?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Synchrony effects regarding mood diurnal fluctuations (i.e., better mood at optimal and worse mood at suboptimal times-of-day, corresponding to the interaction between chronotype* time-of-day) have been studied in adolescents and adults. However, evidence in children is lacking. We investigated the interactive effect of chronotype and time-of-day on primary school children’s momentary emotional states, in a naturalistic setting. From an initial pool of 298 3rd/4th graders (7–11 years-old), 134 Morning (M)-type and Evening (E)-type children were selected using the parental-report Children ChronoType Questionnaire (CCTQ). Potential covariates were assessed based on parental questionnaires. Students completed emotional states’ measures on the first (9 a.m.) and last lessons (4 p.m.) of the school day, in a counterbalanced order. Given the intercorrelations between emotional measures, a composite score of momentary emotional states was determined. There was a small-to-moderate significant interactive effect of chronotype*time-of-day in the overall momentary emotional states score. M/E-types showed better overall momentary emotional states when tested at their optimal time-of-day. Chronotype or time-of-day main effects were non-significant, and the overall momentary emotional states score did not correlate with sleep or psychopathological symptoms. In the present study, children overall momentary emotional states in a naturalistic setting varied depending on chronotype combined with time-of-day.
1. Introduction
The biological clock is essential for the regulation of several psychological and biological functions that exhibit circadian rhythmicity (e.g. mood) (Jankowski and Ciarkowska Citation2008; Jeong Jeong et al. Citation2015; Antypa et al. Citation2016; Cox and Olatunji Citation2019; Montaruli et al. Citation2021). Individual differences in biological rhythms allow for the categorization of individuals according to circadian typologies, also known as chronotype or morningness-eveningness (Gelbmann et al. Citation2012; Au and Reece Citation2017; Bai et al. Citation2021). It has been argued (e.g., Chiu et al. Citation2017) that a distinction must be made between the two concepts. Randler et al. (Citation2017) define morningness-eveningness as a personal preference regarding bedtimes and wake times, whereas chronotype is measured considering these self-reported times on week and free days. However, chronotype can generally be considered synonymous with morningness-eveningness (see, for example, Putilov Citation2017). Thus, despite referring to time-of-day preferences, the term “chronotype” will be used throughout for brevity and consistency. Chronotype can be defined as an individual characteristic which reflects timing or phase differences in underlying circadian rhythms. Based on these differences, individuals can be classified as morning, intermediate, or evening chronotypes. People with a morning chronotype (M-types, also called “larks”) exhibit an earlier sleep-wake schedule and a phase advance (i.e., earlier peaks) in behavioral and physiological functions, contrary to evening-types (E-types, also called “owls”). Intermediate types (I-types) do not have a clear preference for earlier or later times of the day and fall between these two extremes (Biss and Hasher Citation2012; Dagys et al. Citation2012; Díaz-Morales and Escribano Citation2014; Antúnez et al. Citation2015; Arbabi et al. Citation2015; Díaz-Morales et al. Citation2015). Chronotype has been typically measured with self-report questionnaires (Levandovski et al. Citation2013; Au and Reece Citation2017; Randler et al. Citation2017; Cox and Olatunji Citation2019) and considered an indirect measure of circadian rhythm (Levandovski et al. Citation2013). Despite the lack of consensus in the literature regarding the use of chronotype questionnaires as measures of individual circadian phase (Levandovski et al. Citation2013), the scores obtained in these questionnaires have strong to moderate associations with objective measures of circadian rhythmicity (Haraden et al. Citation2017).
In the last decades, previous literature has focused on synchrony and asynchrony effects of chronotype and time-of-day (i.e., moment of the day on which the assessment is conducted) on several aspects of an individual’s diurnal functioning. A synchrony effect can be defined as better functioning (e.g., mood) at the preferred or optimal time-of-day, near or at an individual’s peak arousal (e.g., M-types exhibiting better functioning in the morning) and inferior functioning at non-preferred or suboptimal time-of-day (e.g., E-types exhibiting worse functioning in the morning) (Nowack and Van Der Meer Citation2018). Contrarily, an asynchrony effect occurs when an individual exhibits a better functioning at a non-optimal time-of-day (Hahn et al. Citation2012; Carciofo et al. Citation2014; Díaz-Morales and Escribano Citation2014; Randler et al. Citation2014).
Chronotype and time-of-day synchrony effects have largely been investigated in relation to cognition and performance, but not in-depth in relation to emotional functioning, specifically in respect to diurnal mood fluctuations, despite time-of-day being known to impact psychological functioning (Foster and Kreitzman Citation2017) and chronotype being considered “a modulator of time-of-day effects” (Escribano and Díaz-Morales Citation2014, p.761).
Additionally, very few studies have examined the combined influence of chronotype and time-of-day on momentary emotional states diurnal fluctuations in children, with the majority of research being conducted on adults or adolescents, possibly due to the assumption that most children are M-types or display strong morning tendencies (Randler et al. Citation2009; Zimmermann Citation2016; Haraden et al. Citation2017).
In adolescents and adults, previous research focusing on the synchrony effect of chronotype and time-of-day on emotional state diurnal fluctuations has yield mixed results, with some studies supporting and several other studies not supporting the presence of a synchrony effect of chronotype and time-of-day on emotional state diurnal fluctuations. In general, studies lacking evidence of a synchrony effect demonstrated that positive affect (PA) increased throughout the day, and that M-types experienced higher levels of positive emotions in comparison to E-types, irrespective of time-of-day (Randler et al. Citation2016). For instance, Díaz-Morales et al. (Citation2015) used three repeated measures of the Face Scale (FS) on consecutive weeks and concluded that adolescents reported higher levels of positive mood (pleasantness) later in the school day, regardless of their chronotype. Randler and Weber (Citation2015) also examined the association between mood and chronotype on two separate moments of school day and concluded that PA increased throughout the school day for both chronotypes. No synchrony effect was found for PA, since E-types still exhibited lower levels of PA in the last hour of the school day when compared to their M-type peers. Lastly, in a study with 8th grade students, Bettencourt et al. (Citation2020) reported a significant main effect of time-of-day on momentary mood, PA and state-anxiety, and a main effect of chronotype on these variables (only when psychological symptoms and sleep variables [e.g., sleep length] were not controlled for). The results did not support a significant interactive effect of chronotype and time-of-day on adolescent’s emotional state fluctuations.
However, previous research has also found support for the presence of this synchrony effect across different emotional state variables. In adolescents, Itzek-Greulich et al. (Citation2016) examined the effects of chronotype and time-of-day on the benefits of active labwork on motivation and achievement. Their findings indicated a significant synchrony effect of chronotype and time-of-day on achievement-related emotional states (i.e., joy, interest, and anger) among German adolescents attending the same science course, in the morning or afternoon: E-types exhibited lower levels of joy and interest higher levels of anger when classes occurred at their non-optimal time-of-day (i.e., morning), contrary to M-types.
In adults, Hill and Chtourou (Citation2020) examined the combined effect of chronotype and time-of-day on the relationship between mood states (as measured by the Profile of Mood State [POMS] questionnaire), and physical performance at different times of day (i.e., 8 a.m., 2 p.m. and 8 p.m.). The effects of mood states on performance depended both on chronotype and time-of-day: M-types’ performance varied from morning to afternoon, in accordance with fluctuations in vigour levels, and from afternoon to evening in accordance with fluctuations in fatigue levels, while the opposite was true for E-types, suggesting the presence of a synchrony effect. Additionally, and despite not finding a synchrony effect, Willis et al. (Citation2005) found an interactive effect of chronotype and time-of-day on heart rate variation at rest and in response to stress in E-type adults who completed stress tasks and anxiety measures: compared to M-types, E-types exhibited higher heart rate levels in the afternoon, both at rest and during stress. The authors also failed to find a significant main effect of time-of-day on this heart rate variation, but concluded for a time-of-day main effect on self-reported anxiety levels.
In view of the previously presented findings, comprehensive knowledge regarding the combined impact of chronotype and time-of-day on the diurnal fluctuations of children’s momentary emotional states is needed (Doi et al. Citation2014; Zimmermann Citation2016; Bettencourt et al. Citation2020; Bai et al. Citation2021), given, for instance, the essential role of mood in ensuring an individual’s wellbeing and optimal functioning (e.g., Jankowski and Ciarkowska Citation2008; Birchler-Pedross et al. Citation2009). Moreover, previous research has yield mixed results that might not apply to children, particularly when considering developmental differences in circadian rhythms and sleep (e.g., Hagenauer et al. Citation2009).
Therefore, the main aim of the present study was to investigate potential chronotype and time-of-day interactive effects on momentary emotional states (i.e., momentary mood, PA, NA,Footnote1 and state-anxiety) of M- and E-type primary school children, in a naturalistic school setting, while controlling for the effect of sleep-related variables, psychological symptoms (i.e., confounding variables). Despite the scarce research on this age group (Arbabi et al. Citation2015), our general hypothesis was that differences on children’s momentary emotional states, comparing the first to the last hour of the school day, would be determined by both chronotype and time-of-day, representing a generalized synchrony effect (i.e., better emotional states at optimal times-of-day): M-types and E-types children would exhibit better momentary emotional states at an optimal time-of-day (i.e. morning for M-types, afternoon for E-types) and worst momentary emotional states at suboptimal time-of-day (i.e. morning for E-types, afternoon for M-types).
2. Materials and methods
This study was conducted as a part of a larger research project – True Times: Morningness-eveningness and time-of-day effects on cognitive performances and emotional states: New lessons from children and adolescents (Ref: PTDC/PSI-ESP/32581/2017) and was ethically approved by the Portuguese Ministry of Education and Science (MIME DGE- registration number: 0665200001) and by the ethical comity of the University of Coimbra, following Europe’s General Data Protection Regulation (GDPR). Methods’ description is limited to emotional states’ measurement in children.
2.1 Participants
From an initial pool of 298 participants from the 3rd and 4th grades, a total of 134 M-type (n = 52) and E-type (n = 82) children (53% girls and 47% boys) ranging in age from 8 to 11 years old (M = 8.84, SD = .60) completed the questionnaires. M-types and E-types did not differ by age (t (134) = −1.14, p > .05) or sex ( (1,134) = .30, p > .05).
2.2 Measures
In this study, several parental/self-report measures were used to assess variables of interest. Chronotype was measured using mainly the morningness/eveningness scale (M/E) score of the Children’s ChronoType Questionnaire (CCTQ), which allowed for the classification of students into M- or E-types. Sleep variables and psychopathological symptoms were considered potential covariates and measured using the Children’s Sleep-Waking Pattern questionnaire (PSVC), specific sections of the CCTQ related to sleep schedules and durations, and the Strengths & Difficulties Questionnaire (SDQ). Lastly, children’s momentary emotional states were assessed through Ecological Momentary Assessment (EMA) procedures using the Face Scale (FS), the Anxiety-State Scale from the State-Trait Anxiety Inventory for Children (STAIC-S) and the Positive and Negative Affect Scale for Children (EAPNC). These measures are described below.
2.2.1. Children ChronoType Questionnaire (CCTQ)
The CCTQ (Werner et al. Citation2009; Portuguese version: Couto et al. Citation2014) is 27-item mixed-format parental-report questionnaire for children between the ages of 4 and 11. The questionnaire provides three chronotype measures: midsleep point on free days (MSF), morningness/eveningness scale (M/E) score, and a five-point chronotype (CT) score (corresponding to the questionnaire’s 27th question). In the current study, the M/E scale score (the sum of 10 individual items, from questions 17 to 26) was the main measure used to determine children’s chronotype. Higher scores in the M/E scale correspond to higher eveningness. In the present sample, Cronbach alpha for the morningness/eveningness (M/E) scale was α = .70.
In the current study, instead of the 10th and 90th percentiles indicated by Werner et al. (Citation2009), the 20th and 80th percentiles were used as cutoff points to define children´s chronotype as M- or E-type, respectively, allowing the identification not only of definitive but also moderate M- or E-types. These cut-off points are a modified adaptation, for the present research, of the Horne and Ostberg (Citation1976) criteria and have already been applied in previous studies (e.g., Cruz et al. Citation2016). It is also crucial to mention that for the definitive determination of the participant’s chronotype, the M/E scale’s score was also compared with the answer given to the nominal rating of chronotype (i.e., CT score -CCTQ’s 27th item), to ensure higher classification consistency. Incompatible scores between these two measures determined the exclusion of the participants from the final sample of M and E-type children. M/E scores were considered incompatible with the CT score if a participant classified as M-type by the M/E-scale score was rated by the CT measure as “moderately” or “definitely” E-type; or if a participant classified as E-type by the M/E-scale score was rated by the CT measure as “moderately” or “definitely” M-type.
Other relevant sleep information provided by the CCTQ is, e.g., sleep onset, wake times and sleep durations (Werner et al. Citation2009; Couto et al. Citation2014). The two latter variables were considered potential covariates in this research as well as social jetlag (i.e., the difference between midpoint of sleep on free days and on weekdays (Martínez-Lozano et al. Citation2020) derived from CCTQ’s answers.
2.2.2 Children’s Sleep-Waking Pattern Questionnaire (PSVC)
The PSVC, developed by Clemente (Citation1997), is a 33-item parental-report measure of the child’s sleep and waking behavior. Most items use a 4-point Likert response scale ranging from 1 (never) to 4 (always) or have a yes/no format. The items are organized in different domains [i.e., bedtime behaviors (e.g., sleep latency), overnight behaviors (e.g., night waking), waking behaviors (e.g., rise time on week and free days), daytime behaviors (e.g., daytime sleepiness), and health questions (e.g., medication)] and six subscales: parents help to sleep, parasomnias, sleeping difficulties, afraid of dark, sleep limit setting and daytime sleep consequences (Carvalho Bos et al. Citation2009).
2.2.3 Strengths & Difficulties Questionnaire (SDQ)
The SDQ (Goodman Citation1997; Goodman et al. Citation1998; Portuguese version: Fleitlich et al. Citation2005) is a screening questionnaire that measures the presence of emotional and behavioral symptoms/problems in children and adolescents, shown to discriminate between clinical and community samples (Goodman et al. Citation1998). The scale includes 25 items distributed by 5 domains (Conduct Problems, Hyperactivity/Inattention, Emotional symptoms, Peer problems, and Pro-social Behavior) from which subscale scores can be derived. The items’ 3-point Likert response scale ranges from 0 (Not true) to 2 (Certainly true), except for reversed items. A total scale score can also be obtained from 20 items and ranges from 0 to 40. This questionnaire has different versions, meant to be filled either by the participants themselves (ages 11 and up), by their parents/guardians or by teachers (for children between the ages of 4 and 17 years old) (Goodman Citation1997; Goodman et al. Citation1998; Fleitlich et al. Citation2005). The present study used the parental version. In the current study, Cronbach alfa for the total scale was α = .61.
2.2.4 Faces Scale (FS)
The FS (adapt. from Andrews and Withey Citation1976), composed of seven simple drawings of faces, arranged horizontally, has been used as a simple tool to measure momentary happiness or mood during the day, both in children (e.g., Holder Citation2012) and adolescents (e.g., Díaz-Morales et al. Citation2015), by asking the participant how they fell “in this exact moment”. The alignment of the face’s mouths varies from upturn (suggestive of happiness) to downturned (suggestive of unhappiness). In the present study, answers were scored from 7 to 1, so that higher scores correspond to more upturned faces and are suggestive of a better mood (Díaz-Morales et al. Citation2015; Bettencourt et al. Citation2020).
2.2.5 Positive and Negative Affect Scale for Children (EAPNC)
The EAPNC (Giacomoni and Hutz Citation2006; European Portuguese version: Ameixa Citation2013) is a self-report scale that measures PA and NA in children from ages 8 to 14, through a 5-point Likert scale. It comprises 30 items organized in two scales: NA Scale and PA Scale and is similar to other well-known scales used to assess mood and emotion (e.g., PANAS-C, Watson et al. Citation1988). Two separate scores are obtained, one for each scale. In our study Cronbach alphas were α = .87 and α = .85 for the PA and NA subscales measured in the morning, and α = .86 and α = .72 for the PA and NA subscales measured in the afternoon.
2.2.6 State-anxiety scale from the State-Trait Anxiety Inventory for Children (STAIC)
STAIC (Spielberger Citation1973; Portuguese version: Matias Citation2004) is a measure of state and trait anxiety in children and adolescents. It encompasses two scales of 20 items each: The State-anxiety Scale (STAIC-S) and the Trait-anxiety Scale (STAIC-T). Since our study focuses on momentary emotional states, only the STAIC-S was used. Each item is rated on a 3-point Likert scale, with higher scores denoting higher anxiety stare (Spielberger Citation1973; Matias Citation2004; Azevedo et al. Citation2013). STAIC-S’ internal consistency was α = .87 and α = .88 for the morning and afternoon measurements, respectively.
2.3 Procedures
Data collection occurred in two separate stages between November 2020 and April 2021 on school clusters from central Portugal. First, after written informed consent, parents/guardians filled the CCTQ, used to assess children’s chronotype. To control for sleep variables and psychopathological symptoms, parents/guardians also filled the PSVC and the SDQ. Second, students completed the emotional states measures (FS, STAIC-S, and the EAPNC), on the first (9 a.m.) and last lessons (4 p.m.) of the school day, either by the order “first and last hour of the school day” (i.e., first on the morning and later in the afternoon of the same day) or by the reverse order (i.e., first in the afternoon of one day and on the following morning), in a counterbalanced order, to control for order effects (Seymor Citation2017). Approximately half of the selected participants (n = 58) first responded to the questionnaires in the afternoon, while the remaining participants (n = 76) first filled the questionnaires in the morning. Students were instructed to respond based on their momentary emotional states, regardless of their previous responses. Due to COVID-19 pandemic restrictions, only the children’s teacher was present in the room during the data collection process. Thus, detailed standardized instructions on proper data acquisition were presented to all teachers to ensure rigorous data collection, and the researchers assisted these professionals remotely via telephone or video call. All classrooms where assessments were conducted had natural daylight exposure. Both emotional states’ assessment times (9 a.m. and 4 p.m.) correspond to daylight hours at this latitude in the months of the year considered. Children, teachers, and researchers remained blind for participants diurnal type during all stages of data collection.
2.3.1 Statistical analysis
Statistical analyses were conducted using IBM SPSS (version 22.0). For each scale score (e.g., STAIC-S; PA; NA), if missing values were detected for a given participant, the missing answer was replaced by the mean score obtained by that participant in the remaining items of the same scale. The replacement was performed only if missing values did not surpass 20% of the total number of items of each scale, e.g., up to 2 missing answers in a 10-item scale (Cheema Citation2014). Skewness (Sk) and Kurtosis (Ku) for all ordinal and quantitative variables revealed a non-serious violation of the normality assumption for variables (i.e., skewness < |3| and kurtosis < |8|, cf. Kline Citation2005), except for NA kurtosis (10.06 ≤ |Ku| ≤10.72). Parametric tests did not exclude NA scores given the sample’s size and considering that the violation of normality assumptions is relatively unproblematic in relation to type I errors, namely when skewness is not significantly departed from normality and if variances are homogenic (Blanca et al. Citation2017; Knief and Forstmeier Citation2021). As previously mentioned, only M- and E-type children (n = 134) were included in the analysis. Significance level was set at p < .05. Descriptive statistics (i.e., means and standard deviations) were used for sample characterization. Independent sample t-tests and Chi-square analysis were conducted to examine age and sex differences between chronotypes. Bivariate correlations were used as an exploratory analysis to assess relationships between study variables and to determine potential cofounders. All Pearson correlation coefficients were interpreted according to the following criteria: correlations between .10 ≤ r < .30 were classified as small, between .30 ≤ r < .50 as moderate, and higher than r ≥ .50 as large (Cohen Citation1988).
To analyze the four momentary emotional states scores, by time-of-day and chronotype, a solution was searched to prevent eventual Type-I errors associated with multiple comparisons. Given the reduction in the statistical power and the inflation of type II errors associated with the use of Bonferoni corrections (e.g., Perneger Citation1998; Feise Citation2002; Nakagawa Citation2004), an alternative method to the adjustment of p-values was used. Rather than adjusting the p-value, Feise (Citation2002) recommends that researchers facing the multiple comparisons issue should either select a primary outcome measure or use a global assessment measure. Regarding the first possible solution, an individual measure of emotional states could not be selected as a primary outcome for analysis due to the scarce research in children. Therefore, the second solution was deemed to be the most appropriate.
Thus, a global assessment measure of overall momentary emotional states was determined, from the four primary measures of emotional states collected in the morning (9 a.m.) and in the afternoon (4 p.m.) [(i.e., momentary mood (FS), state-anxiety (STAIC-S), PA (EAPNC), NA (EAPNC)]. This composite measure was obtained by first reversing the NA scale (EAPNC) and STAIC-S scores, so that higher scores corresponded to a better overall emotional experience for all primary measures included. Due to differences in scoring and response options, these primary measures scores were converted into z-scores, allowing for standardization across all four measures. A composite measure of overall momentary emotional states was then determined based on an average of the z-scores for the four scales on each moment of the school day (9 a.m. and 4 p.m.). The reliability of these morning and afternoon composite measures was determined using Cronbach’s alpha and was deemed acceptable (α = .70 for the two measurement moments) (Nunnally, Citation1978). All inter-item correlation values were between .20 and .40,suggesting that primary measures are homogenous and non-redundant (Cohen and Swerdlik Citation2017). Corrected item-total correlations were also determined to evaluate how each primary measure contributed to these two general measures. All corrected item-total correlation values were above .30 (Field Citation2018), meaning that primary emotional states measures were associated with each general measure. Kurtosis and skewness were |3.56| and |1.41|, respectively, in the two overall emotional states measurements (morning; afternoon), indicating no serious violations of normal distribution and thus allowing for the use of ANOVA.
To test our main hypothesis, a two-way mixed-design analysis of variance (ANOVA) with chronotype as between-subjects factor (i.e., M-types and E-types) and time-of-day (i.e., time of assessment) as within-subjects factor (i.e., morning and afternoon) was performed. The analysis of interaction effects was particularly relevant since these constitute an indicator of a potential synchrony effect associated with individual circadian differences. Analysis of covariance (ANCOVA) using the same variables would have been performed, if necessary, to adjust for the effect of significantly associated covariates (i.e., sleep-related variables, psychopathological symptoms).
To comprehend the magnitude of observed effects, partial eta-square values () were used as an effect size measure. These values were interpreted using Cohen’s criteria for eta square: an effect size of
= .01 is considered small,
= .06 moderate and
= 0.14 large (Cohen Citation1988).
3. Results
3.1 Descriptive statistics and correlational analyses
3.1.1 Descriptive statistics
Descriptive statistics (i.e., mean and standard deviation) for primary and overall emotional states, psychopathology, and sleep measures according to chronotype (M-types/E-types) are shown in .
Table 1. Means and standard deviations for chronotype, and primary and overall emotional states measures
Table 2. Means and standard deviations for psychopathology measures
Table 3. Means and standard deviations for sleep measures
3.1.2 Correlations between momentary emotional states, psychopathological symptoms, and sleep variables
Focusing on the association between primary emotional states measures, most correlated, at least, moderately with each other. Thus, a composite z-score measure of overall emotional states could be determined (e.g., Evans Citation1996), as already described (cf. Statistical analysis). Additionally, no significant associations were found between the overall momentary emotional states measure, either in the morning or in the afternoon time-of-day, and all potential covariates, i.e., sleep-wake variables and psychological symptoms, specifically: parents help to sleep, parasomnias, sleeping difficulties, afraid of dark, sleep limit setting and daytime sleep consequences (PSVC subscales); wake times, sleep durations, and social jet lag (from the CCTQ); Conduct Problems, Hyperactivity/Inattention, Emotional symptoms, Peer problems, and Pro-social Behavior (SDQ subscales). These correlation coefficients ranged between |.01| and |.15|, and were in its majority very low (i.e., < |.1|).
3.2 Chronotype, time-of-day, and emotional states
3.2.1 Effects of chronotype and time-of-day on overall momentary emotional states
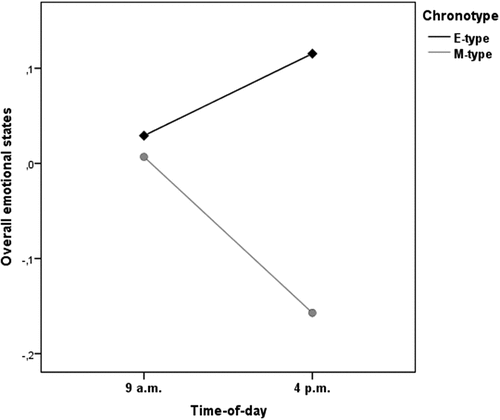
To examine the effect of chronotype and time-of-day on M-and E-type children’s overall momentary emotional states throughout the school day, a 2 × 2 mixed-design ANOVA with chronotype as between-subject factor and time-of-day as within-subjects factor was conducted. There was a statistically significant small to moderate interaction between chronotype and time-of-day on overall momentary emotional states [F (1,127) = 4.83, p < .05, =.04]. This effect indicates that there are differences between M-types and E-types’ overall momentary emotional states according to time-of-day. The main effects of chronotype [F (1,127) = 1.35, p >.05] and time-of-day [F (1,127) =.633, p >.05] were non-significant, suggesting that these variables alone cannot account for the observed differences in children’s overall emotional experience. and summarize the results of the present analysis.
Table 4. Two-way mixed ANOVA for overall momentary emotional states by time-of-day and chronotype
4. Discussion
Considering the role of mood variation on well-being (e.g., Jankowski and Ciarkowska Citation2008; Birchler-Pedross et al. Citation2009; Randler and Weber Citation2015), a better comprehension of underlying factors regarding children’s diurnal mood fluctuations seems to be of clear relevance. This study aimed to investigate the influence of both chronotype and time-of-day in children’s overall momentary emotional states experience during the school day through EMA. There are several methodological strengths to this study. First, the assessment of overall momentary emotional states fluctuations through the employment of a composite measure (as more reliable and valid summary score) captures the net effect of different measures of emotional states more precisely, without the need for multiple comparations or a larger sample size (Evans Citation1996; Feise Citation2002; Ferreira-González et al. Citation2008). Other strengths of the present study are: i) the use of counterbalancing that allowed for the control of order effects; ii) the focus on the present emotional experience; iii) the focus on a specific understudied age group (i.e., primary school children) (Randler et al. Citation2016); iii) assessment of momentary emotional states in the late afternoon (Randler et al. Citation2016); and iv) the use of EMA (that allowed for minimization of memory bias and for the maximization of ecological validity (defined here as a facet of external validity referring to the naturalness of the experimental context and/or its proximity with real-life situations) (Schmuckler Citation2001; Shiffman et al. Citation2008).
4.1 Eveningness in children and the synchrony effect of chronotype and time-of-day on children’s overall momentary emotional states diurnal fluctuations
Several observations can be based on the present findings. First, a considerable amount of children from our sample were E-types (30%), which further validates the notion that not all school-aged children display a morning preference (Randler et al. Citation2009; Zimmermann Citation2016; Haraden et al. Citation2017). Interestingly, our results also seem to coincide with previous studies that have suggested that eveningness in childhood has increased in recent years as a result of changing parental attitudes towards bedtime and due to children’s higher usage of electronic devices in bedrooms for entertainment (Foster and Kreitzman Citation2017). This should be carefully considered in both scientific and clinical settings (Randler et al. Citation2009; Zimmermann Citation2016; Haraden et al. Citation2017; Bai et al. Citation2021). Second, this study contributed to the literature in several ways. The obtained results support our main hypothesis of a generalized synchrony effect in primary school children’s overall momentary emotional states, determined by chronotype and time-of-day combined. Children exhibited significant fluctuations in their momentary emotional states throughout the school day, which followed different patterns for M-types and E-types. In the morning time-of-day, overall momentary mood states were similar in both chronotypes. In the afternoon time-of-day, E-types reported better emotional states while M-types’ emotional states were worse. This result is not entirely consistent with previous research, which has yield mixed results, as previously mentioned. Therefore, while most studies regarding the relationship between chronotype, time-of-day, and momentary emotional states fluctuations in adolescents reported no significant synchrony effects (e.g. Díaz-Morales et al. Citation2015; Randler and Weber Citation2015; Randler et al. Citation2016; Bettencourt et al. Citation2020), previous evidence also supports a synchrony effect of chronotype and time-of-day on momentary emotional states diurnal variability (i.e., better emotional states at optimal times-of-day) in both adolescents and adults (e.g. Willis et al. Citation2005; Itzek-Greulich et al. Citation2016; Hill and Chtourou Citation2020).
Additionally, it is important to highlight that the individual influence of chronotype and time-of-day in children of this age is not straightforward, given that no main effects on overall momentary emotional states were found. The effects of chronotype on momentary emotional states fluctuations are only observable in conjunction with time-of-day, reinforcing the underlying importance of the latter variable in the study of chronotype differences and emotional states fluctuations. Thus, both variables should be accounted for in future studies regarding momentary emotional states fluctuations in children (Willis et al. Citation2005). Lastly, the magnitude of this synchrony effect is small, emphasizing these variables as one of many potential contributors to momentary emotional states diurnal fluctuations (e.g., Foster and Kreitzman Citation2017).
Nevertheless, the present results provide valuable insights regarding the circadian rhythmicity of mood in primary school children that may allow for a better understanding of children’s emotional functioning throughout the school day, which might be relevant for future research investigating the most optimal times for learning and functioning throughout the day (Escribano and Díaz-Morales Citation2014; Díaz-Morales et al. Citation2015; Zarch et al. Citation2018) and the circadian processes associated with affective disorders (Díaz-Morales et al. Citation2015).
4.2 Limitations
Despite its contributions to the present literature, the findings from this study need to be interpreted in the context of its limitations. Chronotype was assessed through subjective parental-report measures, which might be biased and not reflect individual differences, since parents/guardians might not be fully aware of their children’s circadian preferences (Touchette et al. Citation2008; Van der Heijden et al. Citation2018). Objective assessment of chronotype (e.g., actigraphy, dim-light melatonin onset) could have strengthen this study’s findings (Van der Heijden et al. Citation2018), given the limitations of the current chronotype scales (Putilov Citation2017). In the current study, we prioritized the M/E scale as measure of chronotype. Several authors argue that M/E scale’s scores reflect preferences and do not constitute a measure of the phase of entrainment (for a discussion, cf. Levandovski et al. Citation2013). It is important to note that results obtained with the M/E scale of the CCTQ might not be correspond to the results that could have been obtained if other chronotype measures were to be used, namely the CCTQ’s MSFsc, based on the chronotype formula of Roenneberg et al. (Citation2004).
Furthermore, the use of a composite measure might increase the risk for a spurious interpretation of results, particularly when a significant effect is found (Ferreira-González et al. Citation2008). Another disadvantage of the composite measures is the inability to draw conclusions about each measure separately (Freemantle et al. Citation2003). Despite our efforts to control for emotional/behavioral symptoms, we cannot be sure whether the internal consistency of the SDQ (α < .70) may have influenced the current results. Light intensity was not controlled accurately during the assessment sessions, despite all classrooms where exposed to natural daylight. Lastly, we did not control for the effects of pubertal status.
4.3 Implications and future directions
The present findings seem to support the proposal of adjusting school times to chronotype, when possible (e.g., Díaz-Morales et al. Citation2015), and to identify new policies and strategies to better align the social and biological rhythms of school children, besides altering school’s start times, such as preventative educational sleep programs (Escribano and Díaz-Morales Citation2014; Antypa et al. Citation2016; Foster and Kreitzman Citation2017; Nowack and Van Der Meer Citation2018; Gariépy et al. Citation2019). Nonetheless, “little is known about the behavioural and emotional correlates of chronotype in prepubertal children” (Gelbmann et al. Citation2012, p.899). Additional studies are thus needed to further examine the potential presence of a synchrony effect of chronotype and time-of-day in children’s momentary emotional states fluctuations (Jeong Jeong et al. Citation2015; Gariépy et al. Citation2019).
To better understand the impact of both chronotype and time-of-day on children’s emotional experience, future studies could build on existing research by collecting more frequent assessments of overall momentary emotional states, throughout several school days, multiple times in each day and at more extreme morning and afternoon/evening hours of the school day. Future research should also attempt to replicate the present findings in clinical and non-clinical samples. Analyzing the combined effects of chronotype and time of day on other children populations, such as clinical samples, might be important to better understand the emotional and behavioural difficulties in this age group (e.g., Hasler et al. Citation2010).
5. Conclusion
Notwithstanding this study’s limitation and the need for future studies, the present findings suggest that chronotype and time-of-day combined have a significant influence on children’s emotional experience (in terms of their momentary emotional state’s diurnal fluctuation pattern), that we may hypothesize can potentially impact their daily functioning and emotional well-being while engaging in school activities. To the best of our knowledge, this is the first study to explore the combined influence of chronotype and time-of-day in primary school children’s emotional experience in a naturalistic real-life setting. These results were found in children, therefore are not generalizable to other developmental periods (e.g., adolescence).
Acknowledgments
The present study was conducted as a part of a larger research project -True Times: Morningness-eveningness and time-of-day effects on cognitive performances and emotional states: New lessons from children and adolescents (PTDC/PSI-ESP/32581/2017, CENTRO-01-0145-FEDER-032581), cf. https://transparencia.gov.pt/fundos-europeus/beneficiarios-projetos/projeto/CENTRO-01-0145-FEDER-032581. We would like to acknowledge our funding sources: Portugal 2020, Centro 2020, FEDER (UE), and FCT (Fundação para a Ciência e da Tecnologia/Portuguese national funding agency for science, research, and technology).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, A.A.G, upon reasonable request.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes
1. Despite the apparent lack of NA circadian rhythmicity in adults and adolescents (e.g., Randler and Weber Citation2015), NA was included in this study due to insufficient research on the potential daily variation of NA in children (e.g., Arbabi et al. Citation2015) and the results obtained in more recent studies (e.g., Emens et al. Citation2020).
References
- Ameixa G (2013). Estudo de Adaptação e Validação de duas Escalas de Avaliação da Dimensão Emocional Master thesis. Univerity of Algarve. https://core.ac.uk/download/pdf/61523362.pdf
- Andrews FM, Withey SB. 1976. Social indicators of well-being: America’s perception of life quality. New York: Plenum Press.
- Antúnez JM, Navarro JF, Adan A. 2015. Circadian typology is related to resilience and optimism in healthy adults. Chronobiol Int. 32(4):524–530. doi:10.3109/07420528.2015.1008700.
- Antypa N, Vogelzangs N, Meesters Y, Schoevers R, Penninx BWJH. 2016. Chronotype associations with depression and anxiety disorders in a large cohort study. Depress Anxiety. 33(1):75–83. doi:10.1002/da.22422.
- Arbabi T, Vollmer C, Dörfler T, Randler C. 2015. The influence of chronotype and intelligence on academic achievement in primary school is mediated by conscientiousness, midpoint of sleep and motivation. Chronobiol Int. 32(3):349–357. doi:10.3109/07420528.2014.980508.
- Au J, Reece J. 2017. The relationship between chronotype and depressive symptoms: a meta-analysis. J Affect Disord. 218:93–104. doi:10.1016/j.jad.2017.04.021
- Azevedo V, Simões S, Marques M, Cunha M. 2013. O Papel dos estilos educativos parentais na sintomatologia ansiosa de adolescentes do 3° ciclo do ensino básico. J Clin Child Adolesc Psychol. 4(2):31–50. http://revistas.lis.ulusiada.pt/index.php/rpca/article/view/418/398
- Bai S, Karan M, Gonzales NA, Fuligni AJ. 2021. A daily diary study of sleep chronotype among Mexican-origin adolescents and parents: implications for adolescent behavioral health. Dev Psychopathol. 33(1):313–322. doi:10.1017/s0954579419001780.
- Bettencourt C, Tomé B, Pires L, Leitão JA, Gomes AA. 2020. Emotional states in adolescents: time of day X chronotype effects while controlling for psychopathological symptoms and sleep variables. Biol Rhythm Res. 1–22. doi:10.1080/09291016.2020.1783489
- Birchler-Pedross A, Schröder CM, Münch M, Knoblauch V, Blatter K, Schnitzler-Sack C, Wirz-Justice A, Cajochen C. 2009. Subjective well-being is modulated by circadian phase, sleep pressure, age, and gender. J Biol Rhythms. 24(3):232–242. doi:10.1177/0748730409335546.
- Biss RK, Hasher L. 2012. Happy as a lark: morning-type younger and older adults are higher in positive affect. Emotion. 12(3):437–441. doi:10.1037/a0027071.
- Blanca M, Alarcón R, Arnau J, Bono R, Bendayan R. 2017. Non-normal data: is ANOVA still a valid option? Psicothema. 29(4):552–557. doi:10.7334/psicothema2016.383.
- Carciofo R, Du F, Song N, Zhang K. 2014. Chronotype and time-of-day correlates of mind wandering and related phenomena. Biol Rhythm Res. 45(1):37–49. doi:10.1080/09291016.2013.790651.
- Carvalho Bos S, Gomes A, Clemente V, Marques M, Pereira AT, Maia B, Soares MJ, Cabral AS, Macedo A, Gozal D, et al. 2009. Sleep and behavioral/emotional problems in children: a population-based study. Sleep Med. 10(1):66–74. doi:10.1016/j.sleep.2007.10.020.
- Cheema JR. 2014. Some general guidelines for choosing missing data handling methods in educational research. J Mod Appl Stat Methods. 13(2):53–75. doi:10.22237/jmasm/1414814520.
- Chiu W-H, Yang H-J, Kuo P-H. 2017. Chronotype preference matters for depression in youth. Chronobiol Int. 34(7):933–941. doi:10.1080/07420528.2017.1327441.
- Clemente V (1997). Sono e vigília em crianças de idade escolar: hábitos, comportamentos e problemas Master thesis. University of Coimbra.
- Cohen J. 1988. Statistical power analysis for the behavioral sciences. 2nd ed. New York: L. Erlbaum Associates
- Cohen RJ, Swerdlik ME. 2017. Psychological testing and assessment: an introduction to tests and measurements. 9th ed. New York: McGraw-Hill Education.
- Couto D, Gomes A, Azevedo H, Leitao J, Silva C (2014). The European Portuguese version of the children chronoType Questionnaire (CCTQ): reliability and raw scores in a large continental sample [P532]. [Abstract]. 23:160. doi:10.1111/jsr.12213/abstract
- Cox RC, Olatunji BO. 2019. Differential associations between chronotype, anxiety, and negative affect: a structural equation modeling approach. J Affect Disord. 257:321–330. doi:10.1016/j.jad.2019.07.012
- Cruz HMF, Gomes AA, Martins AM, Leitão JA, Clarisse R, Le Floc’h N, Da Silva CF. 2016. Morning-evening types in kindergarten, time-of-day and performance on basic learning skills. Int Online J Educ Sci. 8(5). doi:10.15345/iojes.2016.05.014.
- Dagys N, McGlinchey EL, Talbot LS, Kaplan KA, Dahl RE, Harvey AG. 2012. Double trouble? The effects of sleep deprivation and chronotype on adolescent affect: effects of sleep deprivation and chronotype on adolescent affect. J Child Psychol Psychiatry. 53(6):660–667. doi:10.1111/j.1469-7610.2011.02502.x.
- Díaz-Morales JF, Escribano C. 2014. Consequences of adolescent’s evening preference on school achievement: a review. [Consecuencias de la mayor vespertinidad durante la adolescencia para el funcionamiento psicológico: una revisión]. An. Psicol. 30(3):1096–1104. doi:10.6018/analesps.30.3.167941.
- Díaz-Morales JF, Escribano C, Jankowski KS. 2015. Chronotype and time-of-day effects on mood during school day. Chronobiol Int. 32(1):37–42. doi:10.3109/07420528.2014.949736.
- Doi Y, Ishihara K, Uchiyama M. 2014. Sleep/wake patterns and circadian typology in preschool children based on standardized parental self-reports. Chronobiol Int. 31(3):328–336. doi:10.3109/07420528.2013.852103.
- Emens JS, Berman AM, Thosar SS, Butler MP, Roberts SA, Clemons NA, Herzig MX, McHill AW, Morimoto M, Bowles NP, et al. 2020. Circadian rhythm in negative affect: implications for mood disorders. Psychiatry Res. 293:113337. doi:10.1016/j.psychres.2020.113337
- Escribano C, Díaz-Morales JF. 2014. Daily fluctuations in attention at school considering starting time and chronotype: an exploratory study. Chronobiol Int. 31(6):761–769. doi:10.3109/07420528.2014.898649.
- Evans LD. 1996. A two-score composite program for combining standard scores. Behav Res Methods Instrum Comput. 28(2):209–213. doi:10.3758/BF03204767.
- Feise RJ. 2002. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2(1):8. doi:10.1186/1471-2288-2-8.
- Ferreira-González I, Alonso-Coello P, Solá I, Pacheco-Huergo V, Domingo-Salvany A, Alonso J, Montori V, Permanyer-Miralda G. 2008. Composite endpoints in clinical trials. Rev Esp Cardiol. doi:10.1016/S1885-5857(08)60116-4
- Field A. 2018. Discovering statistics using IBM SPSS statistics. 5th ed. London: SAGE Publications.
- Fleitlich B, Loureiro M, Fonseca A, Gaspar F. (2005). Questionário de capacidades e de dificuldades (SDQ-Por) [strengths and difficulties questionnaire—Portuguese version]. Youth in Mind. [accessed 2017 March 30]. http://www.sdqinfo.org.
- Foster RG, Kreitzman L. 2017. Circadian rhythms: a very short introduction. New York: Oxford University Press. doi:10.1093/actrade/9780198717683.003.0002.
- Freemantle F, Calverkut M, Wood J, Eastaugh J, Griffin C. 2003. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 289(19):2554–2559. doi:10.1001/jama.289.19.2554.
- Gariépy G, Riehm KE, Whitehead RD, Doré I, Elgar FJ. 2019. Teenage night owls or early birds? Chronotype and the mental health of adolescents. J Sleep Res. 28(3):e12723. doi:10.1111/jsr.12723.
- Gelbmann G, Kuhn-Natriashvili S, Pazhedath TJ, Ardeljan M, Wöber C, Wöber-Bingöl Ç. 2012. Morningness: protective factor for sleep-related and emotional problems in childhood and adolescence? Chronobiol Int. 29(7):898–910. doi:10.3109/07420528.2012.686946.
- Giacomoni CH, Hutz CS. 2006. Escala de afeto positivo e negativo para crianças: estudos de construção e validação. Psicol Esc Educ. 10(2):235–245. doi:10.1590/S1413-85572006000200007.
- Goodman R. 1997. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. 38(5):581–586. doi:10.1111/j.1469-7610.1997.tb01545.x.
- Goodman R, Meltzer H, Bailey V. 1998. The strengths and difficulties questionnaire: a pilot study on the validity of the self-report version. Adolesc Psychiatry. 7(3):6.
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. 2009. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 31(4):276–284. doi:10.1159/000216538.
- Hahn C, Cowell JM, Wiprzycka UJ, Goldstein D, Ralph M, Hasher L, Zelazo PD. 2012. Circadian rhythms in executive function during the transition to adolescence: the effect of synchrony between chronotype and time of day. Dev Sci. 15(3):408–416. doi:10.1111/j.1467-7687.2012.01137.x.
- Haraden DA, Mullin BC, Hankin BL. 2017. The relationship between depression and chronotype: a longitudinal assessment during childhood and adolescence. Depress Anxiety. 34(10):967–976. doi:10.1002/da.22682.
- Hasler BP, Allen JJ, Sbarra DA, Bootzin RR, Bernert RA. 2010. Morningness–eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Res. 176(2–3):166–173. doi:10.1016/j.psychres.2009.06.006.
- Hill DW, Chtourou H. 2020. The effect of time of day and chronotype on the relationships between mood state and performance in a Wingate test. Chronobiol Int. 37(11):1599–1610. doi:10.1080/07420528.2020.1786394.
- Holder MD. 2012. The assessment of happiness in adults and children. In: Holder MD, editor. Happiness in children: measurement, correlates and enhancement of positive subjective well-being. Dordrecht: Springer Netherlands; 19–33. doi:10.1007/978-94-007-4414-1_3.
- Horne JA, Ostberg O. 1976. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 4(2):97–110.
- Itzek-Greulich H, Randler C, Vollmer C. 2016. The interaction of chronotype and time of day in a science course: adolescent evening types learn more and are more motivated in the afternoon. Learn Individ Differ. 51:189–198. doi:10.1016/j.lindif.2016.09.013
- Jankowski KS, Ciarkowska W. 2008. Diurnal variation in energetic arousal, tense arousal, and hedonic tone in extreme morning and evening types. Chronobiol Int. 25(4):577–595. doi:10.1080/07420520802261770.
- Jeong Jeong H, Moon E, Min Park J, Dae Lee B, Min Lee Y, Choi Y, In Chung Y. 2015. The relationship between chronotype and mood fluctuation in the general population. Psychiatry Res. 229(3):867–871. doi:10.1016/j.psychres.2015.07.067.
- Kline RB. 2005. Principles and practice of structural equation modelling. 2nd ed. New York: Guilford Press.
- Knief U, Forstmeier W. 2021. Violating the normality assumption may be the lesser of two evils. Behav Res Methods. 53(6):2576–2590. doi:10.3758/s13428-021-01587-5.
- Levandovski R, Sasso E, Hidalgo MP. 2013. Chronotype: a review of the advances, limits and applicability of the main instruments used in the literature to assess human phenotype. Trends Psychiatry Psychother. 35(1):3–11. doi:10.1590/S2237-60892013000100002.
- Martínez-Lozano N, Barraco GM, Rios R, Ruiz MJ, Tvarijonaviciute A, Fardy P, Madrid JA, Garaulet M. 2020. Evening types have social jet lag and metabolic alterations in school-age children. Sci Rep. 10(1). doi:10.1038/s41598-020-73297-5.
- Matias MCS (2004). Aferição do state-trait anxiety inventory for children (STAIC) de spielberger para a população portuguesa [spielberger’s State-trait anxiety inventory for children (STAIC) adaptation for the Portuguese population] Master’s thesis. Badajoz: University of Extremadura.
- Montaruli A, Castelli L, Mulè A, Scurati R, Esposito F, Galasso L, Roveda E. 2021. Biological rhythm and chronotype: new perspectives in health. Biomolecules. 11(4):487. doi:10.3390/biom11040487.
- Nakagawa S. 2004. A farewell to bonferroni: the problems of low statistical power and publication bias. Behav Ecol. 15(6):1044–1045. doi:10.1093/beheco/arh107.
- Nowack K, Van Der Meer E. 2018. The synchrony effect revisited: chronotype, time of day and cognitive performance in a semantic analogy task. Chronobiol Int. 35(12):1647–1662. doi:10.1080/07420528.2018.1500477.
- Nunnally JC. 1978. Psychometric Theory. 2nd ed. New York: McGraw-Hill.
- Perneger TV. 1998. What’s wrong with bonferroni adjustments. Br Med J. 316(7139):1236–1238. doi:10.1136/bmj.316.7139.1236.
- Putilov AA. 2017. Owls, larks, swifts, woodcocks and they are not alone: a historical review of methodology for multidimensional self-assessment of individual differences in sleep-wake pattern. Chronobiol Int. 34(3):426–437. doi:10.1080/07420528.2017.1278704.
- Randler C, Bilger S, Díaz-Morales JF. 2009. Associations among sleep, chronotype, parental monitoring, and pubertal development among German adolescents. J Psychol. 143(5):509–520. doi:10.3200/jrl.143.5.509-520.
- Randler C, Rahafar A, Arbabi T, Bretschneider R. 2014. Affective state of school pupils during their first lesson of the day-effect of morningness-eveningness. Mind Brain Educ. 8(4):214–219. doi:10.1111/mbe.12060.
- Randler C, Weber V. 2015. Positive and negative affect during the school day and its relationship to morningness–eveningness. Biol Rhythm Res. 46(5):683–690. doi:10.1080/09291016.2015.1046249.
- Randler C, Bechtold K, Vogel M. 2016. Chronotype and time of day do not influence mathematical achievement in standardised tests, but impact on affect – results from a field experiment. Int Online J Educ Sci. 8(5). doi:10.15345/iojes.2016.05.006.
- Randler C, Faßl C, Kalb N. 2017. From lark to owl: developmental changes in morningness-eveningness from new-borns to early adulthood. Sci Rep. 7(1):45874. doi:10.1038/srep45874.
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. 2004. A marker for the end of adolescence. Current Biol. 14(24):R1038–R1039. doi:10.1016/j.cub.2004.11.039.
- Schmuckler MA. 2001. What is ecological validity? A dimensional analysis. Infancy. 2(4):419–436. doi:10.1207/s15327078in0204_02.
- Seymor T. 2017. Experimental designs. In: Gorvine B, Rosengren K, Stein L, Biolsi K, editors. Research methods: from theory to practice. New York: Oxford University Press; p. 177–204.
- Shiffman S, Stone AA, Hufford MR. 2008. Ecological momentary assessment. Annu Rev Clin Psychol. 4(1):1–32. doi:10.1146/annurev.clinpsy.3.022806.091415.
- Spielberger CD. 1973. Manual for the State trait anxiety inventory for children. Palo Alto (CA): Consulting Psychologists Press.
- Touchette É, Mongrain V, Petit D, Tremblay RE, Montplaisir JY. 2008. Development of sleep-wake schedules during childhood and relationship with sleep duration. Arch Pediatr Adolesc Med. 162(4):343–349. doi:10.1001/archpedi.162.4.343.
- Van der Heijden KB, Stoffelsen RJ, Popma A, Swaab H. 2018. Sleep, chronotype, and sleep hygiene in children with attention-deficit/hyperactivity disorder, autism spectrum disorder, and controls. Eur Child Adolesc Psychiatry. 27(1):99–111. doi:10.1007/s00787-017-1025-8.
- Watson D, Clark LA, Tellegen A. 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 54(6):1063–1070. doi:10.1037/0022-3514.54.6.1063.
- Werner H, LeBourgeois MK, Geiger A, Jenni OG. 2009. Assessment of chronotype in four- to eleven-year-old children: reliability and validity of the children’s chronotype questionnaire (CCTQ). Chronobiol Int. 26(5):992–1014. doi:10.1080/07420520903044505.
- Willis TA, O’Connor DB, Smith L. 2005. The influence of morningness–eveningness on anxiety and cardiovascular responses to stress. Physiol Behav. 85(2):125–133. doi:10.1016/j.physbeh.2005.03.013.
- Zarch ZN, Sharifi M, Heidari M, Pakdaman S. 2018. Investigating chronotype orientation on daily and weekly rhythm fluctuations in preschoolers working memory performance. Int Clin Neurosci J. 5(4):150–157. doi:10.15171/icnj.2018.27.
- Zimmermann LK. 2016. The influence of chronotype in the daily lives of young children. Chronobiol Int. 33(3):268–279. doi:10.3109/07420528.2016.1138120.