Abstract
Recombinant human TPO (rhTPO) is effective for refractory/relapsed primary immune thrombocytopenia (ITP), but optimal dosing regimen remains elusive. In this multicenter, randomized, controlled trial, a total of 282 adult ITP patients (mean age 47.3 years; 82 men) with a platelet count ≤30 × 109/L or >30 × 109/L with active bleeding randomly received a once daily (QD) subcutaneous injection of 7500 U (n = 64) or 15000 U rhTPO for 14 injections, or 15000 U or 30000 U rhTPO once every other day (QOD) for 7 injections. The primary outcomes included change from baseline in platelet count and total response rate (TRR) on day 14. On day 14, the median increase of platelet count from baseline was the highest in the 15000-U QD group (167.5 × 109/L, interquartile range [IQR] 23.0–295.0 × 109/L), followed by the 30000-U QOD group (57.5 × 109/L, IQR 9.0–190.0 × 109/L) (ANCOVA P < .001; P = .266 with baseline count as a covariate). The TRR on day 14 was also the highest in the 15000-U QD group (63.2%), followed by the 30000-U QOD group (59.7%). The rate of grade 3 and above adverse events did not differ among the four groups. There were no new safety concerns. All 4 regimens are safe and well-tolerated. The 30000-U QOD regimen is practically indistinguishable in efficacy to the 15000-U QD regimen.
Plain Language Summary
What is the context?
Relative thrombopoietin deficiency is implicated in primary immune thrombocytopenia (ITP), which is characterized by increased platelet destruction and impaired megakaryopoiesis.
Patients who are innately unresponsive to or have relapsed after glucocorticoid treatment have limited treatment options.
Recombinant human thrombopoietin (rhTPO) improves treatment response of primary ITP patients when added to high-dose dexamethasone.
What is new?
This trial sought to identify an optimal dosing regimen of rhTPO for patients who had failed or relapsed after glucocorticoid therapy.
Of the 4 regimens, once daily 15000 U rhTPO for 14 injections yielded the greatest median increase in platelet count (167.5 × 109/L) from baseline and attained the highest total response rate on day 14 (63.2%).
30000 U rhTPO once every other day for 7 injections was effective in rapidly increasing platelet counts in the first 7 days.
All 4 regimens were safe and well-tolerated.
What is the impact?
The 30000 U rhTPO once every other day regimen may offer an effective and safe regimen with less frequent injections, but future trials with longer follow-up are needed.
Introduction
Primary immune thrombocytopenia (ITP) is characterized by increased platelet destruction and impaired megakaryopoiesis, and accounts for 80% of all ITP cases [Citation1]. Numerous factors are implicated in the pathogenesis and development of ITP, including relative deficiency of thrombopoietin, a key regulator of megakaryopoiesis and a potent stimulator of platelet production, and dysregulated immune response such as Treg dysfunction [Citation2–4]. Corticosteroids are recommended for initial treatment of newly diagnosed primary ITP but achieve sustained response in <50% of the patients. For patients who are innately unresponsive to or have relapsed after glucocorticoid treatment, second-line options include splenectomy, immunosuppressive agents, rituximab and fostamatinib and thrombopoietin receptor agonists [Citation5,Citation6]. Each of these second-line treatments is associated with distinct limitations, including lack of durable response, frequent relapses after treatment discontinuation and untoward effects [Citation7].
In a recent trial in adult patients with newly diagnosed primary ITP, addition of recombinant human thrombopoietin (rhTPO), a glycosylated full-length peptide TPO (3SBIO, Shenyang, China), to high-dose dexamethasone improved the initial response and increased the rate of sustained response [Citation8]. In an observational study of 31 pregnant women with ITP who had failed corticosteroids and/or intravenous immunoglobulin and were refractory to platelet transfusion, the response rate was 74.2% with 300 U/kg rhTPO for 14 days [Citation9]. This finding is clinically relevant since eltrombopag and romiplostim readily cross the placenta and thus are not recommended for pregnant women; in contrast, rhTPO does not appear to cross the placenta [Citation10,Citation11].
ITP is a chronic disease that requires long-term treatment. Current rhTPO regimens require daily injections, incurring both inconvenience and costs. We conducted a trial in an attempt to identify an optimal dosing regimen that maximizes the efficacy of rhTPO without compromising the safety profile, as well as reducing the need for frequent injections in patients with resistant/relapsed primary ITP. Specifically, we compared the following 4 regimens: 7500-U/QD/14-injection, 15000-U/QD/14-injection, 15000-U/QOD/7-injection, and 30000-U/QOD/7-injection regimens. Pharmacokinetic (PK) and pharmacodynamic (PD) properties were also examined.
Methods
Study design and participants
This multicenter, open label, parallel group randomized controlled trial enrolled adult ITP patients (≥18 years) who failed or relapsed after glucocorticoid therapy. ITP was diagnosed according to the 2016 China Expert Consensus on the Diagnosis and Treatment of Primary Immune Thrombocytopenia in Adults [Citation12]. The main inclusion criteria included: 1) a platelet count ≤30 × 109/L or >30 × 109/L with active bleeding; 2) no contraindication for rhTPO. Patients with a history of thrombosis, failed rhTPO therapy (300 U/kg/d for 14 days) or had inadequate organ function were excluded. Additional exclusion criteria are shown in Protocol.
The trial protocol was approved by the ethics committees of participating hospitals. The trial is registered with clinicaltrials.gov (NCT04089267) and was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. Shenyang Sunshine Pharmaceuticals, Co., Ltd provided rhTPO.
Randomization and procedures
Eligible subjects were randomized at a 1:1:1:1 ratio to receive a once daily (QD) subcutaneous injection of 7500 U or 15000 U rhTPO for 14 injections, or 15000 U or 30000 U rhTPO once every other day (QOD) for 7 injections. Randomization was conducted using a centralized service system.
Outcomes
The primary outcomes were change in platelet count on day 14 from the baseline and TRR as of day 14. The secondary outcomes included change in platelet count at day 7 and 28 from the baseline and total response rate (TRR) as of day 7 and 28. Safety assessments were prespecified. Bleeding events were tabulated both for efficacy and safety and bleeding score was graded using an ITP-specific bleeding scale [Citation13,Citation14]. Treatment response was evaluated as described by Rodeghiero et al [Citation15]. Complete response (CR) and response were based on platelet count on at least 2 occasions more than 7 days apart. Relapse was based on platelet count on at least 2 occasions more than 1 day apart. The TRR was the proportion of patients achieving either CR or response.
PK/PD
Blood samples for PK analyses were collected within 30 min prior to the start of the first injection, immediately and 2, 5, 8, 10, 12, 16, 24, 48, 72, 96, 120, 168, and 216 h after the first rhTPO injection. Plasma rhTPO concentration was measured using a quantitative sandwich ELISA (R&D System, Cat #: DTP00B). Minimum steady state concentration [CSS(min)] was defined as the plasma rhTPO concentration immediately prior to the final rhTPO injection. Accumulation ratio was defined as CSS(min) /rhTPO plasma concentration immediately prior to the second rhTPO injection. For PD analysis, the maximal change from baseline in platelet counts (Emax) and time to Emax (ETmax) were calculated.
Statistical analysis
Sample size calculation was empirical due to limited information in the existing literature. A total of 240 patients were required, with 60 patients in each group. Assuming a drop off rate of 20% and ensuring at least 10 patients in each group in the PK/PD study, we planned to enroll 288 patients (72 per group).
Statistical analyses were prespecified and followed the intention-to-treat (ITT) principle. The full analysis set (FAS) included all patients who underwent randomization, received at least one dose of rhTPO, and had a baseline assessment and at least one post-baseline assessment. Platelet count change from baseline at day 7 and 14 were compared and analyzed using the analysis of covariance (ANCOVA) using the baseline platelet count as a covariate, and with the center effect taken into consideration. Last observation carried forward was used for missing efficacy data. The least square mean (LSM) changes from the baseline platelet count in each group and their 95% confidence interval (95% CI), the LSM differences in efficacy between the 15000-U QD group, the 15000-U QOD group, or the 30000-U QOD and the 7500-U QD group and their 95%CI were calculated. The 7 and 14-day TRR was calculated and differences in the TRR between the 15000-U QD group, the 15000-U QOD group, or the 30000-U QOD and the 7500-U QD groups and their 95%CI were calculated.
The safety set included all patients who received at least one dose of rhTPO and had at least one follow-up safety assessment and was analyzed mainly using descriptive statistics. The PK population comprised all patients who received at least one dose of rhTPO and had at least one measurable rhTPO concentration and no major protocol violation in the PK phase. The Kaplan-Meier plots were used for the time from the start of therapy to platelet count ≥30 × 109/L, 50 × 109/L or 100 × 109/L, the duration (days) during which the platelet count remained below 30 × 109/L during treatment (excluding time in recurrence) and the duration during which the platelet count remained ≥50 × 109/L or 100 × 109/L.
All tests were two-tailed with a level of significance set at α ≤ 0.05.
Role of the funding source
The funder did not contribute to study design, data collection, data analysis, data interpretation, writing of the report, and did not have the opportunity to review and comment on the manuscript before publication. Data were collected by the authors and their research teams. All authors had access to all study data and the first and the corresponding authors were responsible for the final version of the manuscript.
Results
A total of 308 patients were assessed for eligibility and 287 patients were randomized. The FAS included 282 patients, with 64, 76, 70 and 72 patients in the 7500-U QD, 15000-U QD, 15000-U QOD and 30000-U QOD rhTPO groups, respectively (). The demographic and key baseline characteristics (e.g., platelet count and prior pharmacotherapy for ITP) were comparable among the four groups ().
Figure 1. The study flowchart. The full analysis set (FAS) included all patients who underwent randomization, received at least one dose of rhTPO, and had a baseline assessment and at least one post-baseline assessment. The per protocol set (PPS) included patients who met the eligibility criteria and completed the treatment as specified by the trial protocol and completed efficacy evaluation on day 14. The safety set included all patients who received at least one dose of rhTPO and had at least one follow-up safety assessment and was analyzed mainly using descriptive statistics. rhTPO, recombinant human thrombopoietin.
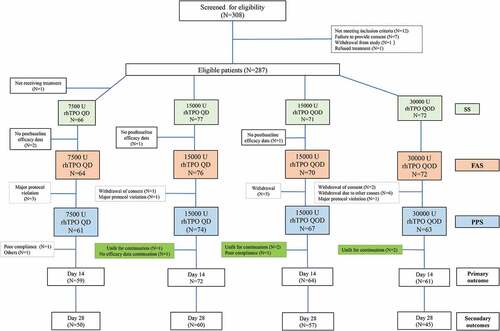
Table I. Patient demographic and baseline characteristics-the full analysis set (FAS).
The increase in platelet count from the baseline was significant on day 7 and diminished toward the end of the 28-day follow-up period. On day 14, the median platelet count was the highest in the 15000-U QD group (188.0 × 109/L, IQR 54.0 × 109-333.0 × 109/L), followed by the 30000-U QOD group (76.5 × 109/L, IQR 24.0 × 109-211.0 × 109/L), whereas the 7500-U QD group had the lowest platelet count (45.5 × 109/L, IQR 17.0 × 109-95.0 × 109/L). The median platelet count was (50.0 × 109/L, IQR 15.0 × 109–101.0 × 109/L) in the 15000-U QOD group (). The change of platelet count from the baseline was also the largest in the 15000-U QD group (167.5 × 109/L, IQR 23.0 × 109–295.0 × 109/L), followed by the 30000-U QOD group (57.5 × 109/L, IQR 9.0 × 109–190.0 × 109/L). The change of platelet count from the baseline was 29.0 × 109/L (IQR 3.0 × 109/L −85.0 × 109/L) in the 15000-U QOD group and 33.5 × 109/L (IQR 7.0 × 109/L −78.0 × 109/L) in the 7500-U QD group, with a significant difference among the four groups (ANCOVA test, P < .001) (). TRR on day 14 was 63.2% (95%CI: 52.3%–74.0%) in the 15000-U QD group, 59.7% (95%CI: 48.4%-71.1%) in the 30000-U QOD group and 50.0% in the remaining two groups (). No difference was observed in the TRR among the groups (Chi-square test, P = .268).
Figure 2. Median platelet count over time (a) and median change in platelet count (b) from baseline on day 7, 14 and 28 in the FAS. Platelet count changes from baseline were analyzed using the analysis of covariance (ANCOVA) in which the baseline platelet count was used as a covariate, groups as a fixed effect, and centers as a random effect. ^day 7, P = .027; *day 14, P < .001; *day 28, P = .388.
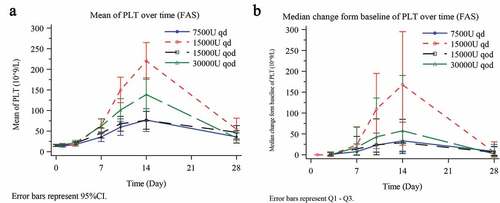
Table II. Primary and secondary efficacy outcomes in the full analysis set (FAS).
Similar to the findings on day 14, the change of platelet count on day 7 from the baseline was the largest in the 15000-U QD group (23.5 × 109/L, IQR 0.5 × 109/L–67.0 × 109/L), followed by the 30000-U QOD group (21.0 × 109/L, IQR 0.0 × 109/L–67.0 × 109/L), with a significant difference among the four groups (ANCOVA test, P = .027). Furthermore, the change of platelet count on day 7 from the baseline was 14.0 × 109/L (IQR −1.0 × 109/L–44.0 × 109/L) in the 15000-U QOD group and 7.5 × 109/L (IQR 0.0 × 109/L–26.0 × 109/L) in the 7500-U QD group (). The TRR was the highest in the 15000-U QD group (42.1%; 95% CI: 31.0%–53.2%), followed by the 30000-U QOD group (36.1%; 95% CI: 25.0%-47.2%), and the lowest in the 7500-U QD group (17.2%; 95% CI: 7.9%–26.4%), with a statistical difference among the groups (Chi-square test, P = .015). The TRR was 50·0% (95% CI 38·3%–61·7%) in the 15000-U QOD group ().
On day 28, platelet count was statistically higher than the baseline in all four groups, but the differences were apparently not clinically relevant (). The TRR did not differ among the four groups (Chi-square test, P = .596) ().
Subgroup analysis showed that overall, at day 14, patients who did not receive platelet transfusion had a greater increase in platelet counts than their counterparts who received platelet transfusion. In addition, the 15000-U QD group showed the greatest increase in platelet counts in patients who did not receive platelet transfusion and had greater changes in platelet counts from baseline than patients who had received platelet transfusion (187.5 × 109/L vs. 14.0 × 109/). In the 30000-U QOD group, patients who did not receive platelet transfusion had greater changes in platelet counts from baseline than patients who had received platelet transfusion (58.0 × 109/L vs. 36.0 × 109/) (Supplementary Table S1).
At baseline, moderate bleeding events were reported in 9.5%, 19.6%, 11.4% and 18.4% of the patients in the 7500-U QD group, 15000-U QD group, 15000-U QOD group and the 30000-U QOD group, respectively. One (2.2%) massive and 2 (4.4%) severe bleeding events were reported in the 15000-U QD group. On day 14, 2 (4.6%) moderate and 1 (2.2%) severe bleeding events occurred in the 15000-U QD group, and 1 (2.0%) moderate bleeding event was reported in the 30000-U QOD group. No other moderate, massive, or severe bleeding events occurred in the four treatment groups (Supplementary Table S2). Overall, the ITP-BAT scores showed a trend of decline over the course of treatment and there was no notable increase in the platelet transfusion volume before and following rhTPO treatment (Supplementary Table S3). In patients with bleeding and baseline platelet count <10 × 109/L, the 15000-U QD group had the largest increase in platelet count from baseline (day 7, 34.3 × 109/L, day 14, 187.7 × 109/L, and day 28, 94.1 × 109/L).
Safety profile did not differ significantly among the four groups. The rate of adverse events (AEs) and serious AEs (SAEs) was 57.6% and 4.6% in the 7500-U QD group, 57.1% and 5.2% in the 15000-U QD, 52.1% and 7.0% in the 15000-U QOD group, 61.1% and 4.2% in the 30000-U QOD group (). Grade 3 and above AEs occurred in 7.6%, 7.8%, 4.2% and 4.2% patients in the four groups, respectively. Additionally, a patient in the 30000-U QOD group had a transient thrombotic event. The platelet count in the patient increased from 11 × 109/L at baseline (3 September 2017) to 294 × 109/L at day 14 (16 September 2017) and treatment was discontinued. No thrombotic events occurred in any other groups. The rate of adverse drug reaction (ADR) was 9.1%, 5.2%, 7.0% and 11.1% in the 7500-U QD, 15000-U QD, 15000-U QOD and 30000-U QOD groups, respectively. All these AEs resolved, most without intervention. AEs causing treatment termination occurred in 3.0%, 2.6%, 1.4% and 4.2% in the four groups, respectively. No death was reported.
Table III. Adverse events in the safety set.
The mean plasma rhTPO concentration was 152.4, 140.8, 232.4 and 136.8 pg/mL at the end of first injection for the 7500-U QD, 15000-U QD, 15000-U QOD and 30000-U QOD group, respectively, and the concentration-time profile of 15000-U QD and 30000-U QOD was largely superimposable from day 1 to day 7 and rhTPO concentration was lower from day 8 to 13 in the 30000-U QOD group. On day 14, the mean plasma rhTPO concentration was 266.5, 786.2, 640.4 and 845.9 pg/mL (Supplementary Figure S1a), with CSS(min) at 325.65, 792.80, 429.40 and 665.95 pg/mL in the 7500-U QD, 15000-U QD, 15000-U QOD and 30000-U QOD groups, respectively (Supplementary Figure S1b). The accumulation ratio was the highest in the 15000-U QD group (2.15), followed by the 30000-U QOD group (1.84). The Emax was the highest in the 15000-U QD group (167.5 × 109/L), followed by the 30000-U QOD group (81.5 × 109/L) (Supplementary Figure S1c). The ETmax did not differ among the four groups. A positive linear correlation was observed between Emax and trough plasma rhTPO concentration irrespective of the groups (Supplementary Figure S1d).
Discussion
This study demonstrated optimal efficacy of rhTPO with the 15000-U QD regimen. On day 14, 57.9% of the patients achieved CR, with a faster time to the target platelet count than the other three regimens. The regimen also had the highest TRR on day 14 (63.2%). More importantly, practically all efficacy and safety measures with the 30000-U QOD regimen were indistinguishable from that obtained with the standard 15000-U QD regimen in the first 7 days, suggesting that the QOD regimen is effective in rapidly increasing platelet counts. These findings support the use of 15000-U QD regimen for rhTPO and encourage the use of the 30000-U QOD regimen. Expecting an increase of adherence due to a 50% reduction in dosing frequency, the 30000-U QOD regimen could bring clinical benefit in daily practice.
TPO is a c-Mpl ligand that specifically stimulates all stages of megakaryopoiesis. Increased platelet count in ITP patients treated with TPO receptor agonists improve Treg function and help restore immune tolerance [Citation16]. It remains to be investigated whether rhTPO also leads to normalized immune response and immune tolerance. In this study, we used a target platelet count ≥30 × 109/L, doubling baseline platelet counts and absence of bleeding for defining treatment response [Citation13,Citation15], and found that the target platelet count ≥30 × 109/L was achieved faster in the 15000-U QD versus the 7500-U QD group (7.5 versus 11.0 days). This finding is consistent with shorter time to response in patients receiving rituximab plus rhTPO versus rituximab alone in patients with corticosteroid-resistant or relapsed ITP (7 versus 28 days) [Citation17].
A platelet count of 20 to 30 × 109/L is a minimum treatment goal in primary ITP and a higher platelet target should be attained for older patients (≥60 years) as they are at increased risk of bleeding, thrombosis, and death [Citation18,Citation19]. Less than 60% of the patients in the 7500-U QD, 15000-U or 30000-U QOD groups achieved a platelet count ≥30 × 109/L. Even with the 15000-U QD regimen, only 63.2% of the patients attained the target platelet count. In other words, nearly four in ten patients failed to respond to rhTPO therapy, suggesting that the dose or dosing interval of rhTPO should be further optimized or should be used in combination with other treatments. Nonetheless, considering that this trial was conducted in patient who failed or relapsed after glucocorticoid therapy, the 14-day TRR of approximately 60% is acceptable in our opinion. Indeed, a higher CR rate has been shown in patients receiving dexamethasone plus rhTPO versus dexamethasone alone in patients with newly diagnosed primary ITP (75.0% vs. 42.7%) [Citation8]. A randomized trial in patients with corticosteroid-resistant or relapsed ITP failed to show significant increase in overall response, but an increased CR rate with the rituximab/rhTPO combination regimen versus rituximab monotherapy (45.4% vs. 25.7%) [Citation17]. Our study was only exploratory, aiming at finding an optimized treatment regimen for ITP.
Better efficacy in the 15000-U QD and 30000-U QOD groups in this trial could be readily explained by the PK/PD characteristics. Both regimens achieved higher CSS(min) (792.80 and 665.95 pg/mL, respectively) and had an accumulation ratio of approximately 2, suggesting effective accumulation of rhTPO. A positive linear correlation between Emax and CSS(min) in these groups adds further support to the rationale of using either the 15000-U QD or 30000-U QOD regimens. However, after a nearly superimposable concentration time profile for the 15000-U QD and 30000-U QOD regimens in the first 7 days of treatment, the 30000-U QOD regimen was less effective in increasing platelet counts compared to the 15000-U QD regimen. Currently, rhTPO is given as a daily injection in clinical studies and in clinical practice [Citation8,Citation20,Citation21]. No other known protocols are reported. Daily administration of an injected dose of rhTPO limits its use as a long-term treatment for ITP. In the current dose finding study, we attempted to find an optimized dosing schedule by including every other day dosing regimen for rhTPO 15000 U and 30000 apart from daily dosing regimen for rhTPO 7500 U and 15000. We showed that both rhTPO 15000 U QD and rhTPO 30000 U QOD rapidly increased platelet counts during the first 7 days, which would be valuable in an emergency setting, but the effect of rhTPO 30000 U QOD fell off after 7 days, indicating the challenge in finding the right dosing regimen beyond once daily dosing for rhTPO monotherapy to sustain long-term efficacy.
RhTPO has been shown to be safe and well tolerated by several studies [Citation22,Citation23]. The fact that no new safety concerns were reported in this trial is consistent with previous reports [Citation8,Citation24]. Notably, the rate of ≥ grade 3 AEs with the 15000-U QD and 30000-U QOD regimen did not differ from that with the 7500-U QD regimen, suggesting that increasing rhTPO dosage in this range does not increase the toxicities.
This trial has several limitations. First, we did not assess quality of life, which is an important measure of the overall success of the treatment in patients with primary ITP. Second, given the nature of this dose finding study, the study sample size is rather limited. There was wide variance in plasma TPO concentration within each group. We consider that the small sample size in each group could contribute to the variance in TPO concentration where overrepresentation of high values could lead to a skewed distribution of data. In addition, the study intervention only lasted for 14 days, and safety and efficacy assessment did not go beyond 28 days and the proportion of patients who completed follow up was low as visit beyond completion of outcome assessment was not mandatory. Benefits with treatment for prolonged period of time are unknown. Indeed, long-term platelet responses remain disappointing with many other treatment (e.g., rituximab), reflecting challenges in long term ITP management [Citation25]. Furthermore, as this is an exploratory study, prespecified statistical analyses did not include correction for multi-group comparisons.
In conclusion, all the rhTPO regimens in the current study are safe and well-tolerated in resistant/relapsed ITP patients. The 15000-U QD regimen, and, to a lesser degree, the 30000-U QOD regimen lead to a faster achievement of target platelet count, a higher TRR as well as a higher platelet count than the 7500-U QD regimen. The 30000-U QOD regimen offers the convenience of less frequent dosing, and rapidly increases platelet counts in the early stage of treatment without increasing AEs. Future trials with longer follow-up are needed to optimize and address the long-term efficacy and safety of rhTPO regimens.
Data sharing statement
Trial data, including patient-level data, are available upon request to the corresponding author.
Supplemental Material
Download PDF (2 MB)Acknowledgments
The authors thank the trial participants as well as the study investigators, research coordinators, and nurses not listed in the authorship. Writing assistance under the direction of the authors was provided by Dr. Bo Cui of the Ivy Medical Editing.
Disclosure statement
Sunqiong Cao is a full-time employee of Shenyang Sunshine Pharmaceuticals Co., Ltd. No potential conflicts of interest were disclosed by other authors. This trial was sponsored by Shenyang Sunshine Pharmaceuticals Co., Ltd.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2022.2157806.
Additional information
Funding
References
- Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, Ghanima W, Godeau B, González-López TJ, Grainger J, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–8. doi:10.1182/bloodadvances.2019000812.
- Nishimoto T, Kuwana M. Cd4+cd25+foxp3+ regulatory T cells in the pathophysiology of immune thrombocytopenia. Semin Hematol. 2013;50:S43–49. doi:10.1053/j.seminhematol.2013.03.018.
- Makar RS, Zhukov OS, Sahud MA, Kuter DJ. Thrombopoietin levels in patients with disorders of platelet production: diagnostic potential and utility in predicting response to TPO receptor agonists. Am J Hematol. 2013;88(12):1041–1044. doi:10.1002/ajh.23562.
- Semple JW, Rebetz J, Maouia A, Kapur R. An update on the pathophysiology of immune thrombocytopenia. Curr Opin Hematol. 2020;27(6):423–429. doi:10.1097/MOH.0000000000000612.
- Miltiadous O, Hou M, Bussel JB. Identifying and treating refractory ITP: difficulty in diagnosis and role of combination treatment. Blood. 2020;135(7):472–490. doi:10.1182/blood.2019003599.
- Vianelli N, Auteri G, Buccisano F, Carrai V, Baldacci E, Clissa C, Bartoletti D, Giuffrida G, Magro D, Rivolti E, et al. Refractory primary immune thrombocytopenia (ITP): current clinical challenges and therapeutic perspectives. Ann Hematol. 2022;101(5):963–978. doi:10.1007/s00277-022-04786-y.
- Kado R, McCune WJ. Treatment of primary and secondary immune thrombocytopenia. Curr Opin Rheumatol. 2019;31(3):213–222. doi:10.1097/BOR.0000000000000599.
- Yu Y, Wang M, Hou Y, Qin P, Zeng Q, Yu W, Guo X, Wang J, Wang X, Liu G, et al. High-dose dexamethasone plus recombinant human thrombopoietin vs high-dose dexamethasone alone as frontline treatment for newly diagnosed adult primary immune thrombocytopenia: a prospective, multicenter, randomized trial. Am J Hematol. 2020;95(12):1542–1552. doi:10.1002/ajh.25989.
- Kong Z, Qin P, Xiao S, Zhou H, Li H, Yang R, Liu X, Luo J, Li Z, Ji G, et al. A novel recombinant human thrombopoietin therapy for the management of immune thrombocytopenia in pregnancy. Blood. 2017;130(9):1097–1103. doi:10.1182/blood-2017-01-761262.
- Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–2247. doi:10.1056/NEJMoa073275.
- Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, Lichtin AE, Lyons RM, Nieva J, Wasser JS, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355(16):1672–1681. doi:10.1056/NEJMoa054626.
- Liu XG, Bai XC, Chen FP, Cheng YF, Dai KS, Fang MY, Feng JM, Gong YP, Guo T, Guo XH, et al. Chinese guidelines for treatment of adult primary immune thrombocytopenia. Int J Hematol. 2018;107(6):615–623. doi:10.1007/s12185-018-2445-z.
- Rodeghiero F, Michel M, Gernsheimer T, Ruggeri M, Blanchette V, Bussel JB, Cines DB, Cooper N, Godeau BH, Greinacher A, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121(14):2596–2606. doi:10.1182/blood-2012-07-442392.
- Khellaf M, Michel M, Schaeffer A, Bierling P, Godeau B. Assessment of a therapeutic strategy for adults with severe autoimmune thrombocytopenic purpura based on a bleeding score rather than platelet count. Haematologica. 2005;90(6):829–832.
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong B, Cooper N, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. doi:10.1182/blood-2008-07-162503.
- Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, Yazdanbakhsh K. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639–4645. doi:10.1182/blood-2010-04-281717.
- Zhou H, Xu M, Qin P, Zhang HY, Yuan CL, Zhao HG, Cui ZG, Meng YS, Wang L, Zhou F, et al. A multicenter randomized open-label study of rituximab plus rhTPO vs rituximab in corticosteroid-resistant or relapsed ITP. Blood. 2015;125(10):1541–1547. doi:10.1182/blood-2014-06-581868.
- Cohen YC, Djulbegovic B, Shamai-Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630–1638. doi:10.1001/archinte.160.11.1630.
- Mahévas M, Michel M, Godeau B. How we manage immune thrombocytopenia in the elderly. Br J Haematol. 2016;173(6):844–856. doi:10.1111/bjh.14067.
- Wang S, Yang R, Zou P, Hou M, Wu D, Shen Z, Lu X, Li Y, Chen X, Niu T, et al. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. Int J Hematol. 2012;96(2):222–228. doi:10.1007/s12185-012-1124-8.
- Mei H, Xu M, Yuan G, Zhu F, Guo J, Huang R, Qin J, Lv T, Qin F, Cai H, et al. A multicentre double-blind, double-dummy, randomised study of recombinant human thrombopoietin versus eltrombopag in the treatment of immune thrombocytopenia in Chinese adult patients. Br J Haematol. 2021;195(5):781–789. doi:10.1111/bjh.17808.
- Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB.Thrombopoietin receptor agonists: undefined years later. Haematologica. 2019;104(6):1112–1123. doi:10.3324/haematol.2018.212845.
- Rodeghiero F, Marranconi E. Management of immune thrombocytopenia in women: current standards and special considerations. Expert Rev Hematol. 2020;13(2):175–185. doi:10.1080/17474086.2020.1711729.
- Arai Y, Jo T, Matsui H, Kondo T, Takaori-Kondo A. Comparison of up-front treatments for newly diagnosed immune thrombocytopenia -a systematic review and network meta-analysis. Haematologica. 2018;103(1):163–171. doi:10.3324/haematol.2017.174615.
- Khellaf M, Charles-Nelson A, Fain O, Terriou L, Viallard JF, Cheze S, Graveleau J, Slama B, Audia S, Ebbo M, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood. 2014;124(22):3228–3236. doi:10.1182/blood-2014-06-582346.
