Abstract
Objective
The aim of the study was to expand the understanding of pathogenesis of adenomyosis-associated pelvic pain.
Material and Methods
We studied 30 (n = 30) biopsy samples obtained after hysterectomy in women with diffuse adenomyosis of grade II-III, accompanied by severe pain syndrome, who did not receive hormonal therapy. The morphologic comparison group comprised 30 (n = 30) biopsy samples obtained from women with adenomyosis, without pain syndrome, operated on for abnormal uterine bleeding, who also did not receive hormone therapy.
Results
The total density of immunological OTR labeling in the adenomyotic lesion foci was 73.7 ± 1.8%, and in the morphological control group it was 35.2 ± 1.4% (p <0.05), which indicates a significant effect of oxytocin as a ureterotonic peptide. Processes of local neurogenesis and growth of nerve fibers was established due to an increase in the expression of the nervous system growth factor NGF in the myometrium stroma, in comparison with biopsy samples of morphological control.
Conclusion: Pelvic pain pathogenesis in women with diffuse adenomyosis compared with the painless form of the disease is an increase in the activity of ureterotonic factors of OTR oxytocin. Compared to the painless form of adenomyosis, the myometrial innervation apparatus of patients with pelvic pain is characterized by a significantly higher expression of nerve growth factor.
Relevance of the problem
The pathogenetic mechanisms underlying the development of adenomyosis-associated pelvic pain (PP) are not yet fully understood to date and require an integrated approach to their analysis to determine the most effective diagnosis and therapy for this condition.
According to recent studies that have contributed to the development of the up-to-date concept of the formation and persistence of PP, a number of key factors are identified that indicate neuroimmunogenic conditionality of PP in endometriosis in general, and in adenomyosis in particular [Citation1,Citation2].
It is proved that the neuronal component of PP is associated with an increase in the density of nerve fibers directly in the area of endometrial tissue damage. It has been established that the expansion of the innervational field in the myometrium due to the formation of heterotopic endometrial foci in women with adenomyosis is accompanied by clinically apparentPP syndrome [Citation3–8].
However, in the case of adenomyosis, the terminal pathophysiological substrate in the development of PP is spasmodic contractions of the myometrium, occurring associated with an already developed and/or progressive local hyperalgesia associated with endometrial heterotopies foci. It is in this connection that a number of biologically active triggers are distinguished, which probably cause not only the intensification of local growth of nerve fibers, but can also directly or indirectly contribute to an increase in spontaneous contractility of smooth myocytes of the myometrium, and underlie pain perception in adenomyosis [Citation9].
Among the secreted neuronal markers, oxytocin, which is a key factor regulating uterine contractility, must be identified. It should be noted that oxytocin directly stimulates the contraction of smooth myocytes of the myometrium and its blood-stream by binding to the same-name receptors – oxytocin receptor (OTR). The role of oxytocin in the development of TB is confirmed by the proven therapeutic effect of its receptor antagonists of the same name. Studies have found that increased expression of OTR in the myometrium and in the connecting uterine zone positively correlates with the amplitude of uterine contractions and can lead to the appearance of pathological and uncoordinated myometrial spasms, accompanied by severe dysmenorrhea and reduced fertility in women of reproductive age [Citation9–12].
OTR expression and morphologic changes in the myometrial architecture of uteri having adenomyosis support the hypothesis that dysperistalsis plays an essential role in the development of endometriosis and dysmenorrhea. In the near future, specific inhibition of this receptor might yield a promising treatment for therapy [Citation10].
Compared with normal endometrium, the immunoreactivity of OTR is significantly increased in the ectopic endometrium. OTR immunoreactivity is positively correlated with the severity of dysmenorrhea and was found to be a significant predictor for dysmenorrhea severity [Citation13].
Locally increased estrogen levels and inflammation cause increased nerve growth factor (NGF) production in the uterus of patients with adenomyosis. Nerve growth factor stimulates the proliferation and increased aromatase expression of endometrium stromal cells from adenomyosis foci, suggesting NGF might contribute to the pathology and etiology of adenomyosis [Citation14].
The aim of the study
To expand the understanding of pelvic pain pathogenesis associated with adenomyosis.
Materials and research methods
In the present study, 60 uterine biopsies were examined after a hysterectomy of 60 women (n = 60) suffering from adenomyosis (ICD-10: endometriosis of the uterus (adenomyosis) N80.0) who gave voluntary informed consent for their inclusion in the study, for the collection of biological material, the study of clinical and laboratory parameters, statistical processing and publication of the findings obtained.
The study design suggested the stratification of patients of the studied cohort with a morphologically verified diagnosis of adenomyosis into 2 groups. Group I (main, n = 30) was comprised of biopsy specimens of female patients with severe PP, caused by diffuse II–III degree adenomyosis, who were not administered hormone therapy before.
Group II (morphological control, n = 30) – uterus biopsy in women with diffuse adenomyosis of II–III degree, without pain syndrome, operated on for abnormal uterine bleeding, who had not previously received hormone therapy either.
Inclusion criteria
Endometriosis of the uterus – adenomyosis (N80.0 according to the ICD-10 classification), pelvic pain, morphologically confirmed diagnosis, and its verified diffuse form.
Exclusion criteria
History of concomitant gynecological diseases of inflammatory and non-inflammatory etiology, accompanied by pelvic pain syndrome: varicose disease, systemic diseases, peritoneal adhesions, interstitial cystitis, myofascial pain syndrome, irritable bowel syndrome, the presence of pelvic pain associated with neurological disorders, a psychogenic pain.
Morphological, immunohistochemical (IHH) studies were performed with the conduct of standardized computer morphometry (Candidate of Medical Sciences, G.A. Demyashkin).
Immunohistochemical (IHC)
The study was carried out after dewaxing and rehydrating paraffin sections according to a standard protocol in an automatic mode in the Bond-Max immunohistostainer (Leica, Germany). Murinalmonoclonal antibodies (Abcam, UK) to OTR (anti-oxytocin receptor antibody, Clone ab217212, 1: 300) and polyclonal to NGF (Anti-NGF antibody, Clone ab140492, 1: 200) were used as primary antibodies.
The semi-quantitative method involved evaluating the results of immunohistochemical reactions using a 3-point system, counting the number of immunopositive cells in 10 randomly selected fields of view at 400× magnification (%): ‘−’ – absence; ‘+’ – weak (5–25% of cells, 1 point); ‘++’ – moderate (25–50% of cells, 2 points); ‘+++’ – profound (≥51% of cells, 3 points).
Computer morphometry
To assess the results of the immunohistochemical reaction and to determine the area of positively stained objects in the field of view was performed using an open-source image analysis computer system – Image J 1.51.
The resulting data were statistically processed using the SPSS 7.5 program for Windows – statistical software package (IBM Analytics, Armonk, NY). The variation series, the sample mean, the standard error of the sample mean (SEM), and the probability of difference were determined.
Research results
The results of a general morphological study of the uterine wall biopsy specimens in women of the cohort under study had shown that microscopic examination of the obtained biopsy specimens in the endometrium revealed uterine glands in all the proliferation phases of the menstrual cycle with uneven contour associated with of non-uniform distribution of glandular structures and endometrial stroma.
Connective endometrial/myometrial zone is determined without clear boundaries and demarcation signs. The eutopic endometrium basal layer largely extends into the submucosal substratum layer, sometimes with the invasion of the middle and subserous layers of the myometrium, and forms uneven areas of prolapse of uterine glands into the myometrium with the formation of foci of ectopic localization of endometrioid tissue containing the glands and endometrial stroma.
According to the morphometric analysis of the epithelial lining of endometrioid heterotopia, no significant differences in the height of the glandular epithelium cells, gland diameter, and their density per unit area in the 1st and 2nd groups have been established – p > .05.
During the immunohistochemical study, it was found that the cytoplasm of smooth muscle cells (SMC) of all myometrium layers showed a moderate (2 points) immunopositive response to antibodies to OTR. However, in smooth myocytes surrounding the adenomyotic lesion areas, an intense (3 points) IHC reaction is detected. According to the morphometric analysis results, the diffuse IHC density for OTR labeling in myometrial MH was 92.2 ± 2.1%, p < .05 (). There was no significant change in the intensity of OTR immunolabelling in the myometrium depending on the menstrual cycle phase (p > .05).
Figure 1. Group I (adenomyosis with PP). Myometrium (IHC Method: antibodies to OTR, counterstaining with hematoxylin, increase ×40). (а) Myometrium section; (b) adenomyosis focal area.
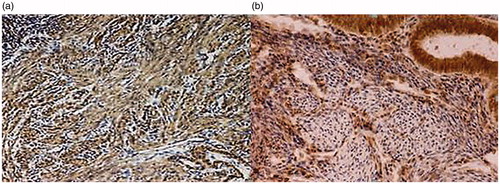
In glandular epithelium cells of endometrioid heterotopies, diffusely located in the myometrium, a pronounced (3 points) cytosolic IHC reaction of glandular epithelium cells to OTR, which is also independent of menstrual cycle phase, is detected. In the surrounding endometrial stroma, single OTR-positive cells are identified, closely adjacent to the myometrium.
The total density of immunological OTR labeling in the foci of adenomyotic lesion was 73.7 ± 1.8% (p < .05), which indicates a significant effect of oxytocin as anureterotonic peptide, aimed at enhancing myometrial dysperistaltic contractions and local vasoconstriction in adenomyosis.
The combination of these pathogenetic factors and hyperplastic change in the morphology of the myometrium around the foci of endometrioid heterotopies does not unreasonably contribute to the development of adenomyosis-associated PP ().
On the contrary, in the morphological control group, a moderate (2 points) positive immunological OTR labeling was found in the SMC cytoplasm is determined in all myometrium layers, also in the SMC surrounding the endometrioid heterotopies foci. The IHC density of the OTR labeling in myometrial SMC was 36.3 ± 2.4%, (p < .05).
In glandular epithelium cells of endometrioid heterotopias, a moderate (2 points) cytosolic IHC response of glandular epithelium cells to OTR is detected, independent of the menstrual cycle phase (p > .05). In the surrounding endometrial stroma, single OTR-positive cells are identified, closely adjacent to the SMC myometrium. The total density of immunological OTR labeling in the adenomyotic lesion foci was 35.2 ± 1.4% (p < .05). This fact indicates a low degree of local oxytocin activity and predisposes to a decrease in the tonic activity of the smooth muscle of the myometrium.
A moderate (2 points) immunopositive response to vascular endothelial growth factor-A (VEGF-A) was detected in the vascular endothelium of myometrium – 38.4 ± 2.5%, (p < .05). In the SMC cytoplasm of the myometrium, this indicator was 11.4 ± 2.1%, 2 points, p < .05. In individual myometrium stroma cells, a weak (1 point) immunopositive response was observed – 9.7 ± 1.3% (p < .05).
In the course of the study, an increase in the processes of local neurogenesis and growth of nerve fibers was established due to an increase in the expression of the nervous system growth factor NGF in the myometrium stroma 2.4 times (57.2 ± 2.3 versus 23.1 ± 1.5%; p < .05), in the stroma and epithelium of glands of the focus of adenomyosis, 2.5 and 2.0 times, respectively (32.3 ± 1.4 against 13.7 ± 1.1% and 10.2 ± 1.1 versus 5.6 ± 1.7%; p < .05), in comparison with biopsy samples of morphological control.
In the cytoplasm of the epithelial lining cells of the ectopic endometrium glands, there was moderate (2 points) and weak (1 point) IHC VEGF-A expression determined – 27.5 ± 1.8% (p < .05). In individual endometrial stroma cells – 6.3 ± 1.4%, 1 point, (p < .05).
The data obtained indicate the absence of active neovasculogenesis processes and a decrease in the potential contractility of smooth myocytes, which predisposes to the inhibition of active spasmodic contractions of the myometrium, thereby not causing irritation of local nociceptive receptors, unlike the painful form of adenomyosis.
Conclusions
Based on the pathogenesis of pelvic pain in women with diffuse adenomyosis compared with the unpainful form of the disease, there is an increase in the activity of uterotonic factors of oxytocin and vasopressin, causing spontaneous dysperistaltic contractions of myometrial spasmodic nature associated with of increased expression of the same name receptors of the myometrium. This is evidenced by an increase in OTR expression in myometrium by 2.5 times (92.2 ± 2.1% versus 36.3 ± 2.4%, p < .05), in adenomyosis foci – by 2.09 times (73.7 ± 1.8% versus 35.2 ± 1.4%, p < .05). Compared to the painless form of adenomyosis, the myometrial innervation apparatus of patients with pelvic pain is characterized by a significantly higher expression of nerve growth factor – 2.4 times (57.2 ± 2.3 versus 23.1 ± 1.5; p < .05), which together increases the area of the local innervation field and triggers the pain syndrome.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Orazov MR, Demyashkin GA, Khamoshina MB, et al. Pelvic pain pathogenesis in external genital endometriosis: treatment options. Obstet Gynecol: News, Opin, Learning. 2017;3(17):117–124.
- Vercellini P, Viganò P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275.
- Orazov MR, Radzinsky VE, Khamoshina MB, et al. Pathological neurogenesis is a key element in the pathogenesis of pelvic pain associated withadenomyosis. Pathol Physiol Experimental Ther. 2018;62(1):59–64.
- Zhang X, Lu B, Huang X, et al. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil Steril. 2010;94(2):730–737.
- Barcena de Arellano ML, Arnold J, Vercellino GF, et al. Influence of nerve growth factor in endometriosis-associated symptoms. Reprod Sci. 2011;18(12):1202–1210.
- Mita S, Shimizu Y, Sato A, et al. Dienogest inhibits nerve growth factor expression induced by tumor necrosis factor-α or interleukin-1β. Fertil Steril. 2014;101(2):595–601.
- Goteri G, Lucarini G, Montik N, et al. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1alpha (HIF-1alpha), and microvessel density in endometrial tissue in women with adenomyosis. Int J GynecolPathol. 2009;28:157–163.
- Radzinsky VE, Orazov MR, Nosenko EN. Expression of vascular endothelial growth factor (VEGF) in the uterus tissues as one of algogenesismechanisms in adenomyosis associated with chronic pelvic pain. Pathol Physiol Experimental Ther. 2016;60(1):32–35.
- Jiang C, Cheng Z. Update of recent studies of adenomyosis-associated dysmenorrhea. Gynecol Minimally Invasive Ther. 2016;5(4):137–140.
- Mechsner S, Grum B, Gericke C, et al. Possible roles of oxytocin receptor and vasopressin-1α receptor in the pathomechanism of dysperistalsis and dysmenorrhea in patients with adenomyosis uteri. Fertil Steril. 2010;94(7):2541–2546.
- Guo SW, Mao X, Ma Q, et al. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril. 2013;99(1):231–240.
- Zhang Y, Yu P, Sun F, et al. Expression of oxytocin receptors in the uterine junctional zone in women with adenomyosis. Acta Obstet Gynecol Scand. 2015;94(4):412–418.
- Nie J, Liu X, Guo SW. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am J Obstet Gynecol. 2010;202(4):346.e1.
- Li Y, Zou S, Xia X, et al. Human adenomyosis endometrium stromal cells secreting more nerve growth factor: impact and effect. Reprod Sci. 2015;22(9):1073–1082.
