Abstract
Purpose
To explore the therapeutic effects of Bu-Shen-Ning-Xin decoction (BSNXD) on POI and the underlying mechanism.
Methods
VCD was used to induce the in vivo and in vitro POI model. HE staining was used to evaluate the pathological state of ovarian tissues. ELISA was used to detect the production of hormones in the serum and granule cells (GCs). An immunohistochemical assay was used to determine the expression of ATG7 and p-AKT in the ovarian tissues. The number of oocytes in POI rats was counted. The mitochondrial membrane potential (MMP) in oocytes and GCs was detected by flow cytometry. A Western blot assay was used to measure the expression of AKT, p-AKT, p-mTOR, mTOR, S6K, p-S6K, ULK1, p-ULK1, Beclin-1, Bcl-2, LC3-II, LC3-I, ATG7, and cleaved Caspase3. The numbers of autophagosomes were detected by transmission electron microscope and autophagic flux assay. The CCK-8 assay was used to detect the cell viability.
Results
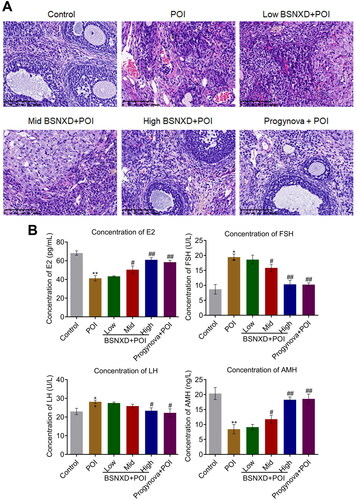
Decreased primary follicles in the ovarian tissues, elevated concentration of FSH, and LH, suppressed concentration of E2 and AMH in the serum, reduced number of oocytes, and mitochondrial dysfunction in oocytes induced by VCD were significantly reversed by BSNXD. Activated autophagy state and inhibited PI3K/AKT/mTOR pathway stimulated by VCD in both ovarian tissues and GCs were dramatically reversed by BSNXD. The protective effect of BSNXD on VCD-treated GCs was abolished by LY294002, an inhibitor of the PI3K/AKT/mTOR pathway.
Conclusion
Our data revealed that BSNXD alleviated POI by regulating autophagy of granule cells through activating PI3K/AKT/mTOR pathway.
摘要
目的
探讨补肾宁心汤(BSNXD)对POI的治疗作用及其潜在机制。
方法
采用VCD诱导体内外POI模型。 HE染色用于评价卵巢组织的病理状态。 ELISA 用于检测血清和颗粒细胞 (GC) 中激素的产生。免疫组织化学测定用于确定卵巢组织中ATG7和p-AKT的表达。计数POI大鼠的卵母细胞数。通过流式细胞术检测卵母细胞和GCs中的线粒体膜电位(MMP)。使用蛋白质印迹法测定 AKT、p-AKT、p-mTOR、mTOR、S6K、p-S6K、ULK1、p-ULK1、Beclin-1、Bcl-2、LC3-II、LC3-I 的表达、ATG7 和裂解的 Caspase3。用透射电镜和自噬通量测定法检测自噬体的数量。 CCK-8测定用于检测细胞活力。
结果
BSNXD可显著逆转VCD诱导的卵巢组织初级卵泡减少、FSH和LH浓度升高、血清E2和AMH浓度抑制、卵母细胞数量减少以及卵母细胞线粒体功能障碍。 BSNXD显著逆转VCD在卵巢组织和 GC 中激活的自噬状态和抑制的 PI3K/AKT/mTOR 通路。 BSNXD 对VCD处理的GCs 的保护作用被 LY294002(PI3K/AKT/mTOR 通路的抑制剂)消除。
结论
我们的数据显示, BSNXD 通过激活 PI3K/AKT/mTOR 通路调节颗粒细胞的自噬, 从而减轻 POI。
Introduction
Premature ovarian insufficiency (POI) is defined as the cessation of menstruation before the expected age of menopause, which is generally more junior than 40. The diagnosis of POI is mainly determined by significantly increased serum FSH level (>40 IU/L) [Citation1]. POI is clinically characterized by menoxenia and symptoms induced by estrogen deficiency, such as hot flashes, night sweats, sexual discomfort, vaginal dryness, and poor sleep [Citation2]. Currently, the main method for treating POI is the supplement or intervention of hormones, such as estrogen, progesterone, androgen, and dehydroepiandrosterone [Citation3]. However, significant side effects will be induced by the therapy of hormones, such as metabolic dysfunction, hyperglycemia, and osteoporosis [Citation4]. Therefore, it is urgent for the development of a novel strategy with minor side effects for the treatment of POI.
Currently, the pathogenesis of POI remains unknown, which is an obstacle to the development of a treating strategy for POI. Recently, follicular atresia and arrest of development induced by autophagy of granule cells (GCs) are found to take part in the pathological mechanism of POI [Citation5]. Autophagy is associated with the degradation of long-lived proteins and organelles by the lysosomal pathway, which is a peculiar phenomenon in eukaryotes [Citation6]. Autophagy is closely related to multiple physiological processes, including development, metabolism, immunoregulation, and senescence, which induces cell death by exceeding self-digestion and degradation of cell morphology [Citation7]. GCs are originated from follicles and responsible for the development of follicles. Whereas the autophagy of GCs is observed in all the stages of follicle development and is considered a critical element for the development and processing of multiple ovarian diseases [Citation8]. Song reported that the storage of primary follicles could be regulated by the autophagy of GCs, which might be a potential pathogenic factor for POI [Citation9]. Therefore, autophagy of GCs will be a promising target for developing strategies for treating POI.
BSNXD is a classical Chinese medicine applied for the treatment of menopause- and aging-related disorders [Citation10], which is mainly composed of Schisandra chinensis, Cornus, Parched white peony root, Poria cocos, Scutellaria baicalensis, Coptis chinensis, Rehmannia Glutinosa, Gelatin beads, and Semen nelumbinis. In addition, Kuntai capsule, the components of which is similar to BSNXD in the present study, is reported to exert significant regulatory effects on PI3K/AKT/mTOR pathway [Citation11]. The protective effect of BSNXD on the POI will be explored in this study to evidence the therapeutic property of BSNXD on POI.
Materials and methods
The preparation of BSNXD
The BSNXD was prepared by obtaining crude water extracts from the mixture of 8 crude herbs listed in according to the Chinese Pharmacopoeia 2005 [Citation12,Citation13].
Table 1. Composition and preparation of BSNXD.
Animal experiments
Seventy-two female 21-day SD rats were divided into 6 groups randomly: control, POI, Low BSNXD + POI, Mid BSNXD + POI, High BSNXD + POI, and Progynova + POI (n = 12/group). Rats in the control group were dosed with 2.5 mL/kg sesame oil (i.p) per day for 15 days, followed by orally administered with normal saline for a consecutive 45 days. The animals in the POI group were dosed with 80 mg/kg 4-vinylcyclohexene diepoxide (VCD, i.p) per day for 15 days, followed by orally administered with normal saline for a consecutive 45 days. In the Low BSNXD + POI group, Mid BSNXD + POI, and High BSNXD + POI groups, animals were dosed with 80 mg/kg VCD (i.p) per day for 15 days, followed by administered intragastrically with 3.74 g/(kg·d), 7.47 g/(kg·d), and 14.5 g/(kg·d) BSNXD for a consecutive 45 days, respectively. In the Progynova + POI group, animals were dosed with 80 mg/kg VCD per day for 15 days, followed by dosed intragastrically with 0.13 mg/(kg·d) Progynova for a consecutive 45 days, which was taken as the positive control. VCD was dissolved in 2.5 mL/kg sesame oil. Serum was achieved from each animal after anesthesia using 3 mL/kg chloral hydras, followed by oophorectomy. Part of the ovarian tissues was fixed in 10% formaldehyde for HE staining and 2.5% glutaraldehyde for the transmission electron microscope, while the rest was stored for molecular experiments. Animals were sacrificed by CO2 inhalation at the end of the experiments.
Ovulation induction and oocyte incubation
Ovulation was induced as described previously [Citation14]. Briefly, after treatments, rats were super-ovulated with 50 IU pregnant mare serum gonadotropin (Sansheng Pharmaceutical Co., Ltd., China). After 48 h, 50 IU human chorionic gonadotropin (Sansheng Pharmaceutical Co., Ltd., China) was administered intraperitoneally to trigger oocytes maturation. Oviducts were harvested 24 h later, followed by being teared with a needle to release oocytes into pre-heated G-MOPSplus medium (Vitrolife, Sweden). Cumulus-free oocytes were harvested from the oocyte-corona-cumulus complex after removing granulosa cells with the addition of 0.3 mg/mL hyaluronidase (Sigma, USA), which were washed thrice with G-MOPSplus and finally incubated at 37 °C in G-IVFplus medium (Vitrolife, Sweden), followed by counting using a flow cytometer (ACEA Biosciences, USA).
The detection of mitochondrial membrane potential (MMP) using a flow cytometry
Cells were incubated at 37 °C for 1 h, followed by centrifugation at 500 g for 5 min. Cells were then resuspended using 1 mL of DPBS and 1 µL of reagent JC-1 was added, followed by 15 min incubation at 37 °C. Then, cells were washed and resuspended in 500 µL of DPBS and then 1 µmol/L of propidium iodide was added prior to analysis. The MMP was evaluated by flow cytometry (ACEA Biosciences, USA) as the geometric mean of fluorescence of the JC-1 aggregates.
Hematoxylin and eosin (HE) staining [Citation15]
The ovarian tissue was removed from formalin solution and washed with tap water for 24 h, followed by being dehydrated with ethanol and transparent with xylene. Then tissues were embedded in paraffin and sliced using a slicer, followed by being stained with hematoxylin and eosin. After being sealed with neutral resin, images were obtained under the microscope, and 4 different fields of vision were selected for each section.
ELISA assay [Citation16]
The level of E2, FSH, LH, and AMH in the serum of each animal and the supernatant of cells was detected using the ELISA commercial kits (Mlbio, Beijing, China). The serum or supernatant was implanted in the 96-well plate together with the standards. Following incubation at 37 °C for 60 min, the medium was removed and conjugate reagents were introduced into wells. After incubation at 37 °C for 60 min. plates were added with the TMB solution and incubated for 15 min, followed by adding the stop solution. Lastly, the microplate reader (LIUYI BIOTECHNOLOGY CO., LTD., Beijing, China) was used to obtain the OD value at 450 nm.
Immunohistochemical analysis [Citation17]
The tissue slides were deparaffinated and rehydrated, which were further introduced with 3% H2O2 for 15 min. Subsequently, slides were blocked utilizing 5% skim milk, followed by incubating with primary antibody against p-AKT (1:500, Affinity, Melbourne, Australia) or ATG7 (1:500, Affinity, Melbourne, Australia). After washing, HRP-conjugated secondary antibody (Affinity, Melbourne, Australia) was added, followed by taking images using a light microscope (NIKON, Japan).
Transmission electron microscope (TEM) [Citation18]
GCs were fixed in 2.5% glutaraldehyde in PBS overnight. Subsequently, cells were embedded in epoxy resin, followed by collecting 50–70 nm ultrathin sections on copper grids. After counterstaining with aqueous uranyl acetate for 1 h, sections were stained with phosphotungstic acid for 60 min and then Reynolds’ lead citrate for 20 min, followed by checked on a TEM (Hitachi, Japan).
For tissues, slides were fixed with 2.5% glutaraldehyde phosphate buffer saline, followed by further fixing using 1% osmium tetroxide. After being dehydrated, slides were incubated with 4.8% uranyl acetate. Subsequently, slides were impregnated utilizing epoxy resins, followed by being contrasted using uranyl acetate. Lastly, slides were checked under a TEM (Hitachi, Japan).
Western blot analysis [Citation19]
After extracting proteins from ovarian tissues and GCs, quantification was conducted on isolated proteins with a BCA kit (Solarbio, Beijing, China), followed by adding 30 μg proteins onto 12% SDS PAGE to be separated. Then, proteins were shifted from the gel to the PVDF membrane, which was further incubated with 5% goat serum for blocking. Afterwards, the sample was introduced with the primary antibody against AKT (1:800, Abcam), p-AKT (1:800, Abcam), p-mTOR (1:800, Abcam), mTOR (1:800, CST), S6K (1:800, CST), p-S6K (1:800, CST), p-ULK1 (1:800, CST), ULK1 (1:800, CST), Beclin-1 (1:800, Abcam), Bcl-2 (1:800, Abcam), LC3-II (1:800, Abcam), LC3-I (1:800, Abcam), ATG7 (1:800, CST), cleaved-Caspase3 (1:800, CST), and GAPDH (1:800, Abcam), followed by adding the secondary antibody (1:2000, Bosterbio, California, USA) and incubated for 90 min. The bands were lastly visualized by exposing to ECL solution and quantified using the Image J software.
The isolation of ovary granulosa cells from rats
Eight female SD rats (7–9 weeks) were administered with 40 U PMSG followed by raising for 2 days. Rats were sacrificed to strip ovarian tissues and obtain follicle. Isolated GCs were kept in DMEM medium supplemented with 5% FBS, followed by being identified using the immunofluorescence assay with the FSH-R antibody. Cells from other parts of ovarian tissues were taken as a negative control (NC).
The preparation of serum containing BSNXD
Twenty rats (7–9 weeks) were divided into two groups: control and BSNXD. The animals in the BSNXD group were administered by gavage with 145 g/(kg·d) BSNXD (10-fold of the high dosage in the pharmacodynamic experiment) for a consecutive 3 days. The animals in the control group were administered by gavage using an equal volume of normal saline for a consecutive 3 days. The blood was collected from the aorta abdominalis 2 h post last dosage followed by collecting the serum after centrifugation. After filtration using 0.2 μm filter, the serum was incubated using water bath at 56 °C for 30 min to remove the antibodies, enzymes, complements, and other bioactivators. Finally, the blank serum and serum containing BSNXD were collected for subsequent experiments.
CCK-8 assay [Citation20,Citation21]
To screen the optimized concentration of VCD to stimulate GCs, GCs were incubated with VCD (0.5–3 mM) for 24 h. Cells were centrifugated at 300 g for 5 min to remove the supernatant, followed by adding 180 μL medium and 10 μL CCK-8 reagent. Following 3 h, the OD value at 450 nm was detected using the microplate reader (VEDENG, Shanghai, China).
Grouping of the in vitro assay
For the in vitro experiments, 5 groups were divided: Control, VCD, VCD + FSH, VCD + BSNXD, and VCD + BSNXD + LY294002. GCs in the control group were incubated with blank serum for 24 h and in the VCD group were incubated with 2 mM VCD for 24 h. The VCD + FSH group was taken as the positive control, in which GCs were incubated with 100 ng/mL FSH [Citation22] and 2 mM VCD for 24 h. GCs in the VCD + BSNXD group were incubated with the serum containing BSNXD for 2 h, followed by incubating with 2 mM VCD for 24 h. Lastly, GCs in the VCD + BSNXD + LY294002 group were treated with 50 μM LY294002 (an inhibitor of the AKT pathway [Citation23]) for 1 h, the serum containing BSNXD for 2 h, and VCD for 24 h, successively.
mRFP-GFP-LC3 fluorescence system assay [Citation24]
After different treating strategies, adenovirus transfection was conducted based on the mRFP-GFP-LC3 kit’s instruction (Hanbio, China), followed by observation under a fluorescence microscope (Olympus, Tokyo, Japan).
Statistical analysis
Mean ± SD was utilized to present the data, which was analyzed by the GraphPad software. The one-way ANOVA method was utilized to analyze data among more than three groups. p < .05 was taken as a significant difference in the present study. p < .01 was taken as an extremely significant difference in the present study.
Results
BSNXD significantly alleviated the POI symptom in animal model
Firstly, the therapeutic effects of BSNXD on POI rats were evaluated. The number of primordial follicles was reduced greatly in the POI group (), which was observed to be elevated in the High BSNXD + POI and Progynova + POI groups. In addition, compared in to control, the concentration of FSH and LH was dramatically promoted and the concentration of E2 and AMH was dramatically declined () in the animals treated with VCD, which were significantly reversed in the Mid BSNXD + POI, High BSNXD + POI, and Progynova + POI groups (*p< .05 vs. Control, **p < .01 vs. Control, #p < .05 vs. POI, ##p < .01 vs. POI).
BSNXD increased the number of oocytes and alleviated the mitochondrial dysfunction of oocytes in POI rats
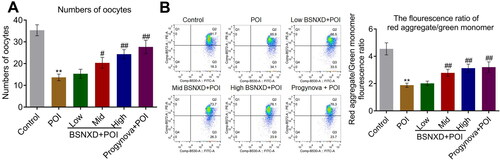
Oocytes were isolated from each animal and the number of oocytes in each group was counted. As shown in , the number of oocytes was declined from 35 to 14 in POI rats, which was greatly increased to 15, 20, and 24 by 3.74 g/(kg·d), 7.47 g/(kg·d), and 14.5 g/(kg·d) BSNXD, respectively. The number of oocytes in the Progynova + POI group was elevated to 27 (**p < .01 vs. Control, #p < .05 vs. POI, ##p < .01 vs. POI). Furthermore, in oocytes, the red aggregate/green monomer fluorescence ratio was dramatically declined in POI rats, which was greatly promoted by 7.47 g/(kg·d), and 14.5 g/(kg·d) BSNXD, as well as Progynova (**p < .01 vs. Control, ##p < .01 vs. POI), suggesting that the declined MMP in oocytes of POI rats was dramatically alleviated by BSNXD.
Figure 2. The declined number of oocytes and the mitochondrial dysfunction in oocytes of POI rats were alleviated by BSNXD. A. The number of oocytes was counted. B. The MMP in oocytes was evaluated by the red aggregate/green monomer fluorescence ratio detected using the flow cytometry (**p < .01 vs. Control, #p < .05 vs. POI, ##p < .01 vs. POI).
BSNXD suppressed the autophagy in the ovarian tissues of POI rats
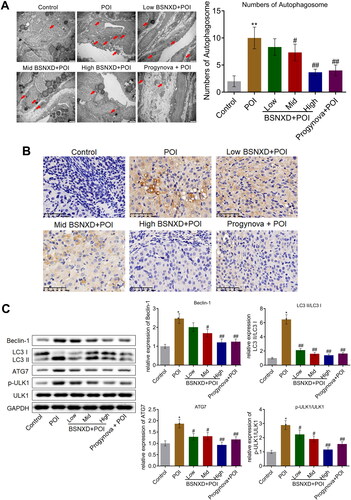
Subsequently, the autophagy state of the isolated ovarian tissues was evaluated. Increased number of autophagosome (red arrow) was observed in the POI group (), which decreased in the Low BSNXD + POI, Mid BSNXD + POI, High BSNXD + POI, and Progynova + POI groups, respectively. Furthermore, the result of immunohistochemical revealed that compared to control, ATG7 was upregulated in the ovarian tissues () in the POI group, which was macroscopically downregulated in the High BSNXD + POI and Progynova + POI groups. The level of Beclin-1, LC3-II/LC3-I, ATG7, and p-ULK1/ULK1 was significantly elevated in the POI group (), which was dramatically inhibited by the treatment of low, medium, and high dosage of BSNXD, and Progynova, respectively (*p<.05 vs. Control, #p < .05 vs. POI, ##p < .01 vs. POI).
Figure 3. The autophagy in the ovarian tissues of POI rats was alleviated by BSNXD. A. The number of autophagosome in ovarian tissues was observed and counted using TEM. B. Immunohistochemical assay was used to determine the expression level of ATG7 in the ovarian tissues. B. The expression of ULK1, p-ULK1, Beclin-1, LC3-II, LC3-I, and ATG7 was evaluated by Western blotting assay (*p<.05 vs. Control, #p < .05 vs. POI, ##p < .01 vs. POI).
BSNXD activated the PI3K/AKT/mTOR pathway in POI rats
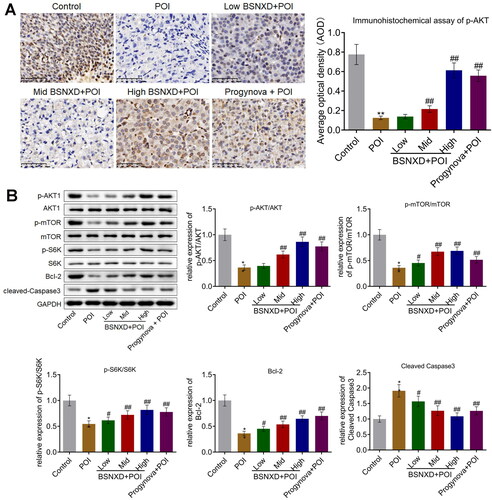
We subsequently investigated the activity of the PI3K/AKT/mTOR signal in ovarian tissues. We found that p-AKT was downregulated in VCD-treated rats (), which was macroscopically upregulated in the High BSNXD + POI, and Progynova + POI groups. The relative expression of p-AKT/AKT, p-mTOR/mTOR, and p-S6K/S6K () was suppressed in VCD-treated rats, which was dramatically promoted in the Low BSNXD + POI, Mid BSNXD + POI, High BSNXD + POI, and Progynova + POI groups, respectively. In addition, compared to control, Bcl-2 was significantly downregulated and cleaved Caspase-3 was pronouncedly upregulated in the POI group, which were pronouncedly abolished in the Low BSNXD + POI, Mid BSNXD + POI, High BSNXD + POI, and Progynova + POI groups (*p<.05 vs. Control, **p < .01 vs. Control, #p < .05 vs. POI, ##p < .01 vs. POI), respectively, indicating a promising anti-apoptotic effect of BSNXD.
Figure 4. BSNXD suppressed the autophagy in the ovarian tissues of POI rats. A. Immunohistochemical assay was used to determine the expression level of p-AKT in the ovarian tissues. B. The expression of AKT, p-AKT, p-mTOR, mTOR, S6K, p-S6K, Bcl-2, and cleaved Caspase3. was evaluated by Western blotting assay (*p<.05 vs. Control, **p < .01 vs. Control, #p < .05 vs. POI, ##p < .01 vs. POI).
BSNXD protected GCs stimulated by VCD by activating PI3K/AKT/mTOR pathway
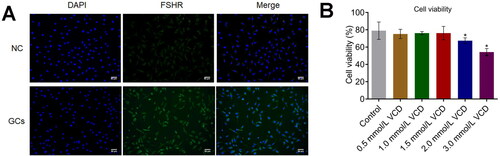
Firstly, the isolated GCs were identified by the immunofluorescence assay. Compared to NC, FSHR () was found highly expressed in isolated GCs, indicating a successful extraction of GCs from the animals. We found that cell viability () was maintained until 2 mM VCD was incubated (*p < .05 vs. Control). To exclude the autologous cytotoxicity of VCD, 2 mM was applied in the subsequent assays.
Figure 5. The identification of isolated GCs and the screening of the optimized concentration of VCD. A. The FSHR immunofluorescence was used to identify the isolated GCs. B. CCK-8 assay was used to detect the cell viability (*p < .05 vs. Control, **p < .01 vs. Control).
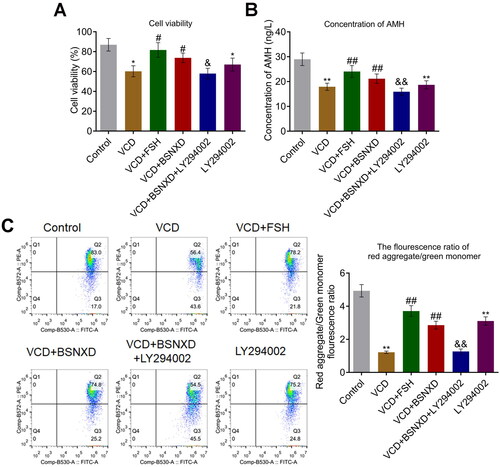
After different treating strategies, cell viability was evaluated by the CCK-8 assay. Cell viability () was dramatically inhibited by the stimulation of VCD and LY294002, which was reversed by FSH and the serum containing BSNXD. Compared to the VCD + BSNXD, cell viability was pronouncedly inhibited in the VCD + BSNXD + LY294002 group. In addition, significantly lower release of AMH () was observed in the VCD and LY294002 group. The declined level of AMH induced by VCD was significantly reversed by FSH and BSNXD, which were greatly abolished by the co-administration of LY294002. Furthermore, the MMP was evaluated by flow cytometry (). The red aggregate/green monomer fluorescence ratio was dramatically declined in the VCD and LY294002 groups, which was greatly elevated by FSH and BSNXD. Compared to the VCD + BSNXD, the red aggregate/green monomer fluorescence ratio was extremely reduced in the VCD + BSNXD + LY294002 group (*p < .05 vs. Control, **p < .01 vs. Control, #p < .05 vs. VCD, ##p < .01 vs. VCD, &p < .05 vs. VCD + BSNXD, &&p < .01 vs. VCD + BSNXD).
Figure 6. GCs stimulated by VCD was protected by BSNXD. A. CCK-8 assay was used to detect the cell viability. B. ELISA was used to detect the production of AMH released by GCs. C. The MMP in GCs was evaluated by the red aggregate/green monomer fluorescence ratio detected using the flow cytometry (*p < .05 vs. Control, **p < .01 vs. Control, #p < .05 vs. VCD, ##p < .01 vs. VCD, &p < .05 vs. VCD + BSNXD, &&p < .01 vs. VCD + BSNXD).
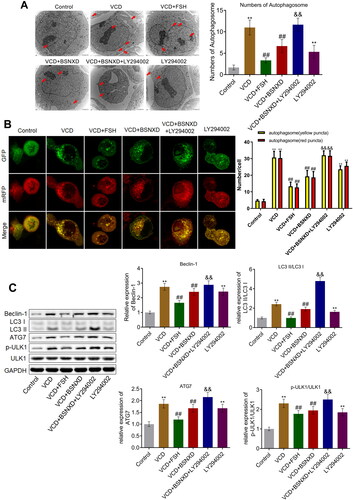
BSNXD ameliorated the autophagy in GCs induced by VCD through activating PI3K/AKT signal pathway
The number of autophagosome (red arrow) was increased greatly in the VCD and LY294002 group, which was declined in the VCD + FSH and VCD + BSNXD groups (). Compared with the VCD + BSNXD group, the number of autophagosome was dramatically increased in the VCD + BSNXD + LY294002 group. In addition, the ratio of red:yellow puncta () was higher in VCD and LY294002 treated GCs, which was declined in the VCD + BSNXD group. The decreased ratio of red:yellow puncta in the VCD + BSNXD group was reversed by the co-administration of LY294002. The level of Beclin-1, LC3-II/LC3-I, ATG7, and p-ULK1/ULK1 () was significantly elevated in the VCD and LY294002 group, which was dramatically reversed by FSH and serum containing BSNXD. Compared to the VCD + BSNXD group, the expression of Beclin-1, LC3-II/LC3-I, ATG7, and p-ULK1/ULK1 was greatly elevated in the VCD + BSNXD + LY294002 group (**p < .01 vs. Control, ##p < .01 vs. VCD, &&p < .01 vs. VCD + BSNXD).
Figure 7. BSNXD ameliorated the autophagy in GCs induced by VCD through activating PI3K/AKT signal pathway. A. The number of autophagosome in GCs was observed and counted using TEM. B. The state autophagy was visualized by mRFP-GFP-LC3 fluorescence system assay. C. The expression of ULK1, p-ULK1, Beclin-1, LC3-II, LC3-I, and ATG7 was evaluated by Western blotting assay (**p < .01 vs. Control, ##p < .01 vs. VCD, &&p < .01 vs. VCD + BSNXD).
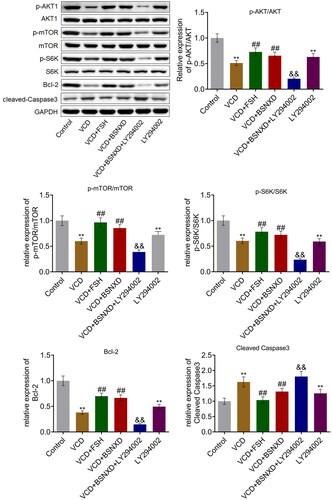
BSNXD activated the PI3K/AKT signal pathway and alleviated the apoptosis state of GCs induced by VCD
The suppressed level of p-AKT/AKT, p-mTOR/mTOR, and p-S6K/S6K in GCs () triggered by VCD or LY294002 was significantly elevated by FSH and serum containing BSNXD, which was further inhibited by the co-administration of LY294002. In addition, the downregulated Bcl-2 and upregulated cleaved Caspase-3 triggered by VCD were abolished by FSH and serum containing BSNXD, which were further abolished by the co-administration of LY294002 (**p < .01 vs. Control, ##p < .01 vs. VCD, &&p < .01 vs. VCD + BSNXD).
Figure 8. BSNXD activated the PI3K/AKT signal pathway and alleviated the apoptosis state of GCs induced by VCD. The expression of AKT, p-AKT, p-mTOR, mTOR, S6K, p-S6K, Bcl-2, and cleaved Caspase3. was evaluated by Western blotting assay (**p < .01 vs. Control, ##p < .01 vs. VCD, &&p < .01 vs. VCD + BSNXD).
Discussion
VCD is classic reagent used for the establishment of POI in the animals [Citation25] and the function of VCD on premature ovarian failure is reported to be related to the regulation of the Rictor/mTORC2 signal pathway, which further results in the inhibition of AKT signal pathway and thereafter the pathogenesis of apoptosis [Citation26]. This study applied VCD to induce POI model and the in vitro model in GCs, which were verified by declined amounts of primary follicles, elevated production of FSH and LH, and declined release of E2 and AMH, along with the inhibition of AKT signaling and the elevated apoptotic state. The results of the declined primary follicles were consistent with the data collected from POI mice intraperitoneal injected with 80 mg/kg VCD [Citation27]. The change on the production of hormones was consistent with the clinical and animal POI characteristics [Citation11,Citation28,Citation29]. After the treatment of BSNXD, these pathological symptoms were greatly alleviated, suggesting a potential therapeutic property of BSNXD on POI.
The formation of autophagosome with a bilayer structure is regarded as the main characteristic of autophagy [Citation30]. Phosphatidylinositol triphosphate kinase complex composed of Vps15, Beclin1, and Atg18 is reported to participate in autophagic vacuoles formation [Citation31]. The Atg12 protein binding system and LC3 lipidation system are the essential ubiquitin-like protein binding systems participating in autophagic vacuoles formation [Citation32]. GCs originate from follicles and regulate the development of follicles. Recently, GC autophagy is found to be associated with the follicular atresia [Citation33]. Our data indicated that both in the ovarian tissues isolated from POI animals and in the VCD-treated GCs, activation of autophagy was observed, which evidenced that the development of POI is accompanied by the autophagy in GCs. By the treatment of BSNXD, the state of autophagy both in vivo and in vitro was significantly alleviated. Furthermore, the severe apoptotic state triggered by VCD was ameliorated by BSNXD.
PI3K/AKT/mTOR signaling is the most classic pathway regulating autophagy, which is found to be associated with the cell proliferation, apoptosis and differentiation [Citation34]. Activated PI3K induces the phosphorylation of IRS-1 to produce PIP3, which combines with AKT and PDK1 to activate AKT. AKT directly initiates the phosphorylation of mTOR, which inhibits autophagy by mediating S6K/ULK1 pathway [Citation35]. Choi [Citation5] confirmed that follicular development and atresia was mediated by the PI3K/AKT/mTOR signaling regulated autophagy in GCs. We verified that PI3K/AKT/mTOR signaling mediated autophagy was exactly involved in the pathogenesis of POI stimulated by VCD. The therapeutic effect of BSNXD on POI animals and the protective effect of BSNXD on VCD-treated GCs were accompanied by the activation of PI3K/AKT/mTOR signaling. Furthermore, the inhibitor of PI3K/AKT/mTOR signaling, LY294002, abolished the protective effect of BSNXD on VCD-treated GCs. BSNXD might exert the therapeutic property on POI by mediating the PI3K/AKT/mTOR signal pathway. In future work, the mechanism will be evidenced by co-administrating the POI rats with both BSNXD and LY294002.
Collectively, BSNXD alleviated POI by regulating autophagy of granule cells via mediating PI3K/AKT/mTOR signaling.
Authors’ contributions
Conception and design of the research: Wenjun Chen.
Acquisition of data: Xiaoqing Dou and Mingxiao Wen.
Analysis and interpretation of data: Xingbei Chen.
Statistical analysis: Qun Zhou.
Drafting the manuscript: Xin Jin.
Revision of manuscript for important intellectual content: Hanyu Chen.
Consent for publication
Consent for publication has been achieved from all authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Qin Y, Jiao X, Simpson JL, et al. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21(6):787–808.
- Tsiligiannis S, Panay N, Stevenson JC. Premature ovarian insufficiency and long-term health consequences. Curr Vasc Pharmacol. 2019;17(6):604–609.
- Paciuc J. Hormone therapy in menopause. Adv Exp Med Biol. 2020;1242:89–120.
- Deligdisch L. Effects of hormone therapy on the endometrium. Mod Pathol. 1993;6:94–106.
- Choi J, Jo M, Lee E, et al. Induction of apoptotic cell death via accumulation of autophagosomes in rat granulosa cells. Fertil Steril. 2011;95(4):1482–1486.
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12.
- Mattiolo P, Yuste VJ, Boix J, et al. Autophagy exacerbates caspase-dependent apoptotic cell death after short times of starvation. Biochem Pharmacol. 2015;98(4):573–586.
- Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–263.
- Song ZH, Yu HY, Wang P, et al. Germ cell-specific Atg7 knockout results in primary ovarian insufficiency in female mice. Cell Death Dis. 2015;6(1):e1589–e1589.
- Wang L, Qiu XM, Hao Q, et al. Anti-inflammatory effects of a Chinese herbal medicine in atherosclerosis via estrogen receptor beta mediating nitric oxide production and NF-kappaB suppression in endothelial cells. Cell Death Dis. 2013;4:e551.
- Zhang H, Qin F, Liu A, et al. Kuntai capsule attenuates premature ovarian failure through the PI3K/AKT/mTOR pathway. J Ethnopharmacol. 2019;239:111885.
- Wang Y, Cui K, Zhao H, et al. Bushen Ningxin Decoction pharmacological serum promotes the proliferation and suppresses the apoptosis of murine osteoblasts through MAPK pathway. J Ethnopharmacol. 2009;122(2):221–226.
- Wang L, Qiu XM, Gui YY, et al. Bu-Shen-Ning-Xin decoction: inhibition of osteoclastogenesis by abrogation of the RANKL-induced NFATc1 and NF-kappaB signaling pathways via selective estrogen receptor alpha. Drug Des Devel Ther. 2015;9:3755–3766.
- Cornejo-Cortes MA, Sanchez-Torres C, Vazquez-Chagoyan JC, et al. Rat embryo quality and production efficiency are dependent on gonadotrophin dose in superovulatory treatments. Lab Anim. 2006;40:87–95.
- Zhang H, Qin F, Liu A, et al. Electro-acupuncture attenuates the mice premature ovarian failure via mediating PI3K/AKT/mTOR pathway. Life Sci. 2019;217:169–175.
- Ma Y, Ren Y, Dai ZJ, et al. IL-6, IL-8 and TNF-alpha levels correlate with disease stage in breast cancer patients. Adv Clin Exp Med. 2017;26(3):421–426.
- Yao H, Xu JK, Zheng NY, et al. Intra-articular injection of magnesium chloride attenuates osteoarthritis progression in rats. Osteoarthritis Cartilage. 2019;27(12):1811–1821.
- Lu X, Bao H, Cui L, et al. hUMSC transplantation restores ovarian function in POI rats by inhibiting autophagy of theca-interstitial cells via the AMPK/mTOR signaling pathway. Stem Cell Res Ther. 2020;11(1):268.
- Guo Y, Zhu X, Sun X. COTI-2 induces cell apoptosis in pediatric acute lymphoblastic leukemia via upregulation of miR-203. Bioengineered. 2020;11(1):201–208.
- Liu P, Feng Y, Dong D, et al. Enhanced renoprotective effect of IGF-1 modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury. Sci Rep. 2016;6:20287.
- Zhao L, Feng Y, Chen X, et al. Effects of IGF-1 on neural differentiation of human umbilical cord derived mesenchymal stem cells. Life Sci. 2016;151:93–101.
- Durlej M, Duda M, Knapczyk K, et al. Effects of transferrin on aromatase activity in porcine granulosa cells in vitro. Folia Histochem Cytobiol. 2008;46(4):423–428.
- Shultz JC, Vu N, Shultz MD, et al. The proto-oncogene PKCiota regulates the alternative splicing of Bcl-x pre-mRNA. Mol Cancer Res. 2012;10(5):660–669.
- Chen J, Yu Y, Li S, et al. MicroRNA-30a ameliorates hepatic fibrosis by inhibiting Beclin1-mediated autophagy. J Cell Mol Med. 2017;21(12):3679–3692.
- Cao LB, Liu HB, Lu G, et al. Hormone-like effects of 4-vinylcyclohexene diepoxide on follicular development. Front Cell Dev Biol. 2020;8:587.
- Chen Z, Kang X, Wang L, et al. Rictor/mTORC2 pathway in oocytes regulates folliculogenesis, and its inactivation causes premature ovarian failure. J Biol Chem. 2015;290(10):6387–6396.
- Van Kempen TA, Gorecka J, Gonzalez AD, et al. Characterization of neural estrogen signaling and neurotrophic changes in the accelerated ovarian failure mouse model of menopause. Endocrinology. 2014;155(9):3610–3623.
- Sun S, Chen H, Zheng X, et al. Analysis on the level of IL-6, IL-21, AMH in patients with auto-immunity premature ovarian failure and study of correlation. Exp Ther Med. 2018;16:3395–3398.
- Zhang H, Luo Q, Lu X, et al. Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice. Stem Cell Res Ther. 2018;9(1):20.
- Wang J, Davis S, Zhu M, et al. Autophagosome formation: where the secretory and autophagy pathways meet. Autophagy. 2017;13(5):973–974.
- Zhou C, Qian X, Hu M, et al. STYK1 promotes autophagy through enhancing the assembly of autophagy-specific class III phosphatidylinositol 3-kinase complex I. Autophagy. 2020;16(10):1786–1806.
- Ichimura Y, Kirisako T, Takao T, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–492.
- Tiwari M, Prasad S, Tripathi A, et al. Apoptosis in mammalian oocytes: a review. Apoptosis. 2015;20(8):1019–1025.
- Zhang DM, Liu JS, Deng LJ, et al. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis. 2013;34(6):1331–1342.
- Yahiro K, Tsutsuki H, Ogura K, et al. DAP1, a negative regulator of autophagy, controls SubAB-mediated apoptosis and autophagy. Infect Immun. 2014;82(11):4899–4908.