Introduction
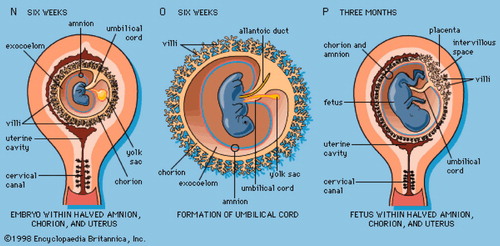
The placenta is a fetal organ, responsible for nutrient and gas exchange between the mother and fetus throughout pregnancy Citation[1]. At day 6.5, gastrulation begins in the posterior region of the embryo. Pluripotent cells located in the epiblast will produce the three germ layers of the embryo and germ cells, as well as the extraembryonic mesoderm of the yolk sac, amnion and allantois Citation[2-5]. Eight days after fertilisation, the trophoectoderm differentiate into two layers, cytotrophoblast and syncytiotrophoblast. This specialised cell type attaches to the maternal endometrium and invade into it differentiating into villous trophoblast and extravillous trophoblast Citation[6]. The villous trophoblastic layer consists of the outer syncytiotrophoblast, and a subjacent layer of proliferative cytotrophoblast cells resting on a fine lamina basalis. The ultrastructure of the syncytiotrophoblast shows minor modifications between 6 and 11 weeks' gestation, but there is a progressive decrease in the prominence of the cytotrophoblast cells, which by the end of the 10th week form a discontinuous layer. From 6 weeks of gestation, some capillaries are already in close proximity to the trophoblastic basal lamina Citation[7],Citation[8]. The extravillous cytotrophoblast cells migrate from the cell columns of the anchoring villi into the maternal endometrium during the first and early second trimesters of pregnancy Citation[9],Citation[10]. These cells start invading between the uterine glands as interstitial trophoblast, and down to the lumens of the vessels as endovascular trophoblast, soon after the blastocyst has implanted. Hustin and Schaaps Citation[11] have demonstrated that the extravillous trophoblast forms a cellular shell at the uterine-intervillous interface, and that extensions of this shell create plugs inside the lumen of most utero-placental arteries.
The amnion
It is a common misconception that the amnion is considered as derived from the extraembryonic endoderm or extraembryonic trophectoderm such as the yolk sac or chorion. Indeed, the amnion is derived from the epiblasts, which eventually give rise to all of the cell types of the embryo. The inner cell mass of the blastocyst differentiates into two layers, the hypoblast and epiblast. Epiblast is the source of all three germ layers. At the same time, the amniotic cavity appears within the epiblast. Cells adjacent to the cytotrophoblast are named amnioblasts, which differentiate to amniotic epithelia. Concomitantly, a layer of extraembryonic mesenchyme around the amniotic epithelium cell layer develops into the amniotic connective tissue. At days 31–35 of gestation, the amnion is made up of three layers. The innermost layer towards the amniotic cavity is the amniotic epithelium. Outside of this is a layer of connective tissue cells. The outermost layer separating the amnion from embryonic coelom consists of a solid, single layer of strongly flattened cells with marked cell borders. This mesothelium-like coating disappears when the amnion and chorion start to bind (at the end of 3 month). The fetal surface of the placenta is covered entirely by the amnion.
The amniotic fluid
The amniotic fluid ensures structure development and growth, cushions and protects the embryo, helps maintain consistent pressure and temperature and permits freedom of fetal movement, important for musculoskeletal development and blood flow Citation[12]. A wide variety of different origins has been suggested for the mixture of cells within amniotic fluid Citation[13]. Cells of different embryonic/fetal origins of all three germ layers have been reported to exist in amniotic fluid Citation[14],Citation[15]. These cells are thought to be sloughed from the fetal amnion, skin, digestive, respiratory and urogenital tracts.
Extraembryonic tissues as an alternative source of stem cells
According to the nature of its main components, the human placenta contains different populations of stem/progenitor cells such as haematopoietic, mesenchymal, trophoblastic, amniotic and embryonic stem cell like cells that remains to be further explored Citation[16].
Hematopoietic stem cells (HSC)
The first evidence of transplantable haematopoietic activity in the placenta can be found in the early transplantation studies by Till, Mc Culloch and Dancis and coworkers Citation[17-19]. The placenta has an important role as haematopoietic organ. During mouse embryogenesis, early haematopoietic progenitor cells were found to be two to fourfold more numerous in the placenta than in fetal liver or yolk sac Citation[20]. These progenitors are capable of forming all haematopoietic cell lineages of the adult animal. The murine placenta is an enriched source of haematopoietic progenitors, including CFU-GM, CFU-GEMM, BFU-E and high-proliferation-potential colony-forming cells Citation[20].
Fetal stem cells enter the maternal circulation as early as 6 weeks' gestation. It has been reported pregnancy associated progenitor cells (PAPCs). These cells have shown to home in areas of placental tissues injury. By the end of pregnancy, all pregnant women have these progenitor cells in their maternal circulation Citation[21]. In early embryonic life, HSC precursors migrate from the aortic-gonadotropinmesonephric region in the hind gut of the developing embryo, forming the fetal liver through the allantois. Migration then occurs from the fetal liver to bone marrow again through the primitive allantois/umbilical cord. These cells therefore are trapped at a very early embryological age and retain the properties of primitive stem cells. In 2006, Zeigler et al. Citation[22], demonstrated that both the allantois and the chorion have haematopoietic potential. They cultured them as explants on stromal cells in the presence of haematopoietic cytokines, or by CFU-C assays following a 2-day explant culture on plastic. Explants formed myeloid lineage cells and progenitors for definitive erythroid cells whereas Runx1-deficient explants did not.
Mesenchymal stem cells (MSCs)
MSCs are spindle-shaped cells that resemble fibroblasts. There are no markers which specifically and uniquely identify MSCs and therefore they are defined by their immunophenotypic profile, by their characteristic morphology; and their extensive capacity for self-renewal while retaining the ability to differentiate into a number of mesenchymal lineages. MSCs express neither haematopoietic markers (e.g. CD45, CD34, CD14) nor endothelial markers (e.g. CD34, CD31, vWF)Citation[23-25]. They do express a large number of adhesion molecules (e.g. CD44 and integrins), some stromal cell markers (e.g. SH-2, SH-3 and SH-4) and some cytokine receptors (e.g. IL-1R, TNF-aR) Citation[23-26]. Placental mesenchymal cells have a number of unique characteristics compared with other mesenchymal cells. They proliferate more quickly in culture than the comparable cells harvested from fetal or adult tissue, at a similar rate to that of mesenchymal amniocytes Citation[27]. First trimester and term placenta contains MSC that can be successfully isolated and expanded in vitroCitation[28],Citation[29]. The villous region contains the mesenchymal cell population Citation[30]. Second and third-trimester amnion represent a rich source for fetal MSCs that may contribute to the presence of MSCs in second-trimester amniotic fluid. Maternal MSCs can be cultured from both second-trimester and term deciduas basalis and deciduas parietalis. The phenotype of these cells is comparable with adult BM-derived MSCs, and the expansion potency is significantly higher than that of MSCs from adult BM Citation[14]. A population of multipotent cells has been isolated from the human term placenta Citation[31]. These placenta-derived multipotent cells (PDMCs) exhibit many markers common to mesenchymal stem cells including CD105/endoglin/SH-2, SH-3, and SH-4 and they lack hematopoietic, endothelial and trophoblastic specific cell markers.
In addition, PDMCs exhibit ESC surface markers of SSEA-4, TRA-1–61 and TRA-1–80. Adipogenic, osteogenic and neurogenic differentiation were achieved after culturing under the appropriate conditions.
Amniotic stem cells
Different origins have been suggested for the mixture of cells within amniotic fluid Citation[13]. Cells of different embryonic/fetal origins of all three germ layers have been reported to exist in amniotic fluid Citation[14],Citation[15]. It has been reported that cells cultured from amniotic fluid as well as placenta provide evidence that they may represent new stem cell sources with the potency to differentiate into different cell types Citation[32]. Interestingly, it has been demonstrated that a subpopulation of cells in amniotic fluid express Oct4 mRNA, which is a well-known pluripotency marker Citation[33]. The fact that certain progenitor cells are found in the amniotic fluid was first reported in 1993, when small, nucleated, round cells identified as haematopoietic progenitor cells were found before the 12th week of gestation Citation[34]. PDSCs can be isolated also from the amniotic membranes, although the placenta seems to be a more abundant source. The stem cells isolated from the amniotic membranes are named as amniotic epithelial (AE) stem cells Citation[35]. AE cells are known to have low level expression of major histocompatibility complex antigens, and a less restricted differentiation potential. Recently, it has been reported that AE cells from term placenta express several pluripotency stem cell surface markers and are able to differentiate into cell types from all three germ layers Citation[35]. Because the amnion differentiates from the epiblast at a time when it retains pluripotency, it is reasonable to speculate that AE cells may have escaped the specification that accompanies gastrulation and may preserve some of the original characteristics. Thus, some AE cells express cell surface antigens characteristic of pluripotent embryonic stem (ES) cells such as SSEA-3, SSEA-4, TRA 1–60 and TRA 1–81. In addition, AE cells express molecular markers of pluripotency, Oct-4 and Nanog, and differentiate into all three germ layers was demonstrated in vitro. The expression of this molecular marker in AE cells suggests that they maintain the pluripotency of the epiblast before the gastrulation and its cytosol-positive staining indicates that their developmental stage may be similar to that of embryonic germ cells (EG cells) Citation[36]. Pluripotency of AE cells has been demonstrated in vitro in different experiments. In 2004, Tamagawa et al. Citation[37], established cell lines from cells isolated from whole amnion, which contain AE cells and AMFs. The cell line HAM-1 was mixed with mouse early embryonic stem cells (EES-7) to form an aggregation chimera. The xenogeneic chimera embryo was maintained until all three primordial germ layers were formed. The contribution of amnion-derived stem cells to all three germ layers of the xenogeneic aggregation chimera was demonstrated. Tamagawa's report is a remarkable demonstration of pluripotency in vitro. When AE cells were cultured as an adherent monolayer for several weeks, they can form spontaneously embryoid bodies-like structures with similar structure to embryoid bodies (EB) described cultures of ES cells. Stem cell markers were examined by immunohistochemistry in the spheroid structures after 24 days of culture. Immunohistofluorescent staining revealed that the cells on the outer edge of each EB-like spheroid structure expressed stem cell-specific cell surface antigens SSEA-3, SSEA-4, TRA 1–60 and TRA 1–81 Citation[35]. The stem cell molecular marker genes, Oct-4 and Nanog, are also predominantly expressed in the cells of the spheroids. Furthermore, because the amnioblast is sometimes also referred to as the amniotic ectoderm, there is a propensity for the AE cells to differentiate towards the neural lineage (ectodermal). This fact was demonstrated by detecting the expression of markers of glial and neuronal progenitor cells Citation[38]. The potential for AE cells to differentiate to endoderm was also reported. In 2000, Sakuragawa et al. Citation[39] showed albumin secretion from cultured human AE cells. In another experiment, Takashima et al. 2004 Citation[40] used hepatocyte growth factor, FGF-2, heparin sodium salt and oncostain M to induce albumin-producing hepatocyte-like cells. The same group also reported that they could induce AE cells to produce insulin and could normalise blood glucose in diabetic model animals following transplantation of the AE cells in to the spleen Citation[41]. AE cells differentiation capability to mesoderm was reported by the demonstration of expression of a-actinin protein in cardiac cells obtained from AE cells Citation[42]. However, AE cells show a senescent nature. After 6–10 passages in culture, AE cells fall into a terminally no dividing state. If AE cells are cultured at very low densities, senescence occurs even earlier. This discrepancy indicates that the senescence mechanism is not simply controlled by telomere length. Integrin-dependent epidermal growth factor receptor (EGFR) activation is one of the signalling mechanisms involved in cell proliferation Citation[43]. AE cell proliferation may depend on the integrin and EGFR-associated molecular complex and cell-to-cell interactions, which are facilitated by higher density culture Citation[44]. The use of de-epithelialised human amniotic membrane has been well documented in ophthalmic surgery for the treatment of Stevens-Johnson syndrome, ocular cicatricial pemphigoid, acute thermal and alkali burns, pterygium surgery and limbal stem cell transplantation Citation[45],Citation[46]. For this application, only the basement membrane of amnion is needed to serve as a biological scaffold for epithelial cell migration as well as for anti-inflammatory properties. The low antigenicity of amnion may be an advantage for amniotic epithelium-derived stem cell transplantation or cell replacement therapy.
In 2007, De Coppi et al. Citation[47] reported the isolation of human and rodent amniotic fluid-derived stem (AFS) cells that express embryonic and adult stem cell markers. Undifferentiated AFS cells represent 1% of the population cells obtained from amniocentesis and are characterised by the expression of the receptor for stem cell factor c-Kit. AFS cells expand extensively without feeders, double in 36 h and are not tumorigenic are broadly multipotent. Clonal human lines were induced to differentiate into cell types representing each embryonic germ layer, for example differentiate into specialised functional population such as neural, hepatic and osteogenic lineages Citation[48-50].
In this reviewed the actual knowledge about the different types of placenta derived stem cells (PDSC). Thus, the PDSC may be one of the different fields of stem cell research in the stem cell biology and regenerative medicine.
References
- Georgiades P, Ferguson-Smith A C, Burton G J. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 2002; 23: 3–19
- Downs K M, Harmann C. Developmental potency of the murine allantois. Development 1997; 124: 2769–2780
- Downs K M, Hellman E R, McHugh J, Barrickman K, Inman K E. Investigation into a role for the primitive streak in development of the murine allantois. Development 2004; 131: 37–55
- Gardner R L, Beddington R S. Multilineage “stem” cells in the mammalian embryo. J Cell Sci Suppl 1988; 10: 11–27
- Loebel D A, Watson C M, De Young R A, Tam P P. Lineage choice and differentiation in mouse embryos and embryonic stem cells. Dev Biol 2003; 264: 1–14
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher S J. Trophoblast differentation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest 2004; 114: 744–754
- Demir R, Kaufmann P, Castellucci M, Erbengi T, Kotowski A. Fetal vasculogenesis and angiogenesis in human placental villi. Acta Ana 1989; 136: 190–203
- Jauniaux E, Jurkovic D, Campbell S, Hustin J. Doppler ultrasound features of the developing placental circulations: correlation with anatomic findings. Am J Obstet Gynecol 1992; 166: 585–587
- Pijnenborg R, Dixon G, Robertson W B, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980; 1: 3–19
- Pijnenborg R, Bland J M, Robertson W B, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983; 4: 397–414
- Hustin J, Schaaps J P. Echographic and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol 1987; 157: 162–168
- Baschat A A, Hecher K. Fetal growth restriction due to placental disease. Semin Perinatol 2004; 28: 67–80
- Medina-Gomez P, Del Valle M. The culture of amniotic fluid cells: an analysis of the colonies, metaphase and mitotic index for the purpose of ruling out maternal cell contamination. Ginecol Obstet Mex 1988; 56: 122–126
- In 't Anker P S, Scherjon S A, Kleijburg-van der Keur C, Noort W A, Claas F H, Willemze R, Fibbe W E, Kanhai H H. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003; 102: 1548–1549
- Prusa A R, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, Bernaschek G, Hengstschlager M. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol 2004; 191: 309–314
- Miki T, Mitamura K, Ross M A, Stolz D B, Strom S C. Identification of stem cell marker-positive cells by immunofluorescence in term human amnion. J Reprod Immunol 2007; 75: 91–96
- Till J E, McCulloch E A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 1961; 14: 212–222
- Dancis J, Jansen V, Gorstein F, Douglas G W. Hematopoietic cells in mouse placenta. Am J Obstet Gynecol 1968; 100: 1110–1121
- Dancis J, Jansen V, Brown G F, Gorstein F, Balis M E. Treatment of hypoplastic anemia in mice with placental transplants. Blood 1977; 50: 663–670
- Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development 2003; 130: 5437–5444
- Cetrulo C L, Cetrulo K J. Placenta and Pregnacy stem cells. Stem Cells Rev 2006; 2: 79–80
- Zeigler B M, Sugiyama D, Chen M Guo Y, Downs K M, Speck N A. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development 2006; 133: 4183–4192
- Pittenger M F, Mackay A M, Beck S C, Jaiswal R K, Douglas R, Mosca J D, Moorman M A, Simonetti D W, Craig S, Marshak D R. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147
- Van den Heuvel R L, Versele S R, Schoeters G E, Vanderborght O L. Stromal stem cells (CFU-f) in yolk sac, liver, spleen and bone marrowof pre- and postnatal mice. Br J Haematol 1987; 66: 15–20
- Majumdar M K, Thiede M A, Mosca J D, Moorman M, Gerson S L. Phenotypic and functional comparison of cultures of marrow derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 1998; 176: 57–66
- Majumdar M K, Keane-Moore M, Buyaner D, Hardy W B, Moorman M A, McIntosh K R, Mosca J D. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 2003; 10: 228–241
- Kaviani A, Perry T E, Barnes C M, Oh J T, Ziegler M M, Fishman S J, Fauza D O. The placenta as a cell source in fetal tissue engineering. J Pediatr Surg 2002; 37: 995–999, discussion 995–999
- Portmann-Lanz C B, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, Surbek D V. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol 2006; 194: 664–673
- Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int 2006; 30: 681–687
- Zhang X, Nakaoka T, Nishishita T, Watanabe N, Igura K, Shinomiya K, Takahashi T A, Yamashita N. Efficient adeno-associated virus-mediated gene expression in human placenta-derived mesenchymal cells. Microbiol Immunol 2003; 47: 109–116
- Yen B L, Huang H I, Chien C C, Jui H Y, Ko B S, Yao M, Shun C T, Yen M L, Lee M C, Chen Y C. Isolation of amniotic stem cell lines with potential for therapy. Stem Cells 2005; 23: 3–9
- Prusa A R, Hengstschlager M. Amniotic fluid cells and human stem cell research: a new connection. Med Sci Monit 2002; 8: 253–257
- Prusa A R, Marton E, Rosner M, Bernaschek G, Hengstschlager M. Oct4 expressing Cells in human amniotic fluid: A new source for stem cell research?. Hum Reprod 2003; 18: 1489–1493
- Torricelli F, Brizzi L, Bernabei P A, Gheri G, Di Lollo S, Nutini L, Lisi E, Di Tommaso M, Cariati E. Identification of hematopoietic progenitor cells in human amniotic fluid before the 12th week of gestation. Ital J Anat Embryol 1993; 98: 119–126
- Miki T, Lehmann T, Cai H, Stolz D, Strom S. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005; 23: 1549–1559
- Jiang Y, Jahagirdar B N, Reinhardt R L, Schwartz R E, Keene C D, Ortiz-Gonzalez X R, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotente of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–49
- Tamagawa T, Ishiwata I, Saito S. Establishment and characterization of a pluripotent stem cell line derived from human amniotic membranes and initiation of germ layers in vitro. Hum Cell 2004; 17: 125–130
- Hori J, Wang M, Kamiya K, Takahashi H, Sakuragawa N. Immunological characteristics of amniotic epithelium. Cornea 2006; 25: 53–58
- Sakuragawa N, Enosawa S, Ishii T, Thangavl R, Tashiro T, Okuyama T, Suzuki S. Human amniotic epithelial cells are promising transgene carriers for allogeneic cell transplantation into liver. J Hum Genet 2000; 45: 171–176
- Takashima S, Ise H, Zhao P, Akaike T, Nikaido T. Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell Struct Funct 2004; 29: 73–84
- Wei J P, Zhang T S, Kawa S, Aiawa T, Ota M, Akaike T, Kato K, Konishi I, Nikaido T. Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant 2003; 12: 545–552
- Takahashi T, Lord B, Schulze P C, Fryer R M, Sarang S S, Gullans S R, Lee R T. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation 2003; 107: 1912–1916
- Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J 1998; 17: 6622–6632
- Schlaepfer D D, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol 1996; 16: 5623–5633
- Dua H S, Gomes J A, King A J, Maharajan V S. The amniotic membrane in ophthalmology. Surv Ophthalmol 2004; 49: 51–77
- Gomes J A, Romano A, Santos M S, Dua H S. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol 2005; 16: 233–240
- De Coppi P, Bartsch G, Jr, Siddiqui M M, Xu T, Santos C C, Perin L, Mostoslavsky G, Serre A C, Snyder E Y, Yoo J J, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007; 25: 100–106
- Kolambkar Y M, Peister A, Soker S, Atala A, Guldberg R E. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol 2007; 38: 405–413
- Perin L, Giuliani S, Jin D, Sedrakyan S, Carraro G, Habibian R, Warburton D, Atala A, De Filippo R E. Renal differentiation of amniotic fluid stem cells. Cell Prolif 2007; 40: 936–948
- Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe S P, Driscoll B, Bellusci S, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells 2008; 26: 2902–2911