ABSTRACT
Binary polymer systems provide significant advantages in the preparation of materials used in biomedical applications. To highlight the importance and need of binary polymer systems in biomedical applications; utilisations of nano-carrier and fibre are discussed in detail in terms of their use as biomaterial, and their potential for further development with focus on dual and sequential drug delivery applications. On the other hand, in fibre technology, creation of binary polymer systems have been investigated using spinning processes such as electrospinning and even more recently innovated pressurised gyration. How these methods can be used to promote the mass production of binary polymer systems with various morphologies and characteristics are elucidated. The effects of different polymer materials, including solvents, mechanical properties, and the rate of degradation of polymers, are discussed. Current polymer blending systems and manufacturing processes are analysed, and technologies for biomaterials are carefully considered with up to date details.
Introduction
Polymers are a critical class of materials owing to their chemical variations and characteristics for biomedical purposes. While natural polymers have modifiable properties where largely a top-down strategy can be adopted, synthetic polymers can be synthesised from bottom-up or can be made suitable for a specific aim by chemical modification [Citation1,Citation2]. However, the complex nature of biological systems and the difficulty of the materials design needed in diagnostic and therapeutic strategies to respond to this complex hierarchy reveal the need to use different polymers together. Polymers used in the health applications have flexibility, strength, biocompatibility, biodegradability, biological activity, cell-inducing, regenerative, and differentiation properties, which can vary depending on the chemical, physical and/or mechanical structure of the polymer [Citation3–7]. While these polymers developed for demand are sometimes prepared as copolymers, they are more often obtained by preparing blend forms of dual (binary) or more polymers.
Copolymers are a broad group of polymers which comprise of at least two different monomer groups (A and B). These different monomers covalently bond to each other. However, the type of copolymer varies upon the A and B monomer groups bonding types (locations) such as, block, random, graft and alternating. On the other hand, blended polymers present materials with improved/reorganised physicochemical properties that are obtained by homogeneously mixing at least two types of polymers. Additionally, blended polymers can be composed of homopolymers and copolymers. If the prepared blended polymer system consists of two different types of polymers, this dual system is called a binary polymer system [Citation7,Citation8].
The use of binary polymer systems provides advantages in many application areas. The basis of these advantages lies in the ability to create a combination by combining the properties of two different types of polymers. The fact that binary polymer systems have adjustable and modifiable properties causes them to have a leading position for applications in biomedical fields. The binary polymers which have been used in the biomedical area must be considered with priority, as the components of the binary polymer characteristics can serve as a therapeutic, diagnostic, or theranostic purposes. Binary polymer systems have application areas such as nano-carriers [Citation9], nanofibres [Citation10], implants [Citation11], catheters [Citation12], scaffolds [Citation13], microfluidic reactors [Citation14,Citation15] etc. (). Following on, the binary system should be designed considering the biological, physical, and structural needs of the application area, as it will interact, regenerate, support (mechanically) and/or replace. Alginate (ALG) [Citation16,Citation17], cellulose [Citation18,Citation19], silk [Citation20,Citation21], chitosan (CS) [Citation22,Citation23], collagen [Citation24,Citation25], keratin [Citation26], gelatine [Citation27,Citation28], poly(lactic acid) (PLA) [Citation29], poly(lactic-co-glycolic acid) (PLGA) [Citation30], poly(ethylene glycol) (PEG) [Citation31], poly(caprolactone) (PCL) [Citation32], poly(vinyl alcohol) (PVA) [Citation33,Citation34] etc. are the most commonly utilised polymers in binary systems for biomedical applications. Additionally, determining the common physicochemical parameters (solubility, melting temperature, viscosity, conductivity etc.) required for the adaptation of binary polymer systems to co-production techniques is of immense importance in the selection of binary polymer pairs to be used together [Citation35].
Figure 1. Schematic representation of (A) binary polymers composed of two different types of polymer molecules, (B) binary polymers systems for particle and fibre applications (i) core shell, (ii) Janus, (iii) blend.
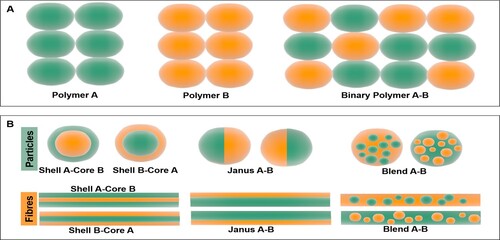
In this review, the advantages of binary polymers are highlighted within the integrated bio-fabrication methods, and the applications are exemplified by given binary polymer pairs. Moreover, binary polymer systems are discussed in order to address the needs of biomedical applications and solutions that can be developed, considering different purposes from physical, chemical and biological aspects, and it is aimed to generate guidelines for researchers interested in binary polymer-based biomedical materials.
Binary polymer systems
Polymers are made up of small molecules called monomers, which bind together to form long chains. In the biomedical field, various chain length polymers can be used for repair in the body or for drug delivery and various other applications [Citation36]. There are two main polymer groups that are classified in terms of their sources, natural and synthetic. Natural polymers have been extensively studied because of their unique biocompatibility and bioactivity, however, problems with physiochemical stability [Citation37] and material batch variations continue to be a limitation [Citation38].
Natural biopolymers, like thermo-responsive polymers, have numerous advantages for biomedical applications. As direct extracellular matrix (ECM) derivatives, polymers including collagen and gelatine provide intrinsic biocompatibility and improve bioactivity in comparison to synthetic polymers. From the available natural sources, ALG, gelatine, CS, and cellulose are readily available and relatively inexpensive. Natural polymers typically produce soft hydrogels which, for certain applications, may not meet the mechanical requirements [Citation39–41]. To achieve significant improvement of mechanical properties and degradation kinetics; chemical modification, copolymerisation or binary natural/synthetic polymer systems can be generated.
Synthetic polymers have generated a significant level of attraction for medical applications. A broad variety of physical and chemical characteristics are being accomplished upon the basis of monomeric groups, polymerisation mechanisms, as well as the production of copolymers composed of various components at different concentrations [Citation41]. Shape memory polymers, for example, have sophisticated mechanical properties that allow them to be easily distorted and then recover to its original position when exposed to a specific stimulation such as pH, temperature, magnetic field, or light. For several applications, synthetic, hydrolytically degrading polymers are desired as an implant or drug release device because their degradation is relatively invariant from patient to patient and for various sites of implantation [Citation42,Citation43]. In comparison to this, enzymatic degradation is the common degradation mode of biopolymers. In tissue engineering, the decomposition strategy is investigated for scaffolds and as a replacement for the ECM, at which the physiological enzymatic turnover of the ECM, is required to disappear [Citation44,Citation45].
Solubility
The concept of polymer blending is an important technique that helps to eliminate the deficiencies of each polymer separately by using at least two different polymers together. The biggest difficulty encountered at this stage is the solubility mismatches of the polymer components in the polymer blend structure. The main reason of the polymer blends immiscibility is thermodynamic incompatibility. Polymer blend homogeneity is directly related to the free energy of blending a polymer mixture. Homogeneous polymer blend pairs should have ΔGm ≤ 0 for miscibility. If the ΔGm > 0, the polymer (pair) solution is an immiscible polymer blend [Citation46]. Additionally, secondary interactions increase the homogeneity in between the polymer blend components which can be listed as hydrogen bonding, ionic and dipole–dipole interactions [Citation47]. These properties can be driven by common solvent systems with suitable physicochemical properties, such as melting point, boiling point, pH (acidity or alkalinity), relative density, surface tension, viscosity, solubility in water and organic solvents. Miscibility (solubility and melting) properties also have a direct impact on drug loading and biomaterial production in binary polymer systems.
Solvents respond differently with polymers, therefore, choosing the right agent is important [Citation48] as certain solvents will completely dissolve a polymer, while others merely partially dissolve or enlarge the polymer [Citation49]. Solvent–polymer interactions may impact the processing of binary polymers by influencing the viscosity and surface tension of a polymer solution [Citation50,Citation51]. The morphology is primarily affected by the polymer solution, and the properties of the solution are usually associated with the solvent class [Citation52]. The morphology is determined by the electrical conductivity of the solution, the dielectric constant, the boiling point, the viscosity, the surface tension, and the activity between the polymer and the solvent [Citation53,Citation54]. As a polymeric fibre production example; solvents with a higher boiling point vaporise gradually, allowing a polymer fibre jet to thin and to be smaller in diameter [Citation55].
A substance dissolves in another when the chemical potential of the blend is less than that of the starting system [Citation56]. It has been found that this mechanism will take place as the entropy increases upon dissolution, unless it is resisted by energetic interactions. This may be the case with non-electrolytes where the pure substances’ binding strength is much greater than that of the mixture. Dissolution will occur if the interactions are approximately equal for all of the substances involved. The solubility parameters of volatile substances can be determined directly from the evaporation enthalpy and the volume of molars. The concept of the solubility parameters helps to find potential solvents rapidly and at a low cost, and to understand many facts about the solubility behaviour. With respect to a given polymer, the thermodynamic efficiency of a solvent determines the value of the second virial coefficient. Expansion coefficients can be determined by the measurement of viscosity or by angular dependence of the scattered light outcomes.
Solubility is also a critical factor in the design of biodegradable binary polymeric drug delivery systems, and it is determined by the chemical composition, structure, and degree of crystallinity of the polymer. Polymer hydrophobicity typically rises with molecular weight, leading to more water-soluble polymers with an increase in backbone branching [Citation57]. Drug release is regulated by surface erosion when the polymer utilised is hydrophobic in nature, and when the polymer backbone has a balance of hydrophobic and hydrophilic functions, degradation may proceed from within the core of the polymer system [Citation58].
Although physical properties at the nanoscale, such as a high surface-to-volume ratio, deliver colloids of polymeric particles stable under physiological circumstances, a broad range of hydrophobic polymers may be developed, a significant amount of hydrophilicity of the constituent polymers is required for macro and microscale polymer therapeutic agents.
Drug miscibility
Fibrous materials and nanoparticles have been extensively studied as vehicles to transport therapeutic agents to target sites because of their benefits, including large surface area, porosity, and structural similarity to the ECM [Citation59–63]. Co-axial electrospinning, for instance, has been used to generate multi-compartmental fibres that enable multiple release states or delivery of multi-drugs [Citation62,Citation64,Citation65]. Polymers need to be carefully chosen to achieve the optimal drug release profiles using polymeric carriers, since the release rates are determined by their degradability, wettability, and diffusivity [Citation66,Citation67]. For degradable polymers, the release mechanism can be more challenging than that of non-degradable drug carriers as their geometry changes during degradation [Citation68,Citation69].
As a controlled release excipient, water-soluble polymers such as poly(ethylene oxide) (PEO) are widely used to regulate drug release and degradation from stable hydrophilic matrix compositions. This is mostly due to the favourable hydration and controlled release abilities of various grade and PEO molecular weight [Citation70]. PEO tends to hydrate and swell when it encounters with liquid, generating a hydrogel surface that controls the ultimate passage of fluids into the matrix and the migration of the therapeutic agents from the active ingredients. Subsequently, due to the emergence of the hydrogel, the pace of liquid consumption reduces, whereas the rate of drug release lowers and extends. The emergence of the hydrogel layer on the surface of a controlled release matrix tablet can be categorised into three phases: (1) the early increase in hydrogel due to the polymer swelling; (2) the maintenance of constant gel layer thickness between the swelling and the frontal dissolution; and (3) the reduction in the gel layer thickness due to the depletion of the glass core. Drug solubility and loading, molecular weight and ratio of polymer, tablet processing method, compression power, and physical configuration of the tablet are all aspects that can affect the release of pharmaceuticals from a swelling matrix tablet. Drug solubility is one of these features that has a big impact on the rate and degree of drug release.
For example, electrospun fibres made from PCL and poly(glyconate) binary polymer blends have been applied as biomaterials in tissue engineering to enhance cell growth, with polymer compositions affecting fibre breakdown and mechanical qualities [Citation71]. Controlling drug release is another promising biological use for electrospun polymer blend fibres [Citation62]. The release rate of teriflunomide from the blending of PLA and poly(butylene adipate) fibres has been modulated by the binary blending of polymer compositions [Citation72]. Moreover, PLGA, PEG-b-PLA and PLA (80/5/15) ternary blended fibres showed controlled delivery of cefoxitin sodium for 7 days comparable to burst release of PLGA fibres in 6 h [Citation73].
Effect on mechanical properties
The mechanical property is a key aspect in the biomedical production processes especially in fibre production applications [Citation74]. Some studies have shown the effect of mechanical properties of the composites created from plastics and fibres which may differ depending on the fibre distribution on the structure, fibre size, fibre content, and fibre matrix adhesion force. Cuvalci et al. [Citation75] investigated an increase in the density of composites as the fibre content increased, for example, the composite density was 1150 kg m–3 at 5.5% fibre volume ratio, whereas it reached the value of 1730 kg m–3 at fibre volume content of 54.9%. An increase in tensile strength was demonstrated with increasing fibre content [Citation76] up to volume ratio of 34.3%. In addition, the tensile strength of the composite decreased as the fibre ratio increased [Citation77], and it reduces to 84 MPa at fibre volume ratio of 54%. Thus, the fibre content in the composites has a beneficial impact on the tensile strength up to 34.3% fibre volume ratio, however, the addition of fibres beyond this level has a detrimental impact on the composite tensile strength.
A study determined the effect of PCL, PLA, and bacterial cellulose (BC) composition on the mechanical properties of such wound dressing constructs, by tensile testing of binary polymer used samples [Citation78]. It was demonstrated that the PLA–PCL binary systems’ ultimate tensile strength varied between 2.2 and 5.6 MPa, while the Young’s modulus values ranged within 3.5–22.3 MPa. PLA is characterised by its high ultimate tensile strength, however, results in low durability, which can be overcome by PCL’s substantial prolongation at break. This study demonstrated the superior mechanical properties by combining beneficial characteristics in their composite products [Citation78]. In this analysis, PLA–PCL polymer blends showed excellent elongation and tensile properties at 50:50 ratios. Across all occurrences, the highest BC ratio in the composite fibres is observed to result in an increase in tensile strength up to 30 wt-%, above this range a decrease in tensile strength is detected [Citation78]. There is an increase in the stiffness of PLA composite systems as the BC content is increased. In PCL systems, however, an adverse trend is seen where the stiffness decreases. The mechanical compatibility of PLA–PCL and the importance of comparative fluctuation among both polymers can justify this reduction. In conjunction with the PLA matrix, the highly elastic nature of the PCL and the bonding forces between the polymers inhibited fibre elongating, due to improved mechanical properties of the composites.
Effect on degradation rate
Modifications in both the chemical structure and physical properties of polymers or polymer-based materials lead to the loss of properties such as tensile strength, colour, shape, etc. under the influence of processing conditions, or one or more environmental parameters such as heat, light, or exposure to chemicals [Citation46,Citation79,Citation80]. Breakage of polymer structure or polymer fragmentation into units that are tiny enough to deteriorate, but comparable to the original substance may cause such loss of characteristics [Citation81]. Thermal, mechanical, hydrolitic, chemical, biological, photolitic, ultrasonication, pollutant contact, radiolytic, and sludge activation are some of the ways polymers can degrade.
In vitro studies have demonstrated that the pH of the solution plays a part in in vitro degradation, and that it is possible to use this as an indicator of its in vivo degradation [Citation82]. For example, high molecular weight PLA has 2–8 years of total resorption time. In some organs, this prolonged presence in vivo may result in inflammation and infection. There is a weak hindering effect of low molecular weight PLAs that are used for drug delivery. They degrade reasonably fast into lactic acid through hydrolysis, which decreases the likelihood of material aggregation in the tissue. For instance, PLA with molecular weight 2000 and 20,000 gmol−1 was used as an artificial antimicrobial release mechanism. The continuous release of antibiotic was found to last for 33 days and more than 3 months in low and high molecular weight implants, respectively [Citation83]. The degradation rate of low molecular weight poly(L-lactide) (PLLA) (60,000 gmol−1) was found to be able to retain mechanical properties for a period of time normally needed for the healing of bone fractures [Citation84].
When exposed to hydrolytic degradation processes, PCL is a long-term durable polymer, and thus demands 2–4 years for comprehensive degradation, reliant on the initial molecular weight of PCL [Citation85]. On the other hand, hydrolytic degradation has been reported to alter the degradability of the polymer matrix by incorporating carbon-based nanomaterials into the polymer matrix [Citation86]. The relation between the degree of crystallinity and the Young’s modulus is extremely important as it defines the mechanical properties and can be controlled by crystallinity [Citation87–89]. Thus, it is very convenient and advantageous to use systems in which binary polymers are used together for a targeted tissue-specific biomedical material development. Considering the different degradation mechanisms and degradation times of binary polymers, it is critical to obtain materials with the potential to degrade at a rate that provides extended drug release, prolonged mechanical strength, and an environment conducive to cell migration and proliferation.
Micro–nano particle production
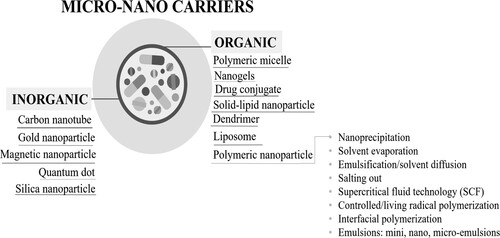
Micro and nano particles are transport materials that have wide potential use in material science and technology. Basically, it is aimed to transport cargo molecules to a targeted area. In biomedical applications, cargo molecules are most often composed of drugs and/or active agent ingredients. Particles (micro and nano-carrier) are divided into two categories: organic and inorganic. Inorganic particles can be exemplified as carbon nanotubes, quantum dots, silica, gold, and magnetic particles. On the other hand, organic particles are classified as: polymeric micelles, dendrimers, drug conjugates, liposomes and polymeric particles [Citation90,Citation91]. Polymeric particles are the most frequently used carriers and can be selected according to the extensive range of physicochemical parameters of the polymers, drug loading capacity/type, targeting and surface modification availability, adjustable degradation, and release profiles [Citation92]. Depending on the purpose of use, polymeric particles can be prepared with single, binary, ternary, or multiple polymer systems.
The main nano-carrier types and the techniques used to synthesise polymeric micro–nano particles are categorised as follows: precipitation, solvent evaporation, emulsification/solvent diffusion, salting out, dialysis, supercritical fluid technology (SCF), interfacial polymerisation, controlled/living radical polymerisation and emulsions: mini emulsion, nano-emulsion, microemulsion (). The most commonly used techniques are reported as emulsion-based and nanoprecipitation [Citation93], and these techniques are reviewed in more detail below for applications in binary polymer systems.
Figure 2. Classification of nano-carriers in terms of material type and polymeric nano-carrier preparation techniques. Nano-carriers are divided into two main groups as organic and inorganic. Polymeric nanoparticles/carriers are a subset of organic nano-carriers, and their preparation methods have been classified in detail as explained in this diagram.
Precipitation
Precipitation is a simple and rapid nanoparticle production technique. The nanoprecipitation technique relies on solubility relationships between the drug, polymer, non-solvent, and the solvent. This method most often involves dissolving a hydrophobic polymer together with a hydrophobic drug in a common organic solvent and thereafter adding to an aqueous solution with an optimised flow and stirring rate [Citation94,Citation95]. Then purification occurs by removing the organic solvent. It has been also reported that, molecular weight of polymer, polymer solution concentration, glass transition properties of polymer, solvent-non solvent ratio and rate of solution mixing are significant in nanoparticle formation in the nanoprecipitation technique [Citation96]. The precipitation technique can be used for binary polymer systems in a step-by-step precipitation method to obtain core–shell binary polymer particles. In the first step the core layer of particles can be precipitated, then the obtained particles can be purified and can be coated as a second layer in the next step. Additionally, different drugs can be loaded to the core and shell structures in each step of forming. If the polymer layer differs, the same drug can be loaded to design a sustained release model. On the other hand, different active ingredients can be easily loaded to create a binary action from a binary polymer system. Han et al. [Citation97] reported a binary polymer system-based nanoprecipitation application for sustained release of ketamine-loaded nanoparticles. Ketamine is an analgesic active molecule which has a short half-life in biological systems. To overcome this limitation, they designed PEG-PLGA nanoparticles and PEG-PLGA/shellac binary polymer systems to increase both drug-loading efficiency and releasing period. It has been demonstrated that, the new binary polymer system increased in vivo drug release up to 21 days with a sustained release profile [Citation97].
Emulsions
Emulsion-based nanoparticle systems involve two immiscible phases and a surfactant. Emulsion solution can be composed of one single (water(w) in oil(o) or oil(o) in water(w)), double (w/o/w or (o/w/o)) and/or multiple emulsions. Additionally, surfactants can be anionic, cationic, or zwitterionic which lowers the surface tension of solution. Emulsion system design depends on the relationship between drug–polymer-target tissue [Citation98]. Bioavailability, biocompatibility, and particle size are the key properties to design nanoparticles with optimal physicochemical aspects. Therefore, a suitable emulsion system is chosen according to the drug type (hydrophilic or hydrophobic), polymer properties (molecular weight, single/copolymer, solubility, in organic phase or water phase, polarity, etc.) and dissolution properties/kinetics of solvents because these parameters directly affect particle yield, size, loading efficiency and release kinetics. Hydrophilic drugs should be loaded into the water phase, while hydrophobic drugs should be loaded into the oil phase [Citation99]. The emulsion system should be designed taking into account whether the polymer used is hydrophilic or hydrophobic. At present, binary polymer systems offer an important role for overcoming dissolution properties and creating sequential and/or dual drug delivery. Additionally, binary polymer systems offer a significant advantage for designing targeted nanoparticles with versatile properties.
Kietzke and co-workers [Citation100] reported the binary polymer nanoparticles synthesis by a mini-emulsion method. In the study, the binary system was composed of polystyrene (PS) and poly(propylene carbonate) (PPC) polymer pair. PS:PPC binary polymer nanoparticles size range was measured as ∼75 nm and the synthesised binary polymer-based nanoparticles exhibited Janus (biphasic) structure (). The Janus-like biphasic nanoparticle formation is related to the immiscible nature of binary PS and PPC polymers. When the binary polymer pair encountered water molecules in the emulsion system, there were no preferences of either polymers to take part in the core or shell of the binary system, thus phase separation and the biphasic structure occurred.
Figure 3. Polystyrene (PS) and poly(propylene carbonate) (PPC) (PS:PPC) binary polymer Janus nanoparticles produced by emulsion method. (A) Transmission electron microscopy (TEM) micrographs of PS:PPC Janus nanoparticles (scale bar = 200 nm) (i) non-stained, (ii) stained, (B) schematic representation of Janus nanoparticles phase separation, and (C) high resolution TEM micrographs of PS:PPC Janus nanoparticles biphasic-binary polymer structure (scale bar = 100 nm). Reproduced from Ref. [Citation100] with permission.
![Figure 3. Polystyrene (PS) and poly(propylene carbonate) (PPC) (PS:PPC) binary polymer Janus nanoparticles produced by emulsion method. (A) Transmission electron microscopy (TEM) micrographs of PS:PPC Janus nanoparticles (scale bar = 200 nm) (i) non-stained, (ii) stained, (B) schematic representation of Janus nanoparticles phase separation, and (C) high resolution TEM micrographs of PS:PPC Janus nanoparticles biphasic-binary polymer structure (scale bar = 100 nm). Reproduced from Ref. [Citation100] with permission.](/cms/asset/0a499b2a-acfc-4285-a402-f516dd7cbd05/yimr_a_2069451_f0003_oc.jpg)
Fibre production techniques relevant to binary polymer systems
Developing polymer binary systems is a versatile strategy for obtaining novel biomaterials with improved properties [Citation101,Citation102]. The addition of the second polymer in a binary system not only provides the original characteristics of the additives to the polymer blend, but also generates novel attributes by tuning the structure of the polymer blend, improving processing and lowering production costs [Citation103–105]. To fabricate functional materials, such as biomaterials using binary polymer fibres, forming must occur on a technologically viable scale, generating fibres with a large surface area and tuneable porosity [Citation38,Citation106]. Such improved properties have been used in a variety of applications, including medicinal delivery and tissue engineering scaffolds [Citation107–110].
Electrospinning
Electrospinning is a process that can generate polymer nanofibres by using electric fields and flow [Citation111–114] ((i)). Binary polymers can be adopted to electrospinning technique by mixing the two different types of polymers, and spinning the mixture to obtain the blended fibres, or using a co-axial needle system and feeding the binary polymer pair separately from the different solution systems. Both techniques allow to load various drugs, such as antibiotics, vitamins, peptides, and proteins, and fibres can be spun to be incorporated into scaffolds [Citation115–117]. The electrospinning method has the ability to control the fibre pore structure and produce nanofibres that provide a high surface-to-volume ratio [Citation118]. These characteristics are highly desirable in biomedical applications including wound dressings, tissue engineering scaffolds, biomedicine, and pharmaceuticals [Citation119,Citation120]. Komur and co-workers [Citation121] produced starch and PCL binary polymer to produce PCL core and starch shell double layer fibres by co-axial electrospinning. The PCL core layer imported mechanical strength to the structure, and the starch shell layer resulted in a cell-friendly surface for wound dressing applications. Additionally, increased starch concentration increased cell viability and decreased the tensile strength of the binary fibre structure. Owing to their high surface-to-volume ratio, electrospun fibres are able to load high amounts of antimicrobial peptides (AMPs) where the release can be modified by altering the type of material properties in the fibres [Citation122]. Electrospinning has attracted much interest in biomedical applications, as this technique can generate biomimetic nanofibrous materials from an extensive range of biologically relevant natural and synthetic polymers. However, this system encounters limitations including poor cell filtration and growth, potential toxicity of chemical residues in electrospun fibres and a slow batch production rate that impedes the progress of its applications [Citation123–125].
Figure 4. Schematic representations of (i) Electrospinning of poly(lactic acid)/chitosan core–shell nanofibres (a) PLA/SDS-CS = 100/0; (b) PLA/SDS-CS = 80/20; (c) PLA/SDS-CS = 70/30; (d) PLA/SDS-CS = 60/40; (e) PLA/SDS-CS = 50/50; (f) PLA/SDS-CS = 40/60 [Citation130] (ii) Centrifugal spinning of poly(acrylonitrile)/PEG fibres (a) pure PAN fibres, (b) PAN/PEG PCM fibres, and PAN/PEG/SiC PCM fibres: (c) SiC 4.0 wt-%, (d) SiC 6.0 wt-%, (e) SiC 8.0 wt-%, (f) SiC 10.0 wt-% and the corresponding energy dispersive spectra (inset in c to f) [Citation131], (iii) Pressurised gyration (a) 0.1 MPa (b) 0.2 MPa (c) 0.3 MPa and core–sheath nanofibre cross-sections [Citation132]. Reproduced from Ref. [Citation130–132] with permission.
![Figure 4. Schematic representations of (i) Electrospinning of poly(lactic acid)/chitosan core–shell nanofibres (a) PLA/SDS-CS = 100/0; (b) PLA/SDS-CS = 80/20; (c) PLA/SDS-CS = 70/30; (d) PLA/SDS-CS = 60/40; (e) PLA/SDS-CS = 50/50; (f) PLA/SDS-CS = 40/60 [Citation130] (ii) Centrifugal spinning of poly(acrylonitrile)/PEG fibres (a) pure PAN fibres, (b) PAN/PEG PCM fibres, and PAN/PEG/SiC PCM fibres: (c) SiC 4.0 wt-%, (d) SiC 6.0 wt-%, (e) SiC 8.0 wt-%, (f) SiC 10.0 wt-% and the corresponding energy dispersive spectra (inset in c to f) [Citation131], (iii) Pressurised gyration (a) 0.1 MPa (b) 0.2 MPa (c) 0.3 MPa and core–sheath nanofibre cross-sections [Citation132]. Reproduced from Ref. [Citation130–132] with permission.](/cms/asset/a420fdf8-b6dc-415f-98db-ef508a81da24/yimr_a_2069451_f0004_oc.jpg)
Centrifugal spinning
Centrifugal spinning is an easy system for converting a spinning solution to micro–nano diameter range fibres [Citation126]. The process is voltage free ((ii)), using centrifugal force to generate bulk fine fibres from melting and/or solution materials used for a selection of applications, i.e. wound dressing materials, tissue engineering scaffolds and medical engineering [Citation127–129]. The process has the ability to generate a high degree of alignment and interconnected fibres, as well as high porosity at a low cost. Depending on the solution and spinning environment, the fibre output spun per minute can vary significantly.
On the other hand, centrifugal spinning fails to control both fibre morphology and pore size. While the fibre diameter is small (in the low micro-range), the fibres are beaded on a string which reduces wider applications. Moreover, when the morphology is ideal, the fibre diameter is large which reduces a high drug loading. Centrifugal fibres cannot achieve fibres with large surface-to-volume ratio as the technique requires optimising spinning variables, thus setting jet and nanofibre movement, which can be a potential limitation. Moreover, centrifugal spinning lacks core–shell and or layer by layer fibre formation. The ease of binary polymer systems adaption to centrifugal spinning technique is only improved by blending the binary polymer pair in single solvent system to obtain blended binary polymer fibres.
Pressurised gyration
Pressured gyration (PG) is an effective manufacturing technique used to produce largely low micro-range diameter fibres [Citation133]. It can be adapted as a technique for spinning nanofibres and nanofibrous structures, that are spun at high speed (36,000 rev min–1) and under high pressure (0.1–0.3 MPa) ((iii) and ), especially with water-soluble polymers causing fibres to erupt, elongate and thin from a cylindrical aluminium pot containing the polymer solution. The large surface-to-volume ratio creates desired conditions for the development of ECM to imitate native tissues, which are promising for wound healing [Citation134] and tissue regeneration applications [Citation135,Citation136]. Moreover, this process has demonstrated a mechanism for rapid release of drugs, such as developing progesterone-loaded nanofibres for vaginal therapeutics for the prevention of pre-term birth [Citation137].
Figure 5. Schematic illustrating the preparation of a binary polymer system. Fibres have been fabricated using pressurised gyration and a scanning electron micrograph (SEM) of the binary fibres is provided (scale bar = 10 µm). Fibres spun at 36,000 rev min–1 and no applied pressure.
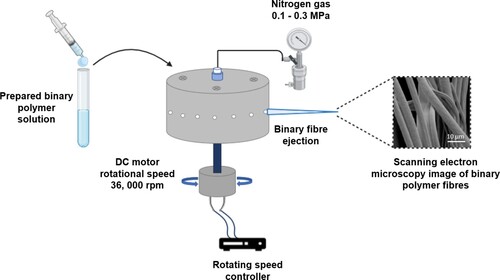
For desired applications, PG enables fibres to be tailored. For example, the fibre diameter can be adjusted using fluid acceleration and enhanced kinetic energy of the evolving jet. As the polymer jet lengthens, jet elongation produces lower diameter fibres, resulting in rapid evaporation of solvents. By varying the gas pressure, the surface topography of the developed fibres can be adjusted. When highly volatile solvents are applied, the ambient temperature drops, and the surface perforations form as water droplets evaporate from the fibre surface. With a greater applied working pressure, the temperature decrease is higher, resulting in faster solvent evaporation and creation of pores. Additionally, by increasing the collection distance, the fibre diameter is decreased. Whereas at greater distances, the jet is allowed to spread further, resulting in smaller diameter fibres. The outcome of the fibre characteristics is substantially influenced by the solution properties such as viscosity and molecular weight, therefore, it is important to confirm the necessary requirements for manufacturing fibres for a specific polymer. Mahalingham et al. [Citation138] reported generating PCL–PVA binary polymers based core–shell fibrous scaffolds for bone tissue engineering (). Hydroxyapatite (HA) molecules embedded into the shell layer of binary polymer system induced cell proliferation. In the core layer, PCL provided the long-time stability and mechanical support, and the HA embedded PVA shell layer ensured the rapid release of active molecule to induce the cell migration and proliferation on the intended application area.
Figure 6. PCL–PVA binary polymer fibres, (A) schematic representation of production set-up of pressurised gyration, (B) fluorescence microscope image of manufactured fibre with core and shell PCL and PVA binary polymer layers, (C) SEM image showing cell-binary PCL–PVA core–shell fibre interactions, and (D) fluorescence microscope image of living cells that interact with PCL–PVA core–shell fibres. Reproduced from Ref. [Citation138] with permission.
![Figure 6. PCL–PVA binary polymer fibres, (A) schematic representation of production set-up of pressurised gyration, (B) fluorescence microscope image of manufactured fibre with core and shell PCL and PVA binary polymer layers, (C) SEM image showing cell-binary PCL–PVA core–shell fibre interactions, and (D) fluorescence microscope image of living cells that interact with PCL–PVA core–shell fibres. Reproduced from Ref. [Citation138] with permission.](/cms/asset/b332789f-2064-4c04-a267-1e4758def0f3/yimr_a_2069451_f0006_oc.jpg)
This ambient temperature method is used to manufacture functional materials from polymeric fibres, such as biomaterials, which must be generated on a technologically significant scale, with a high surface area and tuneable porosity. Due to their inherent flexibility, the desire for ultrafine polymeric fibres is on the increase. It is important that fibres can be mass produced in a consistent, durable, and cost-effective manner in order to be effective in all these application areas. While there are benefits to techniques such as centrifugal spinning, electrospinning and self-assembly, they are not without their limitations. PG has addressed the limitations of other sister spinning techniques and has gained popularity with communities pursuing large-scale manufacturing. It is a more effective process for mass production, but for the low micro–nano diameter fibres.
Drug loading of binary polymer systems
Polymeric drug delivery systems are complex in terms of their preparation and mechanisms of action. While the large number of parameter variables affecting the designed system makes it difficult to obtain the targeted properties, on the other hand, they show the existence of different options to achieve the intended purpose. Presently, binary polymer systems provide a significant advantage by increasing design options in drug delivery system applications. Single drug loading to binary polymer systems with different release kinetics for sustained release and/or dual drug loading with different drug solubility can be easily attained by binary polymer systems for both micro/nanoparticle and micro/nanofibre applications.
Single drug loading
Controlled drug delivery systems (CDDSs) aim to satisfy drug loading efficiency, bioavailability, and biocompatibility. There are numerous examples of drug delivery systems which benefit from binary polymer systems to attain these goals. Khalil and co-workers [Citation139] compared the pharmacokinetics of curcumin loaded PLGA and PLGA–PEG binary polymers. Particle size and encapsulation efficiency parameters did not exhibit a significant difference between these two different systems. However, it has been stated that binary PLGA–PEG nanoparticles increased bioavailability of curcumin 3.5-fold compared to PLGA nanoparticles [Citation139]. In another study, Parveen and Sahoo [Citation140] reported that paclitaxel-loaded PLGA–PEG–CS nanoparticles remained in the bloodstream for a longer time and showed increased anti-proliferative activity compared to PLGA nanoparticles. Mayol et al. [Citation141] studied curcumin loaded PLGA and PLGA-poloxamer blend nanoparticles bioavailability against mesothelioma cells. Their study resulted in enhanced permeability and retention (EPR) on targeted cell lines while inhibiting rapid degradation of curcumin. In another study, 5-fluoruracil loaded CS–PCL blend nanofibres were prepared for anticancer activity [Citation142]. It has been shown that increased CS content increased drug loading efficiency and supported sustained release and prolongation, but in an acidic environment a shortened releasing period was observed. Additionally, increasing CS content decreased the strain in the fibres. As a result, slow degradation was attained with high drug loading efficiency, owing to the optimised binary polymer formulation of CS–PCL fibres.
Dual drug loading
Binary polymer systems exhibit a vital role in dual drug loading systems. Especially in the applications of dual drugs with sequential delivery and different dissolution properties, binary polymers play a key role [Citation143]. Su et al. [Citation144] designed and prepared binary polymer fibres by co-axial electrospinning for modelling dual drug delivery. Rhodamine B and bovine serum albumin (BSA) were loaded as model drugs into different layers of poly(L-lactide-co-caprolactone) (PLLACL) fibres. It was reported that the active ingredient in the core layer exhibited sustained release, while the shell layer exhibited a burst release profile, making this model a powerful candidate for various combinational therapies. Cao and co-workers [Citation145] studied dual drug delivery of combretastatin A4 (CA4) and doxorubicin (DOX) anticancer drugs for two different core–shell nanoparticles contained in binary polymer systems. Poly(vinylpyrrolidone) (PVP)–PLGA and PCL–PLGA core–shell nanoparticles were used throughout the study and PVP–DOX/PLGA–CA4 and PCL–DOX/PLGA–CA4 formulations successfully inhibited HUVECs and B16-F10 cell proliferation [Citation145].
In another study, Mahalingam and co-workers have very recently produced core–shell binary polymer-based fibres by PG [Citation132]. PVP was used as the shell layer and PEO was included in the core. This new method has the ability to be used for dual/sequential drug delivery applications. Also, Silva et al. [Citation146] produced core–shell dual-drug loaded microparticles (). Curcumin loaded PCL microparticles were produced by emulsion technique for the core layer. Then ciprofloxacin (CPx) loaded PVA was used as the shell layer which was produced via spray drying. Resulting core–shell dual drug loaded microparticles were a few micrometres in size and showed very promising outcomes for controlled release applications especially in inhalation therapies.
Figure 7. Core–shell microparticle production via bilayer polymer systems. (i) (a) Process diagram of core–shell microparticles, (b) macroscopic images of dried powders of microparticles, (c–e) SEM images of microparticles with the size distribution graphs given as inset figures, (f) transmission electron micrograph of microparticles. (ii) ATR-FTIR chemical mapping of drug loaded/non-loaded core–shell microparticles. (a) PCL core-PVA shell, (b) PVA shell and drug, (c) PCL core and drug (scale bar = 5 µm), (iii) antibacterial test results against P. aeruginosa, E. coli, S. aureus and B. subtilis. (a) Inhibition zone test results for P. aeruginosa, E. coli, S. aureus and B. subtilis species. (b) SEM images of antibacterial efficiency against P. aeruginosa cells for drug loaded particles (scale bar = 2 µm) [Citation146]. Reproduced from Ref. [Citation146] with permission.
![Figure 7. Core–shell microparticle production via bilayer polymer systems. (i) (a) Process diagram of core–shell microparticles, (b) macroscopic images of dried powders of microparticles, (c–e) SEM images of microparticles with the size distribution graphs given as inset figures, (f) transmission electron micrograph of microparticles. (ii) ATR-FTIR chemical mapping of drug loaded/non-loaded core–shell microparticles. (a) PCL core-PVA shell, (b) PVA shell and drug, (c) PCL core and drug (scale bar = 5 µm), (iii) antibacterial test results against P. aeruginosa, E. coli, S. aureus and B. subtilis. (a) Inhibition zone test results for P. aeruginosa, E. coli, S. aureus and B. subtilis species. (b) SEM images of antibacterial efficiency against P. aeruginosa cells for drug loaded particles (scale bar = 2 µm) [Citation146]. Reproduced from Ref. [Citation146] with permission.](/cms/asset/874c276c-12dc-40b1-a9b5-9db2d1ecbd85/yimr_a_2069451_f0007_ob.jpg)
Binary polymers and biomedical applications
Over the last few decades, the study of polymers and polymer blends has had a huge influence on both industry and academia. These materials have enormous potential as a tool for novel applications [Citation147]. Blending two polymers is a technique for manufacturing a material with specific characteristics, appropriate for highly demanding applications that are often simpler and quicker than the final product’s scratch synthesis [Citation148]. As mentioned in the previous sections, binary polymer systems have a significant impact in many application areas and biomedical applications have priority when considering human health and life. In this section, literature review on binary polymer systems and their applications used in the biomedical field is reported elucidated and critically reviewed ().
Table 1. Binary polymer system pairings for biomedical applications found in the literature.
Alginate
Alginate–gelatine
ALG is a biocompatible, linear binary copolymer made up of monosaccharide units of D-mannuronic acid (M) and its C5 epimer, L-guluronic acid (G), that are covalently bonded by 1–4 glycosidic linkages [Citation180]. In the primary structure, M and G are dispersed in variable amounts throughout the polymer chain to generate heterogenous alternating (MG) and homogeneous (MM or GG) sequences [Citation181–183]. ALG is a naturally occurring anionic polymer found in brown seaweed species like Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera [Citation184,Citation185]. The structural closeness of ALG to the ECM of living tissues allows for a diverse range of biomedical applications, such as wound healing, bioactive agent delivery, and cell transplantation. ALG is also biodegradable because the cross-linking elements release and exchange with monovalent cations in body fluids, causing it to disintegrate gradually in the body. The rate of ALG dissolving can be adjusted by electrochemical reactions [Citation186] of the molecular weight of ALG [Citation187,Citation188]. One significant disadvantage of ALG is that it can gelate into a softer form when exposed to the physical environment, limiting its ability for soft tissue regeneration, and making it unsuitable for use in load-bearing body parts [Citation187]. To address this issue, a variety of elements have been mixed into the ALG structure. Incorporating an adhesive peptide and a natural or synthetic polymer to ALG moieties results in a composite material that not only has superior mechanical properties compared to native ALG, but also has more healing potential and promotes better tissue regeneration [Citation184,Citation187,Citation189–192].
Gelatine is a protein that is made by hydrolysing collagen from animals (such as bovine, porcine, or fish collagen), connective tissues, and bones [Citation193,Citation194]. It has been FDA approved because of its biocompatibility, lack of inflammatory processes in the body, degradability, and lack of toxicity [Citation195]. As a result, it has been used in biomedical applications [Citation196], such as drug delivery [Citation197], gene therapy [Citation198], wound healing [Citation199], tissue engineering [Citation200] and regenerative medicine [Citation201]. Since it retains the bioactive sequences of collagen, gelatine is a popular material for cell hosting. This enables the development of an optimal environment for cell adhesion, migration, proliferation, and differentiation [Citation193,Citation202].
Despite these important benefits, gelatine has certain disadvantages too. Gelatine, for example, transitions from a gel to a solution around 30–40°C, limiting its long-term applications in transplantation [Citation203]. As a result, gelatine can be combined with other polymers, such as ALG, to extend its degradation period and improve its water resistance [Citation204]. Hydrogels, which are a blend of natural polysaccharides (i.e. ALG) and proteins (i.e. gelatine), have recently gained a lot of attention. This is attributed to ALG’s negative properties, such as poor cell adhesion, inefficient ALG cell interactions, and prolonged degradability with unregulated kinetics [Citation205].
The incorporation of ALG that has been initially oxidised to produce ALG dialdehyde (ADA) and then cross-linked with gelatine can be a solution to these restraints. The resulting ALG-gelatine binary hydrogel (ADA-gelatine) can be used to create microcapsules for encapsulating bioactive compounds or cells and drug delivery [Citation206–208], as well as a non-cytotoxic biomaterial with good mechanical strength and biocompatibility in regenerative medicine such, as bone tissue regeneration [Citation209] and as a soft tissue adhesive in wound healing [Citation210]. The microstructure and physicochemical characteristics of the obtained ALG-gelatine binary hydrogels can vary depending on the oxidation degree of the ADA and the cross-linking degree and gelation time of the ADA-gelatine [Citation209–211]. According to Serafin and co-workers [Citation149], ALG microcapsules and ADA-gelatine microcapsules have a greater degradability and demonstrate good cell adhesion, proliferation, and migratory capabilities [Citation195,Citation207,Citation212].
Alginate–chitosan
Chitin and its deacetylated derivative, CS, are a class of linear polysaccharides made up of differing quantities (β1→4) of N-acetyl-2 amino-2-deoxy-D-glucosee (glucosamine, GlcN) and 2-amino-2-deoxy-D-glucose (N-acetyl-glucosamine, GlcNAc) residues [Citation213,Citation214]. CS is found in a limited number of fungus (Mucoraceae) in nature. Primary amine protonation serves to make CS miscible in aqueous acidic media. The quantity of acetylated resides in chitin, on the other hand, is sufficient to avoid the polymer from degrading in aqueous acidic conditions. The fact that chitin and CS are not only abundant in nature, but also harmless and biodegradable is the fundamental driving force behind their development in emerging applications [Citation215]. CS possesses antibacterial [Citation216–219], antifungal [Citation220], mucoadhesive [Citation221], analgesic [Citation220], haemostatic [Citation222], biocompatibility, biodegradability, and nontoxicity properties that have captivated researchers’ interest in recent years, generating significant interest in the field of biomedical applications, according to several studies [Citation223,Citation224].
Despite the fact that ALG and CS biopolymers have been exploited individually in biomedical applications, each has significant drawbacks. The hydrophilic nature of ALG inhibits serum proteins from being accumulated, restricting the ability of anchorage-sensitive cells such as hepatocytes to promote specific cell connections or execute different cell activities like migration, proliferation, and specialised gene expression [Citation225–227]. On the other hand, CS has poor mechanical properties and is difficult to manipulate and mould into a scaffold structure [Citation228,Citation229]. Therefore, cell encapsulation is a difficult process. Thus, an ALG-CS binary system can overcome the single biopolymer limitations.
Dumont et al. [Citation150] investigated acidic aqueous CS acetate solutions. As a result, the antibacterial action may have been caused by both the bioactivity of CS and the acid used to make the solution. Antibacterial efficacy of CS-coated ALG fibres against Gram-negative Escherichia coli (E. coli) species and, more interestingly, Gram-positive Staphylococcus epidermidis were assessed. The results suggest that using CS-coated ALG fibres in wound dressings can combine the wound-healing properties of calcium ALG with the antibacterial activity of CS to combat bacterial infection, notably against antibiotic-resistant and healthcare-associated pathogens.
Alginate–PCL (melt)
ALG has tuneable mechanical characteristics owing to cross-linking with divalent ions like Ca2+ and it can be used in a blend with PCL [Citation230]. The inability of hydrogel to retain a homogeneous 3D structure is its fundamental drawback for tissue engineering. Hydrogels can be combined with synthetic biomaterials to alleviate this challenge. PCL is an FDA-approved polymer with excellent biocompatibility and low hydrolysis degradation. It is also a cost-effective and versatile polymer that is commonly utilised to produce 3D structures for bone regeneration [Citation231]. PCL has excellent rheological properties than many of its resorbable polymer competitors, allowing it to be made and moulded into a wide variety of shapes and structures [Citation231]. Owing to these characteristics, PCL can be formed into scaffolds via 3D printing, electrospinning, and melt-electrowriting (MEW) [Citation232–236]. However, the hydrophobic PCL surface [Citation237] is not suitable for cell adhesion and proliferation, therefore, it must be modified to become more hydrophilic [Citation238]. An additional limitation of PCL is its inability to create bone-forming potentials.
Kundu et al. [Citation151] used the advantages of cell-printing technology to construct pre-tissue by LBL deposition of PCL and chondrocytes enclosed by hydrogels (ALG), with and without transforming growth factor (TGFβ). Findings suggest that the vitality of chondrocytes was not affected by the cell-printing procedure of cells embedded in ALG hydrogels. The created cartilage with the cell-printed PCL–ALG scaffolds had increased ECM and GAG content without an undesirable tissue reaction. A novel cell-printed bio-hybrid scaffold for cartilage regeneration was also developed. The 3D created tissues will have an impact not only in the field of regenerative medicine, but also as an experimental tissue model for cell biology, drug screening, and drug discovery exploration.
Cellulose
Cellulose–PVA
Cellulose is a natural polymer made up of repeating glucose units (C6H10O5)n that is unbranched and is considered to be the most easily and accessible organic material and polysaccharide [Citation239,Citation240]. It is often present in the form of microfibrils in wood and plant cell walls, algae tissue, and the membrane of tunicate epidermal cells [Citation241,Citation242]. Owing to its great physical and mechanical properties, such as biocompatibility [Citation243], low density and biodegradability [Citation244], cellulose and its derivatives allow porosity tuning and interconnectivity that have attracted significant attention for biomedical applications. With the application of hierarchical structure, cellulose generates functionality, versatility, and high specific strength naturally [Citation242,Citation245]. However, cellulose has several less favourable characteristics for use in the biomedical field, such as moisture sensitivity, insolubility in water and most common solvents, and a low resistance to microbial attacks [Citation246,Citation247].
Among the biomaterials designed for cartilage tissue engineering, 3D supports focused on mechanically robust hydrogels are being researched, in order to benefit from their unique characteristics including porosity, pore size and matrix rigidity [Citation248,Citation249]. PVA hydrogel is extensively used in the biomedical field owing to its biocompatibility and non-toxicity, it has a high moisture content and tuneable mechanical behaviour making it an attractive alternative for the formation of synthetic cartilage [Citation250–253]. However, there are several drawbacks to applying PVA-based scaffolds in cartilage tissue engineering, including low biodegradability after cross-linking [Citation254] and a limited ability to facilitate cell adhesion [Citation255]. Apart from improving the biological efficacy of PVA, the manufacturing of these composite scaffolds intends to deliver the engineered construct mechanical features that are consistent to those of the original cartilaginous tissue. Therefore, the binary system of PVA with cellulose can deliver an ideal mechanical effectiveness which has a higher tendency for cell-to-matrix and cell-to-cell activities, allowing the 3D system to effectively resemble in vivo functions and tissue architecture [Citation18,Citation256].
Cellulose–PEG
Cellulose has several distinct properties that make it an excellent material for wound dressings, including non-toxicity, non-carcinogenicity, the ability to retain moisture, absorb exudates from damaged tissue and intensity granulation, as well as high purity and porosity [Citation257–261]. To enhance its efficacy as a wound dressing material, or to supply it with specific qualities or functionalities, techniques have depended on exploiting and improving its natural properties, such as tensile strength, biocompatibility, and water uptake. On the other hand, cellulose lacks numerous desired features, such as antibacterial activity and anti-inflammatory effects [Citation262]. In combining cellulose with other polymers, such as PEG, new properties can be incorporated through the development of binary systems.
PEG is a synthetic and hydrophilic polymer with remarkable solubility properties. It is a biocompatible polymer that has been widely used in the medical industry, such as biomedical applications to enhance wound healing. A study by Cai and Kim [Citation152] has shown that PEG has the ability to penetrate cellulose fibre networks. In terms of fibroblast cell culture, the outcomes reveal that cellulose–PEG polymer binary system has greater biocompatibility than pure cellulose. In vitro studies suggest that it could be exploited as a wound dressing material or tissue regeneration scaffold [Citation152].
Cellulose–collagen
Previous research has shown that collagen is a viable biomaterial for bone tissue regeneration due to its superior biocompatibility, degradability, adhesion, osteogenic induction and low immunogenicity characteristics [Citation263]. Collagen serves as an effective matrix for a variety of cell types, however, alone it may not be singularly sufficient for bone tissue engineering [Citation264]. As a result, collagen must be modified or combined with other polymers to achieve enhanced mechanical characteristics [Citation265]. Cellulose has been widely used as a biomaterial for bone regeneration [Citation266–269]. However, due to its low physicochemical attributes it is limited in its future applications. Thus, cellulose has been regarded as an alternate source for polymer reinforcement in tissue engineering. According to a study by Noh and co-workers [Citation270], binary polymers of cellulose–collagen with a higher cellulose content are more stable, and thus more resistant to contraction in wet conditions, than collagen. It is also known that cellulose content plays a key role in mesenchymal stem cell (MSC) osteogenesis induction, with cellulose–collagen (5:1) being the most potent combination [Citation270].
Chitosan
Chitosan–PCL
CS can be biodegraded into non-toxic residues [Citation271,Citation272], owing to its properties mentioned in Section ‘Alginate-Chitosan’. The rate of breakdown is largely proportional to the polymer’s molecular mass and degree of deacetylation, and it is biocompatible with physiological medium to a certain degree [Citation273,Citation274]. All of these unique characteristics have demonstrated enhanced potential for biomedical applications, such as wound healing [Citation154,Citation155,Citation275–281] and nerve tissue engineering [Citation155]. Moreover, CS can contribute to the formation and structure of granulation tissue by stimulating and modifying the action of inflammatory cells such as neutrophils, macrophages, and fibroblasts, as well as endothelial cells [Citation282,Citation283]. However, pure CS as a biomaterial has structural integrity in moist settings due to swelling [Citation281]. Furthermore, due to the high viscosity of CS solutions, spinning pure CS can be challenging [Citation284].
PCL can be fabricated readily at low voltages and offers the mechanical resistance required for scaffolds in aqueous conditions [Citation285]. The CS–PCL poly-blend fibres, which are formed without chemical cross-linking, have improved mechanical characteristics in both wet and dry environments as well as improved cellular behaviour, and hence would be a better substrate than other PCL–protein structures [Citation286]. In a study by Fahimirad and co-workers [Citation154], PCL–CS–curcumin was functionalised with curcumin CS nanoparticles (NPs), which enhanced the antibacterial efficacy against MRSA by 99.3% and significantly increased antioxidant performance by 89%. The proliferation rate of human dermal fibroblasts (HDF) cells was improved when PCL–CS–curcumin was integrated with the curcumin CSNPs scaffold. As a result, this finding shows that PCL–CS–curcumin electrosprayed with curcumin CSNPs could be used as an effective new wound dressing with substantial antibacterial activity. The micro and nanostructure of CS–PCL nanofibre scaffolds mimics the original ECM in terms of fibre morphology and dimensionality, and it is likely that it acts as an instant reinforcement for keratinocyte and fibroblast migration in the promotion of wound healing and skin repair [Citation287]. Therefore, the wound healing efficiency and ultimate closure, as well as re-epithelialisation, neo-epidermis maturity, and collagen deposition, can be improved with the use of CS–PCL nanofibre scaffolds.
Binary polymer fibrous scaffolds made of synthetic and natural polymers have been explored for nerve regeneration [Citation288–290] to take advantage of the characteristics of CS and PCL. In correlation with this, Cooper and colleagues [Citation155] examined the combination of CS with PCL to generate a mechanically stable polymer for nerve regeneration applications. The thermal degradation of CS and PCL fibres was studied, indicating that CS–PCL is thermally stable, and that neither the CS–PCL material nor the topology of the material generated further cell harm or death [Citation155]. In comparison to CS–PCL film and randomly oriented fibres, highly aligned CS–PCL fibrous scaffolds were found to direct Schwann cells (SC) attachment, resulting in distinctive cell shape required for nerve regeneration. The findings suggest that CS–PCL fibres stimulate chemical and topographical signals for neuritogenesis modulation.
Chitosan–silk fibroin
CS is limited in its applicability due to its low solubility in neutral and alkaline liquids. However, physical, and chemical alterations, as well as the development of new cross-linked CS-based structures, have given it unique functional capabilities that allow it to be used in biosensing [Citation291,Citation292], tissue engineering [Citation293], and medicinal applications [Citation294]. The amino and hydroxyl functional groups of CS can react covalently or non-covalently with various cross-linker reagents such as glutaraldehyde [Citation295], genipine [Citation296], acrylic acid [Citation297], and palladium cations [Citation298], depending on the structure. Indeed, these cross-linking processes have resulted in the development of new cross-linked CS-based composites and hydrogels with varying properties [Citation294,Citation299]. According to previous research, CS hydrogels degrade promptly, which has reduced their use as a biomedical material [Citation300]. Silk fibroin (SF), on the other hand, has demonstrated significant mechanical strength [Citation300]. SF is derived from Bombyx mori silkworm cocoons and has a number of unique properties, including high mechanical strength, low immunogenicity, non-cytotoxicity, non-carcinogenicity, strong biocompatibility, high air permeability, biodegradability and minimal inflammatory reaction [Citation301–305]. Hydrogel [Citation306], film [Citation307], nonwoven textiles [Citation308], nanofibre [Citation309], and 3D porous scaffolds [Citation310] are some of the various shapes and forms that this natural protein can make. Research has shown that combining SF with other materials, such as natural polymers [Citation304,Citation311] and forming SF-based composites can improve SF’s antibacterial property, which is an important component in wound dressing applications. Therefore, the mechanical characteristics of CS biopolymer can be considerably improved by various chemical modification procedures, and that its combination with other polymers [Citation22], such as SF makes it an excellent covering material for wound healing [Citation312].
Cai et al. [Citation313] found that increasing the quantity of SF in CS enhanced the tensile strength of cross-linked nanofibrous membranes from 1.3 to 10.3 MPa. The study revealed that fibroblast growth was facilitated by the CS–SF binary polymer nanofibrous membranes. CS-SF binary polymer nanofibrous membranes increased cell adhesion and growth, according to MTT experiments. The growth of Gram-negative bacteria E. coli was inhibited by binary polymer nanofibrous membranes, according to turbidity measurements [Citation313]. Furthermore, when the ratio of CS increased, the antibacterial activity increased dramatically, considered favourable for CS-SF nanofibrous membranes used as wound dressings.
Chitosan–cellulose
CS nanoparticles show promise as a carrier for anticancer therapeutics, with advantages such as high drug loading capacity and long-term drug release [Citation314]. By mixing with other biopolymers and cross-linking, the degradation of CS can be tailored to acidic environments of tumour tissue [Citation315]. CS-based nano-carriers are usually applied for encapsulation of hydrophilic and hydrophobic pharmaceuticals in several drug delivery systems. In a single cellulose fibril, there are several hundred to thousands of β−1, 4-anhydro-d-glucopyranose units joined by β-d-glycosidic linkages, which are linear, water-insoluble polysaccharides [Citation316]. In the form of fibril aggregates, fibrils, nano-crystallites and nanoscale disordered domains, cellulosic materials use hierarchical structure design that spans from nanoscale to macroscopic dimensions. Cellulose can provide functionality, flexibility, and high specific strength by taking advantage of its hierarchical structure [Citation245]. However, cellulose has a number of limitations as mentioned in Section ‘Cellulose’. To address less desirable qualities or produce new desired characteristics, cellulose can be chemically changed by replacing its native hydroxyl groups with functional groups such as particular acids, chlorides, and oxides [Citation317].
For this reason, Jafari et al. [Citation156] used MTT assays to investigate the in vitro cytotoxicity of free melatonin (MLT) and MLT encapsulated in CS-hydroxypropyl methylcellulose (HPMC) NPs. After 48 h of incubation, both free and encapsulated MLT caused dose-dependent toxicity in MDA-MB-231 breast cancer cells. MLT encapsulated in CS-HPMC NPs was found to have a greater toxicity than free MLT, indicating that encapsulation increased MLT absorption in cancer cells. In an acidic medium (pH 5.5), MLT encapsulated in CS-HPMC NPs demonstrated significantly greater release than in a neutral medium (pH 7.5) [Citation156]. It is suggested that the novel CS-HPMC NPs have a higher efficiency for cancer therapeutic agent delivery in an acidic condition of the tumour tissue. By combining with other biopolymers and cross-linking with tripolyphosphate (TPP) or glutaraldehyde, the degradation of CS can be tailored to the acidic environment of tumour tissue [Citation315].
Collagen
Collagen–gelatine methacrylate
The body’s major structural protein, collagen, is a natural hydrogel [Citation318]. Collagen comes in thirteen different types, with type I being the most prevalent. They all have the same structure of three polypeptides called α-chains that create a triple helix [Citation319]. Type I collagen is an excellent 3D scaffold material for tissue engineering [Citation320] owing to its capacity to self-assemble into a fibrillary gel, chemical alterations, low antigenicity and bioactivity features [Citation321–323]. Gelatine is produced when collagen is chemically or physically denatured or degraded [Citation324]. When gelatine is functionalised with methacrylic groups ((gelatine methacrylate) (GelMA)), photochemical cross-linking with UV light can result in a gelatine gel that is stable at body temperature [Citation325], allowing tissue engineering procedures to be implemented [Citation326–330]. Gelatine has a number of benefits, such as solubility and ease of acquisition [Citation331]. In specific, it has a lower antigenicity when compared to collagen. Furthermore, gelatine maintains an arginine-glycine-aspartic acid (RGD) peptide series that promotes a matrix metalloproteinase (MMP) degradation sequence that stimulates cell enzymatic degradation [Citation325,Citation332]. Nonetheless, since some cross-linking chemicals are hazardous, gelatine’s low melting point and chemical cross-linking may impact its biocompatibility [Citation203]. Fortunately, gelatine’s side chains comprise many active groups, such as –OH, –COOH, –NH2, and –SH. Thus, gelatine can be modified with specific groups to recompense for its limitations.
The proposed hydrogels’ high level of cytocompatibility in terms of angiogenesis is associated with poor printing properties. As a result, Stratesteffen et al. [Citation157] hypothesised that the biological and printing properties of GelMA and type I collagen hydrogel binary polymers could be tuned to produce a material ideal for microvalve-based drop-on-demand bioprinting. Collagen enhances rheological parameters such as viscosity and hydrogel stiffness while also reducing unwanted droplet spreading. The observed capillary-like network creation in GelMA-collagen hydrogels stimulated the fabrication of sophisticated cell-laden 3D structures, helping the creation of 3D-printed pre-vascularised cell-laden hydrogel constructs [Citation157].
Collagen–alginate
ALG must be chemically manipulated or combined with cell-adhesive compounds to enhance attachment features and growth [Citation333–337]. Collagen hydrogels quite often have a reduced matrix stiffness and integrin binding sites for cell-matrix interactions [Citation338–341]. It has been suggested as a polymer that can be mixed with ALG to form a mechanically tuneable hydrogel that allows for cellular attachment [Citation342]. To examine how the hydrogel’s physical and structural properties affect human neuron growth and development, Moxon et al. [Citation158] created an integration of ALG and collagen hydrogel networks as accessible platforms for 3D culture of induced pluripotent stem cell (iPSC) produced neurons. The derived hydrogel matrix is a heterogeneous network of crosslinked ALG and collagen fibrils, which promotes cell attachment, neuronal maturation, and mechanotransducive responses. The ability to tune the mechanical and structural properties of the hydrogel using simple ionic crosslinker concentration modulation has influenced cell phenotype and allowed for optimisation of neuron-specific gene expression. As a result, ALG-collagen blend hydrogels can be used as tailored substrates for investigating neuronal reactions to various mechanical and structural settings, influencing 3D neurogenesis, and examining neuronal behaviour in 3D cell culture models.
Gelatine
Gelatine–PEGDA
Various chemical cross-linking procedures, such as glutaraldehyde [Citation343] and diisocyanate [Citation344,Citation345], have been used to generate appropriate mechanical strength, and a stable gelatine hydrogel. Nonetheless, since the majority of chemical crosslinkers are toxic, their use as cell-laden matrices in tissue engineering is restricted. To enhance the degree of cross-linking and restrict the amount of biodegradation, Wang et al. [Citation159] added poly(ethylene glycol)diacrylate (PEGDA) to a pre-polymer solution. The GelMA-PEGDA binary hydrogel outperformed the pure GelMA hydrogel in terms of mechanical strength, degradation time, diffusion rate and swelling rate. Viability, adhesion, and proliferation were all high in in vitro cell culture tests. Therefore, PEGDA can improve the performance of GelMA hydrogels, and expand their uses as a promising bone regeneration material.
Gelatine–cellulose
Gelatine is a readily available biopolymer that can be electrospun and used as a scaffold for dermal and epidermal tissue engineering [Citation346]. Cellulose has long been used in wound treatments in the form of woven cotton gauze [Citation347]. However, cellulose’s processability is severely constrained due to its low solubility in typical organic solvents [Citation348,Citation349]. Instead, cellulose acetate (CA) is a commercially available, soluble derivative of cellulose that is currently widely used. It has a lower crystallinity, is soluble in a wide range of organic solvents, and is thus easily electrospinnable [Citation350].
According to Vatankhah and colleagues [Citation160], data demonstrates a decreasing trend in porosity with increasing gelatine concentrations, and the pore size in CA-gelatine composite scaffolds reduced dramatically with increased gelatine content. It was suggested that decreasing the pore size caused an increase in the surface area [Citation351,Citation352]. By increasing the gelatine composition in CA-gelatine composite blends, the hydrophilicity of the nanofibrous membrane increased, which provides superior support for cell attachment, adhesion, and proliferation. Electrospun CA-gelatine scaffolds resemble both the morphological and structural properties of normal skin depending on the compositional ratios used. As a result, CA-gelatine 25:75 membranes can be used as tissue engineered implants, while the CA-gelatine 75:25 scaffolds can be used for wound dressing [Citation160].
Silk
Silk–collagen
Silk has the ability to immobilise growth factors through amino acid side shift alterations. Furthermore, chemical modifications can be made to adapt them to a variety of biomedical applications [Citation353–366]. Due to the dominance of hydrophobic domains composed of short side chain amino acids in the primary sequence, silks are typically comprised of β-sheet structures. These structures enable the protein to be packed tightly in stacked sheets of hydrogen bonded anti-parallel networks. To allow cells to deposit new ECM, and restore functional tissue, many biomaterials must degrade at a pace that corresponds to new tissue creation. Furthermore, polymer-based products must be modified by the addition of various natural or synthetic polymers, such as collagen, to improve polymer characteristics [Citation367].
In a study by Zhou et al. [Citation161], SF-collagen tubular scaffolds were electrospun from aqueous solution with the purpose of creating novel vascular tissue engineering alternatives by combining these two materials. The findings reveal that collagen has a better cell attachment and expression ability than SF. The diameter of the fibres increased significantly as the concentration of SF-collagen solution increased. It showed that too much collagen caused the formation of belt-like fibres, and a slight decrease in crystallinity. This work showed that the SF-collagen binary system has more potential in tissue engineering than other natural materials, e.g. used in vascular tissue engineering.
Silk–PVA
SF, unlike other natural polymers, has received significant attention for a variety of biomedical applications due to its ease of chemical modification, slow in vivo degradability, autoclavability and ability to influence structure and function [Citation353,Citation355,Citation368]. To prepare hydrogels with enhanced characteristics, regenerated SF can be combined or chemically cross-linked with other natural or synthetic polymers. PVA is notably beneficial because it allows for the attachment of cell signalling molecules, or drugs via the numerous hydroxyl groups existing on the backbone [Citation369]. The abundance of pendant hydroxyl groups, which can be substituted by a range of substituents, allow PVA to be transformed into multifunctional and multivinyl macromers [Citation370–373].
Numerous researchers are focusing on finding out what enables SF to gel, and one of the most common theories is that the transition to a β-sheet structure is one of the key causes of gelation. The quantity of SF produced can be determined by the hydrogel composition. The PVA and SF result in hydrogels that can release encapsulated model materials in a regulated manner, demonstrating the potential of copolymer networks for drug delivery applications [Citation163]. The findings by Kundu and co-workers [Citation163] suggest that the interaction of silk and embedded drug, associated with diffusion, regulates drug release. As a result, the binary polymer hydrogels can be considered safe drug delivery carriers and hold immense promise as photo-crosslinked gel forming controlled drug delivery systems.
Nonetheless, in order to fabricate composite nanofibres, organic solvents or organic acids such as 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), trifluoroacetic acid, dichloromethane and formic acid must be used. When applied to wounded human skin or tissue, the residual toxic organic solvent or acid in electrospun materials is unacceptable. To address these issues, Zhou et al. [Citation162] created composite nanofibrous membranes of water-soluble N-carboxyethyl CS (CECS)–PVA–SF. CS has a chemical composition that is similar to glycosaminoglycans found in ECM and has been discovered as a suitable potential biomacromolecule for skin scaffolds owing to its haemostatic capacity, which aids wound regeneration [Citation283,Citation374,Citation375]. Following the failure of electrospinning from an aqueous solution of CECS and SF, PVA was chosen as the additive for the binary polymers to produce electrospun nanofibrous mat due to its fibre-forming (increasing viscosity and surface tension), biocompatibility and chemical stability attributes [Citation376].
Findings suggest that increasing the SF content in this binary system from 0 to 8% reduced the average fibre diameter of binary nanofibres from 643 to 126 nm, resulting in a narrower distribution. As a result, wound treatment and skin regeneration materials can be generated with the new electrospun matrices.
PHA
PHA–gelatine
PHAs are divided into two groups: short chain length (SCL) PHAs, which have 3–5 carbon atoms in their monomeric unit, that are generally stiff, crystalline polymers, and medium chain length (MCL) PHAs, which have 6–14 carbon atoms in their monomeric unit, and are generally elastomeric with a bigger amorphous phase composition [Citation377]. PHAs have a broad range of monomeric compositions, resulting in a family of materials with controllable physical properties and degradation rates [Citation378,Citation379]. Poly-3-hydroxybutyrate (PHB) is a biopolymer generated from PHA that is hydrophobic, biodegradable, and biocompatible [Citation379]. These polymers have triggered both long-term and short-term inflammatory reactions. It was discovered that PHAs are required for the engineering of biological tissues and a variety of biomedical applications [Citation380,Citation381]. PHAs with the appropriate improvements offer a great deal of potential to advance tissue engineering, and the development of tissue products for medicinal and therapeutic purposes, such as (1) vascular grafts, (2) heart valves, and (3) nerve tissue engineering [Citation382–393]. PHA has been used alone [Citation394–397] or in combination with other natural and synthetic polymers [Citation398], such as silk, CS, gelatine, PVA, polyglycerol, polyvinylidene fluoride (PVF) and PCL [Citation399–405], to improve mechanical strength and flexibility for tissue regeneration applications like skin, cartilage, tendon, ligament, and bone, among others. In addition, gelatine can provide a suitable environment for cell adhesion, development, and proliferation. Gelatine nanofibres can have a safe and functional structure for tissue engineering due to their biological origin and non-immunogenicity [Citation406].
Sanhueza et al. [Citation164] created scaffolds using PHB microfibres and gelatine nanofibres to produce a more biomimetic architecture for skin regeneration in diabetic wounds, that more closely resembles the properties of the natural ECM. The PHB-microfibre network strengthened and promoted the cross-linking of gelatine nanofibres, which prevented the scaffold from contracting. The generated dual-size gelatine-PHB binary fibre scaffold was biocompatible, encouraged fibroblast attachment, and skin regeneration effectively, with the added benefits of ease of handling and no risk of skin contraction. Gelatine-PHB blend scaffolds also exhibited a significant increase in wound healing rate and overall closure, development of hypodermis, and higher content in hair follicles and sweat glands when compared to non-treated wounds. Ultimately, it is hoped that the gelatine-PHB scaffold proves to be a promising vehicle for more extensive tissue engineering constructs that involve the transport of bioactive constituents with varied polarity to improve skin wound healing.
Poly(3-hydroxybutyrate-co-4-hydroxybutyrate)–gelatine
Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P(3HB-co-4HB)) copolymer is composed of 3HB and 4HB monomers, both of which are found in human metabolites [Citation407]. The biodegradation rate of this copolymer can be controlled by manipulating the 4HB monomer composition. As a result, it is widely used for nanofibre production (electrospinning) and wound healing applications [Citation408]. Although the P(3HB-co-4HB) polymer has outstanding therapeutic benefits, its hydrophobicity prevents it from being used extensively since it does not induce cell adhesion, resulting in ineffective cell colonisation [Citation409]. The polymers are combined with other natural polymers, such as gelatine to provide hydrophilicity, which improves the biological performance of P(3HB-co-4HB) scaffolds. Gelatine is used in the encapsulation of several pharmacological products due to its micro/nanoparticles [Citation410]. Moreover, oral gelatine consumption can also help with bone and joint health [Citation411].
Researchers are looking at using gelatine as a matrix for 3D cell culture and as a material for tissue-engineering scaffolds [Citation412]. However, temperature reversibility [Citation413] of gelatine will limit its applications, such as wound healing treatments where gels must be stable for a specific amount of time before dissolving [Citation414]. To alleviate this challenge and stabilise the gelatine gels, chemical or enzymatic cross-linking is recommended [Citation415]. The study of gelatine-P(3HB-co-4HB) by Azuraini et al. [Citation165] proves that binary polymer scaffolds achieved good biocompatibility properties, implying that the scaffolds produced can support cell growth and proliferation [Citation165,Citation406,Citation416]. These scaffolds are also significantly more hydrophilic than pure P(3HB-co-4HB) copolymer.
PHA–PCL
Innovations in the biosynthesis of natural PHA polyesters are raising the interest in the creation of bioresorbable and highly biocompatible biomaterials with substantial potential in healthcare [Citation417–419]. As a result, the biocompatibility of neat poly(3-hydroxyocttanoate) (P(3HO)) has been demonstrated to be as good as collagen in terms of cell viability, proliferation, and adhesion of neonatal ventricular rat myocytes [Citation420]. A wide range of mechanical properties are provided by a large diversity of PHA monomer units, ranging from rigid and strong biomaterials to very soft and elastomeric materials [Citation381]. MCL PHAs and their copolymers have been notably used to generate healthcare-related materials for applications in cardiac engineering [Citation420] and peripheral nerve engineering [Citation421–424] as a result of their low crystallinity, low glass transition temperature, low tensile strength, and high elongation to break. PHA and blends have been widely explored to tailor their properties to various therapeutic uses, although PHA combined with synthetic origin polymers is rare [Citation422,Citation425,Citation426]. In terms of clinical outcomes, none of the commonly available hollow bioresorbable nerve guidance conduits (NGCs) has yet successfully outperformed an autologous nerve transplant [Citation166]. To address this issue, semicrystalline PCL [Citation427–429] may be combined with a new family of aliphatic polyesters, such as naturally derived PHAs [Citation430,Citation431].
Mendibil and colleagues [Citation166] created a biomimetic NGC by combining natural MCL-PHA poly(3-hydroxyoctanoate-co-3-hydroxydecanoate) (P(3HO-3HD)) with synthetic PCL. According to the findings, P(3HO-3HD)-PCL at a composition of 75:25 appeared as a potential blend useful in the fabrication of hollow NGCs, capable of supporting peripheral nerve regeneration. This system demonstrated a favourable porosity/permeability relationship, providing enhanced nerve regeneration, while maintaining sufficient stiffness, and a low biodegradation rate to support the nerve throughout the regeneration process.
PCL
PCL–PGS
PCL is compatible with a wide range of polymers with great processibility and high thermal stability which enables ease of melt processing [Citation432,Citation433]. PCL’s ease of forming polymer blends make it a desirable material to be used as a supporting device, especially for tissue engineering [Citation434–436]. It is a hydrophobic polymer, and its mechanical characteristics are vastly different from those of healthy tissue. Pure PCL fibres would be undesirable for cornea tissue engineering application. Moreover, poly(glycerol sebacate) (PGS) was established in the last decade as a promising soft tissue engineering scaffold material [Citation437–439]. In biocompatibility investigations, the hydrophilic PGS demonstrated a surprising cellular response when compared to other polyesters such as PLGA [Citation440,Citation441].
PGS–PCL scaffolds were studied by Salehi et al. [Citation167] for cornea tissue engineering applications. A polymer binary system of PGS–PCL is thought to be the most promising. It is reasonable to assume that when a crystalline polymer (PCL) is blended with an amorphous polymer (PGS), the blend ratio affects mechanical properties. The crystallinity of the blend fibres diminish as the PGS content rises. While pure PCL nanofibres had a crystallinity of 57%, the crystallinity of the blend fibres was as low as 17% [Citation167]. The elastic modulus of the fibres decreased as the PGS–PCL mix ratio increased, which corresponded to the decreasing crystallinity. Nano-mechanical characteristics were investigated, and nano-indentation research revealed that surface modulus increases with increasing PGS concentration (contrary to elastic modulus), with the 4:1 blend fibre having the highest surface modulus [Citation167]. Furthermore, the surface moduli were approximately two orders of magnitude greater than the relevant elastic moduli. The fibre-forming PCL is anticipated to be driven into restricted and cross-linked domains near the fibre surface with a rise in PGS content, resulting in the reported dimension and behaviour of the surface moduli.
PCL–collagen
The distinct properties of collagen fibres, as well as their extensive occurrence and relatively easy accessibility, continue to gain the attention of biomedical researchers from numerous fields [Citation442–444]. Polymeric electrospun fibres are currently being used for the differentiation of numerous cell types such as fibroblasts, osteoblasts, chondrocytes, and endothelial cells due to their unique physical features [Citation445–448]. However, cell growth and tissue formation were typically limited to the surfaces of electrospun substrates [Citation449–454]. Synthetic polymers (PCL) can be functionalised with natural polymers (collagen) using a variety of methods, such as simple blending or particular surface modification [Citation455–460].
For instance, Ekaputra et al. [Citation169] developed a scaffold mesh consisting of PCL–collagen binary polymer system for vascularisation applications. This strategy allows the loading and release of angiogenic growth factors, such as vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF) in an electrospun tissue engineering construct that allowed for the recapitulation of primitive endothelial plexus throughout its structure, demonstrating its potential as a totally vascularised 3D scaffold. When compared to PCL fibres alone, meshes constructed of sub-micron sized PCL–collagen fibres have previously shown better adhesion, proliferation and osteogenic potential of bone marrow derived MSC [Citation461]. Furthermore, PCL possesses adequate mechanical characteristics to perform as a tracheal substitute and a slow rate of biodegradation in vivo, which can help overcome drawbacks such as postoperative tracheal softening, and re-stenosis that are associated with rapid deterioration [Citation462–466]. Integrating scaffold materials to manufacture a tracheal construct could be beneficial since one material can offer mechanical integrity, while the other could provide a biocompatible and chondrogenic environment for cells, resulting in a biomimetic design.
Regrettably, the hydrophobicity and lack of porosity of PCL limit its use in tracheal cartilage formation. On the other hand, collagen is a major component of cartilage tissue, it is particularly hydrophilic, and so has the potential to mitigate for PCL’s restrictions in composite materials in terms of cell detachment, proliferation, and chondrogenesis [Citation467,Citation468]. She et al. [Citation168] demonstrated that by using a 3D-printed PCL framework and embedded collagen, a biomimetic tracheal scaffold with a differentiated structure can match both the anatomy and mechanical features of the native trachea [Citation469]. As a result, the PCL scaffold demonstrated reduced cell affinity than the collagen scaffold, which had greater cell affinity [Citation470]. These findings revealed that by using a binary polymer system of PCL and collagen, the differences between the two scaffolds can be exploited to mimic the mechanical properties of the original trachea, while also promoting biomimetic anatomy creation with cartilage rings alternated with PCL ring’s structure. Experiments showed that this polymer blend combination can be used to repair a long-segment tracheal fracture [Citation168].
PEG/PEO
PEG/PEO–PLGA
PEG, also known as PEO, is a versatile, thermoplastic, and crystalline polymer with the general formula (–O–CH2–CH2–)n [Citation471]. PEG polymers typically have molecular weights less than 20,000 gmol−1, whereas PEO polymers have higher molecular weights [Citation472]. Studies have shown that PEG/PEO is soluble in water, ethanol, acetonitrile, benzene, and dichloromethane, but insoluble in diethyl aether and hexane [Citation472]. Moreover, PEO is a non-toxic polymer that is colourless, odourless, and resistant to heat and hydrolysis. It also works as an inert substance to many chemical reagents. PEO is a promising option for biomedical applications, particularly as scaffolds in tissue engineering [Citation473] and biocompatible coatings [Citation474–476], due to its biocompatibility and non-immunogenicity, as well as fascinating physicochemical characteristics. PEO can be integrated with other polymers to improve its properties as a potential biomaterial [Citation477].
Evrova et al. [Citation170] introduced a new method for tuning the physical and mechanical properties of electrospun PLGA fibrous scaffolds, one of the most thoroughly investigated polymers for synthetic scaffold fabrication, without modifying the PLGA polymer chemically [Citation478,Citation479]. When compared to the PLGA-only scaffold, adding PEO to the PLGA scaffolds influenced the mechanical and physical properties of the scaffolds by enhancing strain at break (%) and decreasing Young’s modulus and tensile stress [Citation170]. The increase in strain at break (%) was proportional to the concentration of PEO, which operated as a plasticiser, as previously demonstrated in combination with other polymers [Citation480–482]. Myoblast attachment, proliferation, myoblast fusion and myotube production were all improved using a polymer binary system of PLGA:PEO [Citation170].
PEG/PEO–PHBV
PEO is widely utilised in the biomedical area, particularly in blood-contacting devices, due to its non-immunogenicity and ability to minimise surface protein adsorption [Citation483]. Additionally, it is considerably easier to obtain PEO nanofibres than other polymers such as CS and gelatine, due to its superior solubility, which allows it to dissolve not only in water but also in a variety of organic solvents. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) has strong oxygen permeability and mechanical properties, and the final degradation product is (R)-3-hydroxybutyric acid, which is a naturally occurring component of human blood [Citation484–486]. As a result, PHBV has been widely researched as a biomaterial for a broad variety of applications, including sutures [Citation487], prosthetic devices [Citation488], drug delivery systems [Citation489], and surgical clips [Citation490]. In the case of artificial skin scaffolds, it should be able to serve as a barrier to shield the wound from infection and fluid loss [Citation491,Citation492] while enabling oxygen to pass through [Citation493] and provide a moist environment to stimulate fibroblast activity during the healing process [Citation494]. Due to its high crystallinity, brittle nature, and low hydrophilicity, PHBV has been limited in skin tissue engineering applications. Hence, binary polymer systems of PHBV with other polymers, such as PEO, is critical for improving the drawbacks of PHBV [Citation495–497].
According to Xu and colleagues [Citation171], PHBV–PEO electrospun mats have encouraging mechanical properties for cellular morphogenesis in skin tissue engineering. Furthermore, as the PEO concentration increases, the crystallinity of PHBV–PEO electrospun mats decreases. As a result of blending with a PEO component, all studied PHBV–PEO electrospun mats exhibited desirable and enhanced properties, alleviating PHBV shortcomings (i.e. high crystallinity, low flexibility, brittle nature, and hydrophobicity) [Citation171].
PEG/PEO–PLA
PLA has undergone extensive research to improve its characteristics in order to compete with flexible commodity polymers. Combining PLA with different polymers, altering PLA with plasticisers, and blending PLA with inorganic nano-fillers are all examples of these approaches. PEG is a promising plasticising agent for PLA, as it increases elongation at break by a significant amount [Citation498–503].
An investigation by Banerjee et al. [Citation172] exploited imaging to evaluate the effect of active targeting with a small-molecule prostate-specific membrane antigen (PSMA)-targeting moiety bonded to a PLA–PEG nanoparticle in vivo. Surface functionalisation of PLA–PEG particles with a urea-based PSMA-targeting moiety resulted in a considerable, positive influence on PSMA tumour association, as well as EPR. Particles were linked to tumour epithelium and macrophages. These findings demonstrate that imaging of radiolabelled particles can be used to determine the biodistribution and tumour accumulation of therapeutic targeted particles, with similar size and surface properties, in patients. This technique can therefore be used to select patients that are most likely to respond to treatment. PLA–PEG blend particles incorporated with dispersed docetaxel (DTX) can potentially target the PMSA via surface expression of a low molecular weight targeting ligand that binds to PMSA with a high affinity [Citation504,Citation505].
PLGA
PLGA–PLA
PLA production necessitates the deployment of catalysts under strict temperature, pressure, and pH control, as well as extensive polymerisation durations, all of which result in substantial energy usage [Citation506]. Many approaches have been developed to improve its properties, such as physical blending to generate biodegradable materials with various morphologies and physical properties [Citation507]. The combination of lactic acid and glycolic acid produces PLGA, a copolymer derivative of PLA. Due to their characteristics, they have been widely explored in sustainable drug delivery methods [Citation508]. The lactic acid-glycolic acid content ratio can be adjusted to fine-tune polymeric properties such as degradation rate and Tg [Citation42,Citation479,Citation509].
Polymeric nanoparticles have the ability to alleviate the multi-drug resistance that characterises many anticancer drugs through a mechanism of drug internalisation [Citation510], lowering drug efflux from cells mediated by the P-glycoprotein [Citation511,Citation512]. Musumeci et al. [Citation173] investigated the feasibility of using nanosphere colloidal suspensions as sustained release systems for intravenous administration DTX which enhanced drug solubility. The solvent displacement method was used to create nanospheres from PLA with varying molecular weights and PLGA as biodegradable matrices. This study revealed that the drug was unable to disperse into the external medium through the rigid glassy polymer matrix. As a result, the greater drug release reported in the in vitro assay was attributable to the structural degradation of polymer. The faster interaction of DTX with DPPC liposomes was attributed to the maximum amount of DTX adsorbed on the nanosphere surface, allowing it to be released quickly, as well as the fast degradation of PLA R203 polymer in comparison to PLA R207 due to its low molecular weight. In summary, the results showed that biodegradable polymeric colloidal systems composed of PLA and/or PLGA can entrap DTX and provide sustained drug release.
PLGA–hyaluronic acid
PLGA particles have several benefits in biomedical applications: they can be targeted in vivo with antibodies, and they achieve improved immune response when modified with targeting ligands [Citation42,Citation479,Citation513]. However, problems such as limited drug loading efficiency, challenges regulating encapsulated drug release rates, and/or formulation instability are preventing PLGA from being used more extensively in pharmaceutical products [Citation479,Citation514–518]. Polymer-based scaffolds incorporating diverse biochemical variables have been reported in order to promote diabetic wound healing [Citation519–524]. Not only should an ideal scaffold be biocompatible and bioactive, but it should also facilitate the challenging healing process. To facilitate cell proliferation, they should also be structurally and dimensionally similar to the natural ECM.
To satisfy these needs, Shin and researchers [Citation174] created hyaluronic acid–PLGA core/shell fibre matrices loaded with epigallocatechin-3-O-gallate (EGCG) (hyaluronic acid/PLGA-E) and tested their healing properties in diabetic rats with full-thickness wounds. The results imply that controlled diffusion with PLGA degradation released the hyaluronic acid and EGCG from the matrices in a sustainable manner [Citation174]. Furthermore, an animal investigation showed that the in vivo full-thickness wound healing rate was dramatically accelerated in both normal and STZ-diabetic rats. The finding suggests that the binary system of hyaluronic acid/PLGA-E core/shell fibre matrices are advantageous for diabetic full-thickness wound repair and could be viable candidates for novel scaffolds.
PVA
PVA–PHBH
PVA is derived from the hydrolysis of polyvinyl acetate (PVAc) [Citation525–527] with beneficial properties as stated in Section ‘Cellulose-PVA’. PHB has a high crystallinity of 55–80%, a melting point of 180°C, with stiff, rigid, and brittle properties, as well as mechanical characteristics, all of which are serious limitations in most common applications [Citation528]. Copolymerisation of additional monomer units into the main chains, such as 3-hydroxyvalerate (3HV), 3-hydroxyhexanoate (3HH), and 4-hydroxybutyrate (4HB), considerably increases homopolymer behaviour [Citation400,Citation529,Citation530]. Although both polymers have attractive characteristics, PVA has poor toughness and processability. Blending polymer is one method for combining properties in each polymer material to improve characteristics, material performance, and enhance polymer limitations [Citation531,Citation532].
Rebia et al. [Citation175] carried out a study to evaluate the potential of PHBH–PVA biodegradable binary polymer blend nanofibres as a suitable wound dressing. According to the findings, PHBH–PVA blend nanofibres were immiscible in the crystalline phase, but compatible in the amorphous state due to the presence of a hydrogen interaction between them. As a result, the combination of PHBH and PVA had an influence on the morphological change in response to water. The disintegration rate, cell adhesion, and proliferation were all influenced by the surface properties of the binary polymer nanofibre.
PVA–PGS
PGS has emerged as a promising polymer for tissue engineering applications, particularly in soft tissue applications, such as cardiac [Citation533], nerve [Citation534], blood vessel [Citation535] and cornea tissue engineering [Citation167], due to its elastomeric, mechanical, and biocompatible characteristics. Furthermore, PGS has the advantage of being biodegradable with tuneable properties that become beneficial enzymatically and hydrolytically without any adverse effects from degradation products. Although PGS offers a range of attractive properties, its processability is limited. To cross-link PGS into an insoluble matrix, the polymer must be thermally cured at high temperatures (commonly >110°C) and under vacuum [Citation536]. These factors make it challenging to obtain consistent geometries, limiting the polymer’s application in precision-integrated cells or temperature-sensitive components [Citation440,Citation537–541].
To address this drawback, polymer blends can be formed which are soluble in the similar solvents with the PGS pre-polymer and have a melting temperature higher than the cross-linking temperature [Citation542]. PVA, which satisfies these characteristics and has been adapted to electrospin PGS, is a potential polymer candidate for forming a blend solution with PGS pre-polymer [Citation543,Citation544]. The Young’s modulus of PGS was determined to be 0.21 ± 0.02 MPa in a study by Gultekinoglu et al. [Citation34] and the maximum elongation at break was calculated to be 67 ± 4.6%. PGS can be exploited in soft tissue applications, such as skin, muscle, and ligament tissue engineering. Thus, PGS polymers were generated into fibrous scaffolds for potential soft tissue applications. The resulting fibre structure is 3D, allowing cells to enter the scaffold. Researchers have thereby resolved the processability concerns of PGS polymer, a bio-elastomer with significant potential in tissue engineering applications.
PVA–starch
Starch is a glucose-based natural polymer that is widely available, and it is biodegradable and biocompatible [Citation545]. It partially dissolves in water and can be physically or chemically manipulated. Due to low physicochemical characteristics, such as moisture retention, gel fraction, and water vapour transition rate, polysaccharides cannot be exploited alone [Citation546]. As a result, water-soluble starch can be coupled with synthetic polymers, such as PVA, to generate good mechanical characteristics and form stable hydrogels [Citation547].
Altaf et al. [Citation176] produced an antimicrobial wound dressing with PVA and starch, integrated with essential oils (clove oil, tea tree oil, and oregano oil), that have been cross-linked with glutaraldehyde. The results suggest that the produced binary hydrogels have the ability to deliver a moist environment by decreasing moisture transmission from the wound bed [Citation176]. Increasing the oil concentration enables pores to form and the oil becomes immiscible. FT-IR spectra revealed that PVA–starch blends are adequately cross-linked with essential oils giving amine, hydroxyl, and aether groups, proving the semi-crystalline nature of the membranes made.
PVP
PVP–PVA
PVP, also referred to as polyvidone or povidone [Citation548], is a synthetic polymer composed of linear 1-vinyl-2-pyrrolidone groups. A poly-N-vinylamide structure is included in this polymer, which has a carbon chain with an amide group in the side substituent. PVP has a unique combination of physicochemical properties [Citation549,Citation550], including biocompatibility, thin film forming ability [Citation551], adhesiveness [Citation552], pH stability [Citation549], temperature resistance [Citation553], cross-linking [Citation554], and good complex formation capacity [Citation555]. Moreover, its affinity for complex, both hydrophobic and hydrophilic substances, has made it beneficial as a biomaterial in a variety of substantial medical applications, such as pharmaceutical industry and medicine [Citation556]. PVP is also a common hydrophilization agent used in water purification and dialysis membranes [Citation557,Citation558], as well as for physically stabilising suspensions [Citation559]. One of the most well-studied hydrogels for as a potential prosthetic articular cartilage is PVA [Citation560–562]. PVA hydrogel can be easily processed and manipulated to have a fluid content (65–80%) similar to that of articular cartilage [Citation563]. The 3D network structure of PVA has pores comparable to the native cartilage [Citation564,Citation565] and generates low frictional behaviour [Citation566–568].
Despite the fact that PVP and PVA have many beneficial qualities, PVA is a crystalline polymer, and its strong crystallinity inhibits gas penetration in the polymer matrix due to lower diffusivity, especially in the dry condition. Based on preliminary research by Lilleby Helberg and co-workers [Citation569], it was discovered that blending PVA with a less crystalline polymer, such as PVP, can enhance permeability, as well as benefiting from the PVA’s mechanical strength, increasing the polymer matrix’s capacity to retain water and keep carriers in membranes.
Hydrogels with a PVA–PVP blend have been extensively studied as a cartilage replacement material [Citation570,Citation571]. Inter-chain hydrogen bonding between PVA hydroxyl groups and PVP carbonyl groups enhanced polymer network stability when moderate amounts of PVP (0.5–5%) molecules were added to PVA [Citation571]. By adjusting a set of parameters such as polymer concentration [Citation572], freeze-thawing cycles [Citation573], thawing rate [Citation574], PVA molecular weight [Citation575], and degree of polymerisation [Citation564], the mechanical properties of PVA–PVP hydrogels can be predicted to imitate the mechanical properties of articular cartilage. In an investigation by Kanca et al. [Citation177] PVA–PVP hydrogels produced low coefficients of friction (COF) against articular cartilage and did not harm the articulating cartilage counterface, making them appealing as cartilage imitating materials.
PVP–gelatine
PVP has long been known for its amorphous form, ease of solubility in organic solvents and ability to interact with hydrophilic materials [Citation576]. It has been used in a variety of pharmaceutical applications, including wound dressings, blood plasma compressors, drug coating materials, nanofibre membranes/mats, oral/injectable solutions and disinfectants [Citation555]. The pore-forming ability of PVP offers additional porosity to the scaffold [Citation577]. As an effective therapeutic strategy to imitate various components of natural bone ECM, combining gelatine and PVP can prove to be a desirable biomaterial that can supply the essential environment for cell development and differentiation. Gelatine exhibits efficient absorbency, non-immunogenicity [Citation578], in vitro biocompatibility [Citation579], and thus its ability in the synthesis of scaffolds for bone tissue engineering is being investigated extensively.
The polymer blend of gelatine-PVP composite scaffolds for bone tissue engineering was investigated by Mishra and colleagues [Citation178]. The study reveals that the scaffold has appropriate physicochemical properties. When stimulated with osteogenic medium, enhanced matrix mineralisation was further demonstrated by alizarin red staining and EDX examination of apatite depositions over the scaffold. These findings show that the gelatine-PVP biomimetic binary polymer scaffold, with intrinsic proliferative and osteogenic potential, as well as osteoinductive capability, is suitable for use as a bone graft substitute material.
PVP–PCL
PCL dissolving mechanisms have been investigated to develop alternative solutions to overcome its hydrophobicity [Citation580]. The impact of parameters including molecular weight, morphology and chemical composition has been researched, as well as the impact of polymer crystallinity reduction on an accelerated degradation rate [Citation581]. Blending strategies have also been frequently employed to alter the physical and chemical properties of PCL. A polymer binary system of hydrophobic PCL with a hydrophilic polymer is expected to promote water diffusion to the proximities of PCL chains, speeding up their hydrolytic degradation [Citation582–585].
Tissue engineering and regenerative medicine have recently used synthetic biopolymers such as PCL [Citation586,Citation587] and PVP [Citation588,Citation589] to build substitute tissues for human bodies. PVP can be used as a sacrificial material because of its ability to integrate with a wide range of hydrophilic and hydrophobic materials [Citation590]. As a result, the integration of PCL with a biocompatible water-soluble polymer such as PVP will indeed significantly enhance the composite scaffold’s mechanical characteristics that can result in scaffolds with customisable fibre surface structure and degradation rates [Citation591,Citation592].
In a study by Li et al. [Citation179], the PCL–PVP binary polymer scaffolds were printed in order to determine the presence of PCL and PVP in the printed composite scaffold. Microscale PVL–PVP composite 3D scaffolds with good cell compatibility, as well as high cell density, were created to assist tissue development and proliferation [Citation179]. The innovative E-Jet 3D printing process shows that printing composite synthetic biopolymers for tissue engineering is a viable option.
Concluding remarks and future perspectives
Polymer blends incorporating more than one polymer can be produced and alloyed with different additives, in order to optimise them. This has been helped by the ability to process these blends into various morphologies. For example, the development of micro-to-nano-metre scale polymeric fibres with limitless potential in biomedical applications such as tissue engineering, wound dressings and microbial filtration has helped to drive the demand for polymer blends, in particular binary polymer systems as illustrated in this review. Spinning techniques such as electrospinning, centrifugal spinning, and pressurised gyration have shown significant advantages for the exploitation of polymer blends, especially binary polymer systems. Properties such as fibre size, distribution, and morphology can be tailored using different spinning parameters to suit applications. Each technique and polymer binary system will have its drawbacks; the future, however, may be an integration of binary polymer systems that will allow their desired outcome to be achieved successfully will be exploited, and we forecast extension of the idea to ternary polymer systems and beyond too.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials: review. Prog Polym Sci. 2011;36(9):1254–1276.
- Kumbar S, Laurencin C, Deng M. Natural and synthetic biomedical polymers. Elsevier; 2014. p. 1–402.
- Shoichet MS. Polymer scaffolds for biomaterials applications. Macromolecules. 2010;43(2):581–591.
- Lei B, Guo BL, Rambhia KJ, et al. Hybrid polymer biomaterials for bone tissue regeneration. Front Med. 2019;13(2):189–201.
- Banoriya D, Purohit R, Dwivedi RK. Advanced application of polymer based biomaterials. Mater Today-Proc. 2017;4(2):3534–3541.
- Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8–9):762–798.
- Madhumanchi S, Srichana T, Domb A. Polymeric biomaterials. In: Narayan R, editor. Biomedical materials. 2021. p. 49–100.
- Zhang HM, Zuo M, Zhang XY, et al. Effect of agglomeration on the selective distribution of nanoparticles in binary polymer blends. Compos Part A Appl Sci Manuf. 2021;149:6.
- Grossen P, Witzigmann D, Sieber S, et al. PEG-PCL-based nanomedicines: a biodegradable drug delivery system and its application. J Controlled Release. 2017;260:46–60.
- Lim CT. Nanofiber technology: current status and emerging developments. Prog Polym Sci. 2017;70:1–17.
- Ajili SH, Ebrahimi NG, Soleimani M. Polyurethane/polycaprolactane blend with shape memory effect as a proposed material for cardiovascular implants. Acta Biomater. 2009;5(5):1519–1530.
- Gultekinoglu M, Sarisozen YT, Erdogdu C, et al. Designing of dynamic polyethyleneimine (PEI) brushes on polyurethane (PU) ureteral stents to prevent infections. Acta Biomater. 2015;21:44–54.
- Afjoul H, Shamloo A, Kamali A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: an in vitro, in vivo study. Mater Sci Eng C Mater Biol Appl. 2020;113:110957.
- Sivakumar R, Lee NY. Chemically robust succinimide-group-assisted irreversible bonding of poly(dimethylsiloxane)–thermoplastic microfluidic devices at room temperature. Analyst. 2020;145(21):6887–6894.
- Castanheira A, dos Santos MB, Rodriguez-Lorenzo L, et al. Correction: a novel microfluidic system for the sensitive and cost-effective detection of okadaic acid in mussels. Analyst. 2021;146(24):7748–7749.
- Sibaja B, Culbertson E, Marshall P, et al. Preparation of alginate–chitosan fibers with potential biomedical applications. Carbohydr Polym. 2015;134:598–608.
- Lu JW, Zhu YL, Guo ZX, et al. Electrospinning of sodium alginate with poly(ethylene oxide). Polymer. 2006;47(23):8026–8031.
- Namkaew J, Laowpanitchakorn P, Sawaddee N, et al. Carboxymethyl cellulose entrapped in a poly(vinyl) alcohol network: plant-based scaffolds for cartilage tissue engineering. Molecules. 2021;26(3):578.
- Wu Z, Jiang Y, Li Z, et al. Bacterial cellulose nanofiber distribution on gelatin and silk fibroin scaffolds and the cell behavior. Cellulose. 2021;28(1):91–102.
- Mu X, Agostinacchio F, Xiang N, et al. Recent advances in 3D printing with protein-based inks. Prog Polym Sci. 2021;115:19.
- Chen YJ, Jia ZH, Shafiq M, et al. Gas foaming of electrospun poly(L-lactide-co-caprolactone)/silk fibroin nanofiber scaffolds to promote cellular infiltration and tissue regeneration. Colloid Surf B Biointerfaces. 2021;201:10.
- Eivazzadeh-Keihan R, Radinekiyan F, Aliabadi HAM, et al. Chitosan hydrogel/silk fibroin/Mg(OH)(2) nanobiocomposite as a novel scaffold with antimicrobial activity and improved mechanical properties. Sci Rep. 2021;11(1):13.
- Fu CY, Chuang WT, Hsu SH. A biodegradable chitosan-polyurethane cryogel with switchable shape memory. ACS Appl Mater Interfaces. 2021;13(8):9702–9713.
- San Choi J, Lee SJ, Christ GJ, et al. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29(19):2899–2906.
- Rekulapally R, Udayachandrika K, Hamlipur S, et al. Tissue engineering of collagen scaffolds crosslinked with plant based polysaccharides. Prog Biomater. 2021;10(1):29–41.
- Lv X, Li Z, Chen S, et al. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials. 2016;84:99–110.
- Suarato G, Contardi M, Perotto G, et al. From fabric to tissue: recovered wool keratin/polyvinylpyrrolidone biocomposite fibers as artificial scaffold platform. Mater Sci Eng C Mater Biol Appl. 2020;116:111151.
- Zehnder T, Sarker B, Boccaccini AR, et al. Evaluation of an alginate–gelatine crosslinked hydrogel for bioplotting. Biofabrication. 2015;7(2):025001.
- Bi H, Feng T, Li B, et al. In vitro and in vivo comparison study of electrospun PLA and PLA/PVA/SA fiber membranes for wound healing. Polymers. 2020;12(4):839.
- Chen G, Sato T, Ohgushi H, et al. Culturing of skin fibroblasts in a thin PLGA–collagen hybrid mesh. Biomaterials. 2005;26(15):2559–2566.
- Zhang B, Zhu M, Li Z, et al. Cellular fate of deformable needle-shaped PLGA-PEG fibers. Acta Biomater. 2020;112:182–189.
- Croisier F, Atanasova G, Poumay Y, et al. Polysaccharide-coated PCL nanofibers for wound dressing applications. Adv Healthcare Mater. 2014;3(12):2032–2039.
- Tokarev A, Asheghali D, Griffiths IM, et al. Touch- and brush-spinning of nanofibers. Adv Mater. 2015;27(41):6526–6532.
- Gultekinoglu M, Orturk S, Chen BQ, et al. Preparation of poly(glycerol sebacate) fibers for tissue engineering applications. Eur Polym J. 2019;121:109297.
- Robeson LM. Polymer blends: a comprehensive review. Munich: Hanser Gardner; 2007.
- Namazi H. Polymers in our daily life. Bioimpacts. 2017;7(2):73–74.
- Gu LS, Shan TT, Ma YX, et al. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019;37(5):464–491.
- Song R, Murphy M, Li CS, et al. Current development of biodegradable polymeric materials for biomedical applications. Drug Des Dev Ther. 2018;12:3117–3145.
- Gasperini L, Mano JF, Reis RL. Natural polymers for the microencapsulation of cells. J R Soc Interface. 2014;11(100):20140817.
- Bayram C, Jiang XY, Gultekinoglu M, et al. Biofabrication of gelatin tissue scaffolds with uniform pore size via microbubble assembly. Macromol Mater Eng. 2019;304(11):1970029.
- Maitz MF. Applications of synthetic polymers in clinical medicine. Biosurface Biotribology. 2015;1(3):161–176.
- Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3(3):1377–1397.
- Cameron RE, Kamvari-Moghaddam A. Synthetic bioresorbable polymers. In: Buchanan FJ, editor. Degradation rate of bioresorbable materials. Cambridge: Woodhead Publ; 2008. p. 43–66.
- Amerongen M, Harmsen M, Petersen A, et al. The enzymatic degradation of scaffolds and their replacement by vascularized extracellular matrix in the murine myocardium. Biomaterials. 2006;27:2247–2257.
- Nicolas J, Magli S, Rabbachin L, et al. 3D extracellular matrix mimics: fundamental concepts and role of materials chemistry to influence stem cell fate. Biomacromolecules. 2020;21(6):1968–1994.
- Utracki LA, Wilkie CA. Polymer blends handbook. Dordrecht: Kluwer Academic Publishers; 2002.
- Kim HJ, Peng XY, Shin YS, et al. Blend miscibility of poly(ethylene terephthalate) and aromatic polyesters from salicylic acid. J Phys Chem B. 2021;125(1):450–460.
- Qian YF, Su Y, Li XQ, et al. Electrospinning of polymethyl methacrylate nanofibres in different solvents. Iran Polym J. 2010;19(2):123–129.
- Abbott S. Chemical compatibility of poly(lactic acid): a practical framework using Hansen solubility parameters. In: Auras R, Lim L-T, Selke SEM, Tsuji H, editors. Poly(lactic acid): synthesis, structures, properties, processing, and applications. John Wiley & Sons, Inc; 2010. p. 83–95.
- Veleirinho B, Rei MF, Lopes-da-Silva JA. Solvent and concentration effects on the properties of electrospun poly(ethylene terephthalate) nanofiber mats. J Polym Sci Part B Polym Phys. 2008;46(5):460–471.
- Wu X, Wang L, Yu H, et al. Effect of solvent on morphology of electrospinning ethyl cellulose fibers. J Appl Polym Sci. 2005;97(3):1292–1297.
- Oliveira J, Brichi GS, Marconcini JM, et al. Effect of solvent on the physical and morphological properties of poly(lactic acid) nanofibers obtained by solution blow spinning. J Eng Fibers Fabr. 2014;9(4):117–125.
- Yanilmaz M. Effect of the solvent system on the morphology and performance of nylon 6 nanofibre membranes. Fibres Text East Eur. 2019;27(6):97–101.
- Wannatong L, Sirivat A, Supaphol P. Effects of solvents on electrospun polymeric fibers: preliminary study on polystyrene. Polym Int. 2004;53(11):1851–1859.
- Casasola R, Thomas NL, Trybala A, et al. Electrospun poly lactic acid (PLA) fibres: effect of different solvent systems on fibre morphology and diameter. Polymer. 2014;55:4728–4737.
- Colmenares-Roldan GJ, Quintero-Martinez Y, Agudelo-Gomez LM, et al. Influence of the molecular weight of polymer, solvents and operational condition in the electrospinning of polycaprolactone. Rev Fac Ing Univ Antioquia. 2017;84:35–45.
- Liechty WB, Kryscio DR, Slaughter BV, et al. Polymers for drug delivery systems. Annu Rev Chem Biomol Eng. 2010;1:149–173.
- Kamaly N, Yameen B, Wu J, et al. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663.
- Kaplan JA, Liu R, Freedman JD, et al. Prevention of lung cancer recurrence using cisplatin-loaded superhydrophobic nanofiber meshes. Biomaterials. 2016;76:273–281.
- Sharifi F, Sooriyarachchi AC, Altural H, et al. Fiber based approaches as medicine delivery systems. ACS Biomater Sci Eng. 2016;2(9):1411–1431.
- Sebe I, Szabo P, Kallai-Szabo B, et al. Incorporating small molecules or biologics into nanofibers for optimized drug release: a review. Int J Pharm. 2015;494(1):516–530.
- Chou SF, Carson D, Woodrow KA. Current strategies for sustaining drug release from electrospun nanofibers. J Controlled Release. 2015;220:584–591.
- Balaji A, Vellayappan MV, John AA, et al. An insight on electrospun-nanofibers-inspired modern drug delivery system in the treatment of deadly cancers. RSC Adv. 2015;5(71):57984–58004.
- Dubey P, Gopinath P. Fabrication of electrospun poly(ethylene oxide)–poly(capro lactone) composite nanofibers for co-delivery of niclosamide and silver nanoparticles exhibits enhanced anti-cancer effects in vitro. J Mat Chem B. 2016;4(4):726–742.
- Wei JC, Hu J, Li M, et al. Multiple drug-loaded electrospun PLGA/gelatin composite nanofibers encapsulated with mesoporous ZnO nanospheres for potential postsurgical cancer treatment. RSC Adv. 2014;4(53):28011–28019.
- Morgado PI, Aguiar-Ricardo A, Correia IJ. Asymmetric membranes as ideal wound dressings: an overview on production methods, structure, properties and performance relationship. J Membr Sci. 2015;490:139–151.
- Zhou XJ, Chen L, Wang WZ, et al. Electrospun nanofibers incorporating self-decomposable silica nanoparticles as carriers for controlled delivery of anticancer drug. RSC Adv. 2015;5(81):65897–65904.
- Yuan Y, Choi K, Choi SO, et al. Early stage release control of an anticancer drug by drug-polymer miscibility in a hydrophobic fiber-based drug delivery system. RSC Adv. 2018;8(35):19791–19803.
- Siepmann J, Siepmann F. Modeling of diffusion controlled drug delivery. J Controlled Release. 2012;161(2):351–362.
- Li H, Hardy RJ, Gu X. Effect of drug solubility on polymer hydration and drug dissolution from polyethylene oxide (PEO) matrix tablets. AAPS PharmSciTech. 2008;9(2):437–443.
- Schindler C, Williams BL, Patel HN, et al. Electrospun polycaprolactone/polyglyconate blends: miscibility, mechanical behavior, and degradation. Polymer. 2013;54(25):6824–6833.
- Siafaka PI, Barmbalexis P, Bikiaris DN. Novel electrospun nanofibrous matrices prepared from poly(lactic acid)/poly(butylene adipate) blends for controlled release formulations of an anti-rheumatoid agent. Eur J Pharm Sci. 2016;88:12–25.
- Kim K, Luu YK, Chang C, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Controlled Release. 2004;98(1):47–56.
- Cordin M, Bechtold T, Pham T. Effect of fibre orientation on the mechanical properties of polypropylene–lyocell composites. Cellulose. 2018;25(12):7197–7210.
- Cuvalci H, Erbay K, Ipek H. Investigation of the effect of glass fiber content on the mechanical properties of cast polyamide. Arab J Sci Eng. 2014;39(12):9049–9056.
- Salman SD, Leman Z, Sultan MTH, et al. Influence of fiber content on mechanical and morphological properties of Woven Kenaf reinforced PVB film produced using a hot press technique. Int J Polym Sci. 2016;2016:7828451.
- Zaman I, Ismail AE, Awang M. Influence of fiber volume fraction on the tensile properties and dynamic characteristics of coconut fiber reinforced composite. J Sci Technol. 2010;1:55–71.
- Aydogdu MO, Altun E, Ahmed J, et al. Fiber forming capability of binary and ternary compositions in the polymer system: bacterial cellulose–polycaprolactone–polylactic acid. Polymers. 2019;11(7):1148.
- Jasso-Gastinel CF, Soltero-Martinez JFA, Mendizabal E. Introduction: modifiable characteristics and applications. In: Carlos F. Jasso-Gastinel and José M. Kenny, editors. Modification of polymer properties. Norwich: William Andrew Inc; 2017. p. 1–21.
- Hawkins WL. Polymer degradation. In: Harwood HJ, editor. Polymer degradation and stabilization. Berlin: Springer; 1984. p. 3–34.
- Guillet J, Huber H, Scott J. Biodegradable polymers and plastics. Proceedings of the Second International Scientific Workshop on Biodegradable Polymers and Plastics, Montpellier, 1992. p. 55–70.
- Barbeck M, Serra T, Booms P, et al. Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3D-printed scaffolds combining PLA and biphasic PLA/bioglass components – guidance of the inflammatory response as basis for osteochondral regeneration. Bioact Mater. 2017;2(4):208–223.
- Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications – a comprehensive review. Adv Drug Deliv Rev. 2016;107:367–392.
- Wang Z, Wang Y, Ito Y, et al. A comparative study on the in vivo degradation of poly(L-lactide) based composite implants for bone fracture fixation. Sci Rep. 2016;6(1):20770.
- Sánchez-González S, Diban N, Urtiaga A. Hydrolytic degradation and mechanical stability of poly(ϵ-caprolactone)/reduced graphene oxide membranes as scaffolds for in vitro neural tissue regeneration. Membranes. 2018;8(1):12.
- Kingston C, Zepp R, Andrady A, et al. Release characteristics of selected carbon nanotube polymer composites. Carbon. 2014;68:33–57.
- Dong J, Liu J, Li X, et al. Relationship between the Young’s modulus and the crystallinity of cross-linked poly(ϵ-caprolactone) as an immobilization membrane for cancer radiotherapy. Glob Chall. 2020;4(8):2000008.
- Humbert S, Lame O, Seguela R, et al. A re-examination of the elastic modulus dependence on crystallinity in semi-crystalline polymers. Polymer. 2011;52(21):4899–4909.
- Mileva D, Zia Q, Androsch R. Tensile properties of random copolymers of propylene with ethylene and 1-butene: effect of crystallinity and crystal habit. Polym Bull. 2010;65(6):623–634.
- Chamundeeswari M, Jeslin J, Verma ML. Nanocarriers for drug delivery applications. Environ Chem Lett. 2019;17(2):849–865.
- Lin W. Introduction: nanoparticles in medicine. Chem Rev. 2015;115(19):10407–10409.
- Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41(7):2545–2561.
- Liu Y, Yang GZ, Baby T, et al. Stable polymer nanoparticles with exceptionally high drug loading by sequential nanoprecipitation. Angew Chem Int Ed. 2020;59(12):4720–4728.
- Hornig S, Heinze T, Becer CR, et al. Synthetic polymeric nanoparticles by nanoprecipitation. J Mater Chem. 2009;19(23):3838–3840.
- Gultekinoglu M, Jiang X, Bayram C, et al. Self-assembled micro-stripe patterning of sessile polymeric nanofluid droplets. J Colloid Interface Sci. 2020;561:470–480.
- Zhao C, Melis S, Hughes EP, et al. Particle formation mechanisms in the nanoprecipitation of polystyrene. Langmuir. 2020;36(44):13210–13217.
- Han FY, Liu Y, Kumar V, et al. Sustained-release ketamine-loaded nanoparticles fabricated by sequential nanoprecipitation. Int J Pharm. 2020;581:7.
- Baysal I, Ucar G, Gultekinoglu M, et al. Donepezil loaded PLGA-b-PEG nanoparticles: their ability to induce destabilization of amyloid fibrils and to cross blood brain barrier in vitro. J Neural Transm. 2017;124(1):33–45.
- Eroglu I, Gultekinoglu M, Bayram C, et al. Gel network comprising UV crosslinked PLGA-b-PEG-MA nanoparticles for ibuprofen topical delivery. Pharm Dev Technol. 2019;24(9):1144–1154.
- Kietzke T, Neher D, Kumke M, et al. Phase separation of binary blends in polymer nanoparticles. Small. 2007;3(6):1041–1048.
- Hanumantharao SN, Rao S. Multi-functional electrospun nanofibers from polymer blends for scaffold tissue engineering. Fibers. 2019;7(7):66.
- Prasad K, Bazaka O, Chua M, et al. Metallic biomaterials: current challenges and opportunities. Materials. 2017;10(8):884.
- Chen L, Pan D, He HK. Morphology development of polymer blend fibers along spinning line. Fibers. 2019;7(4):35.
- Luzi F, Torre L, Kenny JM, et al. Bio- and fossil-based polymeric blends and nanocomposites for packaging: structure–property relationship. Materials. 2019;12(3):471.
- Martinova L, Lubasova D. Reasons for using polymer blends in the electrospinning process. International Conference on Nanotechnology – Research and Commercialization (ICONT), Malaysia, Jun 6–9. American Institute of Physics, 2011. p. 115–128.
- Ramakrishna S, Fujihara K, Teo W-E, et al. Electrospun nanofibers: solving global issues. Mater Today. 2006;9(3):40–50.
- Hsu CM, Shivkumar S. N,N-dimethylformamide additions to the solution for the electrospinning of poly(ε-caprolactone) nanofibers. Macromol Mater Eng. 2004;289(4):334–340.
- Li WJ, Laurencin CT, Caterson EJ, et al. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–621.
- Viry L, Moulton SE, Romeo T, et al. Emulsion-coaxial electrospinning: designing novel architectures for sustained release of highly soluble low molecular weight drugs. J Mater Chem. 2012;22(22):11347–11353.
- Gomes ME, Reis RL. Biodegradable polymers and composites in biomedical applications: from catgut to tissue engineering. Part 1 available systems and their properties. Int Mater Rev. 2004;49(5):261–273.
- Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325–347.
- Zhang XW, Lu Y. Centrifugal spinning: an alternative approach to fabricate nanofibers at high speed and low cost. Polym Rev. 2014;54(4):677–701.
- Nagam Hanumantharao S, Rao S. Multi-functional electrospun nanofibers from polymer blends for scaffold tissue engineering. Fibers. 2019;7:66.
- de Cassan D, Sydow S, Schmidt N, et al. Attachment of nanoparticulate drug-release systems on poly(ϵ-caprolactone) nanofibers via a graftpolymer as interlayer. Colloids Surf B. 2018;163:309–320.
- Eriksen THB, Skovsen E, Fojan P. Release of antimicrobial peptides from electrospun nanofibres as a drug delivery system. J Biomed Nanotechnol. 2013;9(3):492–498.
- Li H, Wang M, Williams GR, et al. Electrospun gelatin nanofibers loaded with vitamins A and E as antibacterial wound dressing materials. RSC Adv. 2016;6(55):50267–50277.
- Amariei G, Kokol V, Boltes K, et al. Incorporation of antimicrobial peptides on electrospun nanofibres for biomedical applications. RSC Adv. 2018;8(49):28013–28023.
- Martins A, Reis RL, Neves NM. Electrospinning: processing technique for tissue engineering scaffolding. Int Mater Rev. 2008;53(5):257–274.
- Zafar M, Najeeb S, Khurshid Z, et al. Potential of electrospun nanofibers for biomedical and dental applications. Materials. 2016;9(2):73.
- Haider A, Haider S, Kang IK. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab J Chem. 2018;11(8):1165–1188.
- Komur B, Bayrak F, Ekren N, et al. Starch/PCL composite nanofibers by co-axial electrospinning technique for biomedical applications. Biomed Eng Online. 2017;16(1):1–13.
- Gizaw M, Thompson J, Faglie A, et al. Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications. Bioengineering. 2018;5(1).
- Khorshidi S, Solouk A, Mirzadeh H, et al. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J Tissue Eng Regen Med. 2016;10(9):715–738.
- Chen X, Feng B, Zhu DQ, et al. Characteristics and toxicity assessment of electrospun gelatin/PCL nanofibrous scaffold loaded with graphene in vitro and in vivo. Int J Nanomed. 2019;14:3669–3678.
- Hong J, Yeo M, Yang GH, et al. Cell-electrospinning and its application for tissue engineering. Int J Mol Sci. 2019;20(24):6208.
- Xia L, Lu LL, Liang YX, et al. Fabrication of centrifugally spun prepared poly(lactic acid)/gelatin/ciprofloxacin nanofibers for antimicrobial wound dressing. RSC Adv. 2019;9(61):35328–35335.
- Song JX, Guo J, Liu YF, et al. A comparative study on properties of cellulose/antarctic krill protein composite fiber by centrifugal spinning and wet spinning. Fiber Polym. 2019;20(8):1547–1554.
- Zhang ZM, Duan YS, Xu Q, et al. A review on nanofiber fabrication with the effect of high-speed centrifugal force field. J Eng Fibers Fabr. 2019;14:11.
- Amalorpava Mary L, Senthilram T, Suganya S, et al. Centrifugal spun ultrafine fibrous web as a potential drug delivery vehicle. Express Polym Lett. 2012.
- Li Y, Chen F, Nie J, et al. Electrospun poly(lactic acid)/chitosan core–shell structure nanofibers from homogeneous solution. Carbohydr Polym. 2012;90(4):1445–1451.
- Chen G, Shi T, Zhang X, et al. Polyacrylonitrile/polyethylene glycol phase-change material fibres prepared with hybrid polymer blends and nano-SiC fillers via centrifugal spinning. Polymer. 2020;186:122012.
- Mahalingam S, Huo S, Homer-Vanniasinkam S, et al. Generation of core–sheath polymer nanofibers by pressurised gyration. Polymers. 2020;12(8):1709.
- Heseltine PL, Ahmed J, Edirisinghe M. Developments in pressurized gyration for the mass production of polymeric fibers. Macromol Mater Eng. 2018;303(9):1800218.
- Liu Y, Zhou S, Gao Y, et al. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J Pharm Sci. 2019;14(2):130–143.
- Wang X, Ding B, Li B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater Today. 2013;16(6):229–241.
- Khan PA, Sasikanth K, Nama S, et al. Nanofibers - a new trend in nano drug delivery systems. Pharm Innov J. 2013;2:118–127.
- Brako F, Raimi-Abraham BT, Mahalingam S, et al. The development of progesterone-loaded nanofibers using pressurized gyration: a novel approach to vaginal delivery for the prevention of pre-term birth. Int J Pharm. 2018;540(1):31–39.
- Mahalingam S, Bayram C, Gultekinoglu M, et al. Co-axial gyro-spinning of PCL/PVA/HA core-sheath fibrous scaffolds for bone tissue engineering. Macromol Biosci. 2021;21(10):2100177.
- Khalil NM, do Nascimento TCF, Casa DM, et al. Pharmacokinetics of curcumin-loaded PLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloid Surf B-Biointerfaces. 2013;101:353–360.
- Parveen S, Sahoo SK. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur J Pharmacol. 2011;670(2–3):372–383.
- Mayol L, Serri C, Menale C, et al. Curcumin loaded PLGA–poloxamer blend nanoparticles induce cell cycle arrest in mesothelioma cells. Eur J Pharm Biopharm. 2015;93:37–45.
- Hadjianfar M, Semnani D, Varshosaz J. Polycaprolactone/chitosan blend nanofibers loaded by 5-fluorouracil: an approach to anticancer drug delivery system. Polym Adv Technol. 2018;29(12):2972–2981.
- Kharaghani D, Gitigard P, Ohtani H, et al. Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci Rep. 2019;9(1):1–11.
- Su Y, Su Q, Liu W, et al. Dual-drug encapsulation and release from core–shell nanofibers. J Biomater Sci Polym Ed. 2012;23(7):861–871.
- Cao Y, Wang B, Wang Y, et al. Polymer-controlled core–shell nanoparticles: a novel strategy for sequential drug release. RSC Adv. 2014;4(57):30430–30439.
- Silva DM, Liu R, Goncalves AF, et al. Design of polymeric core-shell carriers for combination therapies. J Colloid Interface Sci. 2021;587:499–509.
- Gomes ME, Reis RL. Biodegradable polymers and composites in biomedical applications: from catgut to tissue engineering. Part 2 systems for temporary replacement and advanced tissue regeneration. Int Mater Rev. 2004;49(5):274–285.
- Graziano A, Jaffer S, Sain M. Review on modification strategies of polyethylene/polypropylene immiscible thermoplastic polymer blends for enhancing their mechanical behavior. J Elastomers Plast. 2019;51(4):291–336.
- Serafin A, Murphy C, Rubio MC, et al. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021;122:111927.
- Dumont M, Villet R, Guirand M, et al. Processing and antibacterial properties of chitosan-coated alginate fibers. Carbohydr Polym. 2018;190:31–42.
- Kundu J, Shim JH, Jang J, et al. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med. 2015;9(11):1286–1297.
- Cai Z, Kim J. Bacterial cellulose/poly(ethylene glycol) composite: characterization and first evaluation of biocompatibility. Cellulose. 2010;17(1):83–91.
- Noh YK, Da Costa AD, Park YS, et al. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesenchymal stem cells. Carbohydr Polym. 2019;219:210–218.
- Fahimirad S, Abtahi H, Satei P, et al. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr Polym. 2021;259:117640.
- Cooper A, Bhattarai N, Zhang M. Fabrication and cellular compatibility of aligned chitosan–PCL fibers for nerve tissue regeneration. Carbohydr Polym. 2011;85(1):149–156.
- Jafari H, Hassanpour M, Akbari A, et al. Characterization of pH-sensitive chitosan/hydroxypropyl methylcellulose composite nanoparticles for delivery of melatonin in cancer therapy. Mater Lett. 2021;282:128818.
- Stratesteffen H, Kopf M, Kreimendahl F, et al. GelMA-collagen blends enable drop-on-demand 3D printablility and promote angiogenesis. Biofabrication. 2017;9(4):045002.
- Moxon SR, Corbett NJ, Fisher K, et al. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater Sci Eng C Mater Biol Appl. 2019;104:109904.
- Wang YH, Ma M, Wang JN, et al. Development of a photo-crosslinking, biodegradable GelMA/PEGDA hydrogel for guided bone regeneration materials. Materials. 2018;11(8):12.
- Vatankhah E, Prabhakaran MP, Jin G, et al. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J Biomater Appl. 2014;28(6):909–921.
- Zhou J, Cao C, Ma X, et al. Electrospinning of silk fibroin and collagen for vascular tissue engineering. Int J Biol Macromol. 2010;47(4):514–519.
- Zhou Y, Yang H, Liu X, et al. Electrospinning of carboxyethyl chitosan/poly(vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int J Biol Macromol. 2013;53:88–92.
- Kundu J, Poole-Warren LA, Martens P, et al. Silk fibroin/poly(vinyl alcohol) photocrosslinked hydrogels for delivery of macromolecular drugs. Acta Biomater. 2012;8(5):1720–1729.
- Sanhueza C, Hermosilla J, Bugallo-Casal A, et al. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater Sci Eng C Mater Biol Appl. 2021;119:111602.
- Azuraini MJ, Huong KH, Khalil H, et al. Fabrication and characterization of P(3HB-co-4HB)/gelatine biomimetic nanofibrous scaffold for tissue engineering application. J Polym Res. 2019;26(11):12.
- Mendibil X, González-Pérez F, Bazan X, et al. Bioresorbable and mechanically optimized nerve guidance conduit based on a naturally derived medium chain length polyhydroxyalkanoate and poly(ϵ-caprolactone) blend. ACS Biomater Sci Eng. 2021;7(2):672–689.
- Salehi S, Bahners T, Gutmann JS, et al. Characterization of structural, mechanical and nano-mechanical properties of electrospun PGS/PCL fibers. RSC Adv. 2014;4(33):16951–16957.
- She YL, Fan ZW, Wang L, et al. 3D Printed biomimetic PCL scaffold as framework interspersed with collagen for long segment tracheal replacement. Front Cell Dev Biol. 2021;9:14.
- Ekaputra AK, Prestwich GD, Cool SM, et al. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (ε-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials. 2011;32(32):8108–8117.
- Evrova O, Hosseini V, Milleret V, et al. Hybrid randomly electrospun poly(lactic-co-glycolic acid):poly(ethylene oxide) (PLGA:PEO) fibrous scaffolds enhancing myoblast differentiation and alignment. ACS Appl Mater Interfaces. 2016;8(46):31574–31586.
- Xu Y, Zou L, Lu H, et al. Preparation and characterization of electrospun PHBV/PEO mats: The role of solvent and PEO component. J Mater Sci. 2016;51.
- Banerjee SR, Foss CA, Horhota A, et al. 111In- and IRDye800CW-labeled PLA–PEG nanoparticle for imaging prostate-specific membrane antigen-expressing tissues. Biomacromolecules. 2017;18(1):201–209.
- Musumeci T, Ventura CA, Giannone I, et al. PLA/PLGA nanoparticles for sustained release of docetaxel. Int J Pharm. 2006;325(1):172–179.
- Shin YC, Shin DM, Lee EJ, et al. Hyaluronic acid/PLGA core/shell fiber matrices loaded with EGCG beneficial to diabetic wound healing. Adv Healthc Mater. 2016;5(23):3035–3045.
- Rebia RA, Rozet S, Tamada Y, et al. Biodegradable PHBH/PVA blend nanofibers: fabrication, characterization, in vitro degradation, and in vitro biocompatibility. Polym Degrad Stab. 2018;154:124–136.
- Altaf F, Niazi MBK, Jahan Z, et al. Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J Polym Environ. 2021;29(1):156–174.
- Kanca Y, Milner P, Dini D, et al. Tribological properties of PVA/PVP blend hydrogels against articular cartilage. J Mech Behav Biomed Mater. 2018;78:36–45.
- Mishra R, Varshney R, Das N, et al. Synthesis and characterization of gelatin-PVP polymer composite scaffold for potential application in bone tissue engineering. Eur Polym J. 2019;119:155–168.
- Li K, Wang DZ, Zhao KP, et al. Electrohydrodynamic jet 3D printing of PCL/PVP composite scaffold for cell culture. Talanta. 2020;211:11.
- Kim HS, Lee CG, Lee EY. Alginate lyase: structure, property, and application. Biotechnol Bioprocess Eng. 2011;16(5):843–851.
- Haug A, Larsen B. Biosynthesis of alginate. Carbohydr Res. 1971;17(2):297–308.
- Smidsrød O, Glover RM, Whittington SG. The relative extension of alginates having different chemical composition. Carbohydr Res. 1973;27(1):107–118.
- Evans LR, Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973;116(2):915–924.
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126.
- Jain D, Bar-Shalom D. Alginate drug delivery systems: application in context of pharmaceutical and biomedical research. Drug Dev Ind Pharm. 2014;40(12):1576–1584.
- Bouhadir KH, Lee KY, Alsberg E, et al. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17(5):945–950.
- Raus RA, Nawawi W, Nasaruddin RR. Alginate and alginate composites for biomedical applications. Asian J Pharm Sci. 2021;16(3):280–306.
- Kong HJ, Kaigler D, Kim K, et al. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules. 2004;5(5):1720–1727.
- Schipani R, Scheurer S, Florentin R, et al. Reinforcing interpenetrating network hydrogels with 3D printed polymer networks to engineer cartilage mimetic composites. Biofabrication. 2020;12(3):035011.
- Gong XY, Dang GY, Guo J, et al. A sodium alginate/feather keratin composite fiber with skin-core structure as the carrier for sustained drug release. Int J Biol Macromol. 2020;155:386–392.
- Ji MZ, Chen H, Yan YG, et al. Effects of tricalcium silicate/sodium alginate/calcium sulfate hemihydrate composite cements on osteogenic performances in vitro and in vivo. J Biomater Appl. 2020;34(10):1422–1436.
- Choe G, Oh S, Seok JM, et al. Graphene oxide/alginate composites as novel bioinks for three-dimensional mesenchymal stem cell printing and bone regeneration applications. Nanoscale. 2019;11(48):23275–23285.
- Del Bakhshayesh AR, Annabi N, Khalilov R, et al. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif Cell Nanomed Biotechnol. 2018;46(4):691–705.
- Nawaz S, Khan S, Farooq U, et al. Biocompatible hydrogels for the controlled delivery of anti-hypertensive agent: development, characterization and in vitro evaluation. Des Monomers Polym. 2018;21(1):18–32.
- Labowska MB, Cierluk K, Jankowska AM, et al. A review on the adaption of alginate-gelatin hydrogels for 3D cultures and bioprinting. Materials. 2021;14(4):858.
- Su K, Wang C. Recent advances in the use of gelatin in biomedical research. Biotechnol Lett. 2015;37(11):2139–2145.
- Thakur G, Rouseau D, Rafanan R. Gelatin-based matrices for drug delivery applications. In: Boran G, editor. Gelatin: production, applications and health implications. 2013. p. 49–70.
- Elzoghby AO. Gelatin-based nanoparticles as drug and gene delivery systems: reviewing three decades of research. J Control Release. 2013;172(3):1075–1091.
- Ndlovu SP, Ngece K, Alven S, et al. Gelatin-based hybrid scaffolds: promising wound dressings. Polymers. 2021;13(17):2959.
- Bello AB, Kim D, Kim D, et al. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Eng Part B Rev. 2020;26(2):164–180.
- Echave MC, Hernáez-Moya R, Iturriaga L, et al. Recent advances in gelatin-based therapeutics. Expert Opin Biol Ther. 2019;19(8):773–779.
- Li JH, Wu CT, Chu PK, et al. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater Sci Eng R Rep. 2020;140:76.
- Sun M, Sun X, Wang Z, et al. Synthesis and properties of gelatin methacryloyl (GelMA) hydrogels and their recent applications in load-bearing tissue. Polymers. 2018;10(11):1290.
- Boccafoschi F, Ramella M, Fusaro L, et al. Biological grafts: surgical use and vascular tissue engineering options for peripheral vascular implants. Amsterdam: Elsevier Science Bv; 2019. p. 310–321.
- Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26(15):2455–2465.
- Balakrishnan B, Jayakrishnan A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 2005;26(18):3941–3951.
- Grigore A, Sarker B, Fabry B, et al. Behavior of encapsulated MG-63 cells in RGD and gelatine-modified alginate hydrogels. Tissue Eng Part A. 2014;20(15–16):2140–2150.
- Dranseikiene D, Schrufer S, Schubert DW, et al. Cell-laden alginate dialdehyde–gelatin hydrogels formed in 3D printed sacrificial gel. J Mater Sci Mater Med. 2020;31(3):5.
- Nguyen TP, Lee BT. Fabrication of oxidized alginate-gelatin-BCP hydrogels and evaluation of the microstructure, material properties and biocompatibility for bone tissue regeneration. J Biomater Appl. 2012;27(3):311–321.
- Yuan L, Wu Y, Fang J, et al. Modified alginate and gelatin cross-linked hydrogels for soft tissue adhesive. Artif Cell Nanomed Biotechnol. 2017;45(1):76–83.
- Sarker B, Papageorgiou DG, Silva R, et al. Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J Mat Chem B. 2014;2(11):1470–1482.
- Ruther F, Distler T, Boccaccini AR, et al. Biofabrication of vessel-like structures with alginate di-aldehyde – gelatin (ADA-GEL) bioink. J Mater Sci Mater Med. 2019;30(1):8.
- Aranaz I, Alcantara AR, Civera MC, et al. Chitosan: an overview of its properties and applications. Polymers. 2021;13(19):3256.
- Li Q, Dunn ET, Grandmaison EW, et al. Applications and properties of chitosan. J Bioact Compat Polym. 1992;7(4):370–397.
- Islam S, Bhuiyan MAR, Islam MN. Chitin and chitosan: structure, properties and applications in biomedical engineering. J Polym Environ. 2017;25(3):854–866.
- Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49(4):780–792.
- Sudarshan NR, Hoover DG, Knorr D. Antibacterial action of chitosan. Food Biotechnol. 1992;6(3):257–272.
- Ong SY, Wu J, Moochhala SM, et al. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29(32):4323–4332.
- Dai TH, Tanaka M, Huang YY, et al. Corrigendum. Expert Rev Anti-Infect Ther. 2013;11(8):866–866.
- Aranaz I, Mengíbar M, Harris R, et al. Functional characterization of Chitin and Chitosan. Curr Chem Biol. 2009;3:203–230.
- Lehr CM, Bouwstra JA, Schacht EH, et al. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78(1):43–48.
- Yang J, Tian F, Wang Z, et al. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J Biomed Mater Res B Appl Biomater. 2008;84B(1):131–137.
- Bhuiyan MAR, Hossain MA, Zakaria M, et al. Chitosan coated cotton fiber: physical and antimicrobial properties for apparel use. J Polym Environ. 2017;25(2):334–342.
- Chou CK, Chen SM, Li YC, et al. Low-molecular-weight chitosan scavenges methylglyoxal and N ϵ-(carboxyethyl)lysine, the major factors contributing to the pathogenesis of nephropathy. Springerplus. 2015;4:312.
- Lee BR, Lee KH, Kang E, et al. Microfluidic wet spinning of chitosan-alginate microfibers and encapsulation of HepG2 cells in fibers. Biomicrofluidics. 2011;5(2):9.
- Yang J, Goto M, Ise H, et al. Galactosylated alginate as a scaffold for hepatocytes entrapment. Biomaterials. 2002;23(2):471–479.
- Lee M, Li WM, Siu RK, et al. Biomimetic apatite-coated alginate/chitosan microparticles as osteogenic protein carriers. Biomaterials. 2009;30(30):6094–6101.
- Weng L, Romanov A, Rooney J, et al. Non-cytotoxic, in situ gelable hydrogels composed of N-carboxyethyl chitosan and oxidized dextran. Biomaterials. 2008;29(29):3905–3913.
- Francesko A, Tzanov T. Chitin, chitosan and derivatives for wound healing and tissue engineering. In: Nyanhongo GS, Steiner W, Gübitz G, editors. Biofunctionalization of polymers and their applications. Berlin: Springer-Verlag Berlin; 2011. p. 1–27.
- Perez RA, Kim M, Kim TH, et al. Utilizing core–shell fibrous collagen-alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng Part A. 2014;20(1–2):103–114.
- Woodruff MA, Hutmacher DW. The return of a forgotten polymer – polycaprolactone in the 21st century. Prog Polym Sci. 2010;35(10):1217–1256.
- Hassanajili S, Karami-Pour A, Oryan A, et al. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;104:109960.
- Felice B, Sanchez MA, Socci MC, et al. Controlled degradability of PCL-ZnO nanofibrous scaffolds for bone tissue engineering and their antibacterial activity. Mater Sci Eng C Mater Biol Appl. 2018;93:724–738.
- Ristovski N, Bock N, Liao S, et al. Improved fabrication of melt electrospun tissue engineering scaffolds using direct writing and advanced electric field control. Biointerphases. 2015;10(1):011006.
- Roder A, Garcia-Gareta E, Theodoropoulos C, et al. An assessment of cell culture plate surface chemistry for in vitro studies of tissue engineering scaffolds. J Funct Biomater. 2015;6(4):1054–1063.
- Paxton NC, Ren JY, Ainsworth MJ, et al. Rheological characterization of biomaterials directs additive manufacturing of strontium-substituted bioactive glass/polycaprolactone microfibers. Macromol Rapid Commun. 2019;40(11):1900019.
- Ren J, Kohli N, Sharma V, et al. Poly-ϵ-caprolactone/fibrin-alginate scaffold: a new pro-angiogenic composite biomaterial for the treatment of bone defects. Polymers. 2021;13:3399.
- Recek N, Resnik M, Motaln H, et al. Cell adhesion on polycaprolactone modified by plasma treatment. Int J Polym Sci. 2016;2016:1–9.
- French AD. Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose. 2017;24(11):4605–4609.
- Seddiqi H, Oliaei E, Honarkar H, et al. Cellulose and its derivatives: towards biomedical applications. Cellulose. 2021;28(4):1893–1931.
- Barikani M, Oliaei E, Seddiqi H, et al. Preparation and application of chitin and its derivatives: a review. Iran Polym J. 2014;23(4):307–326.
- Moon RJ, Martini A, Nairn J, et al. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40(7):3941–3994.
- Sultan S, Mathew AP. 3D printed scaffolds with gradient porosity based on a cellulose nanocrystal hydrogel. Nanoscale. 2018;10(9):4421–4431.
- Fidale LC, Heinze T, El Seoud OA. Perichromism: a powerful tool for probing the properties of cellulose and its derivatives. Carbohydr Polym. 2013;93(1):129–134.
- Ansari F, Sjostedt A, Larsson PT, et al. Hierarchical wood cellulose fiber/epoxy biocomposites – materials design of fiber porosity and nanostructure. Compos Part A Appl Sci Manuf. 2015;74:60–68.
- Dumitriu C, Voicu SI, Muhulet A, et al. Production and characterization of cellulose acetate – titanium dioxide nanotubes membrane fraxiparinized through polydopamine for clinical applications. Carbohydr Polym. 2018;181:215–223.
- Tilki T, Yavuz M, Karabacak Ç, et al. Investigation of electrorheological properties of biodegradable modified cellulose/corn oil suspensions. Carbohydr Res. 2010;345(5):672–679.
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351.
- Mandal A, Clegg J, Anselmo A, et al. Hydrogels in the clinic. Hydrogels in the clinic. Bioeng Transl Med. 2020;5.
- Teodorescu M, Bercea M, Morariu S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: perspectives and challenges. Biotechnol Adv. 2019;37(1):109–131.
- Sánchez-Téllez DA, Téllez-Jurado L, Rodríguez-Lorenzo LM. Hydrogels for cartilage regeneration, from polysaccharides to hybrids. Polymers. 2017;9(12):671.
- Stocco E, Barbon S, Dalzoppo D, et al. Tailored PVA/ECM scaffolds for cartilage regeneration. Biomed Res Int. 2014;2014:12.
- Stocco E, Barbon S, Radossi P, et al. Autologous chondrocytes as a novel source for neo-chondrogenesis in haemophiliacs. Cell Tissue Res. 2016;366(1):51–61.
- Alhosseini SN, Moztarzadeh F, Kargozar S, et al. Development of polyvinyl alcohol fibrous biodegradable scaffolds for nerve tissue engineering applications: in vitro study. Int J Polym Mater Polym Biomat. 2015;64(9):474–480.
- Kim HD, Lee Y, Kim Y, et al. A review of multiscale computational methods in polymeric materials. Polymers. 2017;9(12):16.
- Teodorescu M, Bercea M, Morariu S. Biomaterials of poly(vinyl alcohol) and natural polymers. Polym Rev. 2018;58(2):247–287.
- Khalid A, Ullah H, Ul-Islam M, et al. Bacterial cellulose–TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv. 2017;7(75):47662–47668.
- Li Y, Jiang H, Zheng WF, et al. Bacterial cellulose–hyaluronan nanocomposite biomaterials as wound dressings for severe skin injury repair. J Mat Chem B. 2015;3(17):3498–3507.
- Hu Y, Catchmark JM, Zhu YJ, et al. Engineering of porous bacterial cellulose toward human fibroblasts ingrowth for tissue engineering. J Mater Res. 2014;29(22):2682–2693.
- Klemm D, Schumann D, Udhardt U, et al. Bacterial synthesized cellulose – artificial blood vessels for microsurgery. Prog Polym Sci. 2001;26(9):1561–1603.
- Czaja W, Krystynowicz A, Bielecki S, et al. Microbial cellulose – the natural power to heal wounds. Biomaterials. 2006;27(2):145–151.
- Portela R, Leal CR, Almeida PL, et al. Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb Biotechnol. 2019;12(4):586–610.
- Rodriguez MIA, Barroso LGR, Sanchez ML. Collagen: a review on its sources and potential cosmetic applications. J Cosmet Dermatol. 2018;17(1):20–26.
- Li YB, Liu YZ, Li RH, et al. Collagen-based biomaterials for bone tissue engineering. Mater Des. 2021;210:23.
- Nakayama A, Kakugo A, Gong JP, et al. High mechanical strength double-network hydrogel with bacterial cellulose. Adv Funct Mater. 2004;14(11):1124–1128.
- Zaborowska M, Bodin A, Backdahl H, et al. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010;6(7):2540–2547.
- Yamanaka S, Watanabe K, Kitamura N, et al. The structure and mechanical properties of sheets prepared from bacterial cellulose. J Mater Sci. 1989;24(9):3141–3145.
- Helenius G, Backdahl H, Bodin A, et al. In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res Part A. 2006;76A(2):431–438.
- Torres FG, Commeaux S, Troncoso OP. Biocompatibility of bacterial cellulose based biomaterials. J Funct Biomater. 2012;3(4):864–878.
- Noh YK, Dos Santos Da Costa A, Park YS, et al. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesenchymal stem cells. Carbohydr Polym. 2019;219:210–218.
- Bagheri-Khoulenjani S, Taghizadeh SM, Mirzadeh H. An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohydr Polym. 2009;78(4):773–778.
- Vårum KM, Myhr MM, Hjerde RJ, et al. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr Res. 1997;299(1–2):99–101.
- VandeVord PJ, Matthew HW, DeSilva SP, et al. Evaluation of the biocompatibility of a chitosan scaffold in mice. J Biomed Mater Res. 2002;59(3):585–590.
- Sashiwa H, Aiba SI. Chemically modified chitin and chitosan as biomaterials. Prog Polym Sci. 2004;29(9):887–908.
- Bhattarai N, Li Z, Gunn J, et al. Natural-synthetic polyblend nanofibers for biomedical applications. Adv Mater. 2009;21(27):2792–2797.
- Jana S, Leung M, Chang JL, et al. Effect of nano- and micro-scale topological features on alignment of muscle cells and commitment of myogenic differentiation. Biofabrication. 2014;6(3):035012.
- Shalumon KT, Anulekha KH, Chennazhi KP, et al. Fabrication of chitosan/poly(caprolactone) nanofibrous scaffold for bone and skin tissue engineering. Int J Biol Macromol. 2011;48(4):571–576.
- Zhou M, Qiao W, Liu Z, et al. Development and in vivo evaluation of small-diameter vascular grafts engineered by outgrowth endothelial cells and electrospun chitosan/poly(ε-caprolactone) nanofibrous scaffolds. Tissue Eng Part A. 2013;20.
- Chen SH, Chen CH, Fong YT, et al. Prevention of peritendinous adhesions with electrospun chitosan-grafted polycaprolactone nanofibrous membranes. Acta Biomater. 2014;10(12):4971–4982.
- Liu H, Peng H, Wu Y, et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials. 2013;34(18):4404–4417.
- Wedmore I, McManus JG, Pusateri AE, et al. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60(3):655–658.
- Şenel S, McClure SJ. Potential applications of chitosan in veterinary medicine. Adv Drug Deliv Rev. 2004;56(10):1467–1480.
- Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52(2):105–115.
- Levengood SL, Zhang M. Chitosan-based scaffolds for bone tissue engineering. J Mater Chem B. 2014;2(21):3161–3184.
- Croisier F, Atanasova G, Poumay Y, et al. Polysaccharide-coated PCL nanofibers for wound dressing applications. Adv Healthc Mater. 2014;3(12):2032–2039.
- Bhattarai N, Li ZS, Gunn J, et al. Natural-synthetic polyblend nanofibers for biomedical applications. Adv Mater. 2009;21(27):2792–2797.
- Levengood SL, Erickson AE, Chang FC, et al. Chitosan–poly(caprolactone) nanofibers for skin repair. J Mat Chem B. 2017;5(9):1822–1833.
- Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, et al. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29(34):4532–4539.
- Jiang X, Lim SH, Mao HQ, et al. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223(1):86–101.
- Valmikinathan CM, Defroda S, Yu X. Polycaprolactone and bovine serum albumin based nanofibers for controlled release of nerve growth factor. Biomacromolecules. 2009;10(5):1084–1089.
- Eivazzadeh-Keihan R, Pashazadeh-Panahi P, Baradaran B, et al. Recent progress in optical and electrochemical biosensors for sensing of clostridium botulinum neurotoxin. TrAC Trends Anal Chem. 2018;103:184–197.
- Mohammadinejad A, Oskuee RK, Eivazzadeh-Keihan R, et al. Development of biosensors for detection of alphafetoprotein: as a major biomarker for hepatocellular carcinoma. TrAC Trends Anal Chem. 2020;130(23).
- Eivazzadeh-Keihan R, Maleki A, de la Guardia M, et al. Carbon based nanomaterials for tissue engineering of bone: building new bone on small black scaffolds: a review. J Adv Res. 2019;18:185–201.
- Cheung RCF, Ng TB, Wong JH, et al. Chitosan: an update on potential biomedical and pharmaceutical applications. Mar Drugs. 2015;13(8):5156–5186.
- Zhou ZK, Lin SQ, Yue TL, et al. Adsorption of food dyes from aqueous solution by glutaraldehyde cross-linked magnetic chitosan nanoparticles. J Food Eng. 2014;126:133–141.
- Arteche Pujana M, Pérez-Álvarez L, Cesteros Iturbe LC, et al. Biodegradable chitosan nanogels crosslinked with genipin. Carbohydr Polym. 2013;94(2):836–842.
- Wang YM, Wang J, Yuan ZY, et al. Chitosan cross-linked poly(acrylic acid) hydrogels: drug release control and mechanism. Colloid Surf B Biointerfaces. 2017;152:252–259.
- Zeng M, Yuan X, Yang Z, et al. Novel macroporous palladium cation crosslinked chitosan membranes for heterogeneous catalysis application. Int J Biol Macromol. 2014;68:189–197.
- Depan D, Singh R. Surface modification of chitosan and its implications in tissue engineering and drug delivery. In: Surface modification of biopolymers. New York (NY): Wiley; 2015. p. 20–44.
- Gu Y, Zhu J, Xue C, et al. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials. 2014;35(7):2253–2263.
- Ju HW, Lee OJ, Lee JM, et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int J Biol Macromol. 2016;85:29–39.
- Sakabe H, Ito H, Miyamoto T, et al. In vivo blood compatibility of regenerated silk fibroin. Sen’i Gakkaishi. 1989;45(11):487–490.
- Santin M, Motta A, Freddi G, et al. In vitro evaluation of the inflammatory potential of the silk fibroin. J Biomed Mater Res. 1999;46(3):382–389.
- Guang SY, An Y, Ke FY, et al. Chitosan/silk fibroin composite scaffolds for wound dressing. J Appl Polym Sci. 2015;132(35):7.
- Keirouz A, Zakharova M, Kwon J, et al. High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2020;112:110939.
- Ribeiro VP, Pina S, Oliveira JM, et al. Silk fibroin-based hydrogels and scaffolds for osteochondral repair and regeneration. In: Oliveira JM, Pina S, Reis RL, et al., editors. Osteochondral tissue engineering: nanotechnology, scaffolding-related developments and translation. Cham: Springer International Publishing; 2018. p. 305–325.
- Nguyen TP, Nguyen QV, Nguyen VH, et al. Silk fibroin-based biomaterials for biomedical applications: a review. Polymers. 2019;11(12):1933.
- Balan KK, Sundaramoorthy S. Hydroentangled nonwoven eri silk fibroin scaffold for tissue engineering applications. J Ind Text. 2019;48(8):1291–1309.
- Selvaraj S, Fathima NN. Fenugreek incorporated silk fibroin nanofibers – a potential antioxidant scaffold for enhanced wound healing. ACS Appl Mater Interfaces. 2017;9(7):5916–5926.
- Cengiz IF, Pereira H, Espregueira-Mendes J, et al. Suturable regenerated silk fibroin scaffold reinforced with 3D-printed polycaprolactone mesh: biomechanical performance and subcutaneous implantation. J Mater Sci Mater Med. 2019;30(6):17.
- Cui BL, Zhang CC, Gan B, et al. Collagen-tussah silk fibroin hybrid scaffolds loaded with bone mesenchymal stem cells promote skin wound repair in rats. Mater Sci Eng C Mater Biol Appl. 2020;109:16.
- Jayakumar R, Prabaharan M, Sudheesh Kumar PT, et al. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322–337.
- Cai ZX, Mo XM, Zhang KH, et al. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int J Mol Sci. 2010;11(9):3529–3539.
- Ahmadi Z, Saber M, Akbari A, et al. Encapsulation of Satureja hortensis L. (Lamiaceae) in chitosan/TPP nanoparticles with enhanced acaricide activity against Tetranychus urticae Koch (Acari: Tetranychidae). Ecotoxicol Environ Saf. 2018;161:111–119.
- Işıklan N, Erol ÜH. Design and evaluation of temperature-responsive chitosan/hydroxypropyl cellulose blend nanospheres for sustainable flurbiprofen release. Int J Biol Macromol. 2020;159:751–762.
- Raghav N, Sharma M, Kennedy J. Nanocellulose: a mini-review on types and use in drug delivery systems. Carbohydr Polym Technol Appl. 2021;2:100031.
- Seddiqi H, Oliaei E, Honarkar H, et al. Cellulose and its derivatives: towards biomedical applications. Cellulose. 2021;28(4):1893–1931.
- Friess W. Collagen – biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45(2):113–136.
- Ricard-Blum S, Ville G. Collagen cross-linking. Int J Biochem. 1989;21(11):1185–1189.
- Tan G, Zhou L, Ning C, et al. Biomimetically-mineralized composite coatings on titanium functionalized with gelatin methacrylate hydrogels. Appl Surf Sci. 2013;279:293–299.
- Drzewiecki KE, Parmar AS, Gaudet ID, et al. Methacrylation induces rapid, temperature-dependent, reversible self-assembly of type-I collagen. Langmuir. 2014;30(37):11204–11211.
- Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221(1):1–22.
- Antoine EE, Vlachos PP, Rylander MN. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng Part B Rev. 2014;20(6):683–696.
- Hoch E, Schuh C, Hirth T, et al. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J Mater Sci Mater Med. 2012;23(11):2607–2617.
- Van Den Bulcke AI, Bogdanov B, De Rooze N, et al. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1(1):31–38.
- Chen YC, Lin RZ, Qi H, et al. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater. 2012;22(10):2027–2039.
- Hassanzadeh P, Kazemzadeh-Narbat M, Rosenzweig R, et al. Ultrastrong and flexible hybrid hydrogels based on solution self-assembly of chitin nanofibers in gelatin methacryloyl (GelMA). J Mat Chem B. 2016;4(15):2539–2543.
- Yue K, Trujillo-de Santiago G, Alvarez MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271.
- Schuurman W, Levett PA, Pot MW, et al. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci. 2013;13(5):551–561.
- Jeon O, Wolfson DW, Alsberg E. In-situ formation of growth-factor-loaded coacervate microparticle-embedded hydrogels for directing encapsulated stem cell fate. Adv Mater. 2015;27(13):2216–2223.
- Young S, Wong M, Tabata Y, et al. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109(1–3):256–274.
- Ludwig PE, Huff TJ, Zuniga JM. The potential role of bioengineering and three-dimensional printing in curing global corneal blindness. J Tissue Eng. 2018;9:10.
- Kuo YC, Wang CC. Guided differentiation of induced pluripotent stem cells into neuronal lineage in alginate–chitosan–gelatin hydrogels with surface neuron growth factor. Colloid Surf B Biointerfaces. 2013;104:194–199.
- Bozza A, Coates EE, Incitti T, et al. Neural differentiation of pluripotent cells in 3D alginate-based cultures. Biomaterials. 2014;35(16):4636–4645.
- Frampton JP, Hynd MR, Shuler ML, et al. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed Mater. 2011;6(1):015002.
- Prang P, Müller R, Eljaouhari A, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27(19):3560–3569.
- Matyash M, Despang F, Mandal R, et al. Novel soft alginate hydrogel strongly supports neurite growth and protects neurons against oxidative stress. Tissue Eng Part A. 2012;18(1–2):55–66.
- Heino J. Cellular signaling by collagen-binding integrins. Adv Exp Med Biol. 2014;819:143–155.
- Jokinen J, Dadu E, Nykvist P, et al. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem. 2004;279(30):31956–31963.
- Holder AJ, Badiei N, Hawkins K, et al. Control of collagen gel mechanical properties through manipulation of gelation conditions near the sol–gel transition. Soft Matter. 2018;14(4):574–580.
- Velegol D, Lanni F. Cell traction forces on soft biomaterials. I. Microrheology of type I collagen gels. Biophys J. 2001;81(3):1786–1792.
- Baniasadi M, Minary-Jolandan M. Alginate-collagen fibril composite hydrogel. Materials. 2015;8(2):799–814.
- Yu T, Wang WB, Nassiri S, et al. Temporal and spatial distribution of macrophage phenotype markers in the foreign body response to glutaraldehyde-crosslinked gelatin hydrogels. J Biomater Sci Polym Ed. 2016;27(8):721–742.
- Vijayakumar V, Subramanian K. Diisocyanate mediated polyether modified gelatin drug carrier for controlled release. Saudi Pharm J. 2014;22(1):43–51.
- Subramanian K, Vijayakumar V. Evaluation of isophorone diisocyanate crosslinked gelatin as a carrier for controlled delivery of drugs. Polym Bull. 2013;70(3):733–753.
- Powell HM, Boyce ST. Fiber density of electrospun gelatin scaffolds regulates morphogenesis of dermal–epidermal skin substitutes. J Biomed Mater Res Part A. 2008;84A(4):1078–1086.
- Czaja WK, Young DJ, Kawecki M, et al. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules. 2007;8(1):1–12.
- Qi HX, Hu P, Xu J, et al. Encapsulation of drug reservoirs in fibers by emulsion electrospinning: morphology characterization and preliminary release assessment. Biomacromolecules. 2006;7(8):2327–2330.
- Lee KY, Jeong L, Kang YO, et al. Electrospinning of polysaccharides for regenerative medicine. Adv Drug Deliv Rev. 2009;61(12):1020–1032.
- Tsioptsias C, Sakellariou KG, Tsivintzelis I, et al. Preparation and characterization of cellulose acetate–Fe2O3 composite nanofibrous materials. Carbohydr Polym. 2010;81(4):925–930.
- Murphy CM, O’Brien FJ. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh Migr. 2010;4(3):377–381.
- Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, et al. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29(34):4532–4539.
- Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32(8–9):991–1007.
- Foo CWP, Kaplan DL. Genetic engineering of fibrous proteins: spider dragline silk and collagen. Adv Drug Deliv Rev. 2002;54(8):1131–1143.
- Altman GH, Diaz F, Jakuba C, et al. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416.
- Arai T, Freddi G, Innocenti R, et al. Biodegradation of Bombyx mori silk fibroin fibers and films. J Appl Polym Sci. 2004;91(4):2383–2390.
- Fuchs S, Motta A, Migliaresi C, et al. Outgrowth endothelial cells isolated and expanded from human peripheral blood progenitor cells as a potential source of autologous cells for endothelialization of silk fibroin biomaterials. Biomaterials. 2006;27(31):5399–5408.
- Horan RL, Antle K, Collette AL, et al. In vitro degradation of silk fibroin. Biomaterials. 2005;26(17):3385–3393.
- Hu K, Lv Q, Cui FZ, et al. Biocompatible fibroin blended films with recombinant human-like collagen for hepatic tissue engineering. J Bioact Compat Polym. 2006;21(1):23–37.
- Kim UJ, Park J, Kim HJ, et al. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26(15):2775–2785.
- Li CM, Vepari C, Jin HJ, et al. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27(16):3115–3124.
- Minoura N, Aiba S, Gotoh Y, et al. Attachment and growth of cultured fibroblast cells on silk protein matrices. J Biomed Mater Res. 1995;29(10):1215–1221.
- Motta A, Migliaresi C, Faccioni F, et al. Fibroin hydrogels for biomedical applications: preparation, characterization and in vitro cell culture studies. J Biomater Sci Polym Ed. 2004;15(7):851–864.
- Vepari CP, Kaplan DL. Covalently immobilized enzyme gradients within three-dimensional porous scaffolds. Biotechnol Bioeng. 2006;93(6):1130–1137.
- Arai T, Freddi G, Colonna GM, et al. Absorption of metal cations by modifiedB. Mori silk and preparation of fabrics with antimicrobial activity. J Appl Polym Sci. 2001;80(2):297–303.
- Moy RL, Lee A, Zalka A. Commonly used suture materials in skin surgery. Am Fam Physician. 1991;44(6):2123–2128.
- Grabska-Zielińska S, Sionkowska A. How to improve physico-chemical properties of silk fibroin materials for biomedical applications? Blending and cross-linking of silk fibroin – a review. Materials. 2021;14(6):1510.
- Omenetto FG, Kaplan DL. New opportunities for an ancient material. Science. 2010;329(5991):528–531.
- Bryant SJ, Davis-Arehart KA, Luo N, et al. Synthesis and characterization of photopolymerized multifunctional hydrogels: water-soluble poly(vinyl alcohol) and chondroitin sulfate macromers for chondrocyte encapsulation. Macromolecules. 2004;37(18):6726–6733.
- Martens P, Holland T, Anseth KS. Synthesis and characterization of degradable hydrogels formed from acrylate modified poly(vinyl alcohol) macromers. Polymer. 2002;43(23):6093–6100.
- Martens P, Blundo J, Nilasaroya A, et al. Effect of poly(vinyl alcohol) macromer chemistry and chain interactions on hydrogel mechanical properties. Chem Mater. 2007;19(10):2641–2648.
- Martens P, Anseth KS. Characterization of hydrogels formed from acrylate modified poly(vinyl alcohol) macromers. Polymer. 2000;41(21):7715–7722.
- Mawad D, Poole-Warren LA, Martens P, et al. Synthesis and characterization of radiopaque iodine-containing degradable PVA hydrogels. Biomacromolecules. 2008;9(1):263–268.
- Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24(13):2339–2349.
- No HK, Park NY, Lee SH, et al. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74(1–2):65–72.
- Giménez V, Mantecón A, Cádiz V. Modification of poly(vinyl alcohol) with acid chlorides and crosslinking with difunctional hardeners. J Polym Sci Part A Polym Chem. 1996;34(6):925–934.
- Basnett P, Matharu RK, Taylor CS, et al. Harnessing polyhydroxyalkanoates and pressurized gyration for hard and soft tissue engineering. ACS Appl Mater Interfaces. 2021;13(28):32624–32639.
- Koller M, Maršálek L, de Sousa Dias MM, et al. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017;37:24–38.
- Daranarong D, Chan RT, Wanandy NS, et al. Electrospun polyhydroxybutyrate and poly(L-lactide-co-ϵ-caprolactone) composites as nanofibrous scaffolds. Biomed Res Int. 2014;2014:741408.
- Williams S, Martin D. Applications of polyhydroxyalkanoates (PHA) in medicine and pharmacy. 2005.
- Hazer DB, Kılıçay E, Hazer B. Poly(3-hydroxyalkanoate)s: diversification and biomedical applications. Mater Sci Eng C. 2012;32(4):637–647.
- Sangsanoh P, Israsena N, Suwantong O, et al. Effect of the surface topography and chemistry of poly(3-hydroxybutyrate) substrates on cellular behavior of the murine neuroblastoma Neuro2a cell line. Polym Bull. 2017;74(10):4101–4118.
- Goonoo N, Bhaw-Luximon A, Passanha P, et al. Third generation poly(hydroxyacid) composite scaffolds for tissue engineering. J Biomed Mater Res B Appl Biomater. 2017;105(6):1667–1684.
- Ke Y, Zhang XY, Ramakrishna S, et al. Reactive blends based on polyhydroxyalkanoates: preparation and biomedical application. Mater Sci Eng C. 2017;70:1107–1119.
- Levine AC, Sparano A, Twigg FF, et al. Influence of cross-linking on the physical properties and cytotoxicity of polyhydroxyalkanoate (PHA) scaffolds for tissue engineering. ACS Biomater Sci Eng. 2015;1(7):567–576.
- Ye C, Hu P, Ma MX, et al. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartilage tissue engineering. Biomaterials. 2009;30(26):4401–4406.
- Bian YZ, Wang Y, Aibaidoula G, et al. Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials. 2009;30(2):217–225.
- Qu XH, Wu Q, Liang J, et al. Effect of 3-hydroxyhexanoate content in poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on in vitro growth and differentiation of smooth muscle cells. Biomaterials. 2006;27(15):2944–2950.
- Qu XH, Wu Q, Chen GQ. In vitro study on hemocompatibility and cytocompatibility of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). J Biomater Sci Polym Ed. 2006;17(10):1107–1121.
- Cool SM, Kenny B, Wu A, et al. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composite biomaterials for bone tissue regeneration: in vitro performance assessed by osteoblast proliferation, osteoclast adhesion and resorption, and macrophage proinflammatory response. J Biomed Mater Res Part A. 2007;82A(3):599–610.
- Wang Y, Bian YZ, Wu Q, et al. Evaluation of three-dimensional scaffolds prepared from poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials. 2008;29(19):2858–2868.
- Wang YW, Wu Q, Chen JC, et al. Evaluation of three-dimensional scaffolds made of blends of hydroxyapatite and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for bone reconstruction. Biomaterials. 2005;26(8):899–904.
- Mosahebi A, Fuller P, Wiberg M, et al. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 2002;173(2):213–223.
- Volova T, Goncharov D, Sukovatyi A, et al. Electrospinning of polyhydroxyalkanoate fibrous scaffolds: effects on electrospinning parameters on structure and properties. J Biomater Sci Polym Ed. 2014;25(4):370–393.
- Mottin AC, Ayres E, Orefice RL, et al. What changes in Poly(3-Hydroxybutyrate) (PHB) when processed as electrospun nanofibers or thermo-compression molded film?. Mater Res Ibero Am J Mater. 2016;19(1):57–66.
- Sanhueza C, Acevedo F, Rocha S, et al. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int J Biol Macromol. 2019;124:102–110.
- Sanhueza C, Diaz-Rodriguez P, Villegas P, et al. Influence of the carbon source on the properties of poly-(3)-hydroxybutyrate produced by Paraburkholderia xenovorans LB400 and its electrospun fibers. Int J Biol Macromol. 2020;152:11–20.
- Ray S, Kalia VC. Biomedical applications of polyhydroxyalkanoates. Indian J Microbiol. 2017;57(3):261–269.
- Nagiah N, Madhavi L, Anitha R, et al. Development and characterization of coaxially electrospun gelatin coated poly (3-hydroxybutyric acid) thin films as potential scaffolds for skin regeneration. Mater Sci Eng C Mater Biol Appl. 2013;33(7):4444–4452.
- Asran AS, Razghandi K, Aggarwal N, et al. Nanofibers from blends of polyvinyl alcohol and polyhydroxy butyrate as potential scaffold material for tissue engineering of skin. Biomacromolecules. 2010;11(12):3413–3421.
- Amini F, Semnani D, Karbasi S, et al. A novel bilayer drug-loaded wound dressing of PVDF and PHB/chitosan nanofibers applicable for post-surgical ulcers. Int J Polym Mater Polym Biomat. 2019;68(13):772–777.
- Naghashzargar E, Fare S, Catto V, et al. Nano/micro hybrid scaffold of PCL or P3HB nanofibers combined with silk fibroin for tendon and ligament tissue engineering. J Appl Biomater Funct Mater. 2015;13(2):E156–E168.
- Sadeghi D, Karbasi S, Razavi S, et al. Electrospun poly(hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J Appl Polym Sci. 2016;133(47):9.
- Mirmusavi MH, Zadehnajar P, Semnani D, et al. Evaluation of physical, mechanical and biological properties of poly 3-hydroxybutyrate-chitosan-multiwalled carbon nanotube/silk nano-micro composite scaffold for cartilage tissue engineering applications. Int J Biol Macromol. 2019;132:822–835.
- Heydari P, Varshosaz J, Kharazi AZ, et al. Preparation and evaluation of poly glycerol sebacate/poly hydroxy butyrate core-shell electrospun nanofibers with sequentially release of ciprofloxacin and simvastatin in wound dressings. Polym Adv Technol. 2018;29(6):1795–1803.
- Zhang YZ, Venugopal J, Huang ZM, et al. Crosslinking of the electrospun gelatin nanofibers. Polymer. 2006;47(8):2911–2917.
- Nelson T, Kaufman E, Kline J, et al. The extraneural distribution of γ-hydroxybutyrate. J Neurochem. 1981;37(5):1345–1348.
- Lee YF, Sridewi N, Ramanathan S, et al. The influence of electrospinning parameters and drug loading on polyhydroxyalkanoate (PHA) nanofibers for drug delivery. Int J Biotechnol Wellness Ind. 2016;4:103–113.
- Vigneswari S, Murugaiyah V, Kaur G, et al. Simultaneous dual syringe electrospinning system using benign solvent to fabricate nanofibrous P(3HB-co-4HB)/collagen peptides construct as potential leave-on wound dressing. Mater Sci Eng C Mater Biol Appl. 2016;66:147–155.
- Ma K, Huang D, Cai J, et al. Surface functionalization with strontium-containing nanocomposite coatings via EPD. Colloids Surf B. 2016;146:97–106.
- Alipal J, Pu’ad N, Lee TC, et al. A review of gelatin: properties, sources, process, applications, and commercialisation. 5th International Conference of Chemical Engineering and Industrial Biotechnology (ICCEIB), Aug 9–11. Kuala Lumpur: Elsevier; 2020, p. 240–250.
- Zhang Z, Ortiz O, Goyal R, et al. Chapter 23 – biodegradable polymers. In: Lanza R, Langer R, Vacanti J, editors. Principles of tissue engineering. 4th ed. Boston, MA: Academic Press; 2014. p. 441–473.
- Guo T, Zhang N, Huang J, et al. A facile fabrication of core–shell sodium alginate/gelatin beads for drug delivery systems. Polym Bull. 2019;76(1):87–102.
- Bello AB, Kim D, Kim D, et al. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Eng Part B Rev. 2020;26(2):164–180.
- Yang Z, Hemar Y, Hilliou L, et al. Nonlinear behavior of gelatin networks reveals a hierarchical structure. Biomacromolecules. 2016;17(2):590–600.
- Zhang Y, Ouyang H, Lim CT, et al. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater. 2005;72B(1):156–165.
- Puppi D, Pirosa A, Lupi G, et al. Design and fabrication of novel polymeric biodegradable stents for small caliber blood vessels by computer-aided wet-spinning. Biomed Mater. 2017;12(3):035011.
- Rydz J, Sikorska W, Kyulavska M, et al. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int J Mol Sci. 2015;16(1):564–596.
- Deb P, Deoghare AB, Borah A, et al. Scaffold development using biomaterials: a review. Mater Today Proc. 2018;5(5, Part 2):12909–12919.
- Bagdadi A, Safari M, Dubey P, et al. Poly(3-hydroxyoctanoate), a promising new material for cardiac tissue engineering. J Tissue Eng Regen Med. 2016;12.
- Arslantunali D, Dursun T, Yucel D, et al. Peripheral nerve conduits: technology update. Med Devices. 2014;7:405–424.
- Mathuriya AS, Yakhmi JV. Polyhydroxyalkanoates: biodegradable plastics and their applications. In: Martínez LMT, Kharissova OV, Kharisov BI, editors. Handbook of ecomaterials. Cham: Springer International Publishing; 2019. p. 2873–2900.
- Hazer DB, Bal E, Nurlu G, et al. In vivo application of poly-3-hydroxyoctanoate as peripheral nerve graft. J Zhejiang Univ Sci B. 2013;14(11):993–1003.
- Mozejko-Ciesielska J, Szacherska K, Marciniak P. Pseudomonas species as producers of eco-friendly polyhydroxyalkanoates. J Polym Environ. 2019;27(6):1151–1166.
- Zhang J, Shishatskaya EI, Volova TG, et al. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater Sci Eng C. 2018;86:144–150.
- Możejko-Ciesielska J, Kiewisz R. Bacterial polyhydroxyalkanoates: still fabulous? Microbiol Res. 2016;192:271–282.
- Pawelec KM, Hix J, Shapiro EM, et al. The mechanics of scaling-up multichannel scaffold technology for clinical nerve repair. J Mech Behav Biomed Mater. 2019;91:247–254.
- Jafari M, Paknejad Z, Rad MR, et al. Polymeric scaffolds in tissue engineering: a literature review. J Biomed Mater Res B Appl Biomater. 2017;105(2):431–459.
- Manavitehrani I, Fathi A, Badr H, et al. Biomedical applications of biodegradable polyesters. Polymers. 2016;8(1):20.
- Lizarraga-Valderrama LR, Nigmatullin R, Taylor C, et al. Nerve tissue engineering using blends of poly(3-hydroxyalkanoates) for peripheral nerve regeneration. Eng Life Sci. 2015;15(6):612–621.
- Basnett P, Nigmatullin R, Lukasiewicz B, et al. Polyhydroxyalkanoates: a family of natural polymers, for medical implant development and disease modelling. Transactions of the Annual Meeting of the Society for Biomaterials and the Annual International Biomaterials Symposium, Seattle. 2019. p. 760.
- Charuchinda A, Molloy R, Siripitayananon J, et al. Factors influencing the small-scale melt spinning of poly(ϵ-caprolactone) monofilament fibres. Polym Int. 2003;52(7):1175–1181.
- Cipitria A, Skelton A, Dargaville TR, et al. Design, fabrication and characterization of PCL electrospun scaffolds – a review. J Mater Chem. 2011;21(26):9419–9453.
- Azimi B, Nourpanah P, Rabiee M, et al. Poly (∊-caprolactone) fiber: an overview. J Eng Fibers Fabr. 2014;9(3):74–90.
- Woodruff MA, Hutmacher DW. The return of a forgotten polymer – polycaprolactone in the 21st century. Prog Polym Sci. 2010;35(10):1217–1256.
- Abedalwafa M, Wang FJ, Wang L, et al. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev Adv Mater Sci. 2013;34(2):123–140.
- Gao J, Crapo P, Nerem R, et al. Co-expression of elastin and collagen leads to highly compliant engineered blood vessels. J Biomed Mater Res Part A. 2008;85A(4):1120–1128.
- Redenti S, Neeley WL, Rompani S, et al. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009;30(20):3405–3414.
- Sundback CA, Shyu JY, Wang Y, et al. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26(27):5454–5464.
- Wang Y, Ameer GA, Sheppard BJ, et al. A tough biodegradable elastomer. Nat Biotechnol. 2002;20(6):602–606.
- Lee EJ, Vunjak-Novakovic G, Wang Y, et al. A biocompatible endothelial cell delivery system for in vitro tissue engineering. Cell Transplant. 2009;18(7):731–743.
- Meyer M. Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng Online. 2019;18(1):24.
- Owczarzy A, Kurasiński R, Kulig K, et al. Collagen - structure, properties and application. Eng Biomater. 2020;156:17–23.
- Birk DE, Bruckner P. Collagen suprastructures. In: Brinckmann J, Notbohm H, Müller PK, editors. Collagen: primer in structure, processing and assembly. Berlin: Springer; 2005. p. 185–205.
- Bashur CA, Dahlgren LA, Goldstein AS. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d,l-lactic-co-glycolic acid) meshes. Biomaterials. 2006;27(33):5681–5688.
- Li WJ, Danielson KG, Alexander PG, et al. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67(4):1105–1114.
- Carampin P, Conconi MT, Lora S, et al. Electrospun polyphosphazene nanofibers for in vitro rat endothelial cells proliferation. J Biomed Mater Res Part A. 2007;80A(3):661–668.
- Badami AS, Kreke MR, Thompson MS, et al. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27(4):596–606.
- He W, Ma Z, Yong T, et al. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials. 2005;26(36):7606–7615.
- Jin H-J, Chen J, Karageorgiou V, et al. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials. 2004;25(6):1039–1047.
- Min BM, Lee G, Kim SH, et al. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25(7–8):1289–1297.
- Mo XM, Xu CY, Kotaki M, et al. Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25(10):1883–1890.
- Shin HJ, Lee CH, Cho IH, et al. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J Biomater Sci Polym Ed. 2006;17(1–2):103–119.
- Venugopal JR, Zhang Y, Ramakrishna S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif Organs. 2006;30(6):440–446.
- Jeong SI, Lee AY, Lee YM, et al. Electrospun gelatin/poly(L-lactide-co-ϵ-caprolactone) nanofibers for mechanically functional tissue-engineering scaffolds. J Biomater Sci Polym Ed. 2008;19(3):339–357.
- Yang XB, Roach HI, Clarke NMP, et al. Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification. Bone. 2001;29(6):523–531.
- Li M, Mondrinos MJ, Chen X, et al. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J Biomed Mater Res Part A. 2006;79A(4):963–973.
- Li M, Mondrinos MJ, Chen X, et al. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J Biomed Mater Res A. 2006;79A(4):963–973.
- Jeong SI, Lee AY, Lee YM, et al. Electrospun gelatin/poly(L-lactide-co-ϵ-caprolactone) nanofibers for mechanically functional tissue-engineering scaffolds. J Biomater Sci Polym Ed. 2008;19(3):339–357.
- Yang XB, Roach HI, Clarke NM, et al. Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification. Bone. 2001;29(6):523–531.
- Ekaputra AK, Zhou Y, Cool SM, et al. Composite electrospun scaffolds for engineering tubular bone grafts. Tissue Eng Part A. 2009;15(12):3779–3788.
- Ghorbani F, Moradi L, Shadmehr MB, et al. In-vivo characterization of a 3D hybrid scaffold based on PCL/decellularized aorta for tracheal tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;81:74–83.
- Gao M, Zhang H, Dong W, et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci Rep. 2017;7(1):5246.
- Townsend JM, Ott LM, Salash JR, et al. Reinforced electrospun polycaprolactone nanofibers for tracheal repair in an in vivo ovine model. Tissue Eng Part A. 2018;24(17–18):1301–1308.
- Li Y, Li Q, Li H, et al. An effective dual-factor modified 3D-printed PCL scaffold for bone defect repair. J Biomed Mater Res B Appl Biomater. 2020;108(5):2167–2179.
- Ke DX, Yi HL, Est-Witte S, et al. Bioprinted trachea constructs with patient-matched design, mechanical and biological properties. Biofabrication. 2020;12(1):11.
- Xia D, Jin D, Wang Q, et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair in a goat model. J Tissue Eng Regen Med. 2019;13(4):694–703.
- Omori K, Nakamura T, Kanemaru S, et al. In situ tissue engineering of the cricoid and trachea in a canine model. Ann Otol Rhinol Laryngol. 2008;117(8):609–613.
- Xu Y, Li Y, Liu Y, et al. Surface modification of decellularized trachea matrix with collagen and laser micropore technique to promote cartilage regeneration. Am J Transl Res. 2019;11(9):5390–5403.
- Lin LQ, Xu YW, Li YQ, et al. Nanofibrous Wharton's jelly scaffold in combination with adipose-derived stem cells for cartilage engineering. Mater Des. 2020;186:10.
- Kianfar P, Vitale A, Dalle Vacche S, et al. Enhancing properties and water resistance of PEO-based electrospun nanofibrous membranes by photo-crosslinking. J Mater Sci. 2021;56(2):1879–1896.
- Zarrintaj P, Saeb MR, Jafari SH, et al. Chapter 18 – application of compatibilized polymer blends in biomedical fields. In: Ajitha AR, Thomas S, editors. Compatibilization of polymer blends. Elsevier; 2020. p. 511–537.
- Xu F, Gough I, Dorogin DJ, et al. Nanostructured degradable macroporous hydrogel scaffolds with controllable internal morphologies via reactive electrospinning. Acta Biomater. 2020;104:135–146.
- Rajam S, Ho CC. Graft coupling of PEO to mixed cellulose esters microfiltration membranes by UV irradiation. J Membr Sci. 2006;281(1–2):211–218.
- Aqil A, Vasseur S, Duguet E, et al. PEO coated magnetic nanoparticles for biomedical application. Eur Polym J. 2008;44(10):3191–3199.
- Fusco S, Borzacchiello A, Netti PA. Perspectives on: PEO-PPO-PEO triblock copolymers and their biomedical applications. J Bioact Compat Polym. 2006;21(2):149–164.
- Papagiannopoulos A, Vlassi E, Pispas S, et al. Polyethylene oxide hydrogels crosslinked by peroxide for the controlled release of proteins. Macromol. 2021;1(1):37–48.
- Gentile P, Chiono V, Carmagnola I, et al. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15(3):3640–3659.
- Danhier F, Ansorena E, Silva JM, et al. PLGA-based nanoparticles: an overview of biomedical applications. J Controlled Release. 2012;161(2):505–522.
- Yoon YI, Park KE, Lee SJ, et al. Fabrication of microfibrous and nano-/microfibrous scaffolds: melt and hybrid electrospinning and surface modification of poly(L-lactic acid) with plasticizer. Biomed Res Int. 2013;2013:10.
- Wang BY, Fu SZ, Ni PY, et al. Electrospun polylactide/poly(ethylene glycol) hybrid fibrous scaffolds for tissue engineering. J Biomed Mater Res Part A. 2012;100A(2):441–449.
- Wang H, Feng Y, Zhao H, et al. A potential nonthrombogenic small-diameter vascular scaffold with polyurethane/poly(ethylene glycol) hybrid materials by electrospinning technique. J Nanosci Nanotechnol. 2013;13(2):1578–1582.
- Li X, Liu KL, Wang M, et al. Improving hydrophilicity, mechanical properties and biocompatibility of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] through blending with poly[(R)-3-hydroxybutyrate]-alt-poly(ethylene oxide). Acta Biomater. 2009;5(6):2002–2012.
- Rivera-Briso AL, Serrano-Aroca A. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): enhancement strategies for advanced applications. Polymers. 2018;10(7):28.
- Bossu J, Angellier-Coussy H, Totee C, et al. Effect of the molecular structure of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-3HV)) produced from mixed bacterial cultures on its crystallization and mechanical properties. Biomacromolecules. 2020;21(12):4709–4723.
- Xu YJ, Zou LM, Lu HW, et al. Preparation and characterization of electrospun PHBV/PEO mats: the role of solvent and PEO component. J Mater Sci. 2016;51(12):5695–5711.
- Pillai AB, Kumar AJ, Kumarapillai H. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) in Bacillus aryabhattai and cytotoxicity evaluation of PHBV/poly(ethylene glycol) blends. 3 Biotech. 2020;10(2):10.
- Bonartsev AP, Bonartseva GA, Reshetov IV, et al. Application of polyhydroxyalkanoates in medicine and the biological activity of natural poly(3-hydroxybutyrate). Acta Naturae. 2019;11(2):4–16.
- Sendil D, Gürsel I, Wise DL, et al. Antibiotic release from biodegradable PHBV microparticles. J Control Release. 1999;59(2):207–217.
- Dai ZW, Zou XH, Chen GQ. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) as an injectable implant system for prevention of post-surgical tissue adhesion. Biomaterials. 2009;30(17):3075–3083.
- Hogge J, Krasner D, Nguyen H, et al. The potential benefits of advanced therapeutic modalities in the treatment of diabetic foot wounds. J Am Podiatr Med Assoc. 2000;90(2):57–65.
- Muzzarelli RAA. Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar Drugs. 2011;9(9):1510–1533.
- van der Veen VC, van der Wal MB, van Leeuwen MC, et al. Biological background of dermal substitutes. Burns. 2010;36(3):305–321.
- Nangia A, Gambhir R, Maibach H. Factors influencing the performance of temporary skin substitutes. Clin Mater. 1991;7(1):3–13.
- Siraj S, Sudhakar P, Mallikarjuna B, et al. Factors influencing the performance of temporary skin substitutes. Int J Pharm Sci Rev Res. 2014;25(2):103–109.
- Bianco A, Calderone M, Cacciotti I. Electrospun PHBV/PEO co-solution blends: microstructure, thermal and mechanical properties. Mater Sci Eng C Mater Biol Appl. 2013;33(3):1067–1077.
- Tan SM, Ismail J, Kummerlowe C, et al. Crystallization and melting behavior of blends comprising poly(3-hydroxy butyrate-co-3-hydroxy valerate) and poly(ethylene oxide). J Appl Polym Sci. 2006;101(5):2776–2783.
- Gumus S, Ozkoc G, Aytac A. Plasticized and unplasticized PLA/organoclay nanocomposites: short- and long-term thermal properties, morphology, and nonisothermal crystallization behavior. J Appl Polym Sci. 2012;123(5):2837–2848.
- Pluta M, Paul MA, Alexandre M, et al. Plasticized polylactide/clay nanocomposites. I. The role of filler content and its surface organo-modification on the physico-chemical properties. J Polym Sci Part B Polym Phys. 2006;44(2):299–311.
- Sungsanit K, Kao N, Bhattacharya SN. Properties of linear poly(lactic acid)/polyethylene glycol blends. Polym Eng Sci. 2012;52(1):108–116.
- Hu Y, Hu YS, Topolkaraev V, et al. Crystallization and phase separation in blends of high stereoregular poly(lactide) with poly(ethylene glycol). Polymer. 2003;44(19):5681–5689.
- Gui Z, Xu Y, Gao Y, et al. Novel polyethylene glycol-based polyester-toughened polylactide. Mater Lett. 2012;71:63–65.
- Baiardo M, Frisoni G, Scandola M, et al. Thermal and mechanical properties of plasticized poly(L-lactic acid). J Appl Polym Sci. 2003;90(7):1731–1738.
- Hrkach J, Von Hoff D, Ali MM, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):11.
- Von Hoff DD, Mita MM, Ramanathan RK, et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin Cancer Res. 2016;22(13):3157–3163.
- Lopes MS, Jardini AL, Maciel R. Poly (lactic acid) production for tissue engineering applications. 20th International Congress of Chemical and Process Engineering CHISA, Aug 25–29. Prague: Elsevier Science; 2012. p. 1402–1413.
- Singhvi MS, Zinjarde SS, Gokhale DV. Polylactic acid: synthesis and biomedical applications. J Appl Microbiol. 2019;127(6):1612–1626.
- Gangapurwala G, Vollrath A, De San Luis A, et al. PLA/PLGA-based drug delivery systems produced with supercritical CO2 – a green future for particle formulation? Pharmaceutics. 2020;12(11):1118.
- Shkodra-Pula B, Grune C, Traege A, et al. Effect of surfactant on the size and stability of PLGA nanoparticles encapsulating a protein kinase C inhibitor. Int J Pharm. 2019;566:756–764.
- Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int J Pharm. 2002;233(1):51–59.
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–651.
- Mu L, Feng SS. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. J Controlled Release. 2003;86(1):33–48.
- Swider E, Koshkina O, Tel J, et al. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018;73:38–51.
- Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opin Biol Ther. 2004;4(1):35–51.
- Freitas S, Merkle HP, Gander B. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. J Controlled Release. 2005;102(2):313–332.
- Reinhold SE, Schwendeman SP. Effect of polymer porosity on aqueous self-healing encapsulation of proteins in PLGA microspheres. Macromol Biosci. 2013;13(12):1700–1710.
- Rhee YS, Park CW, Mansour H. Sustained-release injectable drug delivery systems. An invited paper. Pharm Technol Special Issue Drug Delivery. 2010:6–13.
- Tiwari S, Verma P. Microencapsulation technique by solvent evaporation method (study of effect of process variables). Int J Phar Life Sci. 2011;2:998–1005.
- Maruyama K, Asai J, Ii M, et al. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–1191.
- Hinchliffe RJ, Valk GD, Apelqvist J, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2008;24:S119–S144.
- Moura LIF, Dias AMA, Carvalho E, et al. Recent advances on the development of wound dressings for diabetic foot ulcer treatment – a review. Acta Biomater. 2013;9(7):7093–7114.
- Gooyit M, Peng Z, Wolter WR, et al. A chemical biological strategy to facilitate diabetic wound healing. ACS Chem Biol. 2014;9(1):105–110.
- Philandrianos C, Andrac-Meyer L, Mordon S, et al. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns. 2012;38(6):820–829.
- Chattopadhyay S, Raines RT. Collagen-based biomaterials for wound healing. Biopolymers. 2014;101(8):821–833.
- Yang E, Qin X, Wang S. Electrospun crosslinked polyvinyl alcohol membrane. Mater Lett. 2008;62(20):3555–3557.
- Gong XH, Tang CY, Pan L, et al. Characterization of poly(vinyl alcohol) (PVA)/ZnO nanocomposites prepared by a one-pot method. Compos Part B Eng. 2014;60:144–149.
- DeMerlis CC, Schoneker DR. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem Toxicol. 2003;41(3):319–326.
- Gong L, Chase DB, Noda I, et al. Discovery of β-form crystal structure in electrospun poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate] (PHBHx) nanofibers: from fiber mats to single fibers. Macromolecules. 2015;48(17):6197–6205.
- Cheng ML, Chen PY, Lan CH, et al. Structure, mechanical properties and degradation behaviors of the electrospun fibrous blends of PHBHHx/PDLLA. Polymer. 2011;52(6):1391–1401.
- Hosoda N, Tsujimoto T, Uyama H. Green composite of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) reinforced with porous cellulose. ACS Sustain Chem Eng. 2014;2(2):248–253.
- Wang J, Ye L. Structure and properties of polyvinyl alcohol/polyurethane blends. Compos B Eng. 2015;69:389–396.
- Gaaz TS, Sulong AB, Akhtar MN, et al. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules. 2015;20(12):22833–22847.
- Dippold D, Tallawi M, Tansaz S, et al. Novel electrospun poly(glycerol sebacate)–zein fiber mats as candidate materials for cardiac tissue engineering. Eur Polym J. 2016;75:504–513.
- Hu J, Kai D, Ye H, et al. Electrospinning of poly(glycerol sebacate)-based nanofibers for nerve tissue engineering. Mater Sci Eng C. 2017;70:1089–1094.
- Motlagh D, Yang J, Lui KY, et al. Hemocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials. 2006;27(24):4315–4324.
- Pashneh-Tala S, Owen R, Bahmaee H, et al. Synthesis, characterization and 3D micro-structuring via 2-photon polymerization of poly(glycerol sebacate)-methacrylate – an elastomeric degradable polymer. Front Phys. 2018;6:17.
- Chen QZ, Ishii H, Thouas GA, et al. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials. 2010;31(14):3885–3893.
- Frydrych M, Román S, MacNeil S, et al. Biomimetic poly(glycerol sebacate)/poly(l-lactic acid) blend scaffolds for adipose tissue engineering. Acta Biomater. 2015;18:40–49.
- Li Y, Cook WD, Moorhoff C, et al. Synthesis, characterization and properties of biocompatible poly(glycerol sebacate) pre-polymer and gel. Polym Int. 2013;62(4):534–547.
- Chen QZ, Bismarck A, Hansen U, et al. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29(1):47–57.
- Nijst CLE, Bruggeman JP, Karp JM, et al. Synthesis and characterization of photocurable elastomers from poly(glycerol-co-sebacate). Biomacromolecules. 2007;8(10):3067–3073.
- Frydrych M, Chen BQ. Large three-dimensional poly(glycerol sebacate)-based scaffolds – a freeze-drying preparation approach. J Mat Chem B. 2013;1(48):6650–6661.
- Jeffries EM, Allen RA, Gao J, et al. Highly elastic and suturable electrospun poly(glycerol sebacate) fibrous scaffolds. Acta Biomater. 2015;18:30–39.
- Khosravi R, Best CA, Allen RA, et al. Long-term functional efficacy of a novel electrospun poly(glycerol sebacate)-based arterial graft in mice. Ann Biomed Eng. 2016;44(8):2402–2416.
- Zarski A, Bajer K, Kapuśniak J. Review of the most important methods of improving the processing properties of starch toward non-food applications. Polymers. 2021;13(5):832.
- Bursali EA, Coskun S, Kizil M, et al. Synthesis, characterization and in vitro antimicrobial activities of boron/starch/polyvinyl alcohol hydrogels. Carbohydr Polym. 2011;83(3):1377–1383.
- Pal K, Banthia A, Majumdar DK. Preparation of transparent starch based hydrogel membrane with potential application as wound dressing. Trends Biomat Artif Organs. 2006;20:59–67.
- Kariduraganavar MY, Kittur AA, Kamble RR. Chapter 1 – polymer synthesis and processing. In: Laurencin CT, Kumbar S, Deng M, editors. Natural and synthetic biomedical polymers. Oxford: Elsevier; 2014. p. 1–31.
- Kurakula M, Rao G. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): as excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J Drug Deliv Sci Technol. 2020;60:102046.
- Bühler V. Polyvinylpyrrolidone excipients for pharmaceuticals: povidone, crospovidone, and copovidone. Berlin: Springer; 2005.
- Voronova M, Rubleva N, Kochkina N, et al. Preparation and characterization of polyvinylpyrrolidone/cellulose nanocrystals composites. Nanomaterials. 2018;8(12):1011.
- Kathe K, Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian J Pharm Sci. 2017;12(6):487–497.
- Mondal D, Mollick MMR, Bhowmick B, et al. Effect of poly(vinyl pyrrolidone) on the morphology and physical properties of poly(vinyl alcohol)/sodium montmorillonite nanocomposite films. Prog Nat Sci Mater Int. 2013;23(6):579–587.
- Maciejewska BM, Wychowaniec JK, Woźniak-Budych M, et al. UV cross-linked polyvinylpyrrolidone electrospun fibres as antibacterial surfaces. Sci Technol Adv Mater. 2019;20(1):979–991.
- Teodorescu M, Bercea M. Poly(vinylpyrrolidone) – a versatile polymer for biomedical and beyond medical applications. Polym Plast Technol Eng. 2015;54(9):923–943.
- Zhi X, Fang H, Bao C, et al. The immunotoxicity of graphene oxides and the effect of PVP-coating. Biomaterials. 2013;34(21):5254–5261.
- Hayama M, Yamamoto K-I, Kohori F, et al. How polysulfone dialysis membranes containing polyvinylpyrrolidone achieve excellent biocompatibility? J Membr Sci. 2004;234(1):41–49.
- Bolong N, Ismail AF, Salim MR. Spinning effect of polyethersulfone hollow fiber membrane prepared by water or polyvinylpyrrolidone in ternary formulation. 7th International Conference on Membrane Science and Technology (MST) – Sustainable Technology for Energy, Water, and Environment, May 13–15. Kuala Lumpur: John Wiley & Sons; 2009. p. 3–10.
- Kadajji VG, Betageri GV. Water soluble polymers for pharmaceutical applications. Polymers. 2011;3(4):1972–2009.
- Stammen JA, Williams S, Ku DN, et al. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials. 2001;22(8):799–806.
- Maher SA, Doty SB, Torzilli PA, et al. Nondegradable hydrogels for the treatment of focal cartilage defects. J Biomed Mater Res Part A. 2007;83A(1):145–155.
- Bichara DA, Bodugoz-Sentruk H, Ling D, et al. Osteochondral defect repair using a polyvinyl alcohol-polyacrylic acid (PVA-PAAc) hydrogel. Biomed Mater. 2014;9(4):045012.
- Mow VC, Ratcliffe A, Robin Poole A. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97.
- Shi Y, Xiong D. Microstructure and friction properties of PVA/PVP hydrogels for articular cartilage repair as function of polymerization degree and polymer concentration. Wear. 2013;305(1):280–285.
- Gonzalez JS, Alvarez VA. Mechanical properties of polyvinylalcohol/hydroxyapatite cryogel as potential artificial cartilage. J Mech Behav Biomed Mater. 2014;34:47–56.
- Pan Y, Xiong D. Friction properties of nano-hydroxyapatite reinforced poly(vinyl alcohol) gel composites as an articular cartilage. Wear. 2009;266(7):699–703.
- Pan YS, Xiong DS, Ma RY. A study on the friction properties of poly(vinyl alcohol) hydrogel as articular cartilage against titanium alloy. Wear. 2007;262(7–8):1021–1025.
- Li F, Su Y, Wang J, et al. Influence of dynamic load on friction behavior of human articular cartilage, stainless steel and polyvinyl alcohol hydrogel as artificial cartilage. J Mater Sci Mater Med. 2010;21(1):147–154.
- Lilleby Helberg RM, Dai Z, Ansaloni L, et al. PVA/PVP blend polymer matrix for hosting carriers in facilitated transport membranes: synergistic enhancement of CO2 separation performance. Green Energy Environ. 2020;5(1):59–68.
- Joshi A, Fussell G, Thomas J, et al. Functional compressive mechanics of a PVA/PVP nucleus pulposus replacement. Biomaterials. 2006;27(2):176–184.
- Thomas J, Lowman A, Marcolongo M. Novel associated hydrogels for nucleus pulposus replacement. J Biomed Mater Res A. 2003;67A:1329–1337.
- Holloway JL, Lowman AM, Palmese GR. Aging behavior of PVA hydrogels for soft tissue applications after in vitro swelling using osmotic pressure solutions. Acta Biomater. 2013;9(2):5013–5021.
- Holloway JL, Lowman AM, Palmese GR. Mechanical evaluation of poly(vinyl alcohol)-based fibrous composites as biomaterials for meniscal tissue replacement. Acta Biomater. 2010;6(12):4716–4724.
- Wan WK, Campbell G, Zhang ZF, et al. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J Biomed Mater Res. 2002;63(6):854–861.
- Lozinsky V, Damshkaln L, Shaskolskiy B, et al. Study of cryostructuring of polymer systems: 27. Physicochemical properties of poly(vinyl alcohol) cryogels and specific features of their macroporous morphology. Colloid J. 2007;69:747–764.
- Nasouri K, Shoushtari AM, Mojtahedi MRM. Effects of polymer/solvent systems on electrospun polyvinylpyrrolidone nanofiber morphology and diameter. Polymer Science Series A. 2015;57(6):747–755.
- Feng Y, Wang K, Yao J, et al. Effect of the addition of polyvinylpyrrolidone as a pore-former on microstructure and mechanical strength of porous alumina ceramics. Ceram Int. 2013;39(7):7551–7556.
- Dreesmann L, Ahlers M, Schlosshauer B. The pro-angiogenic characteristics of a cross-linked gelatin matrix. Biomaterials. 2007;28(36):5536–5543.
- Shen ZS, Cui X, Hou RX, et al. Tough biodegradable chitosan–gelatin hydrogels via in situ precipitation for potential cartilage tissue engineering. RSC Adv. 2015;5(69):55640–55647.
- Bosworth LA, Downes S. Physicochemical characterisation of degrading polycaprolactone scaffolds. Polym Degrad Stab. 2010;95(12):2269–2276.
- Sekosan G, Vasanthan N. Morphological changes of annealed poly-ϵ-caprolactone by enzymatic degradation with lipase. J Polym Sci Part B Polym Phys. 2010;48(2):202–211.
- Yu L, Dean K, Li L. Polymer blends and composites from renewable resources. Prog Polym Sci. 2006;31(6):576–602.
- García Cruz DM, Coutinho DF, Mano JF, et al. Physical interactions in macroporous scaffolds based on poly(ε-caprolactone)/chitosan semi-interpenetrating polymer networks. Polymer. 2009;50(9):2058–2064.
- Kim G, Park J, Park S. Surface-treated and multilayered poly(ε-caprolactone) nanofiber webs exhibiting enhanced hydrophilicity. J Polym Sci Part B Polym Phys. 2007;45(15):2038–2045.
- Xu F, Cui FZ, Jiao YP, et al. Improvement of cytocompatibility of electrospinning PLLA microfibers by blending PVP. J Mater Sci Mater Med. 2009;20(6):1331–1338.
- Felfel RM, Poocza L, Gimeno-Fabra M, et al. In vitro degradation and mechanical properties of PLA-PCL copolymer unit cell scaffolds generated by two-photon polymerization. Biomed Mater. 2016;11(1):015011.
- Zhang K, Fu Q, Yoo J, et al. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017;50:154–164.
- Ng WL, Yeong WY, Naing MW. Polyvinylpyrrolidone-based bio-ink improves cell viability and homogeneity during drop-on-demand printing. Materials. 2017;10(2):12.
- Chaudhuri B, Mondal B, Ray SK, et al. A novel biocompatible conducting polyvinyl alcohol (PVA)-polyvinylpyrrolidone (PVP)-hydroxyapatite (HAP) composite scaffolds for probable biological application. Colloid Surf B Biointerfaces. 2016;143:71–80.
- Yang Q, Li Z, Hong Y, et al. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. J Polym Sci Part B Polym Phys. 2004;42(20):3721–3726.
- Kim GM, Le KHT, Giannitelli SM, et al. Electrospinning of PCL/PVP blends for tissue engineering scaffolds. J Mater Sci Mater Med. 2013;24(6):1425–1442.
- Yao Q, Cosme JGL, Xu T, et al. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials. 2017;115:115–127.
