Abstract
Changes of mean platelet volume (MPV) and platelet count (PC) could be a marker or a predictor of acute stroke (AS). We conducted a systematic review and meta-analysis of the published literature on the reporting of MPV and PC in AS. Studies were included in accordance with Patient Population or Problem, Intervention, Comparison, Outcomes, and Setting framework. The PRISMA strategy was used to report findings. Risk of bias was assessed with the Newcastle-Ottawa Scale. We included 34 eligible articles retrieved from the literature. PC was significantly lower in AS patients [standardized mean difference (SMD) = − 0.30, (95% CI: − 0.49 to − 0.11), N = 2492, P = .002] compared with controls (N = 3615). The MPV was significantly higher [SMD = 0.52 (95% CI: 0.28–0.76), N = 2739, P < .001] compared with controls (N = 3810). Subgroup analyses showed significantly lower PC in both ischemic stroke (Difference SMD = −0.18, 95% CI: −0.35–0.01) and hemorrhagic stroke (−0.94, −1.62 to −0.25), but only samples by citrate anticoagulant showed significantly lower result for patients compared to controls (−0.36, −0.68 to −0.04). Ischemic stroke patients had higher MPV (0.57, 0.31–0.83), and samples by Ethylenediaminetetraacetic acid (EDTA) anticoagulant showed significantly higher result for patients compared to controls (0.86, 0.55–1.17). PC and MPV appeared to be significantly different between patients with AS and control populations. MPV was significantly higher in ischemic stroke and PC was significantly lower in both ischemic and hemorrhagic strokes. These characteristics might be related to AS and associated with it. It is advisable to pay attention to elapsed time between phlebotomy and hematology analysis, anticoagulant and hemocytometer types in AS.
Systematic review registration
This meta-analysis is registered on the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42017067864 (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=67864).
Introduction
Incidence of stroke in Europe is 95 to 290/100,000 per year and it is the fourth cause of death in the United States [Citation1,Citation2]. The important role of platelets in the pathogenesis of the atherothrombosis and ischemic stroke has been documented [Citation3,Citation4]. Mean platelet volume (MPV) is regarded as a marker of platelet turnover [Citation5]. More immature, thereby larger platelets usually contain more granules, thus releasing more chemokines promoting further platelet aggregation as well as activation. Elevated MPV simultaneously with the elevated PC increases the risk of thrombosis [Citation6]. Significant increased MPV in patients with deep vein thrombosis and isolated elevated PC in patients with pulmonary embolism has been revealed [Citation7].
Modification of platelet function and consequently a hypercoagulable state has been suggested in patients with both ischemic and hemorrhagic strokes [Citation8]. It has been shown that PC and MPV are independent predictors for poor outcome in primary intracerebral hemorrhage (PICH) [Citation9]. There has also been a body of interest for PC and MPV in stroke patients. However, these studies have yielded inconsistent findings. Some groups reported that patients with AS had significantly increased MPV or PC compared with controls [Citation10,Citation11], whereas other studies found decreased MPV or PC values in AS patients [Citation12,Citation13]. Additionally, some studies have observed no association between these parameters and AS [Citation14,Citation15]. Taking these inconsistent findings into consideration, we undertook a meta-analysis to investigate the association between MPV, PC, and AS.
Methods
Protocol and Registration
The present systematic review and meta-analysis was conducted, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation16] and was demonstrated according to the MOOSE (Meta-analysis of Observational Studies in Epidemiology) statement [Citation17]. The systematic review and meta-analysis protocol was registered with the PROSPERO database at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=67864
Search Strategy and Study Selection
Eligibility criteria were in accordance with the Patient Population or Problem, Intervention, Comparison, Outcomes, and Setting inclusion criteria [Citation16]. We modified the PICOS excluding I, where I represents the Interventions, since no interventional studies were included. Studies were included if they (1) studied adult individuals, (2) included a control group, (3) involved the diagnosis or evaluation of acute stroke, (4) included the detailed data about platelet indices in both patient and control groups, and (5) were case-control or cross-sectional studies. There was no language, country or time restriction. We excluded reviews, editorials, commentaries, abstracts, and conference proceedings.
Cerebrovascular events and acute stroke were assessed in this study as the outcomes.
The basis for our analysis was the comprehensive search for the relevant studies published in Medline, EBSCOhost, Web of Science, ScienceDirect, Google Scholar and Scopus from inception to July 2017 (Supplementary file 1). The last search was performed on August 2019 and we included published studies. Google Scholar was searched by Harzing’s Publish or Perish program (Harzing, A.W., 2007, Publish or Perish, available from https://harzing.com/resources/publish-or-perish). The keywords used for systematic searches were as follows: platelet indices, platelet parameters, mean platelet volume, platelet count, stroke, and cerebrovascular disease.
Data Extraction
Two reviewers independently extracted the Data (AHS and FS) by using a standard form and cross-checked. Discrepancies were resolved by consensus or through discussion with the third author (ZC). We used bibliographies of the retrieved articles for other relevant studies. All data were prospectively collected.
After the first search, reviews remained on the list to collect appropriate references. At the second step, we removed all reviews from the list of accepted publications.
We used the Newcastle-Ottawa Scale (NOS) to evaluate the quality of the eligible studies [Citation18]. NOS includes a selection of the study population domain, comparability of the groups’ domain, and ascertainment of the outcome domain. Articles identified as high quality with NOS scores 6–9, whereas scores of 0–5 were considered to indicate poor quality. In those studies that provided only median with range or interquartile range values, mean and standard deviation were calculated according to Wen et al. [Citation19].
Data Synthesis and Statistical Analysis
This study was conducted by following the guidelines of Viechtbauer and Huzsvai and Balogh [Citation20,Citation21]. The meta-analysis involved between-study variances and also covariates for limiting publication biases. Standardized mean differences (SMDs) were estimated for MPV and PC within each study. We also conducted subgroup analysis by a random-effect (RE) meta-analysis for the assessment of heterogeneity using the type of anticoagulant (EDTA or citrate), type of analyzer (Sysmex or Coulter), and type of infarction [ischemic stroke (IS) or PICH] as covariates. Then, a mixed-effect (ME) meta-regression was performed using the type of infarction and type of analytes as the moderator variables. We also performed an outlier detection for MPV regarding storage time and type of analyte. Heterogeneity was quantified using the I2 statistics across studies. Publication bias was assessed through graphical Begg’s funnel plots and Egger’s regression symmetry statistical tests [Citation22]. We performed both adjusted and unadjusted parametric bootstrap sampling with 10,000 replicates of the original data using the normal distribution. All analyses were performed by using the statistical software R 3.3.2 and its metafor package (https://cran.r-project.org).
Results
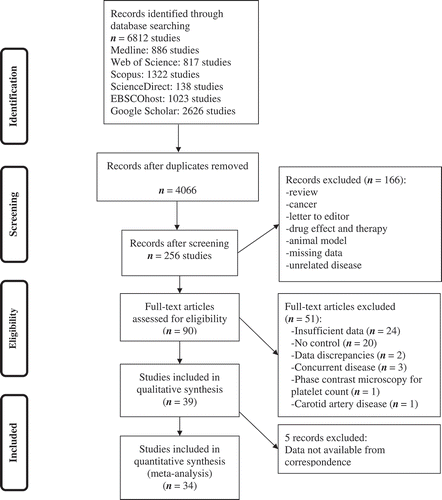
A flowchart diagram of the progression of papers through the review process is shown in . A total of 6812 studies were identified. Supplementary Table I shows search protocols and their details for each database separately. A total of 2746 duplicates (40.3%) were excluded, and 3810 (55.9%) articles were removed after reviewing the title and/or abstract. The remaining 256 articles were assessed for eligibility criteria by screening their titles and abstracts. Following detailed evaluation, 39 studies were selected for qualitative assessment. We corresponded with authors for five studies, but data were not available. Therefore, 34 articles were included in the meta-analysis [Citation10–Citation15,Citation23-Citation50]. Three studies were carried out in the United States, 12 in Europe, ten in Asia, one in Africa, and eight studies were intercontinental. Patient populations ranged from 12 to 384 individuals.
Most of the included studies were about acute ischemic stroke (82%). Thirteen studies did not report the type of analysate (38%), and almost the same number of studies failed to report the type of hematology automated analyzer (41%) ( and more details at Supplementary Table II). Elapse time between phlebotomy and measurement of 20.5% of the studies was more than 2 h. Most of the studies missed to report stroke-related comorbidities ().
Table I. Characteristic of included studies.
The Dersimonian-Laird estimator was used to estimate ME and RE models for PC MPV (Supplementary Table III). Funnel plot indicates publication biases in the RE model, especially for MPV, but the ME model for both variables does not contain publication biases (Supplementary Figure 1). We applied the weighted regression model with multiplicative dispersion and t-values were calculated. Egger’s test provided evidence for publication biases in RE model only for MPV (P < .001), but in case of PC t-statistics show stronger symmetry for the mixed effects model (Supplementary Table IV). There were no false high MPV values during our outlier detection procedure. Bootstrap sampling was used to validate both RE and ME models and to avoid the overfitting of the models in case of a low number of studies (Supplementary Table V).
PC
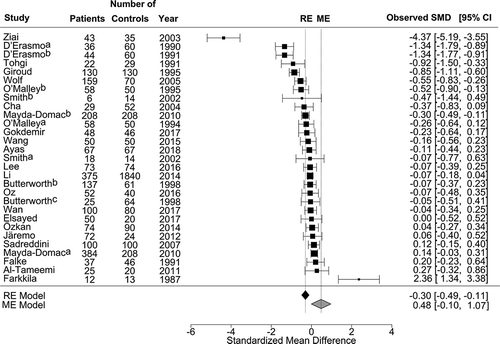
A total of 6107 participants were selected from 24 publications, of which 2492 were assigned to the patient group and 3615 to the control group. Three studies showed that patients with AS had higher PC than controls [Citation24,Citation35,Citation36]. Eleven studies demonstrated lower PC in the patient group [Citation12–Citation14,Citation23,Citation25–Citation27,Citation30,Citation32,Citation35,Citation43], and 11 studies found no difference in PC between patients and controls (, Supplementary Table VI) [Citation11,Citation15,Citation28,Citation29,Citation33,Citation37,Citation41,Citation42,Citation44,Citation46,Citation47]. One study did not provide PC results. The mean PC for all publications was 234 G/L (95% CI: 224–244) for patients and 248 G/L (95% CI 238–259) for controls. There was a marked residual heterogeneity by ME model for PC [χ2 = 230.2; df = 27; I2 = 88.3% (95% CI: 80.0–93.1); P < .001]. The estimated pooled mean difference in PC was 0.48 G/L [Z = 1.61; P = .107] regarding the ischemic stroke for the ME model. It was −0.30 (Z = −3.15, 95% CI: − 0.49– −0.11, P = .002) for the RE model without moderating effects ().
MPV
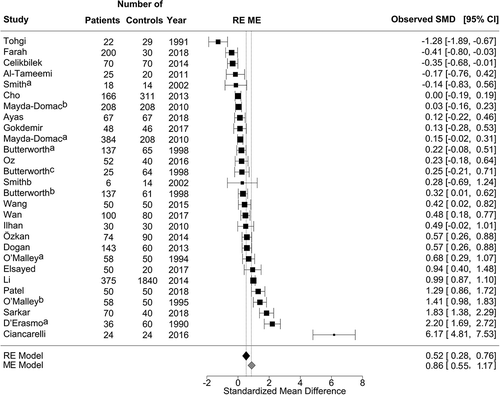
Seventeen results of the 27 studies revealed that patients with stroke had higher MPV than controls [Citation10,Citation12,Citation25,Citation27,Citation28,Citation34,Citation35,Citation39,Citation41–Citation43,Citation46-Citation48,Citation50]. Ten studies found no difference in MPV between patients and controls [Citation14,Citation15,Citation28,Citation29,Citation35,Citation36,Citation38,Citation45,Citation49], and two studies demonstrated a higher MPV in the control group (, Supplementary Table VI) [Citation13,Citation40]. The mean MPV for all publications was 9.8 fL (95% CI 9.4–10.1) for patients and 9.2 fL (95% CI, 8.8–9.6) for controls. There was a marked residual heterogeneity by ME model for MPV [χ2 = 333.5; df = 20; I2 = 94% (95% CI: 89–97); P < .001]. The estimated pooled mean difference in MPV was 0.86 (Z = 5.509; P < .001) for the ME model regarding the type of analyte effect (EDTA compared to Citrate). It was 0.52 fL (Z = 4.29, P < .001) for RE model without moderating effects ().
Subgroup Analysis
We also performed subgroup and sensitivity analyses according to the type of infarction, type of analyzer and type of analyte for MPV and PC to further evaluate the relationship between these parameters. Twelve studies measured complete blood count in 120 min or less, and seven studies assessed blood samples after 120 min. We analyzed them in a separate subgroup. Out of 34 studies which were included in this meta-analysis, three of them had divided their studies into different subgroups () [Citation28,Citation29,Citation35].
In 23 studies about acute ischemic stroke, 2007 cases demonstrated significantly lower PC compared to 3090 control individuals (SMD = −0.18; 95% CI: − 0.35 to −0.01, P < .001) ( panel A, Supplementary Table VII). Six studies about PICH revealed more significant results with 485 patients by comparison with 525 controls (SMD = −0.94; 95% CI: −1.62 to −0.25, P < .001) ( panel B, Supplementary Table VII). Citrated samples for PC measurement showed significantly higher results in 100 patients compared with 173 controls (SMD = −0.36; 95% CI: −0.68 to −0.04, P < .05).
Figure 4. Subgroup analysis for A – platelet count in patients with acute ischemic stroke, or B – platelet count in patients with acute hemorrhagic stroke; and C – mean platelet volume in patients with acute ischemic stroke, or D – mean platelet volume in patients with acute hemorrhagic stroke.
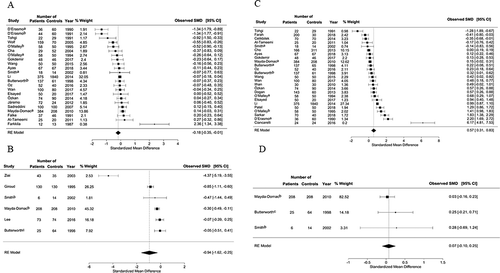
MPV was significantly higher in 2444 patients who suffered acute ischemic stroke compared to 3405 controls (SMD = 0.57; 95% CI: 0.31–0.83, P < .001) ( panel C, Supplementary Table VIII). MPV was lower in patients compared with controls in case of acute hemorrhagic stroke, but this difference was not significant (SMD = 0.07; 95% CI: −0.10–0.25, P > .05) ( panel D, Supplementary Table VIII). MPV from EDTA-anticoagulated samples was measured in 17 studies, and it was significantly higher in 1974 cases compared to 3209 control individuals (SMD = 0.86; 95% CI: 0.55–1.17, P < .001). Citrated samples from 208 cases showed lower MPV compared to 186 controls (SMD = −0.13; 95% CI: −0.69–0.42, P < .001). Sysmex hematology analyzers in 14 studies with 2727 controls measured significantly higher MPV in 1557 patients (SMD = 0.64; 95% CI: 0.28–1.01, P < .001). Coulter hematology analyzers in six studies with 432 patients measured significantly higher MPV compared with 347 controls (SMD = 0.58; 95% CI: 0.08–1.08, P < .001) (Supplementary Table VIII). We also examined MPV results in two subgroups of elapsed time more than 120 min or less than/equal to 120 min. This is the time delay between venipuncture and analysis (Supplementary Table VIII). Compared to control group, MPV was significantly higher in both ≤120 min and >120 min groups (SMD = 0.72; 95% CI: 0.36–1.08, P < .001) and (SMD = 0.70; 95% CI: 0.08–1.32, P < .001), respectively.
Discussion
We conducted a systematic review and meta-analysis of the literature to evaluate PC and MPV in patients with AS. To the best of our knowledge, this is the first meta-analysis assessing results of MPV and PC on AS while considering the type of stroke and some pre-analytical factors.
Larger platelets are more metabolically and functionally active, platelets with high MPV contain more glycogen, ADP, ATP, and reacting more readily to stimulating agents [Citation51]. Platelet dysfunction is described to be associated with PICH [Citation52]. Platelets also have a prominent role in thromboemboli formation that may initiate the symptoms of stroke. Recent meta-analysis showed elevated MPV in different pathological conditions [Citation53,Citation54].
Hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, and smoking habits are among the most important medical pre-conditions and stroke-related comorbidities. Several lines of evidence from the literature suggest that a direct correlation exists between MPV and these traditional cerebrovascular risk factors [Citation55–Citation60]. MPV was significantly higher (0.65 fL) in individuals with coronary artery disease and patients with high MPV had 17% higher chance of having cardiovascular events compared to patients with low MPV [Citation61]. Another meta-analysis concluded that MPV was significantly higher in those with acute myocardial infarction than those without acute myocardial infarction [Citation62].
In our study, significantly higher MPV was found in acute ischemic stroke and PC was significantly decreased in both acute ischemic and hemorrhagic strokes.
Some authors consider a possible use of MPV assessments as a potential biomarker of AS or as a prognostic marker in patients after AS [Citation10,Citation12]. An association of MPV with stroke is possibly due to higher platelet activity, aggregation, and turnover in patients with elevated MPV. It has been shown that increased MPV is linked to more hemostatically active platelets with higher avidity to aggregate [Citation63]. Additionally, increased turnover of platelets causes the release of young, more active and larger platelets from bone marrow [Citation64]. The estimated pooled mean difference of MPV was 0.51 fL and significantly higher in patients with AS than controls. Moreover, MPV was significantly higher in patients than controls after adjustment for the type of analyte or analyzer. It should be acknowledged that these differences are rather small in absolute terms. Therefore, they might reflect some changes of specific characteristics of the platelet population and may also show the presence of more reactive platelets within the blood stream in this group of patients.
Since the normal lifespan of platelets is between 8 and 10 days, the contribution of the elevated MPV in a prothrombotic state and an increased risk of stroke is likely to be present prior to stroke onset. Platelet activation measured by the levels of CD62P (P-selectin) and circulating monocyte-platelet complex was increased in stroke patients [Citation65]. On the other hand, the level of reticulated platelets as a marker of platelet production was the same as controls in stroke patient [Citation66]. These results suggest the presence of continuous stimulation for platelet activation without excessive platelet production after stroke. Monitoring stroke patients revealed constant elevated MPV results post-stroke, even three to 6 months after the event [Citation25,Citation28]. Low number of studies related to acute hemorrhagic stroke and hyporeactive platelets, as determined by significantly decreased platelet aggregation rates induced by ADP [Citation67], could explain partly constant level of MPV in acute hemorrhagic stroke. Lower PC in acute ischemic stroke could be contributed to platelet consumption during acute phase of stroke [Citation13]. Decreased PC number in patients with acute hemorrhagic stroke is difficult to explain. Consumption of platelets to produce effective lower PC requires significantly larger hemorrhage than that usually seen in non-traumatic acute hemorrhagic stroke. Medications such as antiepileptics, or antibiotics may have attributed to lower PC. It seems that modification of PC as an acute phase reactant requires additional time than duration of AS.
Pre-analytical conditions of MPV assessment have a great effect on the results of platelet size. If MPV would apply as a risk factor AS, its measurement should be standardized. This fact is not only important for laboratory, but also for clinicians.
Type of anticoagulant for blood samples has also a significant effect on platelet parameters measured by conventional hematology analyzers [Citation68]. It has been demonstrated previously that the addition of EDTA anticoagulant to the sample causes artificial platelet swelling [Citation69]. However, this modification could be partly explained by the alterations of both platelet cell wall and the membranes lining the canalicular system and the change of phosphorylation patterns of platelet proteins by exposure to EDTA [Citation70,Citation71]. Sixty-one percent of the studies included in this meta-analysis applied EDTA as an anticoagulant and it shows significantly higher MPV for patients. It is important to mention that almost half of the studies failed to report the type of analyte they used for their study. Type of anticoagulant for blood samples could also modify substantially PC results [Citation72]. This phenomenon can be manifested by small-scale platelet micro-aggregate formation with the usage of the citrate anticoagulant [Citation73]. Our results demonstrated that the estimated pooled mean difference of PC was −0.30 G/L in total patients compared to controls.
Hematology analyzers may differ in the method they incorporate for the measurement of the platelet parameters. These constitute mostly impedance or optical methods, and neither of them take into account the shape change of platelets [Citation74]. As a result, these methods do not agree with each other. Sample storage time is another parameter which influences MPV [Citation75]. Although not all studies stated sample storage time in their investigation, MPV was significantly higher than controls in both ≤120 min and >120 min groups. One possibility to increase the chance to harmonize the reporting of MPV is standardizing the elapse time between venipuncture and MPV measurement.
There are some limitations to this study. Several reasons may account for inconsistent results on the association between MPV and stroke in the literature. Pooled estimates demonstrated large heterogeneity. This can be explained by vast differences in pre-analytical factors and demographic characteristics of the studied population. Most of the studies failed to mention these characteristics and it could be a potential source of heterogeneity. Only a limited number of studies evaluated the potential confounding effects of the traditional cerebrovascular risk factors, and pre-analytical factors were profoundly diverse among them. Significant heterogeneity exists among some studies, and the results should be interpreted cautiously. Different results could be related to the time difference between the stroke and blood tests, failure to report the type of analyte or type of analyzer. False results could be obtained as blood samples remain in contact with anticoagulant for a long time. The difference in the analyte used to anticoagulant the samples or assay methods might also partly contribute to the high heterogeneity among the studies.
We recommend future studies design to include a set of standard covariates and pre-analytical factors. This can be achieved by implanting specific time frame to measure the sample results, define the type of anticoagulant in sample tube and specify method of measurement. Lack of thorough documentation of patient-related pre-analytical factors leads to higher variability among studies.
In conclusion, MPV as a part of a routine complete blood count is easily accessible and could be a surrogate marker of platelet turnover. The results of this study emphasize that platelets play a role in the pathogenesis of stroke, PC and MPV appeared to be significantly different from control populations. Acute ischemic stroke demonstrated significantly higher MPV, nonetheless both acute ischemic and hemorrhagic strokes revealed significantly lower PC. These features might be associated with AS and related to its occurrence.
Declaration of interest statement
The authors report no conflicts of interest.
Supplemental Material
Download MS Word (21.5 KB)Supplemental Material
Download MS Word (20.5 KB)Supplemental Material
Download MS Word (15.7 KB)Supplemental Material
Download MS Word (14.1 KB)Supplemental Material
Download MS Word (12.8 KB)Supplemental Material
Download MS Word (14.8 KB)Supplemental Material
Download MS Word (14.9 KB)Supplemental Material
Download MS Word (20.3 KB)Supplemental Material
Download MS Word (209.3 KB)Acknowledgments
This article was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (grant number: BO/00029/19/4).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Bejot Y, Bailly H, Durier J, Giroud M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med 2016;45:e391–e398. DOI:10.1016/j.lpm.2016.10.003
- Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics 2011;8:319–329. DOI:10.1007/s13311-011-0053-1
- Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program 2011;2011:51–61. DOI:10.1182/asheducation-2011.1.51
- Schmalbach B, Stepanow O, Jochens A, Riedel C, Deuschl G, Kuhlenbaumer G. Determinants of platelet-leukocyte aggregation and platelet activation in stroke. Cerebrovasc Dis 2015;39:176–180. DOI:10.1159/000375396
- Martin JF, Trowbridge EA, Salmon G, Plumb J. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res 1983;32:443–460. DOI:10.1016/0049-3848(83)90255-4
- Brown AS, Hong Y, de Belder A, Beacon H, Beeso J, Sherwood R, Edmonds M, Martin JF, Erusalimsky JD. Megakaryocyte ploidy and platelet changes in human diabetes and atherosclerosis. Arterioscler Thromb Vasc Biol 1997;17:802–807. DOI:10.1161/01.ATV.17.4.802
- Kovacs S, Csiki Z, Zsori KS, Bereczky Z, Shemirani AH. Characteristics of platelet count and size and diagnostic accuracy of mean platelet volume in patients with venous thromboembolism. A systematic review and meta-analysis. Platelets 2019;30:139-147.
- Liu L, Lin Z, Shen Z, Zhang G, Li S, Cao P. Platelet hyperfunction exists in both acute non-haemorrhagic and haemorrhagic stroke. Thromb Res 1994;75:485–490. DOI:10.1016/0049-3848(94)90264-X
- Lin CY, Chang CY, Sun CH, Li TY, Chen LC, Chang ST, Wu YT. Platelet count and early outcome in patients with spontaneous cerebellar hemorrhage: a retrospective study. PLoS One 2015;10:e0119109. DOI:10.1371/journal.pone.0119109
- Ciancarelli I, De Amicis D, Di Massimo C, Pistarini C, Ciancarelli MG. Mean platelet volume during ischemic stroke is a potential pro-inflammatory biomarker in the acute phase and during neurorehabilitation not directly linked to clinical outcome. Curr Neurovasc Res 2016;13:177–183. DOI:10.2174/1567202613666160517122109
- Farkkila MA, Rasi V, Tilvis RS, Ikkala E, Viinikka L, Ylikorkala O, Farkkila AM, Miettinen TA. Low platelet arachidonic acid in young patients with brain infarction. Thromb Res 1987;48:721–727. DOI:10.1016/0049-3848(87)90437-3
- D’Erasmo E, Aliberti G, Celi FS, Romagnoli E, Vecci E, Mazzuoli GF. Platelet count, mean platelet volume and their relation to prognosis in cerebral infarction. J Intern Med 1990;227:11–14. DOI:10.1111/joim.1990.227.issue-1
- Tohgi H, Suzuki H, Tamura K, Kimura B. Platelet volume, aggregation, and adenosine triphosphate release in cerebral thrombosis. Stroke 1991;22:17–21. DOI:10.1161/01.STR.22.1.17
- Gokdemir MT, Karakilcik AZ, Gokdemir GS. Prognostic importance of paraoxonase, arylesterase and mean platelet volume efficiency in acute ischaemic stroke. J Pak Med Assoc 2017;67:1679–1683.
- Ayas ZO, Can U. Alteration of mean platelet volume in the pathogenesis of acute ischemic stroke: cause or consequence? Ideggyogy Sz 2018;71:49–56. DOI:10.18071/isz.71.0049
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. DOI:10.1016/j.jclinepi.2009.06.006
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. DOI:10.1001/jama.283.15.2008
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–605. DOI:10.1007/s10654-010-9491-z
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. DOI:10.1186/1471-2288-14-135
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 2010;36:48. doi: 10.18637/jss.v036.i03
- Huzsvai L, Balogh P. Linear models using R. Debrecen, Hungary: Seneca; 2015.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. DOI:10.1136/bmj.315.7109.629
- D’Erasmo E, Celi FS, Acca M, Mazzuoli G. Relationship between platelet and white blood cell counts during the early phase of cerebral infarction. Stroke 1991;22:283. DOI:10.1161/str.22.2.283a
- Falke P, Lindgärde F, Stavenow L. Differences in blood viscosity, glycosylated hemoglobin and platelet count between male patients with carotid territory transient ischemic attacks and minor strokes. Clinical Hemorheology 1991;11:35–40.
- O’Malley T, Langhorne P, Elton RA, Stewart C. Platelet size in stroke patients. Stroke 1995;26:995–999. DOI:10.1161/01.STR.26.6.995
- Giroud M, Creisson E, Fayolle H, Andre N, Becker F, Martin D, Dumas R. Risk factors for primary cerebral hemorrhage: a population-based study–the Stroke Registry of Dijon. Neuroepidemiology 1995;14:20–26. DOI:10.1159/000109775
- O’Malley T, Lahghorne P, Stehart C. Do large platelets cause stroke? Age and Aging 1994;23:P3-c-P3. doi: 10.1093/ageing/23.suppl_1.P3-c
- Butterworth RJ, Bath PM. The relationship between mean platelet volume, stroke subtype and clinical outcome. Platelets 1998;9:359–364. doi: 10.1080/09537109876429
- Smith NM, Pathansali R, Bath PM. Altered megakaryocyte-platelet-haemostatic axis in patients with acute stroke. Platelets 2002;13:113–120. DOI:10.1080/09537100120111559
- Ziai WC, Torbey MT, Kickler TS, Oh S, Bhardwaj A, Wityk RJ. Platelet count and function in spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2003;12:201–206. DOI:10.1016/S1052-3057(03)00075-2
- Cha JK, Jo WS, Shin HC, Bae HR, Ho JM, Kim JW. Increased platelet CD63 and P-selectin expression persist in atherosclerotic ischemic stroke. Platelets 2004;15:3–7. DOI:10.1080/09537100310001644024
- Wolff V, Aleil B, Giroud M, Lorenzini JL, Meyer N, Wiesel ML, Cazenave JP, Lanza F. Soluble platelet glycoprotein V is a marker of thrombosis in patients with ischemic stroke. Stroke 2005;36:e17–19. DOI:10.1161/01.STR.0000155738.02753.4d
- Sadreddini SA, Abolfathi AA, Khandagi R, Talebi M, Lakian A. C-reactive protein, fibrinogen, lipoprotein (a), and lipid profile levels and platelet counts in ischemic stroke patients. Neurosciences (Riyadh) 2007;12:202–206.
- Ilhan D, Ozbabalik D, Gulcan E, Ozdemir O, Gulbacs Z. Evaluation of platelet activation, coagulation, and fibrinolytic activation in patients with symptomatic lacunar stroke. Neurologist 2010;16:188–191. DOI:10.1097/NRL.0b013e318198d8bc
- Mayda-Domac F, Misirli H, Yilmaz M. Prognostic role of mean platelet volume and platelet count in ischemic and hemorrhagic stroke. J Stroke Cerebrovasc Dis DOI:2010;19:66–72. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.003
- Al-Tameemi WF, Ameen AMM. Significance of platelet indices in patients with acute ischemic stroke. Iraqi Journal of Medical Sciences 2011;10:383–389.
- Jaremo P, Eriksson M, Lindahl TL, Nilsson S, Milovanovic M. Platelets and acute cerebral infarction. Platelets 2013;24:407–411. DOI:10.3109/09537104.2012.712168
- Cho SY, Jeon YL, Choi SK, Suh JT, Lee HJ, Park TS. Mean platelet volume in Korean patients with acute ischemic stroke: a gender difference. Platelets 2013;24:75–76. DOI:10.3109/09537104.2012.658109
- Dogan NO, Keles A, Aksel G, Guler S, Demircan A, Bildik F, Kilicaslan I, Karakurt K, Ilhan M. Mean platelet volume as a risk stratification tool in the emergency department for evaluating patients with ischaemic stroke and TIA. J Pak Med Assoc 2013;63:581–584.
- Celikbilek A, Ismailogullari S, Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal 2014;28:27–31. DOI:10.1002/jcla.2014.28.issue-1
- Li B, Liu X, Cao ZG, Li Y, Liu TM, Wang RT. Elevated mean platelet volume is associated with silent cerebral infarction. Intern Med J 2014;44:653–657. DOI:10.1111/imj.12454
- Ozkan B, Arik OZ, Gozukara MY, Sahin DY, Topal S, Uysal OK, Elbasan Z, Epceliden T, Cayli M, Gur M. Mean platelet volume is related with ischemic stroke in patients with sinus rhythm. Blood Coagul Fibrinolysis 2016;27:490–493. DOI:10.1097/MBC.0000000000000108
- Wang D, Zhang FH, Zhao YT, Xiao XG, Liu S, Shi HB, Lin AL, Wang YJ, Han Q, Sun QM. Association of polymorphism in ICAM-1 (K469E) and cytology parameters in patients’ initial blood test with acute ischemic stroke. Genet Mol Res 2015;14:15520–15529. DOI:10.4238/2015.December.1.2
- Lee JM, Siddique J, Kim HC, Green D, Van Horn L, Allison M, Wassertheil-Smoller S, Greenland P. Hemostatic markers and long-term risk of intracerebral hemorrhage in postmenopausal women. J Stroke Cerebrovasc Dis 2016;25:1639–1643. DOI:10.1016/j.jstrokecerebrovasdis.2016.03.013
- Oz II, Yucel M, Bilici M, Serifoglu I, Sayin R, Ilikhan SU, Acikgoz M. Is mean platelet volume a reliable marker to predict ischemic stroke in the follow-up of patients with carotid stenosis? J Stroke Cerebrovasc Dis 2016;25:404–409. DOI:10.1016/j.jstrokecerebrovasdis.2015.10.012
- Elsayed AM, Mohamed AG. Mean platelet volume and mean platelet volume/platelet count ratio as a risk stratification tool in the assessment of severity of acute ischemic stroke. Alexandria Journal of Medicine 2017;53:67–70. DOI:10.1016/j.ajme.2016.03.003
- Wan JL, Ma ZW. The value of mean platelet volume for prognosis of patients with acute cerebral infarction. Clin Lab 2017;63:1801–1807. DOI:10.7754/Clin.Lab.2017.170419
- Patel V, Parmar M, Shah K, Joshi R. A study on mean platelet volume in ischemic cerebrovascular stroke. International Journal of Advances in Medicine 2018;5:316–320. DOI:10.18203/2349-3933.ijam20180474
- Farah R, Samra N. Mean platelets volume and neutrophil to lymphocyte ratio as predictors of stroke. J Clin Lab Anal 2018;32. DOI:10.1002/jcla.22189
- Sarkar RN, Das CK, Bhattacharjee U, Banerjee M. Platelet indices as a marker of severity in non-diabetic nonhypertensive acute ischemic stroke patients. J Assoc Physicians India 2018;66:40–42.
- Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol 1982;50:509–519. DOI:10.1111/j.1365-2141.1982.tb01947.x
- Rajajee V, Brown DM, Tuhrim S. Coagulation abnormalities following primary intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2004;13:47–51. DOI:10.1016/j.jstrokecerebrovasdis.2004.01.002
- Weymann A, Ali-Hasan-Al-Saegh S, Sabashnikov A, Popov AF, Mirhosseini SJ, Nombela-Franco L, Testa L, Lotfaliani M, Zeriouh M, Liu T, et al., Surgery, Cardiology-Group Imcsc-Group IM. Platelets cellular and functional characteristics in patients with atrial fibrillation: a comprehensive meta-analysis and systematic review. Med Sci Monit Basic Res 2017;23:58–86. DOI:10.12659/MSMBR.902557
- Lin WY, Lu X, Fan FJ, Hu Y. Predictive effect of mean platelet volume in patients with portal vein thrombosis: a meta-analysis of case-control studies. Curr Med Sci 2018;38:575–581. DOI:10.1007/s11596-018-1916-z
- Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011;17:47–58. DOI:10.2174/138161211795049804
- Li Z, Wang J, Han X, Yuan J, Guo H, Zhang X, Zheng D, Tang Y, Yang H, He M. Association of mean platelet volume with incident type 2 diabetes mellitus risk: the Dongfeng-Tongji cohort study. Diabetol Metab Syndr 2018;10:29. DOI:10.1186/s13098-018-0333-6
- Varol E, Aksoy F, Bas HA, Ari H, Ozaydin M. Mean platelet volume is elevated in patients with low high-density lipoprotein cholesterol. Angiology 2014;65:733–736. DOI:10.1177/0003319713504024
- Gang L, Yanyan Z, Zhongwei Z, Juan D. Association between mean platelet volume and hypertension incidence. Hypertens Res 2017;40:779–784. DOI:10.1038/hr.2017.30
- Varol E, Icli A, Kocyigit S, Erdogan D, Ozaydin M, Dogan A. Effect of smoking cessation on mean platelet volume. Clin Appl Thromb Hemost 2013;19:315–319. DOI:10.1177/1076029612436675
- Zaccardi F, Rocca B, Pitocco D, Tanese L, Rizzi A, Ghirlanda G. Platelet mean volume, distribution width, and count in type 2 diabetes, impaired fasting glucose, and metabolic syndrome: a meta-analysis. Diabetes Metab Res Rev 2015;31:402–410. DOI:10.1002/dmrr.v31.4
- Sansanayudh N, Numthavaj P, Muntham D, Yamwong S, McEvoy M, Attia J, Sritara P, Thakkinstian A. Prognostic effect of mean platelet volume in patients with coronary artery disease. A systematic review and meta-analysis. Thromb Haemost 2015;114:1299–1309. doi: 10.1160/TH15-04-0280
- Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost 2010;8:148–156. DOI:10.1111/jth.2009.8.issue-1
- Kamath S, Blann AD, Lip GY. Platelet activation: assessment and quantification. Eur Heart J 2001;22:1561–1571. DOI:10.1053/euhj.2000.2515
- Thon JN, Italiano JE Jr. Does size matter in platelet production? Blood 2012;120:1552–1561. DOI:10.1182/blood-2012-04-408724
- McCabe DJ, Harrison P, Mackie IJ, Sidhu PS, Purdy G, Lawrie AS, Watt H, Brown MM, Machin SJ. Platelet degranulation and monocyte-platelet complex formation are increased in the acute and convalescent phases after ischaemic stroke or transient ischaemic attack. Br J Haematol 2004;125:777–787. DOI:10.1111/j.1365-2141.2004.04983.x
- McCabe DJ, Harrison P, Sidhu PS, Brown MM, Machin SJ. Circulating reticulated platelets in the early and late phases after ischaemic stroke and transient ischaemic attack. Br J Haematol 2004;126:861–869. DOI:10.1111/j.1365-2141.2004.05137.x
- Mulley GPHS, Taylor PM, Mitchell JR. ADP-induced platelet release reaction in acute stroke. Thrombosis and Haemostasis 1983;50:3.
- Reardon DM, Hutchinson D, Preston FE, Trowbridge EA. The routine measurement of platelet volume: a comparison of aperture-impedance and flow cytometric systems. Clin Lab Haematol 1985;7:251–257. DOI:10.1111/ijlh.1985.7.issue-3
- White JG. Effects of ethylenediamine tetracetic acid (EDTA) on platelet structure. Scand J Haematol 1968;5:241–254. DOI:10.1111/j.1600-0609.1968.tb01743.x
- Diaz-Ricart M, Brunso L, Pino M, Navalon F, Jou JM, Heras M, White JG, Escolar G. Preanalytical treatment of EDTA-anticoagulated blood to ensure stabilization of the mean platelet volume and component measured with the ADVIA counters. Thromb Res 2010;126:e30–35. DOI:10.1016/j.thromres.2010.04.002
- Gachet C, Hanau D, Spehner D, Brisson C, Garaud JC, Schmitt DA, Ohlmann P, Cazenave JP. Alpha IIb beta 3 integrin dissociation induced by EDTA results in morphological changes of the platelet surface-connected canalicular system with differential location of the two separate subunits. J Cell Biol 1993;120:1021–1030. DOI:10.1083/jcb.120.4.1021
- Kecskemetine G, Csiki Z, Mile M, Zsori KS, Shemirani AH. The clinical significance of pneumatic tube transport system on platelet indices: EDTA or citrate anticoagulant? Int J Lab Hematol 2017;39:e102–e105. DOI:10.1111/ijlh.2017.39.issue-4
- McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol 1990;12:277–285. DOI:10.1111/j.1365-2257.1990.tb00038.x
- Trowbridge EA, Reardon DM, Hutchinson D, Pickering C. The routine measurement of platelet volume: a comparison of light-scattering and aperture-impedance technologies. Clin Phys Physiol Meas 1985;6:221–238. DOI:10.1088/0143-0815/6/3/003
- Mannuss S, Schuff-Werner P, Dreissiger K, Kohlschein P. Magnesium sulfate as an alternative in vitro anticoagulant for the measurement of platelet parameters? Am J Clin Pathol 2016;145:806–814. DOI:10.1093/ajcp/aqw066