. Increased circulating proinflammatory T lymphocytes in children with different forms of anterior uveitis: results from a pilot study. Ocul Immunol Inflamm. 2019;27(5):788–797. doi:10.1080/09273948.2018.1467464.
- Walscheid K, Neekamp L, Heiligenhaus A, et al. Peripheral blood monocytes reveal an activated phenotype in pediatric uveitis. Clin Immunol. 2018;190:84–88. doi:10.1016/j.clim.2017.09.014.
- Kalinina Ayuso V, van Dijk MR, de Boer JH. Infiltration of plasma cells in the iris of children with ANA-positive anterior uveitis. Invest Ophthalmol Vis Sci. 2015;56(11):6770–6778. doi:10.1167/iovs.15-17351.
- Kalinina Ayuso V, Makhotkina N, van Tent-Hoeve M, et al. Pathogenesis of juvenile idiopathic arthritis associated uveitis: the known and unknown. Surv Ophthalmol. 2014;59(5):517–531. doi:10.1016/j.survophthal.2014.03.002.
- Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab). Rheumatology (Oxford). 2011;50(8):1390–1394. doi:10.1093/rheumatology/ker107.
- Wennink RAW, Pandit A, Haasnoot A-MJW, et al. Whole transcriptome analysis reveals heterogeneity in B cell memory populations in patients with juvenile idiopathic arthritis-associated uveitis. Front Immunol. 2020;11:2170. doi:10.3389/fimmu.2020.02170.
- Wildschütz L, Ackermann D, Witten A, et al. Transcriptomic and proteomic analysis of iris tissue and aqueous humor in juvenile idiopathic arthritis-associated uveitis. J Autoimmun. 2019;100:75–83. doi:10.1016/j.jaut.2019.03.004.
- Haasnoot A-MJW, Schilham MW, Kamphuis S, et al. Identification of an amino acid motif in HLA – DR β1 that distinguishes uveitis in patients with juvenile idiopathic arthritis. Arthritis Rheumatol. 2018;70(7):1155–1165. doi:10.1002/art.40484.
- Angeles-Han ST, McCracken C, Yeh S, et al. HLA associations in a cohort of children with juvenile idiopathic arthritis with and without uveitis. Invest Ophthalmol Vis Sci. 2015;56(10):6043–6048. doi:10.1167/iovs.15-17168.
- Wennink RAW, de Boer JH, Hiddingh S, et al. Next-generation HLA sequence analysis uncovers shared risk alleles between clinically distinct forms of childhood uveitis. Invest Ophthalmol Vis Sci. 2021;62(9):19. doi:10.1167/iovs.62.9.19.
- Murray KJ, Szer W, Grom AA, et al. Antibodies to the 45 kDa DEK nuclear antigen in pauciarticular onset juvenile rheumatoid arthritis and iridocyclitis: selective association with MHC gene. J Rheumatol. 1997;24(3):560–567.
- Neuteboom GH, Hertzberger-ten Cate R, de Jong J, van den Brink HG, Feltkamp TE. Antibodies to a 15 kD nuclear antigen in patients with juvenile chronic arthritis and uveitis. Invest Ophthalmol Vis Sci. 1992;33:1657–1660.
- Walscheid K, Hennig M, Heinz C, et al. Correlation between disease severity and presence of ocular autoantibodies in juvenile idiopathic arthritis-associated uveitis. Invest Ophthalmol Vis Sci. 2014;55(6):3447–3453. doi:10.1167/iovs.13-13444.
- Walscheid K, Heiligenhaus A, Holzinger D, et al. Elevated S100A8/A9 and S100A12 serum levels reflect intraocular inflammation in juvenile idiopathic arthritis-associated uveitis: results from a pilot study. Invest Ophthalmol Vis Sci. 2015;56(13):7653–7660. doi:10.1167/iovs.15-17066.
- van den Broek T, Hoppenreijs E, Meerding J, et al. Cytokine profiling at disease onset: support for classification of young antinuclear antibody-positive patients as a separate category of juvenile idiopathic arthritis. Ann Rheum Dis. 2015;74(2):470–472. doi:10.1136/annrheumdis-2014-206424.
- Haasnoot AJW, van Tent-Hoeve M, Wulffraat NM, et al. Erythrocyte sedimentation rate as baseline predictor for the development of uveitis in children with juvenile idiopathic arthritis. Am J Ophthalmol. 2015;159(2):372–7.e1. doi:10.1016/j.ajo.2014.11.007.
- Bou R, Adán A, Borrás F, et al. Clinical management algorithm of uveitis associated with juvenile idiopathic arthritis: interdisciplinary panel consensus. Rheumatol Int. 2015;35(5):777–785. doi:10.1007/s00296-015-3231-3.
- Leverette TJ. Key terms for contracts with grocery firms. Hospitals (Lond). 1980;54:86–91.
- Wennink RAW, Kalinina Ayuso V, Pameijer EM, et al. Improved clinical outcomes in patients with juvenile idiopathic arthritis associated uveitis in the last decade. Acta Ophthalmol. 2022;100(7):781–787. doi:10.1111/aos.15097.
- Giannini EH, Brewer EJ, Kuzmina N, et al. Methotrexate in Resistant Juvenile Rheumatoid Arthritis. N Engl J Med. 1992;326(16):1043–1049. doi:10.1056/NEJM199204163261602.
- Ruperto N, Murray KJ, Gerloni V, et al. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum. 2004;50(7):2191–2201. doi:10.1002/art.20288.
- Reiff A, Shaham B, Wood BP, et al. High dose methotrexate in the treatment of refractory juvenile rheumatoid arthritis. Clin Exp Rheumatol. 1995;13(1):113–118.
- Shea B, Swinden MV, Ghogomu ET, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. J Rheumatol. 2014;41(6):1049–1060. doi:10.3899/jrheum.130738.
- Simonini G, Paudyal P, Jones GT, Cimaz R, Macfarlane GJ. Current evidence of methotrexate efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach. Rheumatology. 2013;52(5):825–831. doi:10.1093/rheumatology/kes186.
- Ferrara G, Mastrangelo G, Barone P, et al. Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting. Pediatr Rheumatol. 2018;16(1):46. doi:10.1186/s12969-018-0255-8.
- Doycheva D, Deuter C, Stuebiger N, Biester S, Zierhut M. Mycophenolate mofetil in the treatment of uveitis in children. Br J Ophthalmol. 2007;91(2):180–184. doi:10.1136/bjo.2006.094698.
- Goebel JC, Roesel M, Heinz C, et al. Azathioprine as a treatment option for uveitis in patients with juvenile idiopathic arthritis. Br J Ophthalmol. 2011;95(2):209–213. doi:10.1136/bjo.2009.173542.
- Chen JL, Abiri P, Tsui E. Recent advances in the treatment of juvenile idiopathic arthritis-associated uveitis. Ther Adv Ophthalmol. 2021;13:2515841420984572. doi:10.1177/2515841420984572.
- Quartier P, Baptiste A, Despert V, et al. ADJUVITE: a double-blind, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated anterior uveitis. Ann Rheum Dis. 2018;77(7):1003–1011. doi:10.1136/annrheumdis-2017-212089.
- Ramanan AV, Dick AD, Benton D, et al. A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE trial). Trials. 2014;15(1):14. doi:10.1186/1745-6215-15-14.
- Simonini G, Druce K, Cimaz R, Macfarlane GJ, Jones GT. Current evidence of anti–tumor necrosis factor α treatment efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach of individual drugs. Arthritis Care Res. 2014;66(7):1073–1084. Preprint at. doi:10.1002/acr.22214.
- Simonini G, Katie D, Cimaz R, Macfarlane GJ, Jones GT. Does switching anti-TNFα biologic agents represent an effective option in childhood chronic uveitis: the evidence from a systematic review and meta-analysis approach. Semin Arthritis Rheum. 2014;44(1):39–46. doi:10.1016/j.semarthrit.2014.03.001.
- Leinonen ST, Aalto K, Kotaniemi KM, Kivelä TT. Anti-adalimumab antibodies in juvenile idiopathic arthritis-related uveitis. Clin Exp Rheumatol. 2017;35:1043–1046.
- Schmeling H. A combination of etanercept and methotrexate for the treatment of refractory juvenile idiopathic arthritis: a pilot study. Ann Rheum Dis. 2001;60(4):410–412. doi:10.1136/ard.60.4.410.
- Kearsley-Fleet L, Baildam E, Beresford MW, et al. P091 Outcomes following switching from originator to biosimilar product in children and young people with JIA. Ann Rheum Dis. 2023;82(Supplement_2):285.2–285. doi:10.1093/rheumatology/kead104.132.
- Iannone C, Marelli L, Costi S, et al. Tocilizumab in juvenile idiopathic arthritis associated uveitis, a narrative review. Children (Basel). 2023;10(3):434. doi:10.3390/children10030434.
- Ramanan AV, Dick AD, Guly C, et al. Tocilizumab in patients with anti-TNF refractory juvenile idiopathic arthritis-associated uveitis (APTITUDE): a multicentre, single-arm, phase 2 trial. Lancet Rheumatol. 2020;2(3):e135–e141. doi:10.1016/S2665-9913(20)30008-4.
- Li Y, Mao X, Tang X, Mao H. Efficacy and safety of anti-TNFα therapy for uveitis associated with juvenile idiopathic arthritis: a systematic review and meta-analysis. Rheumatol Ther. 2021;8(2):711–727. doi:10.1007/s40744-021-00296-x.
- Heiligenhaus A, Klotsche J, Tappeiner C, et al. Predictive factors and biomarkers for the 2-year outcome of uveitis in juvenile idiopathic arthritis: data from the Inception Cohort of Newly diagnosed patients with Juvenile Idiopathic Arthritis (ICON-JIA) study. Rheumatology (Oxford). 2019;58(6):975–986. doi:10.1093/rheumatology/key406.
- Rypdal V, Glerup M, Songstad NT, et al. Uveitis in juvenile idiopathic arthritis: 18-year outcome in the population-based Nordic cohort study. Ophthalmology. 2021;128(4):598–608. doi:10.1016/j.ophtha.2020.08.024.
- Heiligenhaus A, Heinz C, Edelsten C, Kotaniemi K, Minden K. Review for disease of the year: epidemiology of juvenile idiopathic arthritis and its associated uveitis: the probable risk factors. Ocul Immunol Inflamm. 2013;21(3):180–191. doi:10.3109/09273948.2013.791701.
- Cann M, Ramanan AV, Crawford A, et al. Outcomes of non-infectious paediatric uveitis in the era of biologic therapy. Pediatr Rheumatol. 2018;16(1):51. doi:10.1186/s12969-018-0266-5.
- Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143(5):840–846.e2. doi:10.1016/j.ajo.2007.01.033.
- Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2010;117(7):1436–1441. doi:10.1016/j.ophtha.2009.12.003.
- Haasnoot AMJW, Vernie LA, Rothova A, et al. Impact of juvenile idiopathic arthritis associated uveitis in early adulthood. PLoS One. 2016;11(10):e0164312. doi:10.1371/journal.pone.0164312.
- Paroli MP, Speranza S, Marino M, Pirraglia MP, Pivetti-Pezzi P. Prognosis of juvenile rheumatoid arthritis-associated uveitis. Eur J Ophthalmol. 2003;13(7):616–621. doi:10.1177/112067210301300704.
- Woreta F, Thorne JE, Jabs DA, Kedhar SR, Dunn JP. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;143(4):647–655.e1. doi:10.1016/j.ajo.2006.11.025.
- Edelsten C, Lee V, Bentley CR, Kanski JJ, Graham EM. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. British J Ophthalmol. 2002;86(1):51–56. doi:10.1136/bjo.86.1.51.
- Zulian F, Martini G, Falcini F, et al. Early predictors of severe course of uveitis in oligoarticular juvenile idiopathic arthritis. J Rheumatol. 2002;29(11):2446–2453.
- Kalinina Ayuso V, ten Cate HAT, van der Does P, Rothova A, de Boer JH. Male gender as a risk factor for complications in uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2010;149(6):994–999.e5. doi:10.1016/j.ajo.2010.01.016.
- Holland GN, Denove CS, Yu F. Chronic anterior uveitis in children: clinical characteristics and complications. Am J Ophthalmol. 2009;147(4):667–678.e5. doi:10.1016/j.ajo.2008.11.009.
- Sabri K, Saurenmann RK, Silverman ED, Levin AV. Course, complications, and outcome of juvenile arthritis–related uveitis. J Aapos. 2008;12(6):539–545. doi:10.1016/j.jaapos.2008.03.007.
- Chen CS, Roberton D, Hammerton ME. Juvenile arthritis-associated uveitis: visual outcomes and prognosis. Can J Ophthalmol. 2004;39(6):614–620. doi:10.1016/S0008-4182(04)80026-7.
- BenEzra D, Cohen E, Behar-Cohen F. Uveitis and juvenile idiopathic arthritis: a cohort study. Clin Ophthalmol. 2007;1:513–518.
- Gregory AC, Kempen JH, Daniel E, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the systemic immunosuppressive therapy for eye diseases study. Ophthalmology. 2013;120(1):186–192. doi:10.1016/j.ophtha.2012.07.052.
- Orès R, Terrada C, Errera M-H, et al. Laser flare photometry: a useful tool for monitoring patients with juvenile idiopathic arthritis-associated uveitis. Ocul Immunol Inflamm. 2022;30(1):118–128. doi:10.1080/09273948.2020.1792511.
- Davis JL, Dacanay LM, Holland GN, et al. Laser flare photometry and complications of chronic uveitis in children. Am J Ophthalmol. 2003;135(6):763–771. doi:10.1016/S0002-9394(03)00315-5.
- Sijssens KM, Rothova A, Van De Vijver DAMC, Stilma JS, De Boer JH. Risk factors for the development of cataract requiring surgery in uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;144(4):574–579.e1. doi:10.1016/j.ajo.2007.06.030.
- Magli A, Forte R, Rombetto L, Alessio M. Cataract management in juvenile idiopathic arthritis: simultaneous versus secondary intraocular lens implantation. Ocul Immunol Inflamm. 2014;22(2):133–137. doi:10.3109/09273948.2013.834062.
- Foster CS, Rashid S. Management of coincident cataract and uveitis. Curr Opin Ophthalmol. 2003;14(1):1–6. doi:10.1097/00055735-200302000-00001.
- Bohnsack BL, Freedman SF. Surgical outcomes in childhood uveitic glaucoma. Am J Ophthalmol. 2013;155(1):134–142. doi:10.1016/j.ajo.2012.07.008.
- Sijssens KM, Los LI, Rothova A, et al. Long-term ocular complications in aphakic versus pseudophakic eyes of children with juvenile idiopathic arthritis-associated uveitis. British J Ophthalmol. 2010;94(9):1145–1149. doi:10.1136/bjo.2009.167379.
- Yangzes S, Seth N, Singh R, et al. Long-term outcomes of cataract surgery in children with uveitis. Indian, J Ophthalmol. 2019;67(4):490–495. doi:10.4103/ijo.IJO_846_18.
- Mehta S, Linton MM, Kempen JH. Outcomes of cataract surgery in patients with uveitis: a systematic review and meta-analysis. Am J Ophthalmol. 2014;158(4):676–692.e7. doi:10.1016/j.ajo.2014.06.018.
- Sijssens KM, Rothova A, Berendschot TTJM, de Boer JH. Ocular hypertension and secondary glaucoma in children with uveitis. Ophthalmology. 2006;113(5):853–859.e2. doi:10.1016/j.ophtha.2006.01.043.
- Kouwenberg CV, Wennink RAW, Shahabi M, et al. Clinical course and outcome in pediatric idiopathic chronic anterior uveitis. Am J Ophthalmol. 2022;241:198–205. doi:10.1016/j.ajo.2022.04.015.
- Kaur S, Kaushik S, Singh Pandav S. Pediatric uveitic glaucoma. J Curr Glaucoma Pract. 2013;7(3):115–117. doi:10.5005/jp-journals-10008-1147.
- van Meerwijk CLLI, Wieringa WG, de Boer JH, Jansonius NM, Los LI. Factors associated with glaucoma surgery in pediatric non-infectious uveitis. Ocul Immunol Inflamm. 2023;1–6. doi:10.1080/09273948.2023.2166849.
- Flemmons MS, Hsiao Y-C, Dzau J, et al. Icare rebound tonometry in children with known and suspected glaucoma. J Aapos. 2011;15(2):153–157. doi:10.1016/j.jaapos.2010.11.022.
- Gautam Seth N, Yangzes S, Thattaruthody F, Singh R, Bansal R, Raj S, Kaushik S, Gupta V, Pandav SS, Ram J, Gupta A. Glaucoma secondary to uveitis in children in a tertiary care referral center. Ocul Immunol Inflamm. 2019;27(3):456–464. doi:10.1080/09273948.2017.1411517. Epub 2018 Feb 2. PMID: 29394120.
- Merayo-Lloves J, Power WJ, Rodriguez A, Pedroza-Seres M, Stephen Foster C. Secondary glaucoma in patients with uveitis. Ophthalmologica. 1999;213(5):300–304. doi:10.1159/000027443.
- Heinz C, Koch JM, Zurek-Imhoff B, Heiligenhaus A. Prevalence of uveitic secondary glaucoma and success of nonsurgical treatment in adults and children in a tertiary referral center. Ocul Immunol Inflamm. 2009;17(4):243–248. doi:10.1080/09273940902913035.
- Iannucci V, Manni P, Mecarelli G, et al. Childhood uveitic glaucoma: complex management in a fragile population. Applied Sciences. 2023;13(4):2205. doi:10.3390/app13042205.
- Meerwijk CLLIV, Edema AB, Rijn LJV, Los LI, Jansonius NM. Goniotomy for non-infectious uveitic glaucoma in children. J Clin Med. 2023;12(6):2200. doi:10.3390/jcm12062200.
- Zhou M, Wang W, Huang W, Chen S. A nonrandomized controlled study of inflammatory response between uveitic glaucoma and other refractory glaucoma following Ahmed glaucoma valve implantation. Zhonghua Shiyan Yanke Zazhi/Chin J Exp Ophthalmol. 2015;33:241–245.
- Kalinina Ayuso V, Scheerlinck LM, de Boer JH. The effect of an Ahmed glaucoma valve implant on corneal endothelial cell density in children with glaucoma secondary to uveitis. Am J Ophthalmol. 2013;155(3):530–535. doi:10.1016/j.ajo.2012.09.001.
- Wiese K, Heiligenhaus A, Heinz C. Trabekulektomie bei juveniler idiopathischer Arthritis-assoziierter Uveitis: Langzeitergebnisse beim kindlichen Sekundärglaukom. Ophthalmologe. 2014;111(4):330–338. doi:10.1007/s00347-013-2888-9.
- Heinz C, Koch JM, Heiligenhaus A. Transscleral diode laser cyclophotocoagulation as primary surgical treatment for secondary glaucoma in juvenile idiopathic arthritis: high failure rate after short term follow up. Br J Ophthalmol. 2006;90(6):737–740. Preprint at doi:10.1136/bjo.2005.085936.
- Tugal-Tutkun I. Pediatric uveitis. J Ophthalmic Vis Res. 2011;6:259–269.
- Ferrara M, Eggenschwiler L, Stephenson A, et al. The challenge of pediatric uveitis: tertiary referral center experience in the United States. Ocul Immunol Inflamm. 2019;27(3):410–417. doi:10.1080/09273948.2017.1420202.
- Heiligenhaus A, Klotsche J, Niewerth M, et al. Similarities in clinical course and outcome between juvenile idiopathic arthritis (JIA)-associated and ANA-positive idiopathic anterior uveitis: data from a population-based nationwide study in Germany. Arthritis Res Ther. 2020;22(1):81. doi:10.1186/s13075-020-02166-3.
- Heiligenhaus A, Minden K, Tappeiner C, et al. Update of the evidence based, interdisciplinary guideline for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Semin Arthritis Rheum. 2019;49(1):43–55. doi:10.1016/j.semarthrit.2018.11.004.
- Angeles‐Han ST, Lo MS, Henderson LA, et al. Childhood arthritis and rheumatology research alliance consensus treatment plans for juvenile idiopathic arthritis–associated and idiopathic chronic anterior uveitis. Arthritis Care Res (Hoboken). 2019;71(4):482–491. doi:10.1002/acr.23610.
- Foeldvari I, Maccora I, Petrushkin H, et al. New and updated recommendations for the treatment of juvenile idiopathic arthritis–associated uveitis and idiopathic chronic anterior uveitis. Arthritis Care Res (Hoboken). 2023;75(5):975–982. doi:10.1002/acr.24963.
- Solebo AL, Rahi JS, Dick AD, et al. Areas of agreement in the management of childhood non-infectious chronic anterior uveitis in the UK. British J Ophthalmol. 2020;104(1):11–16. doi:10.1136/bjophthalmol-2018-313789.
- Haasnoot A-MJW, Kuiper JJW, Hiddingh S, et al. Ocular fluid analysis in children reveals interleukin-29/Interferon-λ1 as a biomarker for juvenile idiopathic arthritis–associated uveitis. Arthritis Rheumatol. 2016;68(7):1769–1779. doi:10.1002/art.39621.
- Kalinina Ayuso V, de Boer JH, Byers HL, et al. Intraocular biomarker identification in uveitis associated with juvenile idiopathic arthritis. Invest Ophthalmol Vis Sci. 2013;54(5):3709–3720. doi:10.1167/iovs.12-10865.
- Angeles-Han ST, Yeh S, Patel P, et al. Discovery of tear biomarkers in children with chronic non-infectious anterior uveitis: a pilot study. J Ophthalmic Inflamm Infect. 2018;8(1):17. doi:10.1186/s12348-018-0156-5.
- Ramanan AV, Dick AD, Jones AP, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med. 2017;376(17):1637–1646. doi:10.1056/NEJMoa1614160.