Abstract
Objective
To explore the role of isorhamnetin on polycystic ovary syndrome (PCOS) in rats.
Methods
Sprague Dawley (SD) rats were subcutaneously injected with dehydroepiandrosteron (DHEA) to establish PCOS model. Hematoxylin and eosin (H&E) staining and TdT-mediated dUTP Nick-End Labeling (TUNEL) were used to measure histological changes and apoptosis of ovary tissues. The levels of serum hormones and inflammatory factors in ovary tissues were measured by enzyme-linked immuno sorbent assay (ELISA).
Results
In DHEA-induced PCOS rats, the levels of serum glucose, insulin, testosterone and luteinizing hormone (LH) were enhanced, estradiol (E2), sex hormone-binding globulin (SHBG), follicle stimulating hormone (FSH) levels were decreased, inflammatory levels and apoptosis of ovary tissues were increased. Additionally, DHEA increased the body weight, ovary weight, and ovary volume, cystic follicles, and decreased corpus luteum. Moreover, the tumor necrosis factor (TNF) signaling pathway was activated in PCOS rats. The levels of TNF receptor superfamily member 1 A (TNFR1), TNF-α, and fas cell surface death feceptor (FAS) were enhanced in ovary tissues of DHEA induced PCOS rats. Isorhamnetin (ISO) treatment after DHEA modeling markedly reduced serum levels of glucose, insulin, testosterone and LH, increased E2, SHBG, FSH level, decreased inflammatory levels, and inhibited apoptosis and decreased body weight, ovary weight, and ovary volume. The levels of TNFR1, TNF-α, and FAS were markedly decreased after ISO treatment in PCOS rats. Additionally, ISO alone had no significant effect on rats.
Conclusion
Isorhamnetin inhibits inflammatory response to alleviate DHEA-induced PCOS in rats by inactivating the TNF signaling pathway.
Introduction
Polycystic ovary syndrome (PCOS) is clinically manifested by polycystic ovary, abnormal ovulation, and hyperandrogenemia; and is often accompanied by increased risk of metabolic disorders, cardiovascular disease, and diabetes [Citation1–3]. PCOS is a common endocrine disease in women of childbearing age with an incidence of about 10% [Citation1,Citation4]. At present, oral contraceptive [Citation5], letrozole [Citation6], and clomiphene are the main treatments for PCOS to induce ovulation, regulate menstrual cycle, reduce insulin resistance and reduce serum androgen levels [Citation7]. However, existing drug treatments may cause adverse reactions such as ovarian hyperstimulation syndrome, allergic dermatitis, abnormal glucose and lipid metabolism, and gastrointestinal side effects [Citation8,Citation9]. Thus, it is urgent to search new drug for PCOS treatment.
Isorhamnetin (ISO, ) is a natural flavonoid isolated from Hippophae rhamnoides L. with various biological properties [Citation10,Citation11]. Studies have demonstrated that ISO can protect against ischemia reperfusion-induced myocardial injury by attenuating oxidative stress [Citation12]. ISO alleviates high glucose-aggravated OGD/R injury in hippocampal neurons through Akt/SIRT1/Nrf2/HO-1 signaling pathway [Citation13]. Besides, ISO can induce continuous fluid secretion of ocular surface epithelium and relieve the symptoms of dry eye disease in mice [Citation13] and inhibit the inflammation of bronchial epithelial cells by regulating the NF-κB signaling pathway to relieve asthma [Citation14]. More importantly, ISO was reported to affect the estrogen biosynthesis in KGN cells [Citation15]. The study of Li et al. also demonstrated that ISO regulated the steroidogenesis, proliferation, and apoptosis of ovarian granulosa cells [Citation16]. However, it is still unknown whether ISO is effective in the treatment of PCOS.
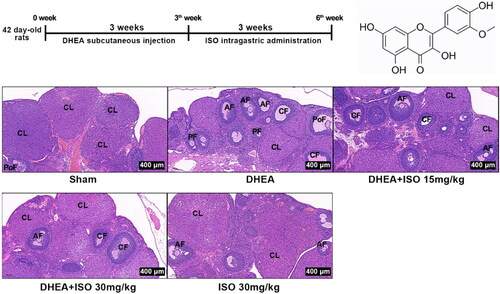
Figure 1. Experimental protocols for PCOS model and ISO treatment (A), chemical structure of ISO (B), and the representative pictures of ovarian tissues after H&E staining (scan bar = 400 μm) (C). CL, corpus luteum; AF, antral follicle; CF, cystic follicles; PF, primary follicle; PoF, preovulatory follicle.
In this study, we detected the serum hormone level, inflammation response, apoptosis, and analyzed the signaling pathways regulated by ISO to explore the effect of ISO in PCOS model rats.
Materials and methods
Materials
Dehydroepiandrosteron (DHEA, T84389, Shanghai yuanye Bio-Technology Co., Ltd). Isorhamnetin (ISO, B21554, Shanghai yuanye Bio-Technology Co., Ltd). Rat Testosterone ELISA Kit (ab285350, Abcam, Shanghai, China). Rat insulin ELISA Kit (SEKR-0033, Solarbio, Beijing, China). Rat Estradiol (E2) ELISA Kit (69-30465, MSK, Wuhan, China). Rat SHBG ELISA Kit (SEA396Ra, USCN, Wuhan, China). Rat luteinizing hormone (LH) ELISA Kit (D731015, Sango Biotech, Shanghai, China). Rat follicle stimulating hormone (FSH) ELISA Kit (D731057, Sango Biotech, Shanghai, China). Rat TNF-α ELISA Kit (ab236712, Rat IL-1β ELISA Kit (ab255730), Rat IL-6 ELISA Kit (ab234570), and Rat IL-18 ELISA Kit (ab213909) were purchased from Abcam (Shanghai, China). Colorimetric TUNEL Apoptosis Assay Kit (C1091), Proteinase K (ST532), RIPA buffer (P0013C), BCA Protein Assay Kit (P0011), ECL Chemiluminescence Kit (P0018S) were purchased from Beyotime (Shanghai, China). Primary antibodies against Bcl-2 (b182858), Bax (ab182733), c-caspase-3 (ab32042), TNFR1 (ab223352), TNF-α (ab215188), GAS (ab82419), GAPDH (ab9485) and secondary antibody (ab6721) were purchased from Abcam (Shanghai, China).
Animals
Total of 40 6-week old female SD rats were housed in an environment with ambient temperature of 25 ± 1 °C and relative humidity of 50%∼60% with a 12 h light/dark cycle. All rats were fed with normal chow diet and tap water ad libitum. The animal experiment was approved by the Animal Ethics Committee of Cangzhou people’s hospital (Approval number: 2020-0625).
PCOS model and grouping
Sixteen of 40 rats were randomly selected as Sham group (Rats were subcutaneously injected with 0.2 ml/d sesame oil for 21 days), and the remaining 24 of 40 rats were used to induce PCOS model. The PCOS model was induced by subcutaneously injection with 60 mg/kg/d DHEA for 21 days [Citation17,Citation18]. After 21 days of DHEA injection, the abdominal aorta blood sample was drawn and the hormonal assay was performed to confirm the induction of PCOS using commercially ELISA kits. After successful PCOS induction, DHEA rats were divided into 3 groups randomly (n = 8/group): DHEA group, DHEA + ISO 15 mg/kg group (intragastric administration of 15 mg/kg ISO for 21 days) and DHEA + ISO 30 mg/kg group (intragastric administration of 30 mg/kg ISO for 21 days). The Sham rats were divided into 2 groups randomly (n = 8/group): Sham group and ISO 30 mg/kg group (intragastric administration with 30 mg/kg/d ISO for 21 days).
From the beginning of the experiment, the weight of the rats was weighed weekly. The experimental protocols for PCOS model and ISO treatment was shown in . After weighing for the last time, the rats were anesthetized with 1% pentobarbital sodium (40 mg/kg) to collect blood sample and then sacrificed by excessive amount of pentobarbital sodium (100 mg/kg) to collect ovaries.
Hematoxylin and eosin (H&E) staining
Ovarian tissues were collected after the rats were sacrificed. The tissues were embedded by paraffin and then cut into 4 μm sections. The sections were deparaffinized in xylene. The sections were soaked in absolute ethanol to elute the xylene and then were soaked in 95%, 85%, and 70% ethanol for hydration. Finally, the sections were stained with hematoxylin for 10 min and eosin for 3 min. The histological changes of ovary were observed under light microscope.
Apoptosis detection
The apoptosis of rat ovary was detected by the TdT-mediated dUTP Nick-End Labeling (TUNEL) apoptosis assay kit. The deparaffinized slices of ovary tissues were hydrated in gradient ethanol, followed by incubation with DNase-free proteinase K. The slices were incubated in 3% H2O2 solution for 20 min at room temperature and then incubated with TUNEL reaction solution at 37 °C for 1 h. Slices were then washed with PBS and visualized by fluorescence confocal microscopy. TUNEL positive cells were counted using Image J (Bio Rad Laboratories).
Serum preparation and hormone detection
The blood was collected from the abdominal aorta after anesthesia. Blood glucose level was measured by Accu-Chek glucometer (ACCU-CHEK Active, Roche Diagnostics GmbH, Germany). The blood was centrifuged at 3000 r/min for 10 min. The supernatant was collected and stored at −80 °C. The levels of serum glucose, insulin, testosterone, SHBG, LH, and FSH were detected by ELISA kits according to the manufacturers. The free androgen index (FAI) was calculated as: Testosterone (nmol/l)/SHBG (nmol/l) × 100.
Pro-inflammatory factors detection
Ovaries were separated after the rats were sacrificed. The tissue homogenate was centrifuged in pre-cooled saline at 3000 RPM for 10 min to collect the supernatant. The levels of TNF-α, IL-1β, IL-6, and IL-18 were detected by ELISA kits.
Western blot
The total proteins were extracted form ovary tissues by using the RIPA buffer. Protein was separated on 10% SDS-PAGE and then transferred to PVDF membranes. The membranes were incubated with primary antibodies (Bcl-2, Bax, c-caspase-3, TNFR1, TNF-α, GAS, GAPDH) overnight at 4 °C. After incubation with secondary antibody for 1 h, the protein bands were detected by ECL Chemiluminescence Kit. GAPDH was used as an endogenous reference. Western blot results were quantified using Image J software (Rawak software Inc.).
Statistical analysis
All data in this study were presented as mean + SD and analyzed by using the GraphPad Prism 7.0 and SPSS 20.0. The difference between two groups was analyzed by Student’s t test. The differences among multiple groups were analyzed by using one-way analysis of variance followed by Bonferroni correction. p < 0.05 was considered to be statistically significant.
Results
DHEA induced PCOS rat model
At three weeks after DHEA injection, the blood glucose and serum levels of hormones were detected to evaluate whether PCOS model was successfully established. Compared with Sham group, the levels of serum glucose, testosterone, and LH were significantly increased in DHEA group (). Conversely, the serum levels of E2, SHBG, and FSH were markedly decreased in DHEA group compared with Sham group (). Additionally, the FAI and LH/FSH ratio were markedly increased in DHEA group compared with Sham group (). These results showed that the PCOS model was successfully established.
Table 1. The blood glucose and serum levels of hormones after DHEA treatment at third week.
Effects of ISO on hormones serum levels in DHEA-induced PCOS rats
At the 6th week, we detected the serum levels of glucose, insulin, E2, testosterone, SHBG, LH, and FSH. The serum levels of glucose, insulin, testosterone and LH were markedly increased () while serum levels of E2, SHBG and FSH was markedly decreased in DHEA-induced PCOS rats (). The FAI and LH/FSH ratio were significantly increased in DHEA rats compared with Sham group (). ISO treatment after DHEA modeling obviously inhibited the serum levels of glucose, insulin, testosterone, LH, FAI and LH/FSH ratio, while enhanced levels of E2, SHBG and FSH. ISO alone had no significant effect on serum glucose, insulin, E2, testosterone, SHEG, LH, and FSH in normal rats ().
Table 2. The serum levels of hormones at sixth week.
Effects of ISO on body weight and ovary weight of DHEA-induced PCOS rats
The body weight, ovary weight, and ovary volume were then analyzed. The body weight of rats was markedly increased after 3 weeks subcutaneous injection of DHEA. At the 6th week, the body weight in DHEA + ISO groups was obviously decreased compared with DHEA group (). The weight and volume of ovary in DHEA + ISO groups were also obviously lower than that in DHEA group (). In Sham group, mature follicles and corpus luteum were observed in ovary tissues. The ovary tissues of DHEA group showed increased cystic follicles and decreased corpus luteum. ISO intragastric administration after DHEA obviously alleviated the injury of ovary tissues, shown as the decreased number of cystic follicles and increased number of corpus luteum (). Compared with Sham group, ISO alone had no effect on body weight, ovary weight, ovary volume, and ovary structure.
Table 3. The body weight, ovary weight, and ovary volume of rats.
Effects of ISO on the levels of inflammation factors in DHEA-induced PCOS rats
The inflammatory changes of ovary tissues were measured by ELISA kits. In comparison with Sham group, the levels of TNF-α, IL-1β, IL-6, and IL-18 were obviously increased (). After ISO treatment, the levels of inflammatory factors were obviously lower in DHEA + ISO groups than that in DHEA group. Besides, ISO alone had no significant effect on inflammatory changes of normal rats ().
Table 4. The levels of TNF-α, IL-1β, IL-6, and IL-18 in ovary tissue homogenate at sixth week.
Effects of ISO on apoptosis of ovarian tissues in DHEA-induced PCOS rats
We then detected the effect of ISO on ovary tissues apoptosis of DHEA-induced PCOS rats. The TUNEL results showed that the TUNEL-positive cells in DHEA group were markedly higher than that in Sham group (). Compared with DHEA group, the TUNEL-positive cells were significantly decreased in DHEA + ISO groups. Additionally, we measured the apoptosis-related proteins in ovary tissues of DHEA rats by western blot (). DHEA modeling obviously suppressed Bcl-2 expression and enhanced levels of Bax and c-caspase-3 in ovary tissues. After IOS treatment, Bcl-2 level was markedly increased while the levels of Bax and c-caspase-3 were decreased (). Besides, ISO alone had no obvious effect on apoptosis of ovary tissues in normal rats.
Figure 2. The representative pictures of apoptosis of ovarian tissues (scan bar = 100 μm) (A), and the protein expression of Bcl-2, Bax, and c-caspase-3 in ovarian tissues (B).
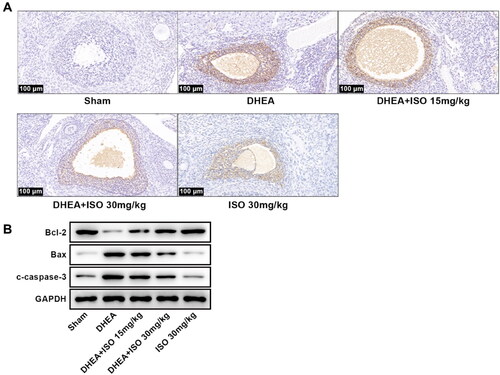
Table 5. The relative expression of apoptosis-related proteins and TNF signaling pathway related proteins in ovary tissue at sixth week.
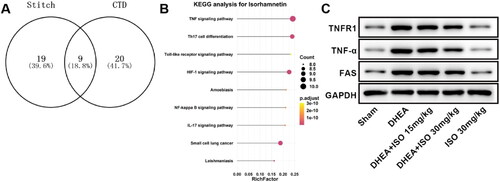
ISO activated the TNF signaling pathway in DHEA-induced PCOS rats
To explore the mechanism of ISO on PCOS, we analyzed the signaling pathways influenced by ISO in PCOS rats. The analysis showed that ISO could regulate the TNF signaling pathway in PCOS (). Compared with Sham group, the levels of TNFR1, TNF-α, and FAS in PCOS rats were obviously enhanced, indicating that PCOS activated the TNF signaling pathway. After ISO treatment, the levels of TNFR1, TNF-α, and FAS were obviously reduced in DHEA + ISO groups compared with DHEA group (, ). Besides, ISO alone had no significant effect on TNF signaling pathway.
Discussion
PCOS is a common disease caused by complex endocrine and metabolic abnormalities in women of childbearing age [Citation19,Citation20]. In present study, we investigated the role of ISO in PCOS rats and found that ISO alleviated PCOS by inhibiting the hormone level, inflammation response, and apoptosis via inactivating the TNF signaling pathway.
The PCOS rat model was successfully developed by administration of DHEA. DHEA is the main precursor of androgen, which can be converted into androstendione and testosterone by bioenzyme in vivo, and does not directly lead to androgen elevation. DHEA-induced PCOS model is one of the most widely used animal models due to its high stability [Citation21]. At 3th week, the changes of hormone levels in serum were evaluated. We found that DHEA markedly increased the levels of serum glucose, testosterone, and LH, while decreased the serum levels of E2, SHBG, and FSH, indicating successful modeling of PCOS. Additionally, DHEA markedly increased cystic follicles and decreased corpus luteum. The results at 6th week showed that ISO effectively alleviated these changes of hormone levels and PCOS induced injury of ovary. These results indicated a protective role of ISO on PCOS.
Hyperandrogenemia is one of the diagnostic components of PCOS, and free testosterone is the sensitive marker of excessive androgen secretion [Citation22,Citation23]. LH and FSH are secreted by the pituitary gland and act mainly on promoting ovulation. LH/FSH imbalance contributes to proliferation of ovarian theca cells, increase of steroidogenesis in women with PCOS, and ultimately leads to hyperandrogenemia [Citation24]. Studies have showed that LH level was dramatically increased while FSH level was decreased in patients with PCOS [Citation25,Citation26]. SHBG is a hepatic glycoprotein involved in the transportation and regulation of androgen [Citation27]. Studies have reported that SHBG can be used as a biomarker of PCOS [Citation28,Citation29]. In PCOS patients, SHBG concentrations are usually low due to elevated androgen levels [Citation30,Citation31]. E2 governs maturation of follicles and improvement of oocyte quality [Citation32,Citation33]. In PCOS cases, inadequate synthesis or disruption in E2 production is the main reason for the damage of oocyte maturation [Citation34]. In addition, PCOS patients are prone to insulin resistance [Citation35,Citation36], resulting in elevated insulin and glucose levels [Citation37,Citation38]. In line with these researches, serum levels of testosterone, LH, glucose, and insulin were markedly increased while serum E2, SHBG, and FSH levels were decreased in PCOS rats in our study. The levels of testosterone, LH, glucose, and insulin were significantly suppressed and serum levels of E2, SHBG, and FSH were enhanced after ISO treatment. These data suggested that ISO alleviated PCOS by regulating serum hormone levels in patients with PCOS.
The interaction of inflammatory factors in PCOS ovary suggests that inflammation is one of the most important risk factors for PCOS [Citation39]. It is reported that the levels of TNF-α and IL-6 in patients with PCOS are significantly higher than those in the controls [Citation40]. Meta-analysis showed that the TNF-α level of PCOS women was significantly higher than that of healthy controls [Citation41]. Compared with the controls matched with body mass index, women with PCOS had higher IL-6 levels [Citation42]. In our study, the levels of TNF-α, IL-6, IL-1β, and IL-18 in DHEA-induced PCOS rats were significantly higher than that in Sham rats. The data were consistent with those researches. It is worth noting that ISO dramatically reduced the levels of these inflammatory factors in ovary tissues of PCOS rats. These findings indicated that ISO could inhibit the inflammatory response of PCOS rats.
TNF-α is an inflammatory catabolic cytokine involved in systemic diseases [Citation43]. Many studies have shown that signal pathways are involved in the pathogenesis of PCOS [Citation44,Citation45]. The expression levels of TNF-α and TNFR2 were significantly increased in PCOS [Citation46]. TNF-α inhibitor etanercept significantly suppressed testosterone production in KGN granulosa cells [Citation47]. TNFR1 is the transmembrane TNF receptor that promotes cell death and inflammation [Citation48]. FAS, the death receptors of the TNF receptor superfamily, which bind to their natural ligands to induce apoptosis, play a key role in PCOS [Citation49,Citation50]. In this study, we found that TNF signaling pathway was activated in PCOS rats. However, ISO markedly suppressed the levels of TNFR1, TNF-α, and FAS in PCOS rats. Moreover, ISO obviously inhibited apoptosis of ovary tissues of PCOS rats. These results suggested that ISO may inactivate the TNF signaling pathway to alleviate apoptosis of ovary and PCOS progress.
Conclusions
This study proved that ISO exerts inhibitory effect on inflammatory response in PCOS rat model. ISO suppresses TNF signaling pathway, thus alleviating the damage of PCOS to ovary. To sum up, ISO has potential therapeutic effect in the prevention and treatment of PCOS.
Authors’ contributions
Conception and design: Fei Yu; Perform research: Fei Yu, Yanfeng Xue, Yunyan Zhao, and Long Zhang; Data analysis and interpretation: Fei Yu, Xiao He and Zheng Liu; Manuscript writing: All authors; Final approval of manuscript: All authors
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval
The experimental protocol of our study was performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Cangzhou people’s hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. Lancet. 2007;370(9588):1–7.
- Katsigianni M, Karageorgiou V, Lambrinoudaki I, et al. Maternal polycystic ovarian syndrome in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1787–1797.
- Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. 2017;232(2):R99–R113.
- Seow K-M, Chang Y-W, Chen K-H, et al. Molecular mechanisms of laparoscopic ovarian drilling and its therapeutic effects in polycystic ovary syndrome. IJMS. 2020;21(21):8147.
- Jin P, Xie Y. Treatment strategies for women with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34(4):272–277.
- Collée J, Mawet M, Tebache L, et al. Polycystic ovarian syndrome and infertility: overview and insights of the putative treatments. Gynecol Endocrinol. 2021;37(10):869–874.
- Cheng X, He B. Clinical and biochemical potential of antioxidants in treating polycystic ovary syndrome. IJWH. 2022;14:467–479.
- Lord JM, Flight IHK, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327(7421):951–953.
- Sharpe A, Morley LC, Tang T, et al. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;12(12):CD013505–CD013505.
- Luo Y, Sun G, Dong X, et al. Isorhamnetin attenuates atherosclerosis by inhibiting macrophage apoptosis via PI3K/AKT activation and HO-1 induction. PLoS ONE. 2015;10(3):e0120259–e0120259.
- Lu X, Liu T, Chen K, et al. Isorhamnetin: a hepatoprotective flavonoid inhibits apoptosis and autophagy via P38/PPAR-α pathway in mice. Biomed Pharmacother. 2018;103:800–811.
- Xu Y, Tang C, Tan S, et al. Cardioprotective effect of isorhamnetin against myocardial ischemia reperfusion (I/R) injury in isolated rat heart through attenuation of apoptosis. J Cellular Mol Med. 2020;24(11):6253–6262.
- Wu Y, Fan L, Wang Y, et al. Isorhamnetin alleviates high glucose-aggravated inflammatory response and apoptosis in oxygen-glucose deprivation and reoxygenation-induced HT22 hippocampal neurons through akt/SIRT1/Nrf2/HO-1 signaling pathway. Inflammation. 2021;44(5):1993–2005.
- Ren X, Han L, Li Y, et al. Isorhamnetin attenuates TNF-α-induced inflammation, proliferation, and migration in human bronchial epithelial cells via MAPK and NF-κB pathways. Anat Rec (Hoboken). 2021;304(4):901–913.
- Lu D-F, Yang L-J, Wang F, et al. Inhibitory effect of luteolin on estrogen biosynthesis in human ovarian granulosa cells by suppression of aromatase (CYP19). J Agric Food Chem. 2012;60(34):8411–8418.
- Li X, Chen H, Zhang Z, et al. Isorhamnetin promotes estrogen biosynthesis and proliferation in porcine granulosa cells via the PI3K/akt signaling pathway. J Agric Food Chem. 2021;69(23):6535–6542.
- Li T, Zhang T, Gao H, et al. Tempol ameliorates polycystic ovary syndrome through attenuating intestinal oxidative stress and modulating of gut microbiota composition-serum metabolites interaction. Redox Biol. 2021;41:101886–101886.
- Guo Z, Chen X, Feng P, et al. Short-term rapamycin administration elevated testosterone levels and exacerbated reproductive disorder in dehydroepiandrosterone-induced polycystic ovary syndrome mice. J Ovarian Res. 2021;14(1):64–64.
- Orisaka M, Hattori K, Fukuda S, et al. Dysregulation of ovarian follicular development in female rat: LH decreases FSH sensitivity during preantral-early antral transition. Endocrinology. 2013;154(8):2870–2880.
- Lee JJM, Yin C. Effects of glycyrrhizic acid (GA) in glucose and lipid homeostasis in pcos female rats. Open Conf Proceed J. 2013;4:170–170.
- Li Y, Zheng Q, Sun D, et al. Dehydroepiandrosterone stimulates inflammation and impairs ovarian functions of polycystic ovary syndrome. J Cell Physiol. 2019;234(5):7435–7447.
- Dumitrescu R, Mehedintu C, Briceag I, et al. The polycystic ovary syndrome: an update on metabolic and hormonal mechanisms. J Med Life. 2015;8(2):142–145.
- Stener-Victorin E, Holm G, Labrie F, et al. Are there any sensitive and specific sex steroid markers for polycystic ovary syndrome? Obstetric Gynecol Surv. 2010;65(6):383–385.
- Ashraf S, Nabi M, Rasool SUA, et al. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: a review. Egypt J Med Hum Genet. 2019;20(1).
- Yu Y, Cao Y, Huang W, et al. β-Sitosterol ameliorates endometrium receptivity in PCOS-Like mice: the mediation of gut microbiota. Front Nutr. 2021;8:667130–667130.
- Huang Y, Zhang X. Luteolin alleviates polycystic ovary syndrome in rats by resolving insulin resistance and oxidative stress. Am J Physiol-Endocrinol Metabol. 2021;320(6):E1085–E1092.
- Kahn SM, Hryb DJ, Nakhla AM, et al. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175(1):113–120.
- Goldštajn M, Toljan K, Grgić F, et al. Sex hormone binding globulin (SHBG) as a marker of clinical disorders [review]. Coll Antropol. 2016;40(3):211–218.
- Deswal R, Yadav A, Dang AS. Sex hormone binding globulin – an important biomarker for predicting PCOS risk: a systematic review and meta-analysis. Syst Biol Reproduct Med. 2018;64(1):12–24.
- Shorakae S, Ranasinha S, Abell S, et al. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin Endocrinol (Oxf). 2018;89(5):628–633.
- Urbano F, Chiarito M, Lattanzio C, et al. Sex Hormone-Binding globulin (SHBG) reduction: the alarm bell for the risk of Non-Alcoholic fatty liver disease in adolescents with polycystic ovary syndrome. Children. 2022;9(11):1748.
- Masjedi F, Keshtgar S, Zal F, et al. Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulosa cells of normal and polycystic ovaries. J Steroid Biochem Mol Biol. 2020;197:105521.
- Pellicer A, Valbuena D, Bauset C, et al. The follicular endocrine environment in stimulated cycles of women with endometriosis: steroid levels and embryo quality. Fertil Steril. 1998;69(6):1135–1141.
- Mazloomi S, Sanoeei Farimani M, Tayebinia H, et al. The association of mitochondrial translocator protein and voltage-dependent anion channel-1 in granulosa cells with estradiol levels and presence of immature follicles in polycystic ovary syndrome. J Reprod Infertil. 2022;23(3):148–159.
- Rojas J, Chávez M, Olivar L, et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth [review]. Int J Reprod Med. 2014;2014(10):719050.
- Broskey NT, Tam CS, Sutton EF, et al. Metabolic inflexibility in women with PCOS is similar to women with type 2 diabetes. Nutr Metab. 2018;15(75):018–0312.
- Siahaan SCP, Santoso, B, Widjiati. Effectiveness of moringa oleifera leaves on TNF-α expression, insulin levels, glucose levels and follicle count in Rattus norvegicus PCOS model. DMSO. 2022;15:3255–3270.
- Hussain L, Aamir N, Hussain M, et al. Therapeutic investigation of standardized aqueous methanolic extract of bitter melon (Momordica charantia L.) for its potential against polycystic ovarian syndrome in experimental animals’ model: in vitro and in vivo studies. Evid-Based Complement Alternat Med. 2022;2022:1–14.
- Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet. 2021;303(3):631–643.
- Ebejer K, Calleja-Agius J. The role of cytokines in polycystic ovarian syndrome. Gynecol Endocrinol. 2013;29(6):536–540.
- Gao L, Gu Y, Yin X. High serum tumor necrosis factor-alpha levels in women with polycystic ovary syndrome: a meta-analysis. PLoS ONE. 2016;11(10):e0164021–e0164021.
- Peng Z, Sun Y, Lv X, et al. Interleukin-6 levels in women with polycystic ovary syndrome: a systematic review and meta-analysis. PLoS ONE. 2016;11(2):e0148531–e0148531.
- Jackman RW, Rhoads MG, Cornwell E, et al. Microtubule-mediated NF-κB activation in the TNF-α signaling pathway. Exp Cell Res. 2009;315(19):3242–3249.
- Oróstica L, Astorga I, Plaza-Parrochia F, et al. Proinflammatory environment and role of TNF-α in endometrial function of obese women having polycystic ovarian syndrome. Int J Obes. 2016;40(11):1715–1722.
- Oróstica L, García P, Vera C, et al. Effect of TNF-α on molecules related to the insulin action in endometrial cells exposed to hyperandrogenic and hyperinsulinic conditions characteristics of polycystic ovary syndrome. Reprod Sci. 2018;25(7):1000–1009.
- Khajouei A, Hosseini E, Abdizadeh T, et al. Beneficial effects of minocycline on the ovary of polycystic ovary syndrome mouse model: molecular docking analysis and evaluation of TNF-α, TNFR2, TLR-4 gene expression. J Reprod Immunol. 2021;144:103289.
- Lang Q, Yidong X, Xueguang Z, et al. ETA-mediated anti-TNF-α therapy ameliorates the phenotype of PCOS model induced by letrozole [research support, Non-U S Gov’t]. PLoS ONE. 2019;14(6):e0217495.
- Fischer R, Kontermann RE, Pfizenmaier K. Selective targeting of TNF receptors as a novel therapeutic approach [review]. Front Cell Dev Biol. 2020;8:401.
- Frankel SK, Cosgrove GP, Cha S-I, et al. TNF-α sensitizes normal and fibrotic human lung fibroblasts to fas-induced apoptosis. Am J Respir Cell Mol Biol. 2006;34(3):293–304.
- Onalan G, Selam B, Baran Y, et al. Serum and follicular fluid levels of soluble fas, soluble fas ligand and apoptosis of luteinized granulosa cells in PCOS patients undergoing IVF [clinical trial randomized controlled trial]. Hum Reprod. 2005;20(9):2391–2395.