ABSTRACT
Clinical studies of children with autism spectrum disorder (ASD) provide evidence for poorer neuropsychological performance within specific domains compared to age, gender, and sometimes IQ-matched controls. Since recent evidence suggests that autistic symptoms form a spectrum that extends into the general population, it was our goal to evaluate the nature of the relationship between autistic traits and neuropsychological performance across the continuum in the general population. We examined neuropsychological performance across five different domains in 1019 6-to-10-year-old children participating in a population-based study of child development. Autistic traits were assessed when the children were 6 years of age using the Social Responsiveness Scale and ASD diagnoses were obtained via medical records. Neuropsychological functioning was measured using the NEPSY-II-NL and included the domains of attention and executive function, memory and learning, sensorimotor functioning, language, and visuospatial functioning. We found that children with higher autistic traits showed significantly lower neuropsychological performance in all domains investigated and that this association remained even after excluding children with the highest autistic traits or confirmed ASD. When comparing 41 children with confirmed ASD diagnosis to typically developing controls, children with ASD showed significantly lower neuropsychological performance across all domains. Taken together, our results suggest that children with both ASD and subclinical autistic traits have lower neuropsychological performance. Thus, this may provide an understanding of why some children without an ASD diagnosis may require some additional assistance within academic settings.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with symptoms in two primary domains: social communication and social interaction impairments, and restricted and repetitive patterns of behavior (APA, Citation2013). With the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), the binary conceptualization of autism has evolved, merging with Asperger syndrome and pervasive developmental disorders, to form a spectrum disorder, with differing levels of autistic symptoms that all fall within the overarching spectrum of ASD (APA, Citation2013). In fact, there is emerging evidence that autistic symptoms form a spectrum that extends into the general population, with some children showing subclinical symptoms of ASD (Constantino & Todd, Citation2000, Citation2003, Citation2005; Ronald, Happe, & Plomin, Citation2005). Twin studies have demonstrated moderate to high rates of heredity of subthreshold autistic traits (Constantino & Todd, Citation2003). Thus, since autistic symptoms form a spectrum within the population, this raises the question whether characteristics associated with ASD, such as poorer cognitive performance, also show a parallel relationship with autistic symptoms along the spectrum.
There have been a number of studies to date that demonstrate a link between ASD and poorer neuropsychological performance (Barron-Linnankoski et al., Citation2015; Hooper, Poon, Marcus, & Fine, Citation2006; Narzisi, Muratori, Calderoni, Fabbro, & Urgesi, Citation2013). However, it is unclear whether the relationship between autistic symptoms and neuropsychological performance extends to subclinical populations. If the underlying neurobiological processes that influence neuropsychological performance also contribute to autistic symptoms, then one would expect a relationship between the continuum of neuropsychological performance and the continuum of autistic traits. Exploring this relationship, not only in clinically diagnosed patients with ASD, but also within the general population, is the primary goal of this study.
Research evaluating the relationship between neuropsychological performance and autistic traits can be classified into two different approaches or categories. The first approach focuses specifically on domains that are most affected in ASD, such as language and executive functioning. The second approach evaluates children with autistic traits using a comprehensive battery of neuropsychological tests. Studies utilizing the first approach have shown general deficits in verbal language and variability in linguistic abilities (Boucher, Citation2012; Eigsti, De Marchena, Schuh, & Kelley, Citation2011; Groen, Zwiers, van der Gaag, & Buitelaar, Citation2008; Pastor-Cerezuela, Fernández-Andrés, Feo-Álvarez, & González-Sala, Citation2016) and lower semantic verbal fluency (Pastor-Cerezuela et al., Citation2016). Studies utilizing DSM-IV criteria have demonstrated that specific aspects of language are universally affected in autism, with comprehension, semantics, and certain facets of morphology being the most affected areas, while articulation and syntax remain less affected (Boucher, Citation2012).
There have also been a number of studies focusing specifically on executive function (EF) in ASD. EF includes planning, inhibition, working, memory, flexibility, initiation, and monitoring actions (Diamond, Citation2013; E. L. Hill, Citation2004; Lezak, Citation2004; Rabbitt, Citation1997). Deficits in this domain are considered to underlie many of the social and nonsocial characteristics of autistic traits (Frith, Citation2004; E. L. Hill, Citation2004). EF differences are also thought to account for symptoms such as rigidity, stereotyped behaviors, adherence to routines, and restricted levels of interest (E. L. Hill, Citation2004; Lopez, Lincoln, Ozonoff, & Lai, Citation2005). It is thought that deficits in EF may provide an explanation as to why individuals with autistic traits have difficulty coping with changes in routines, why they exhibit repetitive actions, and why they benefit from external structure. Numerous studies in children and adults with higher functioning ASD have provided evidence of difficulties in tasks requiring planning and mental flexibility (Ambery, Russell, Perry, Morris, & Murphy, Citation2006; Corbett, Constantine, Hendren, Rocke, & Ozonoff, Citation2009; Geurts, Verté, Oosterlaan, Roeyers, & Sergeant, Citation2004; E. L. Hill & Bird, Citation2006; Kenworthy et al., Citation2005; Miller & Ozonoff, Citation2000; Ozonoff & Jensen, Citation1999; Sinzig, Morsch, Bruning, Schmidt, & Lehmkuhl, Citation2008; Szatmari, Tuff, Finlayson, & Bartolucci, Citation1990; Verté, Geurts, Roeyers, Oosterlaan, & Sergeant, Citation2006).
Studies using the second approach have used neuropsychological batteries, such as the NEPSY to test neuropsychological performance across multiple neuropsychological domains (Korkman, Kirk, & Kemp, Citation1998, Citation2007). Evaluating multiple domains can provide information regarding the specificity of the neuropsychological deficits. The NEPSY-II consists of a total of 32 specific neuropsychological tests within six broad domains. The subtests are designed to assess cognitive abilities related to disorders that are typically diagnosed in childhood and that are required for success in academic settings. Individuals with high-functioning autism have been shown to have significantly poorer performance compared to typically developing children in the domains of attention and executive functions, language, memory and learning, sensorimotor functions, and visuospatial processing (Hooper et al., Citation2006; Narzisi et al., Citation2013; Williams, Goldstein, & Minshew, Citation2006). Williams et al. found that children with higher functioning ASD show greater impairments in complex language, complex memory, sensory-perceptual, and motor domains (Williams et al., Citation2006). More specifically, Barron-Linnankoski et al. found significant group differences in 6 of the 19 NEPSY-II subsets with medium to large effect sizes (Barron-Linnankoski et al., Citation2015). They reported that children with higher functioning ASD performed poorer in the Response Set, Word Generation, Narrative Memory, and Memory for Faces, Imitating Hand Positions, and Design Copying subtests compared to typically developing children. Furthermore, there was also a trend for poorer performance in the Visuomotor Precision subtest.
While there is considerable evidence supporting more global neurocognitive deficits in children with autistic traits, not much is known about neurocognitive performance across the spectrum of autistic symptoms in the general population. Furthermore, many neuropsychological studies have included children and adolescents or children, adolescents, and adults pooled into the same sample, instead of examining neurocognitive performance in narrower age ranges. Pooling participants over large age ranges and adjusting for age in the analyses assumes no developmental differences in cognitive development. Indeed, recent studies in individuals with higher functioning ASD indicate age-related interactions in neurocognitive performance (Happé & Frith, Citation2006; Kuusikko-Gauffin et al., Citation2011). Hence, cognitive studies assessing children with autistic traits within more narrow age ranges are needed. Identifying neuropsychological patterns in children across the whole autism spectrum may provide insights into the need and targets of early intervention.
Thus, the primary goal of this study is to assess the relationship between autistic symptoms and neuropsychological performance across the spectrum using a large population-based study of child development. We applied a three-prong approach that we used previously to study clinical, subclinical, and population effects (Blanken et al., Citation2015). First, we assess ASD/control differences in neuropsychological performance; second we assess the relationship between autistic symptoms and neuropsychological performance in the entire population; and third, we assess the relationship between autistic symptoms and neuropsychological performance after excluding the children with ASD or high autistic symptoms. Based on the previous research, we hypothesize that children with a diagnosis of ASD, tested in a clinical/control analysis, will show global deficits in neuropsychological performance. Furthermore, extending this hypothesis, we predict that a similar relationship will be found assessing the continuous relationship across the spectrum of autistic symptoms. Importantly, we hypothesize that the relationship will hold after excluding children with ASD and the highest autistic traits, which provides evidence that the relationship extends into subclinical populations.
Methods and materials
Participants
This study was embedded in the Generation R Study, a multiethnic population-based cohort investigating children’s health, growth, and development from fetal life onward in Rotterdam, The Netherlands. Overviews of the design and population of the Generation R Study have previously been described (Jaddoe et al., Citation2012; Tiemeier et al., Citation2012).
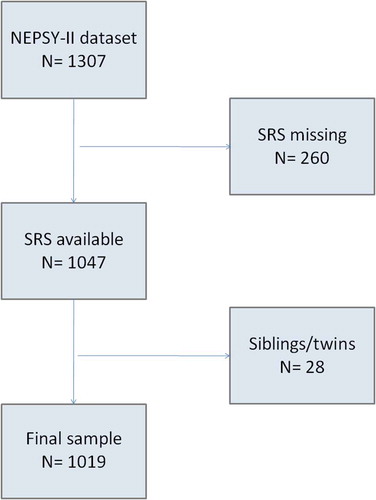
A detailed neuropsychological assessment was performed in a subgroup of the entire Generation R population, as part of a pilot neuropsychology and brain-imaging magnetic resonance imaging (MRI) protocol (White et al., Citation2013). Exclusion criteria of this study included contraindications for the MRI procedure (i.e., pacemaker, ferrous metal implants, and claustrophobia), severe motor or sensory disorders (deafness or blindness), neurological disorders, and moderate-to-severe head injuries with loss of consciousness. From September 2009 to July 2013, 1325 children who were between 6 and 10 years of age from the Generation R Study were invited to participate in this sub-study. A total of 1307 of these children agreed to participate and completed the neuropsychological assessment. A flow diagram of the inclusion is shown in . A total of 260 children were excluded based on missing information on autistic traits. For each sibling or twin pair, one sibling was excluded (n = 17). Thus, the final study sample consisted of 1019 6-to-10-year-old children. Informed consent was obtained from the parents after a complete description of the study was provided. All procedures were approved by the Erasmus Medical Center Medical Ethics Committee.
Neuropsychological functioning
The neuropsychological assessment was performed using subtests of the NEPSY-II-NL. The NEPSY-II-NL is an official and validated Dutch translation and adaptation of the North American NEPSY-II (Brooks et al., Citation2009) . Acceptable to good reliability and validity have been reported for the NEPSY-II (Korkman, Kirk, & Kemp, Citation2010b). The Dutch version of the NEPSY-II is valid for 5-to-12-year-old children. The full NEPSY-II-NL battery consists of 32 tasks. Due to time constraints, we selected a battery of 10 tasks which tapped the 5 domains of attention and executive functioning, memory and learning, sensorimotor functioning, language, and visuospatial processing (White et al., Citation2013).
Since the NEPSY-II-NL does not provide domain-specific summary scores, we used a data reduction technique to derive empirical scores for each domain. Scores within each domain were derived using a principal component analysis on all raw (i.e., non-age-normed) scores from the NEPSY-II-NL and selecting only the first unrotated factor score. Considering the different dimensions of the task in the sensorimotor domain, namely that the completion time and error scores reflect particular strategies (e.g., fast with many errors vs. slow with few errors), we calculated a simple trade-off score by computing the product of a standardized score for completion time and errors.
Autistic traits
To obtain a measure of autistic traits, the Social Responsiveness Scale (SRS) (Constantino, Citation2002) was completed by the mother when the children were approximately 6 years of age (range: 4.89–8.90 years). We utilized the SRS short form, which consists of 18 items scored from 0 (“never true”) to 3 (“almost always true”) and that reflects the parent’s observation of the child’s social behavior over the past 6 months. The 18-item SRS total score has been shown to correlate between 0.93 and 0.99 with the total score of the full SRS version in three different large studies (Constantino & Todd, Citation2003; Daniels et al., Citation2012; White et al., Citation2017). The SRS 18-item measure provides a valid quantitative measure of subclinical and clinical autistic traits and covers both the social communication/interaction and restricted/ repetitive patterns of behavior, interests, or activities. The SRS was not used if more than 25% of the data was missing. When less than 25% missing questions were present, the total score was weighted by the number of non-missing items. In all analyses, SRS scores were square root transformed to approach a normal distribution. To determine a threshold for children with high levels of autistic symptoms, we utilized the cut-off values recommended by the authors of the SRS for screening in population-based studies (consistent with weighted scores of 1.078 for boys and 1.000 for girls) (Constantino, Citation2002).
Autism spectrum disorder diagnoses
Medical records were examined for children who scored screen positive in one or more of several stages of a multifaceted ASD screening procedure (White et al., Citation2017). Screen positive for ASD was based on one of three sources of information. First, all children were formally screened with the SRS with screen positives being those that exceeded the thresholds defined above. Second, to rule out false negatives, children who scored in the top 15% on the total score of the Child Behavior Checklist (CBCL) 1.5–5 total score underwent a more specific screening using the Social Communication Questionnaire (SCQ), a 40-item parent-reported screening instrument for ASD (Berument, Rutter, Lord, Pickles, & Bailey, Citation1999). Scores of 15 or above on the SCQ were considered screen positive (Berument et al., Citation1999). Finally, psychiatric diagnoses and treatment were routinely assessed at all contact moments, including at both the 6 and 9 years of age visit to the research center.
To obtain diagnostic information from the family practice physicians, we sent letters to family practice physicians for children who were screen positive for ASD. In the Netherlands, the general practitioners hold the central medical records, including information on treatment by any medical specialist. A diagnosis of ASD is generally based on clinical consensus by a specialized multidisciplinary team. The diagnostic workup typically involves an extensive developmental case history obtained from parents, as well as school information and repeated observations of the child. All medical records were reviewed by LB, and for questionable cases there was a consensus meeting with Laura Blanken, Frank Verhulst, and Tonya White .
Children’s nonverbal IQ
At the age of 6 years, the children were invited to visit the Generation R research center. During this visit, children’s nonverbal IQ was assessed using two subtests of a well-validated Dutch nonverbal intelligence test: Snijders-Oomen Niet-verbale intelligentie test, revisie. The two subsets were mosaics, which assess spatial visualization abilities, and categories, which assess abstract reasoning abilities. In a different sample of 626 children aged 4½–7½ years, the correlation between total scores derived from the mosaics and categories subsets and IQ scores derived from the complete test was 0.86 (Tellegen, Winkel, Wijnberg-Williams, & Laros, Citation2005). Raw test scores were converted into nonverbal IQ scores using normal values tailored to exact age. Because the NEPSY-II-NL and intelligence test were collected during different study visits, IQ data were available in only 920 of the 1019 children in the final study sample.
Covariates
While all analyses were corrected for gender and age, other variables were included as covariates when they changed the effect estimate (B) by more than 5%. Additional covariates include child ethnicity, which was defined using the ethnicity categorization of “Statistics Netherlands.” Maternal education was defined as highest education completed and household income was defined by the total net monthly income of the household. Information on maternal alcohol use and smoking during pregnancy was obtained using questionnaires from each trimester of pregnancy. Child attention problems, which are known to be highly comorbid with autistic traits, were measured at the age of 6 years using the Attention Problems (AP) syndrome scale of the CBCL-1.5–5 year version (Achenbach & Dumenci, Citation2001).
Statistical analysis
Statistical analyses were performed using the R software package. Chi-square for categorical data and t-tests for continuous variables were used for the nonresponse analysis and to compare differences in the demographic information between typical developing children and those with autistic traits. Linear regression analyses were utilized to assess associations between autistic traits or ASD diagnosis (independent variables) and cognitive performance (dependent variable).
For SRS across the continuum, the analyses were performed in the entire sample using each of the five NEPSY-II domain scores as the dependent variable and SRS as the independent variable, also adjusting for age and sex and the other covariates. Children with ASD and high levels of autistic traits were excluded from the entire sample for the second analysis, and for the case control analysis ASD diagnosis was used as the independent variable. All analyses were corrected for gender and age in the first model. The second model additionally included child ethnicity, maternal education, household income, maternal alcohol use, and smoking during pregnancy as covariates. We had two additional models using the CBCL attention score and nonverbal IQ as covariates. All analyses were corrected for multiple testing using family-wise error.
Results
Sample characteristics and nonresponse analysis
Demographic characteristics of the sample and behavioral and neuropsychological measures are presented, respectively, in and . Supplementary Figure 1 shows a box plot of the individual NEPSY-II performance between children with ASD and typically developing children. A total of 1019 children with a mean age of 7.9 (SD = 1.0, min = 6.0, max = 10.7) are included in the final sample. Children who were not included (n = 260) were more likely to be of non-Dutch origin (χ2 = 15.54, df = 2, p-value = .0004), were slightly younger in age (mean difference = −0.1, t = 1.53, df = 416.37, p-value = .13), had a higher Child Behavior Checklist attention problem score (mean difference = 0.71, t = −3.88, df = 219.23, p-value = .0001), and had a higher nonverbal IQ (mean difference = 5.1, t = −4.7, df = 352.45, p-value <.0001). Mothers of the excluded children had less education (χ2 = 14.71, df = 2, p-value = .0006) and less total income (χ2 = 22.06, df = 2, p-value <.0001). The characteristics of the children included in the nonresponse analysis are presented in Supplementary Table 1.
Table 1. Demographic and clinical characteristics of the study sample.
Table 2. Neuropsychological performance between children with an autism spectrum disorder diagnosis and typically developing children.
ASD/control analyses
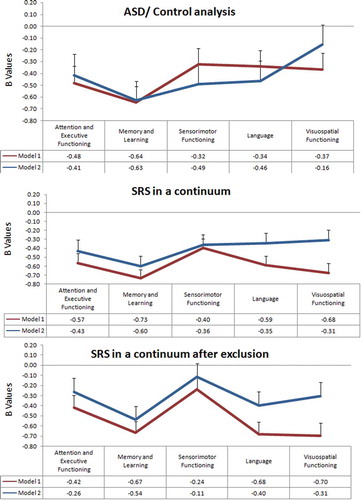
Demographic characteristics of children included in ASD/control analysis are presented in Supplementary Table 2. Linear regression analysis was performed with each of the five neuropsychological domains as the dependent variable and children with an ASD diagnosis as the independent variable. For the first model using age and gender as covariates, children with ASD showed significantly lower performances in all domains ( and (a)). When controlling for child ethnicity, maternal education, household income, maternal alcohol use, and smoking during pregnancy, four of the five domains remained significant, with the exception being the visuospatial domain ().
Table 3. Model adjustments controlling for gender and age (Model I) and five additional covariates (Model II).
Figure 2. NEPSY-II performance across five different neuropsychological domains evaluating (top) an ASD/control analysis, (middle) SRS along the continuum with neuropsychological performance, and (bottom) SRS along the continuum with neuropsychological performance after excluding children with ASD and clinical SRS scores. Model I is adjusted only for age and gender, and Model II is additionally adjusted for age at SRS, gender, child ethnicity, maternal education, household income, maternal alcohol use, and smoking during pregnancy.
Autistic symptoms across the continuum
Linear regression analyses performed in the entire sample for each of the five NEPSY-II domain scores showed significant differences in all domains ( and (b)). An inverse relationship was observed between autistic traits and performance in the five domains (attention and executive functioning, memory and learning, sensorimotor, language, and the visuospatial domain); higher autistic traits are associated with lower performance. Furthermore, all five associations remained highly significant, but with some decrease in the effect estimate, after adjusting for the additional covariates (ethnicity, maternal education, monthly income, maternal alcohol consumption, and smoking during pregnancy).
SRS across the continuum excluding children with ASD and high autistic traits
To test whether children with ASD and high autistic traits drove the relationships between cognitive performance and SRS, we repeated the linear regression analyses excluding children with ASD and high levels of autistic traits. We excluded 41 children with a diagnosis of ASD. In addition, we excluded boys with weighted SRS scores >1.078 and girls with scores >1.000, thresholds that are recommended for screening for ASD in population-based settings (Constantino, Citation2002). Applying these thresholds resulted in additional 18 children excluded, which resulted in a sample of 960 children.
Linear regression analyses between neuropsychological performance and SRS showed effect estimates similar to those using the full sample and all five domains remained significant ( and (c)). After correcting for the additional five covariates, the relation between lower neuropsychological performance and greater autistic traits remained significant in three of the five domains, in memory and learning, sensorimotor functioning, and language domains. The attention and executive functioning and sensorimotor domains were not significant.
Specificity analyses
To examine the specificity of our results related to neuropsychological function, we repeated the analyses adding nonverbal IQ and the attention problem CBCL score to the adjusted models (each added to Model II separately). Interestingly, the model adjusting for IQ showed little change, with four out of the five domains remaining significant (Supplementary Table 3 and Supplementary Figure 2). Children with more autistic traits showed poorer performance in the attention and executive functioning, memory and learning, sensorimotor, and language domains. However, the visuospatial processing domain was not significantly affected.
For the case-control analyses, associations were significant for the memory and learning, sensorimotor functioning, and language domains, but not for the attention and executive functioning and the visuospatial domains (Supplementary Figure 2-B). For the analysis of SRS across the continuum after restricting children with ASD or high autistic traits, the associations remained significant in the following domains: attention and executive Functioning, memory and learning, and language, but not in the sensorimotor and visuospatial domains (Supplementary Figure 2-C).
As for the model where CBCL score was added as a covariate, in the SRS across the continuum analysis, the relationship remained significant in all domains except for the sensorimotor domain, which was no longer significant (Supplemental Table 4 and Supplemental Figure 3-B).
For the case-control analyses and for the SRS after exclusion analyses, the results were consistent with the second model (Supplemental Table 4 and Supplemental Figure 3-A) (Supplemental Table 4 and Supplemental Figure 3-C).
Within sex analyses
As expected, there were highly significant differences in SRS scores (mean difference = 0.151, t = −5.928, df = 966.21, p-value <.0001), with boys having higher scores than girls. Similarly, there were also gender differences in the CBCL attention problem score with boys showing slightly higher scores (mean difference = 0.87, t = −6.7, df = 992, p-value <.0001) and in the nonverbal IQ with boys showing slightly higher scores (mean difference = 1.3, t = −1.42, df = 917.55, p-value = .16). For the NEPSY-II results, boys perform overall better in the visuospatial domain and girls in the attention and executive functioning, language, memory, learning, and sensorimotor domains.
Post hoc analyses using a sex by SRS interaction in the regression model to evaluate gender differences in NEPSY-II performance were analyzed in all domains. At lower SRS scores, girls performed better in attention and executive functioning and language domains compared to boys, but at higher autistic traits the girls’ performance in these domains was significantly lower than the boys, which was a reverse in the pattern. Boys show higher performance in the cognitive domains at higher SRS scores compared to girls, but in the sensorimotor domain, girls show higher performance throughout the entire sample. In the domains of memory and learning and visuospatial functioning, there were no sex-specific differences. Linear regression analyses were also performed separately within sex to see the differences in performance in SRS across a continuum and after excluding the individuals with the highest SRS and a diagnosis of ASD. The results are presented in Supplementary Table 5 and Supplementary Figures 4 and 5.
Discussion
Recent studies suggest that autistic traits fall on a continuum within the general population. Our findings provide support that the relationship between neuropsychological performance and ASD in children also extends across the continuum of autistic symptoms. We tested this relationship using a three-step approach based on our earlier work (Blanken et al., Citation2015). The first step involved an ASD case versus control analysis comparing children with a diagnosis of ASD to children without an ASD diagnosis. This step allows for a direct comparison with the literature, since studies of ASD diagnosis versus controls are the most widely reported in the literature. The second step was to test for a linear relationship between autistic traits and NEPSY-II performance in the entire sample. Finally, we excluded both children with a diagnosis of ASD and children with high autistic symptoms and found that, even after excluding these children, the significant relationship between SRS scores and neuropsychological performance remained. Our results provide support for a relationship along the continuum between autistic symptoms and neuropsychological performance in school-age children who encompass children with subclinical autistic traits.
We found that the direction of the association between autistic traits and neuropsychological functioning along a continuum was negative, with a decrease in neuropsychological performance as autistic traits increased (). The relationship remained significant even after adjusting for confounding variables (age, gender, child ethnicity, maternal education, household income, maternal alcohol use, and smoking during pregnancy). Interestingly when we controlled for the CBCL attention scale, the attention and executive functioning domain continued to be significant for ASD/control (which implies that it is driven by high SRS), but loses significance after children with high SRS and an ASD diagnosis are excluded (the impairment here would be driven by children with high CBCL). This means that this domain is affected separately by SRS and CBCL.
There have been a number of studies comparing clinical samples of children with ASD to controls that have used the NEPSY-II. Consistent with our findings, these studies report lower neuropsychological performance in children with an ASD diagnoses. In a study of 30 high-functioning ASD children (28 boys) aged 6–11 years compared to age and gender matched typically developing children, one study found significantly lower performance in the NEPSY-II subsets of Response Set (attention and executive functioning domain), Word Generation (language domain), and Narrative Memory (memory and learning Domain) (Barron-Linnankoski et al., Citation2015). Similarly, another studied 22 high-functioning children with ASD between the ages of 5 and 16 years compared with matched typically developing controls and found significantly lower global performance in the NEPSY-II (Narzisi et al., Citation2013).
Domain-specific research in ASD has mostly focused on the domains of language, attention, and executive functioning. These studies have consistently reported that children with autistic traits show impairments in these domains (Ambery et al., Citation2006; Boucher, Citation2012; Corbett et al., Citation2009; Boucher, Citation2012; Geurts et al., Citation2004; Groen et al., Citation2008; E. L. Hill & Bird, Citation2006; Kenworthy et al., Citation2005; Miller & Ozonoff, Citation2000; Ozonoff & Jensen, Citation1999; Pastor-Cerezuela et al., Citation2016; Sinzig et al., Citation2008; Szatmari et al., Citation1990; Verté et al., Citation2006). Our ASD patients versus control analyses were consistent with the literature, which is important to demonstrate, as our goal was to extend these findings across the spectrum of autistic symptoms.
Considering the consistency of our ASD patient/control analyses with the literature, we then asked the questions whether first a relationship exists between autistic symptoms and neuropsychological performance along a continuum in the population and second whether the relationship extended to subclinical populations. When testing for a linear relationship within the entire population (SRS in a continuum analyses), we found that cognitive performance decreased in four of the five cognitive domains as autistic traits increase. The exception was in sensorimotor functioning, which did not fit a linear relationship with autistic symptoms. Even though it does not fit the linear relationship, it does follow a similar pattern even though there is a loss of significant after exclusion of children with a high SRS and ASD diagnosis (Model II, regulated for nonverbal IQ, regulated for CBCL attention score). This suggests that impairments in the sensorimotor function are only prominent/visible at higher autistic traits. There is a particular decrease in the effect estimate after adjusting for nonverbal IQ and CBCL, which is expected because sensorimotor performance is related to nonverbal IQ and attention score.
Our findings support a link between the domains of attention and executive function, memory and learning, language, and visuospatial functioning over the spectrum of autistic symptoms.
We also performed additional analyses (models III and IV) using the CBCL attention problem score and nonverbal IQ as additional covariates, respectively. Controlling for the CBCL attention problem score significantly altered the effect estimate in several domains, including the attention and executive function, sensorimotor, and visuospatial processing domains. Neither the memory and learning domain nor the language domain was significantly affected after controlling for the CBCL attention score. Thus, these domains may involve more specific cognitive neurobiological pathways and thus are less influenced by attention problems. Interestingly, controlling for nonverbal IQ had only minor effects on the results. Controlling for nonverbal IQ reduced the effect estimate in the visuospatial functioning domain for the ASD/control analysis and in the sensorimotor domain for the exclusion-SRS-continuum analysis, but the other domains remained significant.
Since the prevalence of ASD has strong gender differences, we examined sex differences in our sample. We found that girls with lower SRS scores performed better in several neuropsychological domains compared to boys. On the contrary, at high SRS scores the performance reversed, with girls showing significantly greater impairment in these domains, making their autistic traits appear more severe. This may reflect that girls with ASD symptoms have greater cognitive impairments than boys with increasing autistic symptoms and thus may require additional services or adaptations within the school.
There are a number of strengths of our study, including the large population-based sample that allowed us to not only assess cognitive performance using an ASD/control analysis, but also assess the relationship between cognitive performance and autistic traits along the spectrum in the general population. Second, since the sample size was quite large, it provided the opportunity to adjust for a number of potential confounding factors. Also, it has been observed that autistic traits are commonly accompanied by attention problems and lower IQ, so we performed secondary analyses to assess whether the performance would change related to these variables. Finally, we corrected for multiple testing using family-wise error and the results remained significant.
There are also several limitations to our study. First, the measurements of autistic traits, neuropsychological functioning, and IQ were not performed at the same time; thus, there was a short time difference between the two measurements. However, there is evidence that autistic traits are relatively stable over time (Constantino et al., Citation2009). Second, the nonresponse analyses showed that there were differences between participants and nonparticipants regarding specific demographic factors. This may limit the generalizability of the findings to the general population. However, based on the demographic characteristics of the excluded group, it is likely that we are missing children with more severe autistic traits, which would likely result in more significant findings. Third, we utilized an abbreviated version of the NEPSY-II rather than the full battery of 32 subtests, and thus we may miss certain neuropsychological domains that are either preserved or more impaired in children with ASD or autistic traits. However, we utilized 10 tests of the NEPSY-II that covered 5 core neuropsychological domains. Finally, we did not utilize a standardized instrument to measure quality of life in the children. Understanding the role in which autistic symptoms and neuropsychological performance relate to quality of life along a continuum would be an important goal for future research.
In conclusion, our findings support a relationship between autistic traits and neuropsychological performance across the continuum in most neuropsychological domains. This relationship was such that greater autistic traits are associated with lower cognitive performance. Furthermore, this relationship between cognition and autistic traits showed a continuous relationship extending into the general population, including children with subclinical autistic traits. This suggests that the underlying neurobiology of this relationship involves either global mechanisms, since many different brain regions are involved in the multiple cognitive domains, or, alternatively, there is a link between ASD and a fundamental neuropsychological process. Processes such as encoding, for example, serve as a building block in neuropsychological processes and involved in many different neuropsychological tasks. Thus, deficits in encoding could influence multiple cognitive domains. Evidence exists for encoding abnormalities in children with ASD (Southwick et al., Citation2011). Furthermore, since we found a “dose-dependent” relationship between autistic traits and cognitive performance, it suggests that the underlying neurobiology of the relationship between autistic symptoms and neuropsychological performance is also “dose dependent.”
Another major contributor to a possible common neurobiological pathway for both autistic traits and neuropsychological impairments may be brain-derived neurotrophic factor (BDNF). BDNF is involved in activity-dependent synaptic plasticity and there have been a number of studies linking BDNF with ASD (Armeanu, Mokkonen, & Crespi, Citation2017; Bryn et al., Citation2015; Kasarpalkar, Kothari, & Dave, Citation2014; Saghazadeh & Rezaei, Citation2017; Wang et al., Citation2015). Within the context of a genetic predisposition for ASD, coupled with environmental factors that result in over excitation of neurons, BDNF levels increase dramatically, resulting in the presence of a fatty coating around the neurons and megalencephaly (Merzenich, Van Vleet, Nahum, Citation2014). These factors could impair the efficient orchestration of brain function and have consequences on global brain function. Thus, having global affects on the brain would in turn affect multiple neuropsychological domains, especially in attention and executive functioning, language and memory, and learning domains. Further research should determine whether there is a dose-dependent relationship between BDNF levels, autistic symptoms, and neuropsychological performance.
It will be helpful for educators and school psychologists to be aware that even in the absence of an ASD diagnosis, children with greater autistic traits may have mild cognitive performance difficulties. In light of the inherent plasticity of the developing brain, it may be possible to identify children at risk and provide cognitive training to enhance outcomes. A recent example is Merzenich’s Fast-For-Word (Merzenich et al., Citation2014) treatment, which has a goal to improve language and learning skills in autistic children by tapping the greater brain plasticity in development. It is an empirical treatment that has shown promising results with improvement in children with autistic traits. Our research provides a scientific basis for engaging children even with subclinical autistic symptoms to hopefully optimize the cognitive outcomes in these children.
Supplementary Tables 1-6 and Supplementary Figures 1-5
Download PDF (885.3 KB)Supplementary material
Supplementary material can be accessed here.
Additional information
Funding
References
- Achenbach, T. M., & Dumenci, L. (2001). Advances in empirically based assessment: Revised cross-informant syndromes and new DSM-oriented scales for the CBCL, YSR, and TRF: Comment on Lengua, Sadowksi, Friedrich, and Fischer (2001). Journal of Consulting and Clinical Psychology, 69(4), 699–702.
- Ambery, F. Z., Russell, A. J., Perry, K., Morris, R., & Murphy, D. G. (2006). Neuropsychological functioning in adults with Asperger syndrome. Autism: The International Journal of Research and Practice, 10(6), 551–564.
- [APA], American Psychiatric Association (2013). The diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing.
- Armeanu, R., Mokkonen, M., & Crespi, B. (2017). Meta-analysis of BDNF levels in autism. Cellular and Molecular Neurobiology, 37(5), 949–954.
- Barron-Linnankoski, S., Reinvall, O., Lahervuori, A., Voutilainen, A., Lahti-Nuuttila, P., & Korkman, M. (2015). Neurocognitive performance of children with higher functioning autism spectrum disorders on the NEPSY-II. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence, 21(1), 55–77.
- Berument, S. K., Rutter, M., Lord, C., Pickles, A., & Bailey, A. (1999). Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry, 175, 444–451.
- Blanken, L. M., Mous, S. E., Ghassabian, A., Muetzel, R. L., Schoemaker, N. K., El Marroun, H., … White, T. (2015). Cortical morphology in 6- to 10-year old children with autistic traits: A population-based neuroimaging study. The American Journal of Psychiatry, 172(5), 479–486.
- Boucher, J. (2012). Research review: Structural language in autistic spectrum disorder – characteristics and causes. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(3), 219–233.
- Brooks, B. L., Sherman, E. M., & Strauss, E. (2009). NEPSY-II: A developmental neuropsychological assessment. Child Neuropsychology, 16(1), 80–101; Second Edition.
- Bryn, V., Halvorsen, B., Ueland, T., Isaksen, J., Kolkova, K., Ravn, K., & Skjeldal, O. H. (2015). Brain derived neurotrophic factor (BDNF) and autism spectrum disorders (ASD) in childhood. European Journal of Paediatric Neurology: EJPN: Official Journal of the European Paediatric Neurology Society, 19(4), 411–414.
- Constantino, J. N. (2002). Social Responsiveness Scale (SRS), manual. Los Angeles: Western Psychological Services.
- Constantino, J. N., Abbacchi, A. M., Lavesser, P. D., Reed, H., Givens, L., Chiang, L., … Todd, R. D. (2009). Developmental course of autistic social impairment in males. Development and Psychopathology, 21(1), 127–138.
- Constantino, J. N., & Todd, R. D. (2000). Genetic structure of reciprocal social behavior. The American Journal of Psychiatry, 157(12), 2043–2045.
- Constantino, J. N., & Todd, R. D. (2003). Autistic traits in the general population: A twin study. Archives of General Psychiatry, 60(5), 524–530.
- Constantino, J. N., & Todd, R. D. (2005). Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry, 57(6), 655–660.
- Corbett, B. A., Constantine, L. J., Hendren, R., Rocke, D., & Ozonoff, S. (2009). Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research, 166(2–3), 210–222.
- Daniels, A. M., Rosenberg, R. E., Anderson, C., Law, J. K., Marvin, A. R., & Law, P. A. (2012). Verification of parent-report of child autism spectrum disorder diagnosis to a web-based autism registry. Journal of Autism and Developmental Disorders, 42(2), 257–265.
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168.
- Eigsti, I. M., De Marchena, A. B., Schuh, J. M., & Kelley, E. (2011). Language acquisition in autism spectrum disorders: A developmental review. Researcher Autism Spect Diseases, 5, 681–691.
- Frith, U. (2004). Emanuel Miller lecture: Confusions and controversies about Asperger syndrome. Journal of Child Psychology and Psychiatry, and Allied Disciplines. doi:10.1111/j.1469–7610.2004.00262.x
- Geurts, H., Verté, S., Oosterlaan, J., Roeyers, H., & Sergeant, J. (2004). How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of Child Psychology and Psychiatry, and Allied Disciplines. doi:10.1111/j.1469-7610.2004.00276.x
- Groen, W. B., Zwiers, M. P., van der Gaag, R. J., & Buitelaar, J. K. (2008). The phenotype and neural correlates of language in autism: An integrative review. Neuroscience and Biobehavioral Reviews, 32(8), 1416–1425.
- Happé, F., & Frith, U. (2006). The weak coherence account: Detailed-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders, 36, 5–25.
- Hill, E. L. (2004). Executive dysfunction in autism. Trends in Cognitive Sciences, 8(1), 26–32.
- Hill, E. L., & Bird, C. M. (2006). Executive processes in Asperger syndrome: Patterns of performance in a multiple case series. Neuropsychologia, 44, 2822–2835.
- Hooper, S. R., Poon, K. K., Marcus, L., & Fine, C. (2006). Neuropsychological characteristics of school-age children with high-functioning autism: Performance on the NEPSY. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence, 12(4–5), 299–305.
- Jaddoe, V. W., van Duijn, C. M., Franco, O. H., van der Heijden, A. J., van Iizendoorn, M. H., de Jongste, J. C., … Hofman, A. (2012). The Generation R Study: Design and cohort update 2012. European Journal of Epidemiology, 27(9), 739–756.
- Kasarpalkar, N. J., Kothari, S. T., & Dave, U. P. (2014). Brain-derived neurotrophic factor in children with autism spectrum disorder. Annals Neuroscience, 21(4), 129–133.
- Kenworthy, L. E., Black, D. O., Wallace, G. L., Ahluvalia, T., Wagner, A. E., & Sirian, L. M. (2005). Disorganization: The forgotten executive dysfunction in high-functioning autism (HFA) spectrum disorders. Developmental Neuropsychology, 28(3), 809–827.
- Korkman, M., Kirk, U., & Kemp, S. (1998). NEPSY: A developmental neuropsychological assessment. San Antonio, TX: Psychological Corporation.
- Korkman, M., Kirk, U., & Kemp, S. (2010b). Technischehandleiding NEPSY-II-NL [Clinical and interpretive scoring manual NEPSY-II- NL]. Enschede, The Netherlands: Ipskamp.
- Korkman, M., Kirk, U., & Kemp, S. L. (2007). NEPSY-II. Clinical and interpretative manual. San Antonio, TX: Psychological Corporation.
- Kuusikko-Gauffin, S., Jansson-Verkasalo, E., Carter, A., Pollock-Wurman, R., Jussila, R., Mattila, M.-L., & Irma, M. (2011). Face memory and object recognition in children with high functioning autism or Asperger syndrome and in their parents. Research in Autism Spectrum Disorders, 5, 622–628.
- Lezak, M. (2004). Neuropsychological assessment (4th ed.). New York, NY: Oxford University Press.
- Lopez, B., Lincoln, A., Ozonoff, A., & Lai, Z. (2005). Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders, 35. doi:10.1007/s10803-005-5035-x
- Merzenich, M. M., Van Vleet, T. M., & Nahum, M. (2014). Brain plasticity-based therapeutics. Frontiers in Human Neuroscience, 8, 1-16. doi:–10.3389/fnhum.2014.00385.
- Miller, J. N., & Ozonoff, S. (2000). The external validity of Asperger disorder: Lack of evidence from the domain of neuropsychology. Journal of Abnormal Psychology, 109, 227–238.
- Narzisi, A., Muratori, F., Calderoni, S., Fabbro, F., & Urgesi, C. (2013). Neuropsychological profile in high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(8), 1895–1909
- Ozonoff, S., & Jensen, J. (1999). Brief report: Specific executive function profiles in three neurodevelopmental disorders. Journal of Autism and Developmental Disorders, 29(2), 171–177.
- Pastor-Cerezuela, G., Fernández-Andrés, M. I., Feo-Álvarez, M., & González-Sala, F. (2016). Semantic verbal fluency in children with and without autism spectrum disorder: Relationship with chronological age and IQ. Frontiers in Psychology. doi:10.3389/fpsyg.2016.00921
- Rabbitt, P. (1997). Introduction: Methodologies and models in the study of executive function. In P. Rabbitt ( Ed.), Methodology of frontal and executive function (pp. 1–38). Hove: Psychology Press.
- Ronald, A., Happe, F., & Plomin, R. (2005). The genetic relationship between individual differences in social and nonsocial behaviours characteristic of autism. Developmental Science, 8(5), 444–458
- Saghazadeh, A., & Rezaei, N. (2017). Brain-derived neurotrophic factor levels in autism: A systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 47(4), 1018–1029.
- Sinzig, J., Morsch, D., Bruning, N., Schmidt, M. H., & Lehmkuhl, G. (2008). Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child and Adolescent Psychiatry and Mental Health, 2. doi:10.1186/1753–2000–2–4
- Southwick, J. S., Bigler, E. D., Froehlich, A., Dubray, M. B., Alexander, A. L., Lange, N., & Lainhart, J. E. (2011). Memory functioning in children and adolescents with autism. Neuropsychology, 25(6), 702–710.
- Szatmari, P., Tuff, L., Finlayson, A. J., & Bartolucci, G. (1990). Asperger´s syndrome and autism: Neurocognitive aspects. Journal of the American Academy of Child and Adolescent Psychiatry, 29, 130–136.
- Tellegen, P. J., Winkel, M., Wijnberg-Williams, B., & Laros, J. A. (2005). Snijders-OomenNiet-Verbaleintelligentietest: SON-R 2.5–7: Handleiding en Verantwoording [Snijders-Oomen non-verbal intelligence test: SON-R 2.5-7]. Amsterdam, The Netherlands: Boom Testuitgevers.
- Tiemeier, H., Velders, F. P., Szekely, E., Roza, S. J., Dieleman, G., Jaddoe, V. W., … Verhulst, F. C. (2012). The Generation R Study: A review of design, findings to date, and a study of the 5-HTTLPR by environmental interaction from fetal life onward. Journal of the American Academy of Child and Adolescent Psychiatry, 51(11), 1119–1135 e1117.
- Verté, S., Geurts, H. M., Roeyers, H., Oosterlaan, J., & Sergeant, J. A. (2006). Executive functioning in children with an autism spectrum disorder: Can we differentiate within the spectrum? Journal of Autism and Developmental Disorders, 36, 351–372.
- Wang, M., Chen, H., Yu, T., Cui, G., Jiao, A., & Liang, H. (2015). Increased serum levels of brain-derived neurotrophic factor in autism spectrum disorder. Neuroreport, 26(11), 638–641.
- White, T., El Marroun, H., Nijs, I., Schmidt, M., van der Lugt, A., Wielopolki, P. A., … Verhulst, F. C. (2013). Pediatric population-based neuroimaging and the Generation R Study: The intersection of developmental neuroscience and epidemiology. European Journal of Epidemiology, 28(1), 99–111.
- White, T., Muetzel, R. L., El Marroun, H., Blanken, L. M. E., Jansen, P., Bolhuis, K., … Tiemeier, H. (2017). Paediatric population neuroimaging and the Generation R Study: The second wave. European Journal of Epidemiology. doi:10.1007/s10654-017-0319-y
- Williams, D. L., Goldstein, G., & Minshew, N. J. (2006). Neuropsychological functioning in children with autism: Further evidence for disordered complex information-processing. Child Neuropsychology, 12, 279–298.