Abstract
Thrombus formation is highly dependent upon the physico-chemical environment in which it is triggered. Our ability to understand how thrombus formation is initiated, regulated, and resolved in the human body is dependent upon our ability to replicate the mechanical and biological properties of the arterial wall. Current in vitro thrombosis models principally use reductionist approaches to model the complex biochemical and cellular milieu present in the arterial wall, and so researcher have favored the use of in vivo models. The field of vascular tissue engineering has developed a range of techniques for culturing artificial human arteries for use as vascular grafts. These techniques therefore provide a basis for developing more sophisticated 3D replicas of the arterial wall that can be used in in vitro thrombosis models. In this review, we consider how tissue engineering approaches can be used to generate 3D models of the arterial wall that improve upon current in vivo and in vitro approaches. We consider the current benefits and limitations of reported 3D tissue engineered models and consider what additional evidence is required to validate them as alternatives to current in vivo models.
Introduction
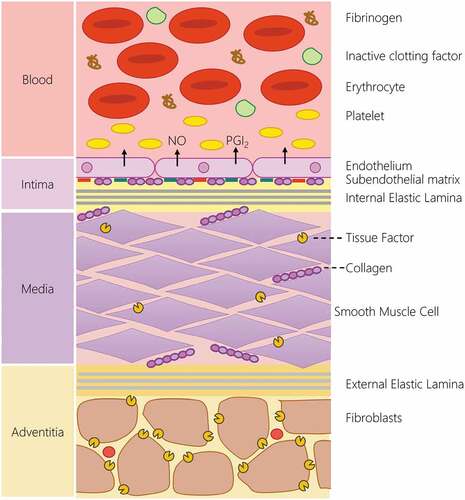
The process of thrombus formation is a carefully choreographed dance that occurs through the dynamic interaction of the bloodstream and the vessel wall. The integrity of the arterial wall is continuously monitored by the flowing bloodstream at the interface between these tissues. In the healthy, uninjured artery, the bloodstream is exclusively exposed to the anti-thrombotic intimal lining that keeps the hemostatic system quiescent through the production and expression of a range of platelet (nitric oxide, prostacyclin, CD39) and coagulation inhibitors (Tissue factor pathway inhibitor, thrombomodulin, endothelial protein C receptor, heparin-like proteoglycans) by the endothelial monolayer found in this arterial layer () [Citation1]. Upon damage, the endothelial layer is interrupted, locally removing this brake to the hemostatic system. The bloodstream is then exposed to the pro-thrombotic subendothelial matrix comprised of collagen, fibronectin and laminin produced by the endothelial and smooth muscle cells [Citation2]. This elicits platelet activation upon the surface of the damaged artery. Additionally, tissue factor expressed by medial smooth muscle cells and adventitial fibroblasts trigger the activation of the extrinsic pathway of coagulation [Citation3]. This allows the targeted activation of platelets and the coagulation cascade to produce a blood clot at the point of injury, preventing excessive blood loss. This response is further patterned by local changes in hemodynamics around the growing thrombus, which will alter platelet recruitment to the growing thrombus [Citation4,Citation5]. Therefore to best understand the processes that regulate thrombus formation, it is important to study this in the physical and chemical conditions found within the human body. However, the practical and ethical limitations of studying thrombus formation directly in volunteers has meant that scientists have had to develop alternative biomimetic.
Figure 1. The structure of the blood-arterial wall interface is the key regulator of hemostatic response. In the uninjured blood vessel, the hemostatic system is kept quiescent by the physical and chemical barrier produced by the endothelial lining. The endothelial lining produces a range of endogenous inhibitors of platelets and the coagulation cascade to prevent activation of the hemostatic system. The intimal layer also physically separates blood from the pro-thrombotic constituents of the medial and adventitial layer of the artery. This includes the presence of pro-aggregatory molecules such as collagen, fibronectin and laminin in the subendothelial matrix, and the production of tissue factor by smooth muscle cells in the medial layer as well as adventitial fibroblasts. These systems help prevent the unwanted activation of the hemostatic system. In the event of vascular damage, the loss of the endothelial lining locally removes the inhibitory effect of this lining and allows blood to interact with the pro-aggregatory and pro-coagulant component of the arterial wall to initiate thrombus formation.
Advances in intravital microscopy have allowed us to study thrombus formation in vivo in other species. These in vivo models involve surgical exposure of accessible vessels under anesthesia in the chosen animal species and tracking the thrombotic response after induction of arterial or venous damage using a range of different methods of chemical or physical injury [Citation6,Citation7]. However, these responses may not replicate those seen in humans due to known differences in hemodynamics, platelet molecular physiology and coagulation profile between human and common model species [Citation8–10]. Previous research has also demonstrated that in vivo models require significant standardization to obtain consistent results, as data obtained can be significantly affected by the type and extent of injury-induced [Citation11,Citation12], the vessel bed targeted [Citation13], the strain and age of mice used [Citation6], types of anesthesia used [Citation14], as well as the skill of the researcher [Citation7,Citation15]. The impact of inter-lab differences in experimental set-up can be seen in the reporting of conflicting results when mouse strain and injury type were the same [Citation16,Citation17]. As different experimental approaches emphasize different aspects of thrombus formation, it is unclear which set-up best represents in vivo human thrombus formation. This uncertainty will limit the translational potential of any results identified. A previous review of cardiovascular treatments tested in animal models found that only 21% of positive results were successfully replicated in clinical trials [Citation18]. Given the great animal and financial costs of conducting these in vivo studies and failed clinical trials, there is a need for an effective humanized in vitro thrombosis model that could better predict translational success of new treatments for hemostatic disorders, whilst reducing the number of animals used in research.
The need for such models is critical for enhancing our understanding of the pathological mechanisms and treatment of both arterial and venous thrombosis. Whilst both involve unwanted blood clotting, arterial and venous thrombosis occur in parts of the circulatory system with significantly different structures –Arterial thrombosis is elicited by atherosclerotic plaque formation and rupture, whilst venous thrombosis is elicited by venous stasis within the pockets of venous valves. Thus in vitro models will need to accurately reproduce the differing vascular geometries, rheology, mechanical and cellular properties of the different sides of the blood circulation that contribute to these distinct pathologies. Although there are currently excellent in vitro models to study venous thrombosis currently being produced [Citation19], in this review we will focus on the
Current in vitro arterial thrombosis models
The modeling of thrombus formation in vitro involves the perfusion of freshly-donated human blood under physiological flow conditions over a simulacrum of the arterial wall held within a flow chamber [Citation20]. Recently this has been achieved through use of microfluidic flow chambers as a basis to create physiological and pathological flow patterns over the model of the arterial wall. By minimizing the blood volumes, thrombogenic substrates and drugs needed for an experiment, whilst producing a more easily standardized model of thrombus formation, these thrombosis-on-a-chip models provide a practical and cost-effective alternative to current in vivo models [Citation21,Citation22]. The success of these models of human in vivo thrombus formation will be determined by their ability to accurately replicate the pro- and anti-thrombotic properties of the arterial wall. Traditionally in vitro thrombosis models have used simple coatings of the flow chamber with fibrillar type I or III collagen from equine or bovine sources either alone or in combination with lipidated human tissue factor to reproduce the core biochemical properties of the subendothelial matrix [Citation20]. This provides a simplified thrombogenic substrate to elicit activation of platelets and the extrinsic coagulation, but overlooks other pro-thrombotic subendothelial components that contribute to thrombus formation and patterning [Citation3]. These 2D acellular models of thrombus formation provide a reductionist approach to in vivo thrombus formation that does not include the controlling influence of the anti-thrombotic properties of the remaining endothelial wall, and likely overemphasizes the prothrombotic components of the arterial wall. For example, many studies use channels fully coated with the thrombogenic substrates. This can lead to an overexposure of blood to tissue factor, which is normally only exposed and de-encrypted at points of injury [Citation23]. Additionally, the accumulation of platelet-derived thromboxane A2 and adenosine nucleotides play a key role in thrombus growth downstream. These models over-estimate the importance of these signaling pathways in the thrombotic response seen [Citation20,Citation24]. It has been recommended to use a micropatterned flow chamber in which there is an isolated patch of the thrombogenic substrate to limit these effects [Citation20].
In the last decade, there has been a rapid growth in the development of endothelialised microfluidic flow chambers as an alternative approach to modeling in vivo thrombosis [Citation25,Citation26]. The microfluidic flow chambers are coated with subendothelial matrix components such as fibronectin, collagen or laminin to facilitate endothelial cell attachment, which can then be cultured to create fully endothelialised channels for use in flow studies, with complex 3D geometries that can act as exquisite structural replicas of the microvasculature and coronary circulation. For example, one previous study has used computed tomography angiography data to produce accurate 3D replicas of the human coronary artery [Citation27]. These models have been used to study the effect of channel geometry or endothelial cell activation in microvascular occlusion, arterial thrombosis and thrombo inflammation [Citation27–30]. However, by only recreating the intimal layer of the arterial wall, these models cannot be used as tools to investigate hemostatic or atherothrombotic reactions, whose reactions depend on the presence of subendothelial structures of the arterial wall.
The coating of endothelial cells or thrombogenic substrate to glass and plastic in flow chambers, creates a channel with mechanical properties that can impact thrombus formation. These surfaces are significantly stiffer (>1 GPa) than the native arterial wall (34 kPa) [Citation31]. The stiffness of the culture surface has previously been demonstrated to alter the glycocalyx and permeability of endothelial cultures [Citation32,Citation33]. Previous studies have also demonstrated that platelets are mechanosensitive and responsive to the stiffness of the arterial wall [Citation34]. This mechanical sensitivity alters platelets adhesive and pro-coagulant responses to type I collagen when conjugated to polyacrylamide gels with both of these properties increasing at matrix stiffnesses above 5 kPa [Citation35]. Furthermore, the lack of compliance of these stiff channels will also act to exaggerate the pressure buildup in the flow channels upon thrombus initiation, which has been demonstrated to impede the development of occlusive thrombi [Citation36]. A more compliant construct could dampen blood pressure rises and better facilitate thrombus growth. Thus, replica subendothelial matrices with mechanical properties like the native artery will assist in the simulation of thrombus formation in the in vivo environment.
Producing 3D arterial wall replicas using vascular tissue engineering
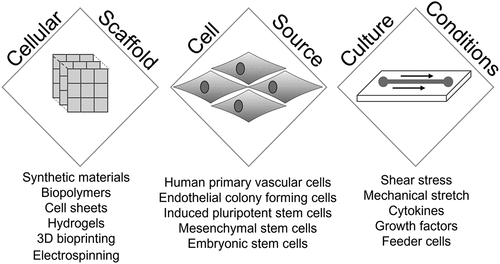
Tissue engineering utilizes a multidisciplinary approach to develop human tissues for the replacement of damaged tissues and organs in patients. These skills and techniques can also be harnessed to generate tissues to create 3D human arterial wall substitutes with the functional properties of the native tissue for use in in vitro alternatives to current in vivo experiments. The generation of any engineered human tissue involves the successful combination of 3 principal components - a source of the requisite cell(s) required to generate the target tissue, a scaffold that can support the growth of the implanted cells and its subsequent transplantation into the human body, and an appropriate physical, chemical and cellular environment in which to grow and mature the tissue to produce the optimal structural and functional properties () [Citation37]. Whilst developing a structurally accurate replica of the arterial wall is desired to improve the physiological relevance of any model, this ambition must be counterbalanced against the resultant manufacturing process, ensuring it is widely accessible and cost effective [Citation7]. A model system that can only be made and used by specialized groups, is unlikely to provide a feasible alternative to current in vivo models that are widely used by groups around the world [Citation7]. When considering accessibility, it is important to think about the required cost, resources and researcher time needed to produce a vessel replica. A feasible model therefore requires an easy and rapid method to produce vessel constructs that is both reproducible between labs and at a scale, to ensure research projects are not impeded by developmental delays. These concerns significantly alter design options beyond the need to just produce an accurate biological replica.
Biofabrication of tissue engineered vascular grafts can be achieved using a variety of 3D scaffolds to produce artificial arteries with the biological and mechanical properties of the native human tissue. This includes the use of biopolymer hydrogels, electrospinning, decellularized of human or animal arteries, and 3D bioprinting [Citation37]. Each of these methods provide synthetic, artificial, or endogenous scaffolds that provide key biochemical and mechanical signals to the cultured cells. These signals support the attachment, differentiation and proliferation of the seeded cells to produce replicas of the intimal, medial and adventitial layers of the artery (). As a multi-layered structure there are two broad approaches that can be used to produce a 3D biomimetic arterial model. This has been achieved by producing a 3D scaffold which replicates the subendothelial and contains cell from the medial and adventitial layers of the artery, and then subsequently culturing an endothelial monolayer atop of this scaffold [Citation38–40]. Alternatively, the model can be made by producing multiple independent layers and then combining together [Citation41,Citation42]. Currently, type 1 collagen hydrogels are the most commonly used method to produce a model of the medial layer of the artery [Citation38–42]. Due to the abundant presence of this molecule in the arterial wall, this provides a biomimetic scaffold that can be produced using simple and well characterized methods of production, without the need for any specialist equipment. Hydrogels can be produced through treating cell-containing mixtures of soluble monomers and triggering fibrillogenesis through initiating shifts in pH or temperature or initiating enzymatic digestion of the fibril precursors. As insoluble fibers form, this creates a phase shift in the polymer solution into a hydrogel. For vascular tissue engineering both fibrin and collagen hydrogels are commonly used. These can be set into molds to provide the desired 3D structure that can then be seeded with the desired vascular cells. These can create tissues which have stiffness akin to those found in vivo. For example, type I collagen hydrogels have been produced to have a stiffness of around 3–15 kPa, depending on the collagen concentration used [Citation43]. Although this is slightly below the stiffness of the native human tissue, this can be increased through crosslinking or plastic compression of the hydrogel [Citation44]. However, it is difficult to precisely pattern these scaffolds and generate constructs with high cellular density. Artificial matrices can also be generated through electrospinning, which uses high voltages to charge polymer solutions to create nanofibers that better replicate the structure of the subendothelial matix [Citation45]. Electrostatic repulsion caused by the applied voltage can trigger an elongated stream of fluid to be driven out of the droplet which can be collected onto frames to form nanofibre meshes. These can be used directly or further coated to facilitate cell seeding and proliferation on the surface. These have been utilized to either produce 2D sheets on which an endothelial monolayer is cultured for an intimal layer structure, or can be used to create multilayer structures for production of an entire arterial wall structure [Citation42,Citation45,Citation46]. This however requires expensive equipment to perform and may not be an easy process to standardize between labs. 3D bioprinting provides a promising alternative to allow precise layer-by-layer deposition of a bioink made of a synthetic or biopolymer solution containing the desired vascular cells. This allows the creation of micropatterned multicellular 3D tissues through its precise control of the spatial distribution of cells to accurately replicate the cellular structures of the native tissue [Citation47]. However, whilst it has been utilized for tissue engineered vascular grafts, it has yet to be fully explored for use in in vitro thrombosis models.
The availability of primary vascular endothelial, smooth muscle and fibroblast cells with well-defined culturing conditions provide an accessible, cost-effective and reliable method for producing sufficient cell numbers for tissue-engineered arterial constructs. These have been utilized in most previous studies to produce 3D arterial wall models for use in in vitro thrombosis studies [Citation38–42]. However, the well-known issues with inter-batch variability of primary cells may impact on the reproducibility of findings initiated from the generated constructs. This has led other groups to use a variety of stem cells to generate tissue engineered substrates for in vitro thrombosis models [Citation48,Citation49]. Whilst these offer an opportunity to provide a consistent cell source, there are issues concerning their use, including the additional time and cost of triggering differentiation or reprogramming of stems cells during the development of the construct, and the heterogeneity of cell subsets produced, which will require careful optimization and validation for use in a model system [Citation50]. Alternatively, recent studies have utilized endothelial colony forming cells to generate patient-specific endothelial lining which will be a key precursor for adapting these models for personalized medical uses - however the additional time costs in isolating and culturing these progenitor cells prior to seeding (2–3 weeks), precludes its use as a wide scale alternative to in vivo studies at present [Citation51].
The cellular components of 3D tissue engineered arterial constructs provide key functional properties. For example, previous studies have utilized real-time assessment of human platelet calcium signals exposed to a 3D tissue engineered arterial construct to assess the efficacy of the luminal surface of a tissue engineered arterial construct in eliciting hemostatic responses when exposed to washed human platelet suspension. Whilst the full arterial construct inhibited thrombin-evoked calcium signals, an intimal free-construct triggered platelet activation [Citation46]. Therefore identifying the ability of the construct to recreate both the anti- and pro-thrombotic properties of the intact and “damaged” arterial construct. This correlated with decreased and increased platelet aggregation under physiological flow conditions [Citation42]. The prothrombotic effect of the subendothelial layers of the arterial construct were elicited by the endogenously produced pericellular matrix (which contained type I and III collagen) by the encapsulated smooth muscle cells, and not the collagen hydrogel itself that was found to be inert [Citation46].
Optimizing the culture of cells within the arterial constructs is therefore essential in producing the optimal balance of anti- and pro thrombotic responses. The maturation of cells on the scaffolds can also be supplemented by exogenous chemical and physical stimulation of the cells seeded within the vascular constructs. Cellular proliferation and differentiation can also be influenced by the scaffold through its structure, mechanical properties, biochemical composition and nanotopography [Citation52]. The sensitivity of cellular behavior to minor alterations in these scaffold properties will require carefully defined manufacturing procedures to ensure reproducible model production between groups. The use of bioreactor systems to stimulate physiological blood flow conditions during the culture period can improve the replication of in vivo tissues as the phenotypic properties of both seeded endothelial and smooth muscle cells are dependent on the presence of shear forces passing over the intimal surface as well as the cyclical mechanical strain exerted by the changes in arterial blood pressure that occur through the cardiac cycle [Citation53,Citation54]. For instance, the exposure to laminar flow conditions helps to prevent the expression of a pro-inflammatory endothelial cell phenotype, whilst mechanical stimuli also help maintain smooth muscle cells in a contractile phenotype [Citation55,Citation56]. The disadvantage of utilizing these systems are the potential barrier it might represent to the widespread uptake of this method due to the cost of bioreactors systems and the expertise needed to use them. Therefore, a future challenge will be to develop cost-effective methods to facilitate continuous perfusion of tissue engineered arterial construct throughout the development period. Recent work has shown that chemical agonist of mechanosensitive channels can induce the cellular responses seen in endothelial cells cultured under flow conditions–suggesting that chemical supplementation of the culture media could be used as an alternative to bioreactor systems [Citation57].
The challenges of using tissue engineered arterial constructs within in vitro thrombosis models
There are now a range of methods that have been or could be successfully used to produce 3D tissue engineered human arterial constructs that could be utilized in in vitro thrombosis models. These models will need to be scrutinized experimentally to assess whether they can be considered a valid alternative to current in vivo models. This would include demonstrating that the construct can:
When uninjured, resist thrombus formation when perfused with human blood under physiological flow conditions for a prolonged period.
Able to support a robust and reproducible thrombotic response when subject to chemical or mechanical injury
Replicate the anti-thrombotic effect of clinically-approved inhibitors of coagulation and platelet function when blood is perfused under the same conditions over human arterial samples obtained from surgical procedures
Replicate the normal core-shell thrombus structure observed in vivo [Citation58].
Be modified to produce models of vascular pathologies, such as those currently being developed for atherosclerosis [Citation59].
Be able to be grown within specialist vascular microenvironments related to key thrombotic pathologies, such as within tumors or the central nervous system, or within medical devices such as dialysis or ECMO machines.
Demonstrate the capability to scale up the blood flow through the microfluidic device from the low flux levels that are typically used in single microfluidic channels to create (patho)physiological shear rates, toward clinically-relevant blood flow rates that allow investigators to more accurately simulate the in vivo environment.
If multiple models can demonstrate these properties, there are currently limited methods in place to compare the effectiveness of these models. Assays have been reported for testing anti- and pro-aggregatory properties of the tissue engineered constructs [Citation45], however a remaining challenge is to develop additional standardized, quantitative methods that can be used to directly compare the anti- and pro-thrombotic properties of tissue engineered arterial constructs. These methods will allow us to assess the most effective models for further development, as well as assess inter-lab consistency in the biological responses observed to ensure reproducibility of response to ensure widespread confidence in the validity of results observed.
A previous study of opinion papers from investigators assessing stroke models found that, despite the overwhelming failure of pre-clinical in vivo models to predict success in clinical trials, there is still significant investigator resistance to the use of in vitro alternatives [Citation60]. Building community confidence in any developed alternative remains a significant challenge in replacing current in vivo models. This will require engagement with researchers, journal editors, grant funders and medical regulators to produce agreed success criteria.
Vascular tissue engineering has provided powerful new ways to produce new humanized in vitro thrombosis models with more realistic replicas of the arterial wall, however future development of the techniques to produce and validate these models will be required to provide a widely-accepted alternative to current in vivo thrombosis models. Given the rapid advances of vascular tissue engineering this will be an exciting field of future study.
Acknowledgements
Jacob Ranjbar was supported by a PhD studentship co-funded by the British Heart Foundation and the National Centre for the Replacement Refinement & Reduction of Animals in Research (NC/R001642/1).
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Neubauer K, Zieger B. Endothelial cells and coagulation. Cell Tissue Res. 2022;387:391–7. doi: 10.1007/s00441-021-03471-2.
- Watson SP. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des. 2009;15:1358–1372. doi: 10.2174/138161209787846702.
- Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle–derived tissue factor is critical for arterial thrombosis after ferric chloride–induced injury. Blood. 2009;113(3):705–713. doi: 10.1182/blood-2007-05-090944.
- Nesbitt WS, Westein E, Tovar-Lopez FJ. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955.
- Westein E, van der Meer AD, Kuijpers MJ, Frimat JP, van den Berg A, Heemskerk JW. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci USA. 2013;110:1357–1362. doi: 10.1073/pnas.1209905110.
- Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series). Arterioscler Thromb Vasc Biol. 2007;27:2079–2093. doi: 10.1161/ATVBAHA.107.142810.
- Denis CV, Dubois C, Brass LF, Heemskerk JWM, Lenting PJ. Towards standardization of in vivo thrombosis studies in mice. J Thromb Haemost. 2011;9:1641–1644. doi: 10.1111/j.1538-7836.2011.04350.x.
- Perlman RL. Mouse models of human disease: an evolutionary perspective. Evol Med Public Heal. 2016;2016:170–176. doi: 10.1093/emph/eow014.
- Ware J. Dysfunctional platelet membrane receptors: from humans to mice. Thromb Haemost. 2004;92:478–485. doi: 10.1160/TH04-05-0308.
- Siller-Matula JM, Plasenzotti R, Spiel A, Quehenberger P, Jilma B. Interspecies differences in coagulation profile. Thromb Haemost. 2008;100:397–404. doi: 10.1160/TH08-02-0103.
- Cooley BC. Murine arterial thrombus induction mechanism influences subsequent thrombodynamics. Thromb Res Elsevier Ltd. 2015;135:939–943. doi: 10.1016/j.thromres.2015.02.016.
- Hechler B, Nonne C, Eckly A, Magnenat S, Rinckel J-Y, Denis CV, Freund M, Cazenave J-P, Lanza F, Gachet C. Arterial thrombosis: relevance of a model with two levels of severity assessed by histologic, ultrastructural and functional characterization. J Thromb Haemost. 2010;8:173–184. doi: 10.1111/j.1538-7836.2009.03666.x.
- Whinna HC. Overview of murine thrombosis models. Thromb Res. 2008;122(S1):S64–69. doi: 10.1016/S0049-3848(08)70022-7.
- Sashindranath M, Sturgeon SA, French S, Craenmehr DDD, Selan C, Freddi S, Johnson C, Cody SH, Nesbitt WS, Hamilton JR, et al. The mode of anesthesia influences outcome in mouse models of arterial thrombosis. Res Pract Thromb Haemost. 2019;3:197–206. doi:10.1002/rth2.12184.
- Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92:486–494. doi: 10.1055/s-0037-1613739.
- Fay W, Eitzman D, Shapiro A, Madison E, Ginsburg D. Platelets inhibit fibrinolysis in vitro by both plasminogen activator inhibitor-1-dependent and -independent mechanisms. Blood. 1999;83:351–356. doi: 10.1182/blood.V83.2.351.351.
- Konstantinides S, Schäfer K, Thinnes T, Loskutoff DJ. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in Mice. Circulation. 2001;103:576–583. doi: 10.1161/01.CIR.103.4.576.
- Vyas MV, Gros R, Hackam DG. Translation of cardiovascular animal models to human randomized trials. Am J Cardiol. 2020;137:141. doi: 10.1016/j.amjcard.2020.10.027.
- Rajeeva Pandian NK, Walther BK, Suresh R, Cooke JP, Jain A. Microengineered human vein-chip recreates venous valve architecture and its contribution to thrombosis. Small. 2020;16:e2003401. doi: 10.1002/smll.202003401.
- Mangin PH, Gardiner EE, Nesbitt WS, Kerrigan SW, Korin N, Lam WA, Panteleev MA. Subcommittee on Biorheology. In vitro flow based systems to study platelet function and thrombus formation: recommendations for standardization: communication from the SSC on Biorheology of the ISTH. J Thromb Haemost. 2020;18:748–752. doi: 10.1111/jth.14717.
- Pandian NKR, Mannino RG, Lam WA, Jain A. Thrombosis-on-a-chip: prospective impact of microphysiological models of vascular thrombosis. Curr Opin Biomed Eng. 2018;5:29–34. doi: 10.1016/j.cobme.2017.12.001.
- Neeves KB, Onasoga AA, Hansen RR, Lilly JJ, Venckunaite D, Sumner MB, Irish AT, Brodsky G, Manco-Johnson MJ, Di Paola JA. Sources of variability in platelet accumulation on type 1 fibrillar collagen in microfluidic flow assays. PLoS One. 2013;8:e54680. doi: 10.1371/journal.pone.0054680.
- Chen VM, Hogg PJ. Encryption and decryption of tissue factor. J Thromb Haemost. 2013;11 Suppl 1:277–284. doi: 10.1111/jth.12228.
- Govindarajan V, Zhu S, Li R, Lu Y, Diamond SL, Reifman J, Mitrophanov AY. Impact of tissue factor localization on blood clot structure and resistance under Venous Shear. Biophys J. 2018;114:978–991. doi: 10.1016/j.bpj.2017.12.034.
- Mannino RG, Qiu Y, Lam WA. Endothelial cell culture in microfluidic devices for investigating microvascular processes. Biomicrofluidics. 2018;12:042203. doi: 10.1063/1.5024901.
- Coenen DM, Mastenbroek TG, Cosemans JMEM. Platelet interaction with activated endothelium: mechanistic insights from microfluidics. Blood. 2017;130:2819–2828. doi: 10.1182/blood-2017-04-780825.
- Costa PF, Albers HJ, Linssen JEA, Middelkamp HHT, van der Hout L, Passier R, van den Berg A, Malda J, van der Meer AD. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab Chip. 2017;17:2785–2792. doi: 10.1039/C7LC00202E.
- Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, Ware RE, Fletcher DA, Lam WA. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122:408–418. doi: 10.1172/JCI58753.
- Mannino RG, Myers DR, Ahn B, Wang Y, Rollins M, Gole H, Lin AS, Guldberg RE, Giddens DP, Timmins LH, et al. Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions. Sci Rep. 2015;5:12401. doi:10.1038/srep12401.
- Dupuy A, Hagimola L, Mgaieth NSA, Houlahan CB, Preketes-Tardiani RE, Coleman PR, Passam FH. Thromboinflammation model-on-A-chip by whole blood microfluidics on fixed human Endothelium. Diagnostics (Basel). 2021;11:203. doi: 10.3390/diagnostics11020203.
- Lundkvist A, Lilleodden E, Siekhaus W, Kinney J, Pruitt L, Balooch M. Viscoelastic properties of healthy human artery measured in saline solution by AFM-based indentation technique. MRS Online Proc Lib. 1996;436:353–358. doi: 10.1557/PROC-436-353.
- Mahmoud M, Cancel L, Tarbell JM. Matrix stiffness affects Glycocalyx expression in cultured Endothelial cells. Front Cell Dev Biol. 2021;9:731666. doi: 10.3389/fcell.2021.731666.
- Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761.
- Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, Ahn B, Hardy ET, Kee MF, Kumar S, et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc Natl Acad Sci USA. 2014;111:14430–14435. doi: 10.1073/pnas.1322917111.
- Kee MF, Myers DR, Sakurai Y, Lam WA, Qiu Y. Platelet mechanosensing of collagen matrices. PLoS One. 2015;10:e0126624. doi: 10.1371/journal.pone.0126624.
- Berry J, Peaudecerf FJ, Masters NA, Neeves KB, Goldstein RE, Harper MT. An “occlusive thrombosis-on-a-chip” microfluidic device for investigating the effect of anti-thrombotic drugs. Lab Chip. 2021;21:4104–4117. doi: 10.1039/D1LC00347J.
- Devillard CD, Marquette CA. Vascular tissue engineering: challenges and requirements for an ideal large scale blood vessel. Front Bioeng Biotechnol. 2021;9:721843. doi: 10.3389/fbioe.2021.721843.
- Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109.
- Poventud-Fuentes I, Kwon KW, Seo J, Tomaiuolo M, Stalker TJ, Brass LF, Huh D. A human vascular injury-on-a-chip model of Hemostasis. Small. 2021;17:e2004889. doi: 10.1002/smll.202004889.
- Menon NV, Tay HM, Wee SN, Li KHH, Hou HW. Micro-engineered perfusable 3D vasculatures for cardiovascular diseases. Lab Chip. 2017;17:2960–2968. doi: 10.1039/C7LC00607A.
- Hasan A, Paul A, Memic A, Khademhosseini A. A multilayered microfluidic blood vessel-like structure. Biomed Microdevices. 2015;17:88. doi: 10.1007/s10544-015-9993-2.
- Njoroge W, Hernández ACH, Musa FI, Butler R, Harper AGS, Yang Y. The combination of tissue-engineered blood vessel constructs and parallel flow chamber provides a potential alternative to in vivo drug testing models. Pharmaceutics. 2021;13:340. doi: 10.3390/pharmaceutics13030340.
- Avendano A, Chang JJ, Cortes-Medina MG, Seibel AJ, Admasu BR, Boutelle CM, Bushman AR, Garg AA, DeShetler CM, Cole SL, et al. Integrated biophysical characterization of Fibrillar Collagen-based hydrogels. ACS Biomater Sci Eng. 2020;6:1408–1417. doi: 10.1021/acsbiomaterials.9b01873.
- Sarrigiannidis SO, Rey JM, Dobre O, González-García C, Dalby MJ, Salmeron-Sanchez M. A tough act to follow: collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater Today Bio. 2021;10:100098. doi: 10.1016/j.mtbio.2021.100098.
- Rickel AP, Deng X, Engebretson D, Hong Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021 Oct;129:112373. doi:10.1016/j.msec.2021.112373.
- Musa FI, Harper AG, Yang Y. A real-time monitoring system to assess the platelet aggregatory capacity of components of a tissue-engineered blood vessel wall. Tissue Eng Part C Methods. 2016;22:691–699. doi: 10.1089/ten.tec.2015.0582.
- Chen EP, Toksoy Z, Davis BA, Geibel JP. 3D Bioprinting of vascularized tissues for in vitro and in vivo applications. Front Bioeng Biotechnol. 2021;9:664188. doi: 10.3389/fbioe.2021.664188.
- Cochrane A, Albers HJ, Passier R, Mummery CL, van den Berg A, Orlova VV, van der Meer AD. Advanced in vitro models of vascular biology: human induced pluripotent stem cells and organ-on-chip technology. Adv Drug Deliv Rev. 2019;140:68–77. doi: 10.1016/j.addr.2018.06.007.
- Mallone A, Gericke C, Hosseini V, Chahbi K, Haenseler W, Emmert MY, von Eckardstein A, Walther JH, View Vogel V, Weber B, et al. Human induced pluripotent stem cell-derived vessels as dynamic atherosclerosis model on a chip. Biorxiv. doi:10.1101/2020.11.27.401034.
- Cai Q, Liao W, Xue F, Wang X, Zhou W, Li Y, Zeng W. Selection of different endothelialization modes and different seed cells for tissue-engineered vascular graft. Bioact Mater. 2021;6:2557–2568. doi: 10.1016/j.bioactmat.2020.12.021.
- Mathur T, Singh KA, Pandian NKR, Tsai SH, Hein TW, Gaharwar AK, Flanagan JM, Jain A. Organ-on-chips made of blood: endothelial progenitor cells from blood reconstitute vascular thromboinflammation in vessel-chips. Lab Chip. 2019 Jul 23;19:2500–2511. doi:10.1039/C9LC00469F.
- Greiner AM, Sales A, Chen H, Biela SA, Kaufmann D, Kemkemer R. Nano-and microstructured materials for in vitro studies of the physiology of vascular cells. Beilstein J Nanotechnol. 2016;7:1620–1641. doi: 10.3762/bjnano.7.155.
- Selden C, Fuller B. Role of bioreactor technology in tissue engineering for clinical use and therapeutic target design. Bioengineering. 2018;5:32. doi: 10.3390/bioengineering5020032.
- Mertsching H, Hansmann J. Bioreactor technology in cardiovascular tissue engineering. In: Kasper C, van Griensven M, Pörtner R, editors. Bioreactor systems for tissue engineering. Advances in biochemical engineering/biotechnology. Vol. 112. Berlin, Heidelberg: Springer; 2019.
- Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630.
- Bachmann BJ, Bernardi L, Loosli C, Marschewski J, Perrini M, Ehrbar M, Ermanni P, Poulikakos D, Ferrari A, Mazza E. A novel bioreactor system for the assessment of Endothelialization on deformable surfaces. Sci Rep. 2016;6:38861. doi: 10.1038/srep38861.
- Davies JE, Lopresto D, Apta BHR, Lin Z, Ma W, Harper MT. Using Yoda-1 to mimic laminar flow in vitro: a tool to simplify drug testing. Biochem Pharmacol. 2019;168:473–480. doi: 10.1016/j.bcp.2019.08.013.
- Pound P, Ram R. Are researchers moving away from animal models as a result of poor clinical translation in the field of stroke? An analysis of opinion papers. BMJ Open Sci. 2020;4:e100041. doi: 10.1136/bmjos-2019-100041.
- Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739.
- Chen J, Zhang X, Millican R, Lynd T, Gangasani M, Malhotra S, Sherwood J, Hwang PT, Cho Y, Brott BC, et al. Recent progress in in vitro models for Atherosclerosis studies. Front Cardiovasc Med. 2022;8:790529. doi: 10.3389/fcvm.2021.790529.