ABSTRACT
The effect of intervention activities on the chemistry content knowledge of 92 preservice chemistry teachers (PSCT) was examined via a pre and post true/false with confidence level test focusing on ionisation energy values and the use of a common alternative conception (AC). Data were collected from three cohorts of PSCT each engaged in a one year full-time university-based teacher education programme. Comparison of pre-and post-test responses and discrimination differences between responses for each PSCT were used to identify the use of the AC and hence the efficacy of the intervention activities. Interviews with 14 of these PSCT allowed triangulation of the data. Findings suggest that the activities allow 71% of PSCT to identify this AC as non-scientific or become more confident in doing so. Interview data indicate that this rejection of this AC is sustained for 9 of the 11 PSCT whose test data indicated improvement. These data have implications for teacher education such that 2 hour intervention sessions can offer the opportunity for PSCT to develop their subject matter knowledge. As the accurate application of electrostatic concepts to electrons has wider application to bonding, these interventions offer PSCT more accurate chemical subject knowledge resources to draw on in their teaching.
Introduction
Recently reports into effective education systems have highlighted the importance of the level of subject expertise of teachers (Barber & Mourshed, Citation2007). Furthermore sustained improvement in education is linked with increasing the academic entry requirements for teachers and with their on-going professional development (Mourshed, Chijioke, & Barber, Citation2010). It was long accepted that good teacher subject knowledge is important (Druva & Anderson, Citation1983) but Wayne and Youngs (Citation2003) review of US research, questions if there is a direct linkage between a teachers’ academic background and their pupils’ performance for science education, although finds such a linkage for mathematics. One difficulty here is in recognising if the higher level qualifications of the science teacher match the science specialist area they are teaching and this is a similarly noted in the UK with many science teachers teaching upper high school science outside their degree subject. Nonetheless according to Wallace (Citation2005, p. 175) exemplary science teachers ‘are sure of their own science content knowledge’. Given that good subject knowledge is considered a characteristic of exemplary teaching, one recent measure to secure further improvement in school teaching in England and Wales, was the removal of state funding for teacher training for graduates who did not achieve a second class honours bachelors level degree (TSO, Citation2010) (equivalent to secured marks 50+/US GPA >3.0/German better than ‘average’/Australian ‘credit’). This increase in entry level requirements was accompanied by the introduction of new teacher standards which preservice teachers must meet to qualify, explicitly requiring teachers to ‘have a secure knowledge of the relevant subject(s) and curriculum areas’ (Crown, Citation2011, p. 11). UK science teachers generally qualify for teaching via a one year postgraduate course involving ‘methods’ and ‘practicum’ aspects similar to France, Cyprus and Finland (2 years). In England qualification by a school-based training year is becoming more commonplace and the development of subject and pedagogical knowledge (PK) is often supported by a teaching colleague in a mentoring role (Evagorou, Dillon, Viiri, & Albe, Citation2015).
However, accepting that a high level of content (subject) expertise is beneficial for teaching and recognising the complexity of the interplay between content (subject), PK and other skills (Kind, Citation2009), there is no consensus on the necessary level of knowledge that could be recognised as ‘secure’. Learned associations in the UK (professional associations for scientists), the Royal Society of Chemistry, the Institute of Physics, the Royal Society of Biology, the Royal Society and the Association for Science Education recommend that initial teacher education (preservice training) provide for the development of an appropriate level of knowledge for specialist sciences such that it is ‘at a level well above that which they will teach’ (SCORE, Citation2015, p. 2). This is mirrored in U.S.A. by the National Science teachers association’s (NSTA) position statement requiring preservice teachers to ‘Develop robust science knowledge and skills beyond the depth and breadth needed for teaching a curriculum based on the National Science Education Standards at the grade levels they are preparing to teach’ (NSTA, Citation2004, p. 1). Similarly Singapore’s education system requires initial teacher preparation programmes to ensure trainees study extensively both the subject and the teaching of the subject (Goodwin, Citation2012).
Kind and Kind (Citation2011) probed the content knowledge (CK) of preservice science teachers on a typical university led initial teacher training course in the England, all of which were science graduates and found that many used descriptive and anthropomorphic statements rather than giving causal explanations. Furthermore it was only when the preservice teachers were challenged regarding their knowledge that they were able to recognise their need to develop deeper conceptual understanding. As these intuitive and non-causal ideas continue to be used by academically well qualified graduates, preservice education must take steps to develop trainees’ conceptual knowledge (Kind, Citation2014). Kind and Kind (Citation2011) suggest that pre-and post-intervention studies of preservice teachers should provide the necessary insights to support and target this development. This paper considers such an intervention for preservice chemistry teachers (PSCT) which aims to go beyond identifying specific deficiencies in subject matter knowledge (SMK) by examining how they may be remedied.
Theoretical framework
There is widespread acceptance that required teacher knowledge goes beyond subject matter understanding. It is more how accurate SMK can be appropriately structured and organised to allow learners to grasp scientific laws, principles and concepts. Shulman (Citation1986) distinguished different aspects of teacher knowledge he termed CK to include subject matter content knowledge, pedagogical content knowledge (PCK) and curricular knowledge which together provide the knowledge which enables teachers to select and use the most appropriate concepts and representations for learners. Further studies have led to complex models of these different aspects of this knowledge and their interconnections. For example, Abell (Citation2007), where PCK acts as the mitigating knowledge which is both determined by and determines the science SMK, PK and knowledge of context drawn upon in teaching. The complexity of this interplay has made it difficult to recommend specifically how expert teaching knowledge can be developed, but that accurate SMK is needed, is agreed. In order for a teacher to develop strong PCK they need accurate SMK so that they are able to identify and question inaccuracies and offer explanations and representations that support the scientific ideas to be learnt. Preservice teachers need to be aware of where their SMK weaknesses and strengths lie so that they can draw on and develop these as appropriate (Kind, Citation2014).
SMK can also be further considered as involving knowledge of the facts, concepts and principles and how these are inter-related in their fields, known as substantive knowledge. For example, in chemistry this would involve knowledge of the properties of electrons and how they interact with nuclei in order to form bonds. It would also include knowledge of standard representations and models. However the nature of these models and the validity of the principles involved would be another aspect of this knowledge termed syntactic knowledge (Shulman, Citation1986). The focus in this study is on the substantive chemistry knowledge (SCK) of PSCT.
Theoretical background
CK for science teaching
Expert teachers are able to recognise and understand pupils’ difficulties with grasping new subject matter ideas and then they draw on their PCK to restructure or present these ideas in ways that enable pupils to grasp them (Hashweh, Citation1987). However Sanders, Borko, and Lockard’s (Citation1993) observations of experienced teachers reveal that they lose this capacity when teaching outside their specialism. Furthermore, Käpylä, Heikkinen, and Asunta (Citation2009) concluded that strong CK allowed teachers to teach pupils more expertly, when they examined Biology teachers’ CK regarding photosynthesis and plant growth and their capacity to handle pupils’ conceptual issues.
However the knowledge of high school science differs from that developed during academic studies (Deng, Citation2007). The curriculum determines this school science and as teachers become more experienced this school science knowledge becomes the main CK source often at the expense of their academic knowledge (Arzi & White, Citation2007). For example, advanced high school curriculums include using heuristic principles like Le Châtelier's principle to predict the behaviour of reversible reactions such that the principle is taught without acknowledgement of its limitations or conceptualisations of the processes that underpin it (Wheeldon, Akinson, Dawes, & Levinson, Citation2012).
There is a significant body of research (Driver & Erickson, Citation1983; Driver, Squires, Rushworth, & Wood-Robinson, Citation1994; Gilbert & Watts, Citation1983) that has uncovered students use of non-scientific ideas in explanations but as these ideas seem to perpetuate is it is likely that teachers pass these on to their students as theirs did to them as there is evidence that science teachers teach as they were taught (Trumper, Citation2001). Therefore in order to make further progress developing science teaching, preservice teachers need to be made aware of the non-scientific ideas that they hold so that these can be replaced with the scientific ones. Such SCK allows effective development of PK which for chemistry teachers links observable chemical phenomena to visualisations of the sub-microscopic entities used to explain these changes and their symbolic representations. It also includes an understanding of which models offer the better resources for particular explanations and hence can better support pupil learning (Coll & Treagust, Citation2003).
SCK of ionisation energy
Explaining differences in ionisation energy values is an area where pupils and their teachers alike use ACs (Taber & Tan, Citation2011). The use of these ideas is replicated across continents with little variation between pupils around the world. Many students and their teachers explain variations in ionisation energy values using arguments that reveal ACs about the electrostatic properties of the electrons and nucleus of an atom, others use ‘stability’ as the cause without connecting this to thermodynamic factors that might support such arguments (Taber & Tan, Citation2007; Tan et al., Citation2008).
ACs used in explaining ionisation energy values
The most common AC used in explanations involves allowing the nucleus to redirect its attractive power so that when an electron is removed the attraction between nucleus and an electron increases due to the increase in net ‘ionic’ charge. Using Coulomb’s Law applied to a point charge for the nucleus and similarly as a point charge for the average location of electron properties within the probabilistic orbital spaces available to it (not necessarily valid for an electron, but approximates well); the force to remove a particular electron depends on the mutual attraction between it and the nucleus. Therefore, removing an electron does not change the magnitude of the charge on an electron or on the nucleus and hence the cause for the change in subsequent ionisation energy must lie elsewhere. Nonetheless, this ‘conservation of force’ idea is often used to explain the general trend of increasing subsequent ionisation energies as noted by Taber (Citation1998) who described this idea as a ‘conservation of force’ misconception.
Tan, Taber, Goh, and Chia (Citation2005) similarly note that explanations for ionisation energy values require competing factors (nuclear charge, repulsion between electrons) to be weighed appropriately. They further suggest that a better understanding of ionisation energy values relies on a good understanding of electrostatics and Coulomb’s law.
Another issue is to do with the terminology used in explaining ionisation energy. Explanations that involve the decrease in attraction between an outer electron and the nucleus due to the ‘shielding’ by inner electrons, are readily accepted as appropriate. But the ‘shielding’ here can be attributed to the inner electrons somehow ‘blocking’ the attraction as in the signified meaning of shielding rather than the ‘active’ repulsion between electrons acting additionally to the attraction between an electron and nucleus such that the net force required to remove an outer electron is less. Here the electrostatic properties are hidden in the semiotics of the shielding term (Niebert, Marsch, & Treagust, Citation2012).
As the ideas used to explain ionisation energy values are also applicable to bonding concepts where similarly ACs are used by both pupils and their teachers, this area offers the opportunity to identify and challenge PSCT use of such ideas, hence deepening their wider chemical understanding and SCK. This paper considers how intervention activities undertaken during preservice teacher training affected the explanatory arguments used by these next generation teachers and hence their SMK.
Intervention activities
The author as a teacher educator used the intervention activities during half day university-based taught sessions (of about 2 hours) as part of the PSCT initial teacher education course – the Postgraduate Certificate in Education (PGCE). This UK course requires 9 months of full-time study combining two school-based periods of practice (6 months) and higher education institution (HEI)-based work interspersed around the school-based periods. The interventions aimed to support PSCT in rejecting the ‘conservation of force’ AC and replacing this with scientific causal arguments. Given that explanations of ionisation energy depend on electrostatic ideas, the first activity was used to develop a better understanding of electrostatics and involved reviewing Coulomb’s law and the effects of charge and distance on the electrostatic attraction/repulsion between charges.
Most PGCE chemistry sessions at this HEI involve approaches that blend both development of subject matter understanding and pedagogical approaches. In keeping with this, PSCT were informed that the purpose of the session was to develop their understanding of ionisation energy values and also to evaluate the potential of diagnostic testing for formative assessment. During the session the typical responses to the test items were shown and considered in terms of their diagnostic value. PSCT considered how such data could be used to inform teaching via targeted tasks for example. They also identified difficulties with such an approach and potential improvements.
First intervention activity: review of electrostatics and their application to atoms
The author as a PGCE course tutor and teacher, used 10 minutes of direct instruction, involving Socratic questioning in order to establish the electrostatics of atoms, especially regarding the mutuality of the interaction between an electron and the nucleus, probing the meaning of effective nuclear charge and developing this to recognise the effect of repulsive interactions between the electrons and hence effects on the distribution of electrons around the nucleus.
Further review of Coulomb’s law involved considering the two factors that make a difference to the magnitude of the mutual attraction between electron and nucleus; the distance between the charges and the magnitude of the charges, in order to develop understanding that for subsequent ionisation of a particular atom, the number of protons and hence nuclear charge does not change and the charge on each individual electron also remains the same and so the attraction between an electron and the nucleus is not affected by these charges, but rather by changes in the distance between them. (Given the quantisation of electron properties treating electrons as point charges is questionable, but the data values approximate well to considering them as such for average shell/orbital distance from the nucleus.). Final questioning involved the PSCT explaining how the removal of another electron could affect the distance between the remaining electrons and the nucleus and hence the subsequent ionisation energy values.
The second intervention activity: the bonfire analogy
Tan et al. (Citation2008) suggest that using a bonfire analogy also helps with supporting ideas about the attraction at a particular distance between nucleus and an electron being the same regardless of the number of electrons at the same distance, by analogy with the heat experienced by people around a bonfire. The fire cannot direct the heat experienced by people according to the numbers of people at a fixed distance from it. This then potentially offers the opportunity to raise the question as to why subsequent ionisation energies for electrons removed from within the same shell increase? To support the use of this analogy the Focus-Action-Reflection (FAR) approach to effective teaching using analogies was followed (Harrison & Treagust, Citation2000). Here the ‘Focus’ aspects of the approach are met by the familiar experience of heat from a bonfire such that this analogue offers a counter to the alternative concept that just ‘less electrons’ (less overall negative charge) is sufficient to account for increases in attraction between outer electrons and nucleus during subsequent ionisation.
This activity asks PSCT to use this analogy to explain the attraction between the nucleus and an electron and the effects of electron/electron repulsions. Groups of two or three PSCT discussed and completed the activity together. The author as their tutor offered prompts to support the use and mapping of the analogy, as they reviewed the target/analogue features by forming a table of these relationships, drawing on the example of atom and solar system (Harrison & Treagust, Citation2000). This also served to provide the ‘Action’ aspects of the FAR approach, to support the use of the analogy so that unshared attributes are not carried into explanations drawn from the analogy (Niebert et al., Citation2012). The identification of these shared and unshared attributes was prompted by the activity materials (Appendix 1).
The author trialled this approach with earlier cohorts of PSCT noting which attributes needed to be considered carefully and hence which to prompt and support thinking about during the subsequent sessions providing the final aspect of the FAR approach.
shows some of the shared and unshared attributes which are important to consider when using this analogy.
Table 1. Examples of potential bonfire analogy attributes.
During the activities as the author circulated, reflection on the usefulness and applicability of the analogy was sought. Additionally, in the plenary session some students offered their evaluations of the analogy and potential difficulties including those of careful mapping of attributes and potential improvements. This provided the final reflective stage of the FAR approach.
Research question
This study considers the following research question in order to consider the efficacy of these intervention activities in developing PSCT’ SCK: How effectively do interventions address the use of the alternative ‘conservation of force’ conception?
Methodology
In order to examine the efficacy of the intervention activities a pre- and post- intervention test methodology was employed which was triangulated via interviews. Tests and intervention activities were taken and carried out during half day training sessions (of about 2 hours). Each training session was part of the HEI-based study and consisted of the pre-intervention test; when data were collected via ‘Promethean Activ Vote®’ (Promethean, Citation2008) an electronic voting system from numbered handsets. PSCT were informed that the purpose of the session included development of their understanding of ionisation energies and also considering the use of formative assessment methods. The first intervention followed the initial test. PSCTs then completed two of three activities before responding to the same electronic test (post- intervention test).
One activity was the second intervention activity described above. Only this activity and the Coulombs Law explanation are considered here, as these apply directly to developing scientific ideas and addressing the AC of ‘conservation of force’ which some test items aim to identify being used. Other test items relate to using ideas about stability to explain ionisation energy values and an intervention activity regarding this idea was also completed by many but not all PSCT. Only data from PSCT who completed the bonfire analogy activity is considered here. During the activities, the author circulated groups clarifying the activities and answering questions. A small sample of these PSCT who had previously been interviewed prior to the training sessions was re-interviewed at least six weeks after the session.
The participant sample
Three academic year cohorts of PSCT attended the half day training sessions. These 95 PSCT were required to attend the sessions and prior to the pre-intervention test their permission was sought to retain and use the anonymised data collected via the electronic pre- and post-intervention tests for research. Handsets were numbered and selected by PSCT and hence responses were anonymised from the outset. All PSCT gave permission for their anonymised data from the electronic tests to be used. Also a convenience sample of 14 PSCT were interviewed prior to and after the training sessions. They volunteered to participate in the interviews and they provided their electronic test number to allow the data to be triangulated.
Data collection
Pre- and post-intervention tests
The electronic voting system was used to collect true or false responses to statements regarding ionisation energy values which included use of common ACs or non-causal ideas. Statements were taken from Taber’s (Citation2002) version of a true/false diagnostic quiz. The statements were displayed and participants prompted to indicate if they think it ‘true’ or ‘false’. This was followed by a confidence prompt using a Likert scale (1–4). Participants were encouraged to use the 3 or 4 option to indicate some level of ‘guessing’ and the 1 and 2 options to indicate their confidence in their reasoned response. Taylor and Kowalski (Citation2004) found that such true/false with confidence level (TFCL) instruments allowed misconceptions to be identified as AC statements were often identified as correct with more confidence than the correct ones were.
and indicate the science ideas needed to correctly identify if the statement is true or false and for statements 3, 6 and 9 how the incorrect true/false response might show the use of this AC. The potential effect of each activity in terms of developing understanding and hence in supporting PSCT in confidently identifying if a statement is true or false is also summarised. This analysis was carried out by both the author and an advanced level high school Chemistry teacher who similarly considered the activities and the statements to infer how they might affect the use of causal ideas and hence allow confident selection of the appropriate true/false response. Responses to statements 1, 7 and 10 are also considered as these statements do not correspond to commonly known ACs and hence these responses with their associated confidence level can be used to discriminate between responses that are incorrect and ones symptomatic of the use of the AC.
Table 2. The TFCL electronic test statements which use the ‘conservation of force’ AC and how each relates to the potential impact of the interventions.
Table 3. The TFCL electronic test statements which do not use ACs to allow for discrimination and how each relates to the potential impact of the interventions.
Analysis of data
Analysis of data from TFCL electronic questionnaire:
The use of true/false response instruments is understandably rare when considering the likelihood of random error or guessing responses skewing results, leading to a lack of valid data. Confidence levels provided with each ‘true’ or ‘false’ response were used to allow the ‘guessing’ factor to be identified and accounted for. Using a framework developed from Khan, Davies, and Gupta’s (Citation2001) any responses which indicated ‘very unsure’ character were considered as guessing regardless of correctness of response and the confident correct or incorrect used as indicators for assigning a scientific understanding or use of ACs. This hierarchy of response allows an ordinal scale to be used to reflect these different types of underlying reasoning as shown in .
Table 4. Framework showing the relationship between confidence and accuracy of true/false responses and also the coding and ordinal scales used for analysis.
In order to consider the efficacy of the activities the change in responses to the statements was evaluated. Favourable outcomes would be indicated by improving use and confidence in scientific concepts/rejection of AC and maintenance of scientific concepts/rejection of AC when already held/rejected (a positive change in position on the ordinal score).
As the responses have non-normal distributions, non-parametric quantitative analysis methods are needed. Wilcoxon Signed Rank tests were carried out to compare the differences in responses as an indicator of variance in these ordinal data sets (Muijs, Citation2011).
Validity and reliability
In order to check that the use of a TFCL test allowed the use of ACs to be identified, the relative accuracy of PSCT’ confidence judgements and performance was considered by comparing this accuracy of judgement for correct and incorrect items for items where ACs are likely to be used compared with items where this is unlikely. This can be measured by a discrimination index (Schraw, Citation2009). Millar (Citation2013) used a similar approach with preservice teachers to identify the use of ACs regarding electricity and Forces. Equation (1) shows how this discrimination index ‘d’ can be calculated for each PSCT’s responses to the statements.(1) where nc is the total number of statements answered correctly, ni is the total number of statements answered incorrectly, Ci,correct is the confidence level for a correct answer and Ci,incorrect is the confidence level for an incorrect answer.
Positive d values occur when an individual is more confident about their correct responses compared to their incorrect ones and negative values indicate when an individual is more confident about their incorrect responses compared to their correct ones. Here if the statements using the ‘conservation of force’ AC are being incorrectly but confidently answered then the discrimination index will be negative (minimum value −4.00). In order to determine if the statements are identifying the use of the AC, the discrimination index ‘d’ value for responses to statements which are included in order to note the use of this AC (S3, 6 and 9) was compared with discrimination indices for responses to other statements (S1, 7 and 10). This allows the difference to be considered in terms of statistical significance. Furthermore analysis of similarities or differences between these discrimination indices before and after intervention will allow potential changes in the metacognitive judgement of the PSCT to be considered after the intervention activities.
A Wilcoxon signed ranks test using SPSS (IBM, Citation2013) for PSCT discrimination indices before and after intervention () indicates that there is a statistically significant difference between the indices and the effect size for this change is large, such that median index values, indicate that the most common overall response pattern for the statements revealing potential use of the AC was of confident giving of incorrect answers compared to confident giving of correct answers to statements that did not involve ideas associated with common ACs. This provides content validity for the TFCL questionnaire in identifying the use of the ‘conservation of force’ AC.
Table 5. Comparison of discrimination indices for non-AC statements with AC statements.
A comparison of the responses collected from each of the cohorts of PSCT was conducted to consider the possible effects of the different times of the tests and interventions being administered and also to consider if cohorts provide consistent responses and so offer reliability. Five sets of tests were administered in total across the three cohorts of PSCT (two of these cohorts were large and so for their PGCE training session they were split into two half cohort groups one with a morning session and one with an afternoon session). Each of these five TFCL test groups’ initial responses was compared to each other via a Kruskal–Wallis independent samples test for non-parametric data. The responses to each statement for S3, S6, S9 and S10 were compared using SPSS (IBM, Citation2013). No statistically significant differences between the cohorts’ responses were found.
Interviews
In order to triangulate data collected from the electronic questionnaire and to determine the ideas PSCT used to explain ionisation energy values, individual semi-structured interviews of approximately 20 minutes were used. Analysis of interview responses aimed to characterise explanatory arguments used and allow identification of the use of the AC and/or scientific ideas. The interview protocols included: An explanation of the variation in ionisation energy values shown in graphs of the subsequent ionisation energy values for an oxygen atom (pre-intervention) and a Fluorine atom (post- intervention).
Interview data
Interviews were audio recorded and detailed contemporaneous notes taken in order to record the meaning making gestures (hand waving and pointing), without the distraction of a video camera. After the interviews the contemporaneous notes were edited, adding detail from the audio recording to ensure the connection between different ideas used in explanations was accurate in the notes. As coding using this protocol compared with that of transcribed interviews did not offer additional reliability, the edited notes were used as the primary data sources (Wheeldon, Citation2012).
Interpreting the interview data
Data were examined via content analysis to determine the types of causality argument used in explanations. This method of analysis was confirmed as suitable by a high level of inter-rater reliability between coding of the ideas by the researcher and an advanced level high school chemistry teacher. As interviewees form a convenience sample of the wider cohorts, a Kruskal–Wallis independent samples test for non-parametric data was used to compare the responses to each statement for the pre- and post- tests for those interviewed compared with those that were not. For each statement no statistically significant difference in distribution of responses was found. Similarly an analysis of the discrimination of interviewees compared with the wider cohorts also showed no statistically significant differences. Therefore data from this sample can offer triangulation.
Findings
How effectively do interventions address the use of the alternative ‘conservation of force’ conception?
Analysis via non-parametric analysis using a related sample Wilcoxon signed rank test administered via SSPS (IBM, Citation2013) for the changes in the ordinal categories of responses for each statement S3, S6 and S9 was carried out and is summarised in .
Table 6. Showing significance and effect size and median scores (on an ordinal scale of 1–7) for S3, S9 & S9 for differences in pre- and post-test.
The medium effect sizes (Cohen, Citation1988) combined with significant statistical differences in responses as shown in and the direction of change in the median score together show that the participants decrease in their incorrect use of this AC or increase in rejecting this as a causal argument for each statement from pre-test to post-test and so indicate that the intervention activities address this AC. While there is some argument whether such medium effect sizes are of practical value in educational research, Xitao Fan (Citation2001) suggests that given the difficulty of multiple factors affecting responses in educational settings such medium-sized effects are potentially meaningful both practically and significantly. This practical meaningfulness can be noted by considering the median score value for the pre- and post-tests in terms of the responses which the ordinal numerical categories represent. However as such median values are just that, some response changes involve less confident rejection of the AC or no change or indeed increased confidence in using the AC as well as those that show increasing confidence in the rejection of the AC.
The interventions are effective in allowing many PSCT to reject the ‘conservation of force’ AC. Furthermore changes in the discrimination indices of responses to the items that involve this AC before and after intervention activities also offer a sense of the changes in the metacognition of the PSCT regarding their responses to the statements. summarises these changes when analysed using a related samples Wilcoxon signed rank test administered via SSPS (IBM, Citation2013). The changes before and after intervention are statistically significant and show a large effect size for a change from confident use of the AC to confident rejection of it. The discrimination index improvement after the interventions for statements that do not involve common ACs is not statistically significantly. The changes in discrimination for the responses to the AC statements are such that after intervention there is no significant difference between discrimination across all statements.
Table 7. Changes in discrimination pre and post-intervention activities.
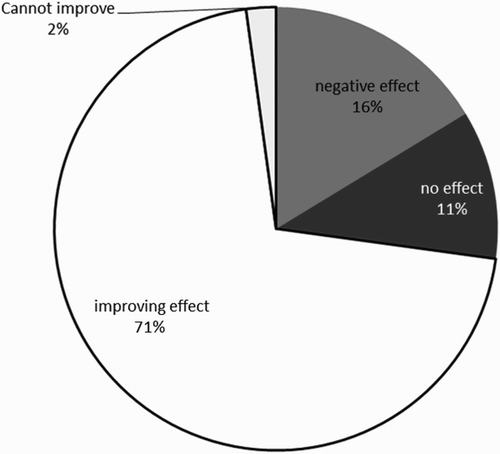
Overall the effect of the intervention on responses to all the statements probing the use of this ‘conservation of force’ AC is illustrated in .
Insights from interviews
Another important consideration is that the interventions’ effects might be for PSCT to identify the AC as non-scientific and hence be effective in changing responses to statements using the AC but not result in the replacement of this conception with scientific ones beyond the interventions session itself and here it is useful to use the additional information gathered via interview to consider changes in interview explanations and hence the possible effects of the interventions in the longer term.
Data from the interview sample reveal that initially the interventions lead to 11 of the 14 (79%) PSCT improving in their rejection of the AC compared to 71% for the whole cohort which is promising for the effectiveness of these interventions.
To illustrate the effect of the interventions, extracts from Syeda’s (pseudonym) interviews are given below (Syeda and four other PSCT’ responses to the pre-intervention tests indicate the use of the AC and they also use this in their pre-intervention interviews – see ). After intervention they no longer use this AC in TFCL tests or in interview explanations. From the first interview, when asked to describe the increasing general trend in subsequent ionisation energy values:
Syeda (pseudonym): Electrostatic attraction between nucleus and electrons increases because there is one more positive for each electron that is lost.
I: Is it getting more positive?
Syeda: It is getting more positive? You mean overall charge remaining the same? The overall positive charge is the same; It is the number of electrons lost, negatives that is being lost.
I: Why does the electrostatic attraction change?
Syeda: Because the remaining electrons are experiencing a greater attraction from the nucleus
I: Why?
Syeda: Because there is an unbalance in the number of protons and electrons.
Table 8. Effects of interventions on the ideas used to explain ionisation energies.
In the post- intervention interview Syeda explains subsequent ionisation energy in a rather different way:
Using the Bonfire Analogy; as electrons are lost, the circle gets smaller, more closer [sic] to the nucleus. Comparing to the Bonfire Analogy fire and people holding hands [around it], as electrons are lost they come closer to the Bonfire.
For some interviewees (4 of the 14) the pre-intervention TFCL test itself is the only time the AC is noted, perhaps being encouraged by the nature of the statements. Taber (Citation1998) suggests that this particular argument may arise as a spontaneous explanation in certain contexts. Through my questioning of advanced level high school pupils and PSCT, I have noted the AC often arises when they are asked to explain subsequent ionisation energies but usually only after a pause when pressed as to why it requires more energy for each subsequent electron removed. I suggest that for these chemists it provides a causality that seems in keeping with similar scientific arguments like the subsequently rising electron affinity energies which are often explained by the need to provide more energy to do the work of adding an electron to an increasing negatively charged ion. As an inverse argument for ionisation energy it does seem to have an initial plausibility (Taber & Tan, Citation2011).
As a further example, I will consider Mary’s (pseudonym) interviews. Prior to the pre-interventions test, during interview Mary did not explain the general trend in subsequent ionisation energy explicitly using the AC. However in her interview 3 months after the interventions she explained the general trend of increasing ionisation energies:
Amount of protons in the nucleus stays the same, so same amount of the positive charge pulling them. As [you] remove each electron they can get closer to the nucleus and then it is harder to remove them.
I used to think that when you took an electron away the nuclear charge would then be shared out over the remaining electrons, so each would be pulled more. The Bonfire [Analogy] told me that’s not the cause.
However interview data indicate that only 9 of 11 (82%) PSCT retain this immediate rejection of this AC in the longer term (at least six weeks) indicating that although a two-hour session with PSCT may allow a long term improvement PSCT’ CK in this area of applying electrostatic ideas appropriately to an atom, as shown in , there is an important group of PSCT where the interventions are not effective.
Discussion
Data suggest that more than 60% (60.5%) of PSCT in this study use the ‘conservation of force’ AC. This is consistent with similar findings (Taber & Tan, Citation2011). These interventions allowed many PSCT to recognise that these ideas are not consistent with science electrostatic causal explanations for changes in ionisation energy values. Furthermore for those that did not initially use this AC, their confidence in rejecting it increased.
The analogy may be most useful when analogical relations are constructed rather than simply noted by target/analogue comparison. Wilbers and Duit (Citation2006) suggest when the features of the target need to be developed and understood then correspondence between target and analogue is difficult to examine. Rather it is these constructed relations which provide the analogy with plausibility. In this study, the suggestion that the target atom feature of ‘electron shells’ could find an analogue feature ‘circles of people holding hands’ around the bonfire’, resulted in both the interviewed PSCT from that session using the analogy to explain subsequent ionisation in their post-intervention interview (Syeda and Shirley). Here the ‘circles of people holding hands’ provides an embodied experience in the analogue which makes this aspect of the analogy available to make sense of the collapsing electron shells of the target (Niebert et al., Citation2012).
These two-hour training sessions offer the opportunity to break the cycle of future chemistry teachers teaching as they were taught (Taber & Tan, Citation2011). This can reduce the use of this AC which the literature shows has also been used by PSCT in Singapore (Tan & Taber, Citation2009) and pupils in the UK, Singapore, New Zealand, China, Spain and USA (Tan et al., Citation2008). These intervention activities offer broader improvements in the PSCT SMK as the development of the appropriate application of the electrostatics to electrons as part of these activities, applies beyond ionisation energy concepts; for example, to bonding where common descriptions of covalent bonds as ‘a shared pair of electrons’ disguises their electrostatic character. This offers the opportunity to improve the wider chemistry subject matter of PSCT which can allow better future teaching of these wider fundamental chemistry concepts (Park & Light, Citation2009). These teachers can better relate observable phenomena to the sub-microscopic entities and the models used to explain them as the PSCT are drawing on more accurate CK (Coll & Treagust, Citation2003).
This study is limited as data are from one institution and focused on one particular AC. However as the use of the AC is consistent with that of PSCT more widely at other intuitions and in other countries, it offers a potential approach for the CK development during preservice training, which allows a common AC to be recognised as such and not perpetuated via teaching.
For a number of PSCT these interventions appear to have no effect (11%) or a negative effect (16%). Such resistant ideas are commonly noted in science education literature and perhaps for these PSCT the length of time that they have held these ideas may also increase the resistance to change (in the case of these PSCT this is likely to be greater than 4 years) (Taber & Tan, Citation2011). An issue with this AC is that it does provide a means by which increasing subsequent ionisation energies can be accounted for and as it is common for students to be asked to describe the trend, but it is rare they are asked to explain why, a spontaneous simplistic explanation which is similar to that for subsequent electron affinities offers an initial plausibility. Kapon and diSessa (Citation2012) considered how selection of simple explanatory principles or e-prims (explanatory primitives) for use with analogies was affected by pragmatic goals, such that when prior use of the e-prim had yielded correct answers this experience would prompt this argument to be used again. This appears to be the case here.
Furthermore, Ahmed (pseudonym) an interviewee who did not show rejection of the ‘conservation of force’ AC, offers a potential insight into how the Bonfire analogy might be used to support this AC. In his initial interview prior to the intervention session he described the increasing charge on the ion as being responsible for the increasing attraction between the remaining electrons and nucleus, but at interview 6 weeks after the interventions he uses the Bonfire analogy: ‘If someone leaves the circle [round the fire], then the circle gets closer’. Here the causal idea is given via the analogy, However he goes on: ‘..heat from the fire is distributed equally for the remaining ones, people. Using that analogy for the force of the nucleus’.
Here one of the issues with the analogy itself comes to the fore, as the analogical ‘heat’ is radiated from the bonfire according to the rate of combustion and so is a property of the bonfire alone whereas for the target ‘attraction between nucleus and electron’, the nucleus does not intrinsically provide force but rather the attraction is due to interactions between both the nucleus and electrons. As Gentner’s (Citation1983) structure mapping theory indicates, this difference in source analogue and target, can encourage an incorrect mapping of ‘only the bonfire affecting the heat radiated’ to ‘the nucleus only causing the attraction’.
This mixed use of appropriately and inappropriately mapped attributes raises the possibility that the embedded AC may even result in Ahmed’s use of the analogy to support it.
Neurobiological processing of mapping activities (Rohrer, Citation2005) provides evidence that when imagining a phenomena this activates the same neutral structures as thinking about or experiencing the analogous behaviour. Niebert et al. (Citation2012) therefore contend that the experiencing of the analogous relationships provides the embodied understanding on which the target concept relies. Here the experience of wanting to experience heat from a fire may have resulted in a mapping of the idea that less people around that fire means that they can be closer to it (analogous electrons move closer to the nucleus as the repulsions between fewer electrons allow this). However with that idea of all the people drawing closer to a desired warmth, perhaps those of social conventions of sharing the extra warmth now available between them fairly rather than one or two people getting much closer may also activate the sharing out of the heat explanation rather than noting that heat from the fire is radiated evenly in all directions and so the distance from it determines the heat experienced. In the analogy the energy available to be transferred is dependent on the characteristics of the fire rather than the interaction between the fire and the person. In the target atom, the attraction between charged nucleus and electron is due to the interaction of their charges and their relative location to one another. Here only the locational aspects are mapped well between analogue and target.
Potvin (Citation2017) has noted that when explanations could draw on ACs (even when these are not used in the argument presented) the neutral structures associated with these ACs are used, but the responses from them are inhibited. Thus rather than such ideas being replaced they are rather only used for explanation when they are appropriate and inhibited as needed. Here the fruitful use of considering the increasing charge on an anion causing increased repulsion between it and an electron can account for increasing subsequent electron affinities but this explanatory idea needs to be inhibited so that it does not lead to an increasing positive charge on a cation resulting in increased subsequent ionisation energies (as here the actual positive charge of the nucleus has not increased). This ability to identify models and explanations with fruitful features and hence reject those that do not, has long been acknowledged as characteristic of expert scientists and results in the expert being able to select the most suitable and simplest model or explanation appropriate for the situation (Grosslight, Unger, Jay, & Smith, Citation1991).
Ahmed’s taking of an analogy to support an embedded idea (AC) is consistent with Wakabayashi and Guskin’s (Citation2010) findings and their argument that these embedded ideas are deeply resistant to change as also noted in science education literature (e.g. Chi, Citation1992). Furthermore Dole (Citation2000) suggested that the more strongly a belief was held, the more it reduced comprehension of new material which was in conflict with the prior belief. Kapon and diSessa (Citation2012) consider how the prior knowledge about the target affects the evaluation of the plausibility and applicability of the analogy. In Ahmed’s explanation; he knows that removal of another electron results in a further increase for the next subsequent ionisation energy even if the electrons are removed from the same shell. Thus an explanatory primitive; the idea of ‘sharing out’ in this case ‘force’, which has generated the correct trend of increasing values for subsequent ionisation values, is mapped onto the analogy, it results in heat being distributed evenly by the fire intentionally rather than heat radiating from the fire source evenly in all directions. Ahmed likely knows that the fire is not intentionally sharing out heat but as the sharing out of a force over fewer electrons seems to offer a simple and plausible explanation for the increase in subsequent ionisation energy, it may result in him not carefully examining the application of this idea to the familiar analogue. His pragmatic goal of having a plausible argument for the increasing subsequent ionisation energy (one he has used before) has superseded recognising that the change in distance he has already noted would increase the heat experienced in the analogy and hence mapped on to the target would result in an increase in attraction between the now closer outer electrons and the nucleus. This reveals just how important teacher prompts are in ensuring the analogue properties are appropriately mapped and making explicit where shared attributes are valuable for explanation, as well as unshared attributes being acknowledged to avoid alternative ideas being applied.
Implications
This study indicates the potential of short preservice training sessions to develop PSCT’ CK even when this involves addressing commonly used ACs and hence offers the opportunity to prevent such ACs being passed on to the next generation of chemistry students and improving their chemical knowledge.
Analogies in science and science education are necessary thinking tools, but ones which are often introduced without consideration of the nature of that tool. For PSCT these intervention activities offer opportunities for them to consider how analogies may work well but also when they do not. As noted in the discussion above the analogy proved most effective when a particular feature ‘people holding handing in circles’ was introduced to the bonfire analogue to support a target concept of contracting shells of electrons. This raises two implications; firstly that this added feature needs to be prompted as one to be explored when using the analogy and secondly, the need for a collective review of the analogy; clarifying the properties of heat which is an abstract concept in its own right although its effects are an everyday experience and considering in depth the good and poor attribute mapping. This would allow explicit recognition of the useful and non-useful explanations supported, to allow an increased retention of the scientific causal ideas and a long lasting rejection (inhibition) of the AC thinking considered here. This review offers the opportunity for the intended conceptual reconstruction via a plausible fruitful alternative (Duit & Treagust, Citation2012).
Chemistry teachers need to be provided with the opportunity preservice, to examine and develop their CK and also their use and understanding of analogies as noted above. In the UK, there is a current drive towards school-based teacher preparation, which involves little or no input from science education specialists who are engaged in research. Therefore, this study provides implications that such teacher training will likely result in these sorts of ACs persisting, being unknowingly passed on by school teachers providing training. Improving teachers’ use of analogies and examination of them is also important as they are used in textbooks often without acknowledging them as analogies or considering the potential areas where the analogies do not work well. This research indicates the importance of the role of HEIs in providing specialist teacher educators who are aware of these conceptions and the value of and issues with analogies and who can provide interventions to develop teachers’ CK and PCK.
Acknowledgements
The author would like to thank all the PSCT who kindly agreed to be interviewed and also those that allowed their responses to the TFCL tests to be used here. I would also like to thank the two anonymous reviewers for their thoughtful and helpful comments on earlier versions of this article.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Abell, S. K. (2007). Research on science teacher knowledge. In S. K. Abell, & N. G. Lederman (Eds.), Handbook of research on science education (pp. 1105–1150). Mahwah, NJ: Lawrence Erlbaum.
- Arzi, H. J., & White, R. T. (2007). Change in teachers’ knowledge of subject matter: A 17-year longitudinal study. Science Education, 92(2), 221–251. doi: 10.1002/sce.20239
- Barber, M., & Mourshed, M. (2007). How the world’s best performing school systems came out on top. London: McKinsey and Company.
- Chi, M. T. H. (1992). ‘Conceptual change within and across ontological categories: Examples from learning and discovery in science’. In R. N. Giere (Ed.), Cognitive models in science (vol. XV, pp. 129–186). Minneapolis: University of Minnesota Press.
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: L. Erlbaum Associates.
- Coll, R., & Treagust, D. (2003). Investigation of secondary school, undergraduate, and graduate learners’ mental models of ionic bonding. Journal of Research in Science Teaching, 40(5), 464–486. doi: 10.1002/tea.10085
- Crown. (2011). Teachers’ Standards. Guidance for school leaders, school staff and governing bodies. Retrieved June 3, 2015, from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/301107/Teachers__Standards.pdf
- Deng, Z. (2007). Knowing the subject matter of a secondary school science subject. Journal of Curriculum Studies, 39(5), 503–535. doi: 10.1080/00220270701305362
- Dole, J. A. (2000). ‘Readers, texts and conceptual change learning’. Reading & Writing Quarterly, 16, 99–118. doi: 10.1080/105735600277980
- Driver, R., & Erickson, G. (1983). ‘Theories-in-action: Some theoretical and empirical issues in the study of students’ conceptual frameworks in science’. Studies in Science Education, 10, 37–60. doi: 10.1080/03057268308559904
- Driver, R., Squires, A., Rushworth, P., & Wood-Robinson, V. (1994). Making sense of secondary science: Research into children’s ideas. London: Routledge.
- Druva, C. A., & Anderson, R. D. (1983). Science teacher characteristics by teacher behavior and by student outcome: A meta-analysis of research. Journal of Research in Science Teaching, 20, 467–479. doi: 10.1002/tea.3660200509
- Duit, R. H., & Treagust, D. F. (2012). Conceptual change: Still a powerful framework for improving the practice of science instruction. In K. C. D. Tan, & K. Mijung (Eds.), Issues and challenges in science education research (pp. 43–54). New York: Springer.
- Evagorou, M., Dillon, J., Viiri, J., & Albe, V. (2015). Preservice science teacher preparation in Europe: Comparing preservice teacher preparation programs in England, France, Finland and Cyprus. Journal of Science Teacher Education, 26, 99–115. doi: 10.1007/s10972-015-9421-8
- Fan, X. (2001). Statistical significance and effect size in education research: Two sides of a coin. The Journal of Educational Research, 94(5), 275–282. doi: 10.1080/00220670109598763
- Gentner, D. (1983). Structure-mapping: A theoretical framework for analogy. Cognitive Science, 7(2), 155–170. doi: 10.1207/s15516709cog0702_3
- Gilbert, J. K., & Watts, D. M. (1983). ‘Concepts, misconceptions and alternative conceptions: Changing perspectives in science education’. Studies in Science Education, 10, 61–98. doi: 10.1080/03057268308559905
- Goodwin, A. L. (2012). Teaching as a profession: Are we there yet? In C. Day (Ed.), The Routledge international handbook of teacher and school development (pp. 44–56). Abingdon: Taylor & Francis.
- Grosslight, L., Unger, C., Jay, E., & Smith, C. (1991). Understanding models and their use in science: Conceptions of middle and high school students and experts. Journal of Research in Science Teaching, 28, 799–822. doi: 10.1002/tea.3660280907
- Harrison, A. G., & Treagust, D. F. (2000). A typology of school science models. International Journal of Science Education, 22(9), 1011–1026. doi: 10.1080/095006900416884
- Hashweh, M. Z. (1987). Effects of subject matter knowledge in the teaching of biology and physics. Teaching and Teacher Education, 3, 109–120. doi: 10.1016/0742-051X(87)90012-6
- IBM. (2013) IBM SPSS Statistics (Version 22.0.0.0) [Computer program]
- Kapon, S. & diSessa, A. A. (2012). Reasoning through instructional analogies. Cognition and Instruction, 30(3), 261–310. doi: 10.1080/07370008.2012.689385
- Khan, K. S., Davies, D. A., & Gupta, J. K. (2001). Formative self-assessment using multiple true-false questions on the Internet: Feedback according to confidence about correct knowledge. Medical Teacher, 23(2), 158–163. doi: 10.1080/01421590031075
- Kind, V., & Kind, P. M. (2011). Beginning to teach chemistry: How personal and academic characteristics of preservice science teachers compare with their understandings of basic chemical ideas. International Journal of Science Education, 33(15), 2123–2158. doi: 10.1080/09500693.2010.542498
- Kind, V. (2009). A conflict in your head: An exploration of trainee science teachers’ subject matter knowledge development and its impact on teacher self-confidence. International Journal of Science Education, 31(11), 1529–1562. doi: 10.1080/09500690802226062
- Kind, V. (2014). A degree is not enough: A quantitative study of aspects of preservice science teachers’ chemistry content knowledge. International Journal of Science Education, 36(8), 1313–1345. doi: 10.1080/09500693.2013.860497
- Käpylä, M., Heikkinen, J-P, & Asunta, T. (2009). Influence of content knowledge on pedagogical content knowledge: The case of teaching photosynthesis and plant growth. International Journal of Science Education, 31(10), 1395–1415. doi: 10.1080/09500690802082168
- Millar, R. (2013). Assessing beginning teachers’ subject knowledge: How reliable is self-audit? Paper presented at the ESERA conference, Nicosia.
- Mourshed, M., Chijioke, C., & Barber, M. (2010). How the world’s most improved school systems keep getting better. London: McKinsey and Company.
- Muijs, D. (2011). Doing quantitative research in education with SPSS. London: SAGE.
- Niebert, K., Marsch, S., & Treagust, D. (2012). Understanding needs embodiment: A theory-guided reanalysis of the role of metaphors and analogies in understanding science. Science Education, 96(5), 849–877. doi: 10.1002/sce.21026
- NSTA. (2004). Position statement: Science teacher preparation. Retrieved January 15, 2016, from http://www.nsta.org/docs/PositionStatement_TeacherPrep.pdf
- Park, E. J., & Light, G. (2009). ‘Identifying atomic structure as a threshold concept: Student mental models and troublesomeness’. International Journal of Science Education, 31(2), 233–258. doi: 10.1080/09500690701675880
- Potvin, P. (2017). The coexistence claim and its possible implications for success in teaching for conceptual “change”. European Journal of Science and Mathematics Education, 5(1), 55–66.
- Promethean. (2008). Promethean Activ software Expression edition (Version 1.0.4522) [Computer program].
- Rohrer, T. (2005). Image schemata in the brain. In B. Hampe, & J. Grady (Eds.), From perception to meaning: Images schemas in cognitive linguistics (pp. 165–196). Berlin: Mouton de Gruyter.
- Sanders, L. R., Borko, H., & Lockard, J. D. (1993). Secondary science teachers’ knowledge base when teaching science courses in and out of their area of certification. Journal of Research in Science Teaching, 30(7), 723–736. doi: 10.1002/tea.3660300710
- Schraw, G. (2009). Measuring metacognitive judgments. In D. J. Hacker, J. Dunlosky, & A. C. Graesser (Eds.), Handbook of metacognition in education (pp. 415–429). New York, NY: Routledge, Taylor & Francis.
- SCORE. (2015). Principles for routes into teaching the sciences at secondary level. Retrieved June 3, 2015, from http://www.score-education.org/media/16294/final20score20ite20position20paper20-20designed.pdf
- Shulman, L. S. (1986). Those who understand: Knowledge growth in teaching. Educational Researcher, 15(2), 4–14. doi: 10.3102/0013189X015002004
- Taber, K. S. (1998). The sharing-out of nuclear attraction: Or I can’t think about Physics in Chemistry. International Journal of Science Education, 20(8), 101–1001. doi: 10.1080/0950069980200807
- Taber, K. S. (2002). Chemical misconceptions—prevention, diagnosis and cure, volume II: Classroom resources. London: RSC.
- Taber, K. S., & Tan, K. C. D. (2007). Exploring learners’ conceptual resources: Singapore A level students’ explanations in the topic of ionisation energy. International Journal of Science and Mathematics Education, 5, 375–392. doi: 10.1007/s10763-006-9044-9
- Taber, K. S., & Tan, K. C. D. (2011). The insidious nature of ‘hard-core’ alternative conceptions: Implications for the constructivist research programme of patterns in high school students’ and pre-service teachers’ thinking about ionisation energy. International Journal of Science Education, 33(2), 259–297. doi: 10.1080/09500691003709880
- Tan, K.-C. D., Taber, K. S., Goh, N.-K., & Chia, L.-S. (2005). The ionisation energy diagnostic instrument: A two-tier multiple choice instrument to determine high school students’ understanding of ionisation energy. Chemistry Education Research & Practice, 6(4), 180–197. doi: 10.1039/B5RP90009C
- Tan, K.-C. D., & Taber, K. S. (2009). Ionization energy: Implications of preservice teachers’ conceptions. Journal of Chemical Education, 86(5), 623–629. doi: 10.1021/ed086p623
- Tan, K. C. D., Taber, K. S., Liu, X., Coll, R. K., Lorenzo, M., Li, J., … Chia, L. S. (2008). Students’ conceptions of ionisation energy: A cross cultural study. International Journal of Science Education, 30(2), 263–283. doi: 10.1080/09500690701385258
- Taylor, A. K., & Kowalski, P. (2004). Naive psychological science: The prevalence, strength and sources of misconceptions. The Psychological Record, 54, 15–25. doi: 10.1007/BF03395459
- Trumper, R. (2001). ‘A cross-college age study of science and nonscience students’ conceptions of basic astronomy concepts in preservice training for high-school teachers’. Journal of Science Education and Technology, 10(2), 189–195. doi: 10.1023/A:1009477316035
- TSO. (2010). The importance of teaching – The Schools White Paper 2010. Retrieved June 3, 2015, from http://webarchive.nationalarchives.gov.uk/20130401151715/https://www.education.gov.uk/publications/eOrderingDownload/CM-7980.pdf
- Wakabayashi, T., & Guskin, K. (2010). The effect of an ‘‘unsure’’ option on early childhood professionals’ Pre- and post-training knowledge assessments. American Journal of Evaluation, 31(4), 486–498. doi: 10.1177/1098214010371818
- Wallace, J. (2005). Reading accounts: Central themes in science teachers’ descriptions of exemplary teaching practice, Ch.8. In S. Alsop, L. Bencze, & E. Pedretti (Eds.), Analysing exemplary science teaching (pp. 171–182). Maidenhead: Open University Press.
- Wayne, A., & Youngs, P. (2003). Teacher characteristics and student achievement gains: A review. Review of Educational Research, 73, 89–122. doi: 10.3102/00346543073001089
- Wheeldon, R., Akinson, R., Dawes, A., & Levinson, R. (2012). Do high school chemistry examinations inhibit deeper level understanding of dynamic reversible chemical reactions? Research in Science and Technological Education, 30(2), 107–130. doi: 10.1080/02635143.2012.692362
- Wheeldon, R. (2012). Examining preservice teachers’ Use of atomic models in explaining subsequent ionisation energy values. Journal of Science Education and Technology, 21, 403–422. doi: 10.1007/s10956-011-9333-0
- Wilbers, J., & Duit, R. (2006). Post-festum and heuristic analogies. In P. J. Aubusson, A. G. Harrison, & S. M. Richie (Eds.), Metaphor and analogy in science education (pp. 37–49). Dordrecht, NL: Springer.
Appendix 1: Bonfire analogy stimulus materials
The Bonfire Analogy
We can compare the attraction an electron experiences to the heat one receives from a bonfire
The Bonfire heat experienced is dependent on:
How big the bonfire is
The distance one is away from the bonfire
Whether one is blocked (screened/shielded) from the bonfire
Tasks
Consider the effect of how many people are present at the same distance away from the bonfire on the heat experienced.
Use this analogy to explain the attraction between nucleus and an electron.
Use the table below to explain the key parts of the analogy and note when the analogy breaks down.
Use the example for the solar system analogy of an atom to help you think about analogies (Harrison & Treagust, Citation2000, p. 1018):
The Bonfire Analogy