Abstract
In this research, the hapten di-n-octyl 4-aminophthalate (DOAP) was designed and synthesised successfully. It was used to couple with carrier proteins, bovine serum albumin (BSA) and ovalbumin (OVA) by diazotization reaction for immunogen (DOP–BSA) and coating antigen (DOP–OVA), respectively. Rabbits were immunised with DOP–BSA; polyclonal antiserum was raised and determined by competitive indirect enzyme-linked immunosorbent assay (ciELISA). After optimisation, a ciELISA was established. The quantitative working range for DOP was 5–75 ng/mL with the detection limit of 1.9±0.1 ng/mL and the IC50 of 19.2±1.1 ng/mL. The optimised ELISA had cross-reactivity of 22.6%, 17.6% and 21.2% with di-iso-octyl phthalate (DIOP), di-n-butyl phthalate (DBP) and di-hexyl phthalate (DHP), respectively. The result of the detection of polyvinyl chloride (PVC) samples showed that the immunoassay we developed had high-accuracy contrast with high-performance liquid chromatography electrospray ionisation tandem mass spectrometry analysis and it could be qualified to determine di-n-octyl phthalate (DOP) residue in PVC samples.
Introduction
Phthalates, primarily as additives to polyvinyl chloride (PVC) plastics, are widely existent environment chemical pollutants in our daily lives. Extensive use of these chemicals resulted in their appearance in various environmental matrices, such as water (drinking water, surface water and waste water), perfume, cosmetic, dust, air, sludge and soil.
Phthalates, also known as endocrine disrupting chemicals (EDC) (Castillo & Barcelo, Citation1997) have endocrine-disrupting properties. It showed that phthalates had estrogenic effects that disrupt the pathways of testicular differentiation in genetically male animals (Higuchi, Palmer, Gray, & Veeramachaneni, Citation2003; Jobling, Reynolds, White, Parker, & Sumper, Citation1995; Moore, Rudy, Lin, Ko, & Peterson, Citation2001; Mylchreest, Cattley, & Foster, Citation1998) and therefore had reproductive effects (Gray et al., Citation2000). As one of the phthalates, di-n-octyl phthalate (DOP) can lead to serious health problems. In 2005, the European Union banned the use of six phthalates including DOP in child products. In addition, DOP was also listed as priority pollutant by the World Health Organization (WHO) and China.
Several methods have been established for determination of phthalates in different environmental matrices. The analysis of phthalates is mostly performed by gas chromatography (GC) and liquid chromatography (LC) (Brossa, Marce, Borrull, & Pocurull, Citation2005; Luks-Betlej, Popp, Janozska, & Paschke, Citation2001; Penalver, Pocurull, Borrull, & Marce, Citation2000; Polo, Llompart, GarcIa-Jares, & Cela, Citation2005; Silva et al., Citation2004). The most common and important detector for phthalate analysis is mass spectrometric detection (Castillo, Oubina, & Barcelo, Citation1998; Gimeno, Marc, & Borrull, Citation2003; Kato, Silva, Needham, & Calafat, Citation2005). These traditional instrument analysis methods are often extremely time-consuming and expensive, and therefore not suitable for routine analysis of a large number of samples.
Enzyme-linked immunoassay and sensor-based immunoassay are more efficient, cost-effective, simple and reliable methods for identifying and quantitating the presence of the target analyte in samples (Chen et al., Citation2009; Hao et al., Citation2009; Li et al., Citation2008; Ma et al., Citation2009; Peng et al., Citation2009; Xie et al., Citation2009; Xu, Peng, & Xu, Citation2008; Xu, Yu, Chu, Peng, & Jin, Citation2006; Yue et al., Citation2009). Several immunoassays for phthalates have been developed (Goda et al., Citation2000; Ius, Bacigalupo, Meroni, Pistill, & Roda, Citation1993; Zhang, Wang, & Zhuang, Citation2006, Citation2007). In this research, we developed sensitive and quantitative indirect enzyme immunoassay for DOP. After the optimisation, we validated the immunoassay for the analysis of DOP in PVC samples and achieved a good agreement with high-performance liquid chromatography electrospray ionisation tandem mass spectrometry (HPLC-ESI-MS/MS) analysis.
Experimental
Reagents and materials
DOP, di-iso-octyl phthalate (DIOP), di-hexyl phthalate (DHP), di-n-butyl phthalate (DBP), n-octanol and reduced iron powder were obtained from Sinopharm Chemical Reagent Company (Beijing, China). 4-nitrophthalic acid (4-NPA) was obtained from Taixing Sunmy Fine Chemical Company (Taixing, China). Bovine serum albumin (BSA, MW 67,000) and Ovalbumin (OVA, MW 45,000) were purchased from Boao Biotechnology Company (Shanghai, China). Complete and incomplete Freund's adjuvant, enzyme immunoassay-grade HRP-labelled goat anti-rabbit immunoglobulin and Tween-20 were obtained from Sigma (St. Louis, MO, USA). All the other reagents were of analytical grades.
Thin-layer chromatography (TLC) was performed on 0.2 mm coated silica gel F254 on glass sheets (Qingdao, Shandong, China). The dialysis membrane (MW cutoff 12,000–14,000) was obtained from Huamei Biotechnology Company (Luoyang, Henan, China). Polystyrene 96-well microtiter plates were obtained from Costar (Corning, MA, USA).
Instruments
Multiskan microplate reader was obtained from Thermo Labsystem (Helsinki, Finland). Accela HPLC and TSQ Quantum Access mass spectrometer were obtained from Thermo Electron Corporation (Shanghai, China).
Hapten synthesis and characterisation
Since DOP does not have any suitable functional group to be coupled with carrier proteins, we designed and synthesised the hapten with amine group according to the following procedures. The synthetic route for this hapten is illustrated in .
Figure 1. Synthetic route for hapten DOAP: (a) di-n-octyl 4-nitrophthalate (DONP) and (b) di-n-octyl 4-aminephthalate (DOAP).
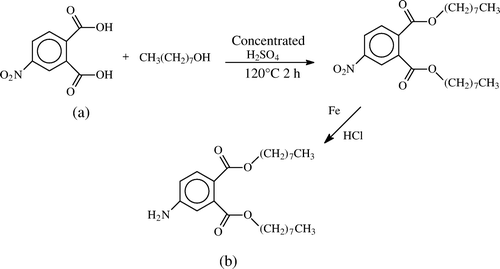
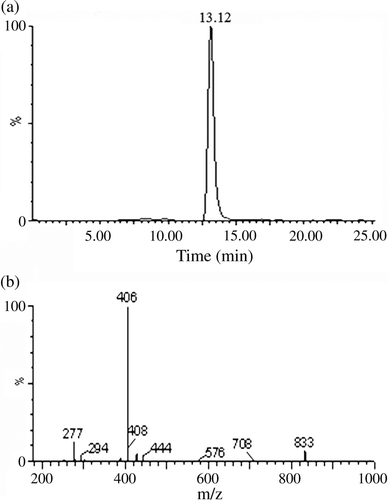
Synthesis of di-n-octyl 4-nitrophthalate (DONP): 21.1 mg (0.12 mol) of 4-NPA was added to a 100-ml volume three-neck flask and then 13.02 mg (0.1 mol) of n-octanol and 6 ml of benzene were added. After that, concentrated sulphuric acid (1.6 ml) was added slowly to the above stirred solution and it was heated to reflux (120°C) for 2 h, and the solvent was evaporated under reduced pressure. The oily residue obtained was added into cold water and the solid mixture was washed with 10% aqueous Na2CO3. The crude product was obtained from the upper layer. The resulting dark-red crystals of DONP were recrystallised from ethanol. TLC: R f=0.89 (acetic acid: n-hexane 1:15). UV: λmax=253 nm. LC-MS: positive ion scan mode, peak 1: [M + H]+=436; peak 2: [M + Na]+=458. It had a good agreement with DONP molecular weight of 435 ().
Figure 2. LC-MS analysis of DOAP: (a) the positive ions LC spectrum of DONP and (b) the mass spectrum of DONP.
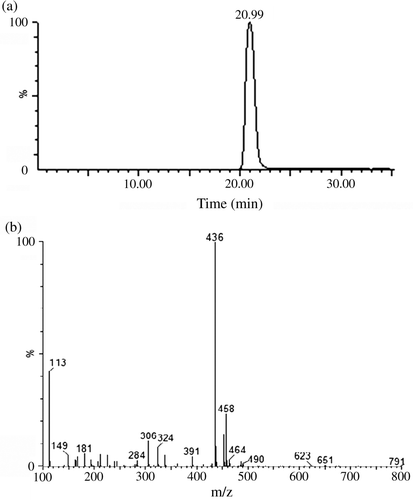
Synthesis of hapten (di-n-octyl 4-aminephthalate – DOAP): 392 mg (7 mmol) of reduced iron powder was added to a 50-ml volume round flask and then 2 ml of water and 1 mmol of ammonium chloride were added. After heated in a water bath for 15 min, 609 mg (1.4 mmol) of DONP dissolved in 16.8 ml methanol was added to the above solution and heated to reflux for 5 h. Before the reaction solution was cooled to room temperature, it was filtered to remove the remaining iron powder. The above filtrate was distilled under reduced pressure to get the salmon pink crude product. After the crude product dissolved in hot methanol was cooled to low temperature, 473.8 mg (1.17 mmol) of DOAP was obtained. TLC: R f=0.65 (acetic acid: n-hexane 1:15). UV: λmax1=232 nm, λmax2=288 nm. The LC spectrum identified that the DOAP we synthesised had a purity of more than 99%. LC-MS: positive ion scan mode, the peak of molecular weight of 406 for mass spectrum could be interpreted as molecular weight of DOAP plus one hydrogen ion, that is [M + H]+=406. It had a good agreement with dioctyl 4-aminephthalate molecular weight of 405 ().
Antigen synthesis
DOAP of 93.2 mg (0.23 mmol) was dissolved in a mixture of 2 ml DMF with 0.2 ml of 1 mol/L hydrochloric acid and cooled to 4°C. Then, 0.14 ml of 1 mol/L NaNO2 solution was added in drops and stirred for 1 h at 4°C. The reaction solution was monitored with potassium iodide starch paper. The NaNO2 solution was stopped being added to the above solution when the potassium iodide starch paper turned blue-grey. After that, BSA (150 mg, 0.023 mmol) or OVA (102 mg, 0.023 mmol) in 6 ml of borate buffer (pH = 9.20) was added dropwise. The pH of the reaction solution was adjusted to 9.20 by adding 1 mol/L sodium hydroxide solution. The mixture was stirred at 4°C overnight. The conjugate was dialysed in water that was changed with fresh water twice a day for three days at 4°C.
Immunisation
Two New Zealand white rabbits (1#, 2#) weighing about 1.5 kg were chosen for raising polyclonal antibodies. Routinely, 2 mg of immunogen dissolved in 1 ml of physiological saline was emulsi?ed with 1 ml Freund's complete adjuvant (1:1, v/v) and injected subcutaneously at multiple sites for the primary immunisation. For booster immunisations, 1 mg of the immunogen emulsified with Freund's incomplete adjuvant were given (1:1, v/v) after 2 weeks intramuscularly. One week after each booster immunisation, the rabbit was bled through ear vein. The blood was allowed to coagulate for about 1 h at room temperature and then stored at 4°C overnight. The antiserum was isolated by centrifugation (4000 rpm, 15 min) and sodium azide was added as a preservative at a final concentration of 0.02%. Antiserum was then divided into aliquots and stored at −70°C until use (Peng et al., Citation2008; Wang, Peng, Chen, & Xu, Citation2008).
Screening of antiserum
Checkerboard assays were used to select appropriate concentrations of coating antigens and antibodies, and to measure affinity of antibodies. We employed the assay similar to conventional procedure (Peng, Xu, Jin, Chu, & Wang, Citation2006; Tian et al., Citation2008). Microplates were coated with DOP–OVA (1/100–1/25,600, 100 µL/well) in carbonate buffer (50 mM, pH 9.6) at 4°C overnight. The microplates were washed three times with PBST (10 mM PBS containing 0.05% Tween-20, pH 7.4) and were blocked by 0.1% gelatine in carbonate buffer (50 mM, pH 9.6, 200 µL/well) for 2 h at 37°C. After another washing step, 100 µL/well of antiserum previously diluted (1/500–1/9600) with PBST (containing 0.1% gelatine) was added. After incubation at 37°C for 30 min, the plates were washed and the goat anti-rabbit IgG–HRP conjugate (diluted 1:3000 in PBST with 0.1% gelatine 100 µL/well) was added. The mixture was incubated at 37°C for 30 min, and after one more washing step, 100 µL/well of a TMB solution (1 ml of 0.06% TMB–Glycol and 5 ml citrate–acetate buffer, pH 5.5) was added and incubated at 37°C for 30 min. The reaction was stopped by adding 100 µL of 2 M H2SO4 and absorbance was read at 450 nm.
Enzyme-linked immunosorbent assay (ELISA) procedure
The procedure of the competitive assay was described similarly by our group (Li et al., Citation2009a, Citation2009b; Peng, Chen, Chen, Xu, & Jin, Citation2008). The assay conditions were the same as the procedure described above with only a small change in the competition step: 100 µL/well of antiserum was replaced by 50 µL/well of antiserum and 50 µL/well of standard analyte. The standard analyte concentrations ranged from 0.5 to 500 ng/ml. The absorbance was measured at 450 nm and recorded. Standard curves were obtained by plotting absorbance against the analyte concentration.
Optimisation of enzyme-linked immunosorbent assay (ELISA) conditions
A set of immunoassay parameters (coating method, methanol content, ionic strength, pH of solvent, competitive time, blocking time, colouration time) were studied sequentially to improve sensitivity and stability of the immunoassay. These experiments were carried out by using the indirect protocol described above. The antibody from 1# rabbit diluted to 1/1600 in PBST and the coating antigen DOP–OVA diluted to 1/2000 was chosen as the working concentration. The criteria used to evaluate immunoassay performance were maximum absorbance (Amax), IC50 and Amax/IC50.
Effect of coating method
Three coating methods were employed (4°C, overnight; 37°C, 2 h; 37°C, 2 h then 4°C overnight) for the test.
Effect of the ionic strength
As to ELISA, ionic strength of the solution was an important factor. Thus, in our protocol, different PBS solutions (5, 10, 20, 40 mM PBS) were used as ELISA solution system.
Effect of the pH
The pH of the solution was another important factor for ELISA. PBS solutions ranging from pH 6.0 to 9.5 were used as the working solutions of the competitive immunoassay.
Effect of the methanol content
Various water–methanol concentration solvents were used to dissolve DOP for assay optimisation. For this test, standard DOP solutions were prepared in PBS containing methanol of varying concentration levels (5, 10, 20 and 40%) to improve the sensitivity.
Effect of competition and colouration time
As to immunoassay, the time for competitive and colouration step was an important factor that could affect the sensitivity and stability. Herein, we employed different competition time (0.5, 1 and 2 h) and colouration time (10, 20 and 30 min) for the optimisation of immunoassay reaction time.
Determination of cross-reactivity
Several phthalates were tested for cross-reactivity using the indirect ELISA procedure described above. The cross-reactivity values were calculated by using the formula: cross-reactivity%=(IC50 of DOP/IC50 of related compound)×100.
Analysis of real samples
The PVC sample with a thickness of 0.5–2 mm was cut into 5×5 mm fragment. PVC sample of 0.5–5.0 g of above 5×5 mm was weighed and wrapped with filter paper. Then it was put into a soxhlet extractor and extracted with organic solvent (CHCl3:CH3OH – 2:1) in water bath at 84±2°C for about 7.5 h. The extracted solution was transferred to a measuring flask and dissolved by methanol with a total volume of 25 ml. Then the solution was centrifuged at 17,000 rpm for 10 min and the supernatant was used for ELISA and HPLC-ESI-MS/MS analysis (Li et al., 2009a, 2009b).
Results and discussion
Hapten design and characterisation
The synthesis of appropriate hapten is the initial and critical step for the establishment of effective ELISA for low molecular weight chemicals, which affects the specificity and sensitivity of antibodies for the target analyte (Kuang et al., Citation2009). In designing hapten, it is preferable to retain functional groups that are unique for the target analyte. DOP has apparently two important antigenic determinants that are n-octanol group and aromatic ring. We selected the 4-NPA to synthesise DOAP as the hapten that would mostly expose the two important antigenic determinants and provide the desired amino group for protein conjugation. However, to make sure the two carboxyl groups of 4-NPA react with n-octanol completely, we employed acidic condition to heated to reflux (120°C) for 2 h to carry out esterification reaction.
Screening of antiserum
The antiserum of terminal rabbit blood was screened by using coating antigen (DOP–OVA) by a checkerboard titration method. The result showed that the titre value of pAb 1# was 1/48,000 and titre value of pAb 2# was 1/12,000. Both the antiserums obtained showed reasonably high recognition for the coating antigen. Although both lower IC50 values were obtained, they showed slightly different specificity degree for the analyte DOP. The pAb 1# exhibited the better inhibition for the analyte and was selected as the assay reagent.
Enzyme-linked immunosorbent assay (ELISA) optimisation
In order to determine the DOP residue in PVC samples, it is important to develop a sensitive ELISA. For this purpose, the effects of several factors were investigated and evaluated as listed in . As shown in , all the standard deviation (SD) values are allowable which means that the results are significant and reliable.
Table 1. Effects of the factors on the performance of ELISA.
Effect of coating method
Coating DOP–OVA at 4°C overnight gave lower IC50 value and higher Amax and Amax/IC50 value than coating at 37°C for 2 h and at 37°C, 2 h then 4 °C, overnight. So coating method of 4°C overnight was selected.
Effect of the ionic strength
The PBS ionic strength increasing from 5 to 40 mM resulted in higher Amax value. But only in 10 mM condition gave a lower IC50 and a higher Amax/IC50 value, indicating that only suitable ionic strength was beneficial to antigen/antibody reaction. Thus, 10 mM ionic strength of assay buffer was selected.
Effect of the assay buffer pH
To determine the effect of pH on the assay, the phosphate buffer was used in the range of pH 6.0–9.5. As shown in , the ELISA was more sensitive under neutral or slightly alkaline conditions than slightly acidic condition. Both under acidic condition (pH 6.0) and alkaline condition (pH 8.5 and 9.5) gave lower Amax, lower Amax/IC50 and higher IC50. Based on the sensitivity of the assay, pH 7.4 was chosen as optimised pH condition.
Effect of the methanol content
The effects of different methanol concentration on the ELISA system were evaluated. clearly indicates that methanol concentration significantly influenced assay performance. Both under lower methanol concentration (10%) and higher methanol concentration (30%) gave lower Amax/IC50 and higher IC50; only 20% methanol concentration contributed to improve the sensitivity of the assay.
Effect of competition and colouration time
As we are aware, more competition and colouration time would lead to higher Amax because of more reaction time resulted in more antibody adsorbed on the microplate. As to Amax/IC50 and IC50, just in a suitable reaction time, we could get a higher Amax/IC50 and lower IC50. As to competition time, 1 h was mostly sensitive with a higher Amax/IC50 and lower IC50 and for colouration time, we got the best result at 20 min.
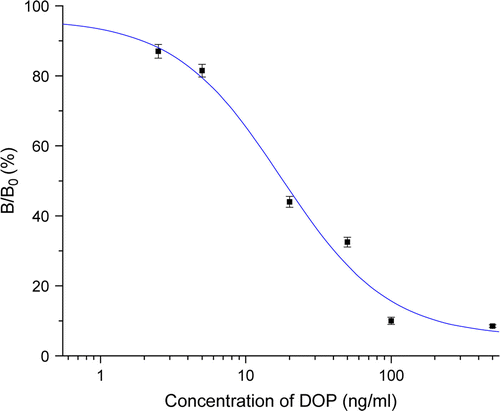
On the basis of the optimal conditions, we developed sensitive ELISA for the DOP residue as follows: the DOP–OVA conjugate as a coating antigen was coated onto the plate and placed at 4°C overnight and then the plate was blocked with 0.1% gelatine at 37°C for 2 h. The antiserum 1# was diluted 1600-fold and competed with the target analyte dissolved in PBS (10 mM, 20% methanol, pH 7.4) at 37°C for 1 h. At last for colouration step, 20 min was employed. For the ELISA solution system, pH 7.4, 10 mM PBS was used. shows a typical calibration curve obtained under these optimum conditions. The IC50 value of the assay was 19.2±1.1 ng/ml with the detection limit of 1.9±0.1 ng/ml and quantitative working range of 5–75 ng/ml.
Cross-reactivity
To determine the speci?city of the optimised ELISA, several phthalates were tested for cross-reactivity. shows the cross-reactivity that was found by the immunoassay we developed. The assay shows a high cross-reactivity with DONP and DOAP, which was used as haptens in our research. The assay also has cross-reactivity with DIOP, DBP and DHP for 22.6%, 17.6% and 21.2%, respectively. Since these compounds have the same aromatic structure and similar alkyl groups with DOP, the cross-reactivity with DOP is understandable. To some extent, the immunoassay we developed was qualified for multi-residues detection.
Table 2. Cross-reactivity of structure related compounds to DOP determined by competitive indirect ELISA.
Real sample analysis
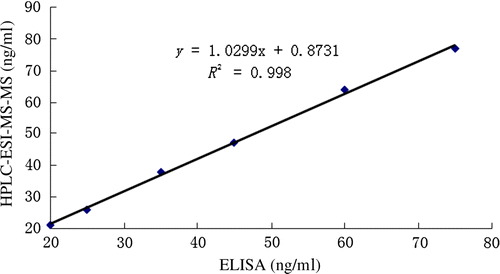
To evaluate the optimised ELISA, the PVC sample analysis was employed. As shown in , the correlative equation has a slope of 1.0299 and related coefficient of 0.998. The result identifies that the immunoassay we developed has a good agreement with HPLC-ESI-MS/MS analysis and it could be qualified to determine DOP residue in PVC samples.
Conclusion
We design and synthesise the hapten for DOP which have elicited the sensitive antibody for DOP. Furthermore, based on the sensitive antibody, an effective ciELISA was developed. Not only it can be used to screen DOP residue itself, but it can also detect DIOP, DBP and DHP residues. According to real samples analysis, the immunoassay has high accuracy contrast with HPLC-ESI-MS/MS analysis. It would be useful to monitor phthalate residues in various environmental matrices. Therefore, the ELISA we developed may become a new convenient and economical analytical tool to detect these residues in future.
References
- Brossa , L. , Marce , R.M. , Borrull , F. and Pocurull , E. 2005 . Determination of endocrine disruptors in environmental water samples by stir bar sorptive extraction-liquid desorption-large volume injection-gas chromatography . Chromatographia , 61 : 61 – 65 .
- Castillo , M. and Barcelo , D. 1997 . Analysis of industrial effluents to determine endocrine-disrupting chemicals . Trends in Analytical Chemistry , 16 : 574 – 583 .
- Castillo , M. , Oubina , A. and Barcelo , D. 1998 . Evaluation of ELISA kits followed by liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry for the determination of organic pollutants in industrial effluents . Environmental Science & Technology , 32 : 2180 – 2184 .
- Chen , W. , Peng , C.F. , Jin , Z.Y. , Qiao , R.R. , Wang , W.Y. , Zhu , S.F. , Wang , L. , Jin , Q. and Xu , C. 2009 . Ultrasensitive immunoassay of 7-aminoclonazepam in human urine based on CdTe nanoparticle bioconjugations by fabricated microfluidic chip . Biosensors and Bioelectronics , 24 : 2051 – 2056 .
- Gimeno , R.A. , Marc , R.M. and Borrull , F. 2003 . Determination of plasticizers by high-performance liquid chromatography and atmospheric pressure chemical ionization mass spectrometry in water and sediment samples . Chromatographia , 58 : 37 – 41 .
- Goda , Y. , Kobayashi , A. , Fukuda , K. , Fujimoto , S. , Ike , M. and Fujita , M. 2000 . Development of the ELISA for detection of hormone-disrupting chemicals . Water Science and Technology , 42 : 81 – 88 .
- Gray , L.E Jr. , Ostby , J. , Furr , J. , Price , M. , Veeramachaneni , D.N.R. , Parks , L. 2000 . Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat . Toxicological Sciences , 58 , 350 365 .
- Hao , X.L. , Kuang , H. , Li , Y.L. , Yuan , Y. , Peng , C.F. , Chen , W. , Wang , L.B. and Xu , C. 2009 . Development of an enzyme-linked immunosorbent assay for the α-cyano pyrethroids multi-residue in Tai lake water . Journal of Agricultural and Food Chemistry , 57 : 3033 – 3039 .
- Higuchi , T.T. , Palmer , J.S. , Gray , L.E. and Veeramachaneni , D.N.R. 2003 . Effects of dibutyl phthalate in male rabbits following in utero, adolescent, or postpubertal exposure . Toxicological Sciences , 72 : 301 – 313 .
- Ius , A. , Bacigalupo , M.A. , Meroni , G. , Pistill , A. and Roda , A. 1993 . Development of a time-resolved fluoroimmunoassay for phthalate esters in water . Fresenius Journal of Analytical Chemistry , 345 : 589 – 591 .
- Jobling , S. , Reynolds , T. , White , R. , Parker , M.G. and Sumper , J.P. 1995 . A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic . Environmental Health Perspectives , 103 : 582 – 585 .
- Kato , K. , Silva , M.J. , Needham , L.L. and Calafat , A.M. 2005 . Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry . Analytical Chemistry , 77 : 2985 – 2991 .
- Kuang , H. , Wu , Y.N. , Hou , X.L. , Miao , H. , Zhang , G. , Shen , J.Z. and Lu , C. 2009 . Synthesis of derivatives and production of antiserum for class specific detection of pyrethroids by indirect ELISA . International Journal of Environmental Analytical Chemistry , 89 : 423 – 437 .
- Li , Y.L. , Hao , X.L. , Ji , B.Q. , Xu , C.L. , Chen , W. , Shen , C.Y. and Ding , T. 2009a . Rapid determination of 19 quinolone residues in spiked fish and pig muscle by HPLC tandem mass spectrometry . Food Additives and Contaminants , 26 : 306 – 313 .
- Li , Y.L. , Ji , B.Q. , Chen , W. , Liu , L.Q. , Xu , C.L. , Peng , C.F. and Wang , L. 2008 . Production of new class-specific polyclonal antibody for determination of fluoroquinolones antibiotics by indirect competitive ELISA . Food and Agricultural Immunology , 19 : 251 – 264 .
- Li , Z.K. , Zhu , Y.Y. , Yin , G. , Peng , C.F. , Chen , W. , Liu , L.Q. , Yin , L.-M. and Xu , C.-L. 2009b . Development of an indirect enzyme-linked immunosorbent assay for the organophosphorus pesticide paraoxon-methyl . Immunological Investigations , 38 : 510 – 525 .
- Luks-Betlej , K. , Popp , P. , Janozska , B. and Paschke , H. 2001 . Solid-phase microextraction of phthalates from water . Journal of Chromatography A , 938 : 93 – 101 .
- Ma , W. , Chen , W. , Qiao , R.R. , Liu , C.Y. , Yang , C.H. , Li , Z.K. , Xu , D. , Peng , C. , Jin , Z. , Xu , C. , Zhu , S. and Wang , L. 2009 . Rapid and sensitive detection of microcystin by immunosensor based on nuclear magnetic resonance . Biosensors and Bioelectronics , 25 : 240 – 243 .
- Moore , R.W. , Rudy , T.A. , Lin , T.M. , Ko , K. and Peterson , R.E. 2001 . Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer di (2-ethylhexyl) phthalate . Environmental Health Perspectives , 109 : 229 – 237 .
- Mylchreest , E. , Cattley , R.C. and Foster , P.M.D. 1998 . Male reproductive tract malformations in rats following gestational and lactational exposure to di (n-butyl) phthalate: an antiandrogenic mechanism? . Toxicological Sciences , 43 : 47 – 60 .
- Penalver , A. , Pocurull , E. , Borrull , F. and Marce , R.M. 2000 . Determination of phthalate esters in water samples by solid-phase microextraction and gas chromatography with mass spectrometric detection . Journal of Chromatography A , 872 : 191 – 201 .
- Peng , C.F. , Chen , Y.W. , Chen , H.Q. , Xu , C.L. and Jin , Z.Y. 2008 . A rapid and sensitive enzyme-linked immunosorbent assay (ELISA) method and validation for progestogen multi-residues in feed . Journal of Animal and Feed Sciences , 17 : 434 – 441 .
- Peng , C.F. , Chen , Y.W. , Chen , W. , Xu , C.L. , Kim , J.M. and Jin , Z.Y. 2008 . Development of a sensitive heterologous ELISA method for analysis of acetylgestagen residues in animal fat . Food Chemistry , 109 : 647 – 653 .
- Peng , C.F. , Li , Z.K. , Zhu , Y.Y. , Chen , W. , Yuan , Y. , Liu , L.Q. , Li , Q. , Xu , D. , Qiao , R. , Wang , L. , Zhu , S. , Jin , Z. and Xu , C. 2009 . Simultaneous and sensitive determination of multiplex chemical residues based on multi-color quantum dot probes . Biosensors and Bioelectronics , 24 : 3657 – 3662 .
- Peng , C.F. , Xu , C.L. , Jin , Z.Y. , Chu , X.G. and Wang , L.Y. 2006 . Determination of anabolic steroid residues (medroxyprogesterone acetate) in pork by ELISA and comparison with liquid chromatography tandem mass spectrometry . Journal of Food Science , 71 : C44 – C50 .
- Polo , M. , Llompart , M. , García-Jares , C. and Cela , R. 2005 . Multivariate optimization of a solid-phase microextraction method for the analysis of phthalate esters in environmental waters . Journal of Chromatography A , 1072 : 63 – 72 .
- Silva , M.J. , Slakman , A.R. , Reidy , J.A. , Preau , J.L. Jr. , Herbert , A.R. , Samandar , E. , Needham , L.L. , Calafat , A.M. 2004 . Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction . Journal of Chromatography B , 805 , 161 167 .
- Tian , Z. , Yu , D.H. , Zhang , Y.Y. , Guo , J.G. , Peng , C.F. , Chen , Z.X. and Xu , C.L. 2008 . Development and optimization of an indirect enzyme-linked immunosorbent assay for 19-nortestosterone . Journal of Animal and Feed Sciences , 17 : 253 – 267 .
- Wang , L.Y. , Peng , C.F. , Chen , W. and Xu , C.L. 2008 . A direct enzyme-linked immunosorbent assay for hexoestrol residues . Food and Agricultural Immunology , 19 : 61 – 75 .
- Xie , H.L. , Ma , W. , Liu , L.Q. , Chen , W. , Peng , C.F. , Xu , C.L. and Wang , L. 2009 . Development and validation of an immunochromatographic assay for rapid multi-residues detection of cephems in milk . Analytica Chimica Acta , 634 : 129 – 133 .
- Xu , C.L. , Yu , D.H. , Chu , X.G. , Peng , C.F. and Jin , Z.Y. 2006 . A chemiluminescence enzyme immunoassay (CLEIA) for the determination of 19-nortestosterone residues in aquatic products . Analytical Letters , 39 : 709 – 727 .
- Xu , L.G. , Peng , C.F. and Xu , C.L. 2008 . Development of the ELISA for detection of microcystin by using magnetic nanometer particle enrichment antibody . Toxicology Letters , 180 : S120 – S120 .
- Yue , N. , Ji , B.Q. , Liu , L.Q. , Tao , G.J. , Eremin , S.A. and Wu , L.H. 2009 . Synthesis of olaquindox metabolite, methyl-3-quinoxaline-2-carboxylic acid for development of an immunoassay . Food and Agricultural Immunology , 20 : 173 – 183 .
- Zhang , M.C. , Wang , Q.E. and Zhuang , H.S. 2006 . A novel competitive fluorescence immunoassay for the determination of dibutyl phthalate . Analytical and Bioanalytical Chemistry , 386 : 1401 – 1406 .
- Zhang , M.C. , Wang , Q.E. and Zhuang , H.S. 2007 . Determination of dibutyl o-phthalate by antigen-coated competitive fluorescence immunoassay . Analytical Letters , 40 : 127 – 137 .