Abstract
Hen egg is one of the most frequent causes of food allergy in infants and adults. Ovalbumin (OVA) has been identified as a major egg allergen. In order to detect OVA in foods, a highly sensitive sandwich enzyme-linked immunosorbent assay (ELISA) based on two monoclonal antibodies (mAbs) was established. The 2 mAbs were selected out of 17 murine hybridomas secreting OVA-specific antibody. Using mAb17 as the capture antibody and mAb15 as the detection antibody, the detection limit of the ELISA method was 0.51 ng/mL, and the linear dynamic range was between 1.95 and 500 ng/mL. The recovery ranged from 85.6 to 115.2%, whereas the intra- and inter-assay coefficients of variation were less than 8.6 and 13.9%, respectively. Sample analysis verified that the produced anti-OVA mAb and the developed ELISA may provide a valuable tool for the sensitive determination of OVA in processed foods and for future studies on the mechanism of how OVA functions in anaphylaxis.
Introduction
Egg, a common food material, is widely used in the food industry. However, egg is one of the most frequent causes of food allergy, especially in children (Eggesbo, Botten, Halvorsen, & Magnus, Citation2001). The major allergens in egg white include ovalbumin (OVA), ovomucoid, ovotransferrin and lysozyme, with OVA accounting for 54% of egg white protein (Hoffman, Citation1983; Mine & Rupa, Citation2004; Mine & Yang, Citation2008; Sampson, Citation2004). Thus, OVA may be used as an indicator for the detection of egg allergen.
The development of a detection method for OVA would be useful for both the food industry and consumer protection agencies. However, the detection of allergens in food products is very difficult. Most foods are processed in various conditions, such as baking and boiling. These processing conditions can change the solubility, structure and bioactivity of allergen proteins (Bu, Luo, Zheng, & Zheng, Citation2009; Fu, Maks, & Banaszewski, Citation2010; Mine & Zhang, Citation2002). In addition, allergens in food products are often present in trace amounts and can be masked by the food matrix. During the past few years, several methods have been developed for the identification and quantification of food allergens, including high performance liquid chromatography, polymerase chain reaction, mass spectrometry (MS), surface plasmon resonance (SPR) and enzyme-linked immunosorbent assay (ELISA) (Hird, Lloyd, Goodier, Brown, & Reece, Citation2003; Matthias, Citation2001; Monaci & Visconti, Citation2009; Poms, Klein, & Anklam, Citation2004; Xu, Ye, Wu, & Ying, Citation2010). ELISA can provide high sensitivity and specificity without sophisticated equipment, and it is the most commonly used method for food allergen analysis. Various ELISA-based methods have been developed for the detection of several food allergens (Fuller, Goodwin, & Morris, Citation2007; Mariager, Solve, Eriksen, & Brogren, Citation1994; Sletten, Lovberg, Moen, Skarpeid, & Egaas, Citation2005). Obtaining an antibody with high affinity and specificity is the key factor in the ELISA technique. In this study, we attempted to generate anti-OVA monoclonal antibodies (mAbs), and develop a mAb-based sandwich ELISA for the detection of OVA in foods.
Materials and methods
Chemicals and materials
Ovalbumin, PEG 1450, HAT supplement (containing hypoxanthine, aminopterin and thymidine) (50×), HT supplement (containing hypoxanthine and thymidine) (100×), complete and incomplete Freund's adjuvant and enzyme immunoassay-grade horseradish peroxidase (HRP)-labelled goat anti-mouse immunoglobulin were obtained from Sigma (St. Louis, MO, USA). Polyvinyl alcohol (PVA) and gelatin were obtained from Beijing Biodee Biotechnology Co., Ltd. (China). Fetal bovine serum and 1640 basal medium were purchased from SunShine Biotechnology Co., Ltd. (Nanjing, China). 3,3′,5,5′-tetra methyl benzidine (TMB) and HRP were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China). All other reagents and chemicals were obtained from the National Pharmaceutical Group Chemical Reagent Co., Ltd. (China).
Apparatus
A CO2 incubator and wellwash were obtained from the Thermo Electron Corporation (Shanghai, China). A high-speed refrigerated bench-top centrifuge was purchased from Heal Force Development Ltd. (China). ELISA plates were purchased from Yi Camry Experimental Equipment Co., Ltd. (Xiamen, China). Cell culture plates were purchased from Corning Incorporated (Beijing, China). A Multiskan microplate reader was obtained from Thermo Labsystem (Helsinki, Finland).
Food samples
Food samples were purchased from a local supermarket and included five types of egg, four types of processed food with a declaration that the food components contained traces of egg, and two types of processed food with no declaration about the presence of egg or egg components in the list of ingredients.
Buffers and solutions
Cell culture media used in this study are: (1) 90% 1640 basal medium: mixture of 90 mL 1640 basal medium and 10 mL fetal bovine serum; (2) 80% 1640 basal medium: mixture of 80 mL 1640 basal medium and 20 mL fetal bovine serum; (3) HAT medium: mixture of 98 mL 80% 1640 basal medium and 2 mL HAT (50×); (4) HT medium: mixture of 99 mL 80% 1640 basal medium and 1 mL HT (100×).
Enzyme-linked immunosorbent assay reaction solutions used in this study are: (1) PBS: 0.01 M phosphate buffered saline pH 7.4; (2) coating buffer: 0.01 M phosphate buffered saline, pH 8.0; (3) blocking buffer: coating buffer containing 0.1% (w/v) PVA; (4) washing buffer: 0.01 M PBS (pH 7.4) containing 0.05% (v/v) Tween 20; (5) antibody dilution buffer: PBS containing 0.1% (w/v) gelatin, 5% (w/v) trehalose and 0.05% (v/v) Tween 20; (6) substrate buffer: mixture of 18 µL of 30% H2O2 and 100 mL 0.1 M citrate phosphate buffer (pH 5.0); (7) TMB solution: 60 mg TMB dissolved in 100 mL glycol; (8) TMB substrate solution: 5:1 (v/v) mixture of substrate buffer and TMB solution; (9) stop solution: 2 M sulphuric acid.
Preparation of mAbs
Five female BALB/c mice (7 weeks old) were selected. Routinely, 200 µL of 1:1 (v/v) mixture of Freund's complete adjuvant and normal saline (NS) containing 0.5 mg/mL of immunogen (OVA) was injected at multiple sites for the first immunisation. Three weeks later, 50 µg of the immunogen emulsified with Freund's incomplete adjuvant was injected subcutaneously three times at intervals of 3 weeks for the booster immunisation. Seven days after the fourth immunisation, serum titres were detected by indirect ELISA. The mouse with the highest titre was selected and was injected intraperitoneally with 100 µL 0.5 mg/mL immunogen solution. Three days later, the mouse was killed and splenocytes were isolated. The splenocytes were then fused with Sp2/0 murine myeloma cells (Chinese Academy of Sciences, Shanghai), and PEG 1450 acted as the fusogen. Hybridoma cells were plated onto eight 96-well microtitre plates. The cell culture incubator was maintained at 37°C and 5% CO2. After culturing in HAT medium, only the fusion cells had grown. The positive cells were selected by indirect ELISA and then subcloned three times by limiting dilution. mAbs were obtained from the ascites of mice injected intraperitoneally with screened hybridoma cells.
HRP labelling of mAbs
The mAbs to OVA were labelled with HRP. Five milligram of HRP was dissolved in 0.5 mL ultrapure water, and mixed with 0.5 mL 0.06 M NaIO4 at 4°C. After 30 min, 0.5 mL 0.16 M glycol was added to the mixture which was allowed to stand for 30 min at room temperature. To this solution, 5 mg purified antibody was added, and the pH was adjusted to 9.0 with 0.05 M carbonate buffer (pH 10.0). After incubating for 20 h, 0.2 mL 5 mg/mL NaBH4 solution was added to the mixture, which was allowed to stand for 2 h at 4°C. Then an equal volume of saturated ammonium sulphate solution was added drop-wise to the mixture which was allowed to stand for 30 min at 4°C. Finally, the mixture was centrifuged at 5000 g for 15 min. The supernatant was discarded. The precipitate was re-dissolved in 0.01 M PBS (pH 7.4), and dialysed with 0.01 M PBS (pH 7.4) at 4°C overnight. All reactions were carried out in the dark. The conjugate was purified using a Sephadex G25 gel filtration column (GE Healthcare Life Sciences), then aliquoted and stored at −20°C.
Sandwich ELISA
The 96-well microplates were coated with anti-OVA mAb diluted in coating buffer (100 µL per well) and incubated at 4°C overnight. After washing three times with 250 µL/well of washing buffer, free binding sites in the wells were blocked with 200 µL blocking buffer, and the plates were incubated for 2 h at 37°C. After washing again, 100 µL/well of a serial dilution of OVA standard solution or sample extract solution was added. Following incubation for 1.5 h at 37°C and washing four times, 100 µL/well HRP-labelled anti-OVA mAb was added to each well, and the plates were incubated for 1 h at 37°C. After another five washes, 100 µL/well of a freshly prepared TMB substrate solution was added and reacted for 15 min at 37°C in the dark. The reaction was stopped by the addition of 2 mol/L sulphuric acid (50 µL/well), and the absorbance was measured at 450 nm with a microplate reader. All measurements were performed in triplicate.
Indirect ELISA
Indirect ELISA was carried out to detect the serum titres and screen the hybridoma cell lines for anti-OVA activity. The ELISA plates were coated with 100 µL/well of OVA in coating buffer and incubated at 37°C for 2 h. The plates were washed three times with washing buffer, carbonate buffer was added to the wells (200 µL/well), and the plates were incubated for 2 h at 37°C. After another washing step, the cell supernatant or mouse serum we established was diluted with an antibody dilution buffer, added to the wells (100 µL/well) and the plates were incubated for 30 min at 37°C. After washing four times, HRP-labelled goat anti-mouse immunoglobulin was diluted using PBS buffer at ratio 1:4000 (v/v) and 100 µL/well was added and the plates were incubated for 30 min at 37°C. After washing five times, 100 µL/well of a freshly prepared TMB substrate solution was added and reacted for 15 min at 37°C in the dark. The reaction was stopped with 2 mol/L sulphuric acid (50 µL/well), and the absorbance was measured at 450 nm with a microplate reader.
Preparation of sample solution
Solid samples
The solid samples were first pestled, then 10 mL 0.01 M PBS (pH 7.4) was added to 1 g of sample. The homogenate was shaken for 12 h at 4°C for extraction, and then centrifuged at 6000 g for 15 min. The supernatant was centrifuged at 6000 g for 15 min and then collected as the food sample solution and examined immediately.
Sample solutions
The sample solutions were diluted 10-fold in 0.01 M PBS at pH 7.4 and examined immediately.
Recovery analysis of spiked sample by sandwich ELISA
Ovalbumin-free wheat flour was spiked with OVA at 2.5, 5, 50 and 250 ng/g. The spiked sample was considered a solid sample and treated the same as those in the “solid sample” category, which was not diluted. Each spiked sample solution was analysed by the developed sandwich ELISA.
Results
Production of mAbs to OVA
Hybridoma cells were produced by fusion of the Sp2/0 murine myeloma cells and the spleen cells isolated from a mouse immunised against OVA. One week after cell fusion, hybridoma cell growth was observed in the 96-well cell culture plates. The supernatant culture fluid from the wells was tested for anti-OVA activity by indirect ELISA. Cell lines with high affinity for OVA were selected and subcloned three times by limiting dilution. Subcloning and screening were carried out three times, and 17 hybridoma cells which showed strong affinity for OVA were obtained and named mAb1–mAb17. mAbs were then obtained from the ascites of mice injected intraperitoneally with screened hybridoma cells.
Combination screening and characterisation of mAbs to OVA
All HRP-conjugated mAbs were tested using the sandwich ELISA. Using one anti-OVA mAb as the capture antibody and the other 16 anti-OVA mAbs labelled with HRP as the detection antibody, there were 272 combinations. We detected the ratio of OD450 by adding 50 ng/mL OVA and OD450 by adding 0 ng/mL OVA in the sandwich ELISA. The combination with the highest ratio was seen as the best matching pairs of antibodies. Using our detection method, when mAb17 was used as the capture antibody and mAb15-HRP was used as the detection antibody, the ratio was highest (data not shown). Therefore, in subsequent experiments we used mAb17 as the capture antibody and mAb15-HRP as the detection antibody. In addition, the two generated mAbs, mAb15 and mAb17, were identified to be IgG1 and κ light chain.
Establishment of sandwich ELISA specific for the determination of OVA based on mAb against OVA
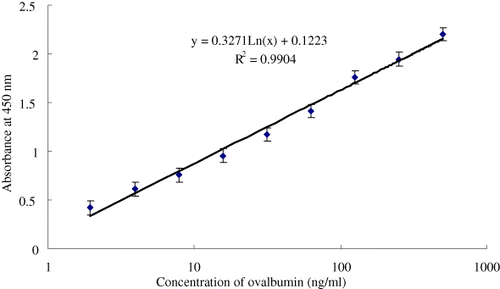
Commercially purified OVA was used as the standard. Using anti-OVA mAb17 as the capture antibody and anti-OVA mAb15-HRP as the detection antibody, we established a sandwich ELISA for OVA. shows the calibration curve for the determination of OVA using the developed sandwich ELISA. The limit of detection (LOD) of OVA was determined to be 0.51 ng/mL, and the linear dynamic range was from 1.95 to 500 ng/mL.
Recovery tests and validation studies
Recovery tests and validation studies were performed using OVA-spiked OVA-free wheat flour. shows the results of inter- and intra-assay laboratory validation for the developed ELISA. Four concentration levels of OVA (2.5, 5, 50 and 250 ng/mL) were used to spike the OVA-free wheat flour samples. Reproducibility showed that the variation coefficients ranged from 4.82 to 8.63% for intra-assay, and from 8.54 to 13.9% for inter-assay. For inter-assay reproducibility, spiking was performed in six independent experiments on six different days. Recovery studies showed that the recoveries ranged from 85.6 to 111.2% for the intra-assay. In the inter-assay, the recoveries varied from 87.3 to 115.2%. These results indicated that the mAb-based sandwich ELISA gave accurate and reliable reproducibility.
Table 1. Recovery of ovalbumin (OVA) by quantitative sandwich ELISA using anti-OVA mAb15 and mAb17.
Application to food products
To evaluate the developed ELISA, 11 types of commercial foods were analysed using the optimised ELISA (). The OD450 of the food samples (S) were compared with the blank value (N). The result was positive when the S/N ≥2.1. Seven foods showed positive results. Four types of food samples gave negative results. It can be seen that duck egg and goose egg showed cross-reactivity. However, pigeon egg and quail egg did not show cross-reactivity. Thus, the developed ELISA can also be used as a tool to obtain qualitative data on duck egg and goose egg. In addition, all four commercially processed foods including egg protein showed positive results. The other two types of foods (peanut and pea) gave negative results. Therefore, the established ELISA method is a reliable and sensitive method for determining OVA in processed foods.
Table 2. Detection of OVA in the commercial foods.
Discussion
Hen egg is one of the most frequent causes of food allergy in infants and adults. OVA has been identified as a major egg allergen. Because avoidance is the only efficient cure for food allergies at this time, extraordinary care to exclude all egg-containing foods from the diet is very important for patients with egg allergy (Eigenmann, Citation2003). A sensitive detection method for OVA is needed in order to prevent egg allergies. There have been several previous attempts to develop a sensitive ELISA for the detection of egg allergens (Li et al., Citation2008; Vidal, Gautron, & Nys, Citation2005). Obtaining an antibody with high affinity and specificity is the key factor. In this research, 17 mAbs were prepared and labelled with HRP. Through combination screening, using anti-OVA mAb17 as the capture antibody and anti-OVA mAb15-HRP as the detection antibody, the detection limit of the developed sandwich ELISA method was 0.51 ng/mL, and the linear dynamic range was between 1.95 and 500 ng/mL. We evaluated OVA levels in wheat flour, and our data showed that the developed ELISA method have good precision, accuracy and repeatability. We also demonstrated the application of this ELISA method for the detection of OVA in food products. Duck egg and goose egg were recognised by both anti-OVA mAb15 and mAb17, whereas pigeon egg and quail egg were not. Thus, the developed ELISA can also act as a tool to obtain qualitative data on duck egg and goose egg. In addition, all four commercially processed foods including egg protein showed positive results. Therefore, the established ELISA with high sensitivity can be used as an effective method for the quantification of OVA in food.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (21071066, 20835006, 91027038, 21101079, 21175034), the Key Programs from MOST (2012BAC01B07, 2012BAD29B05, 2012AA06A303, 2012BAD29B04, 2011BAK10B07, 2011BAK10B05, 2011BAK10B01, 2010AA06Z302, 2010DFB3047, 2009BAK61B04, 2011ZX08012-001, 2010GB2C100167, 2012BAK17B10, 2012BAK08B01), and grants from Natural Science Foundation of Jiangsu Province, MOF and MOE (BE2011626, BK2010001, BK2010141, 201110060, 201110016, 201110061, 201210036, 201310135, 311002).
References
- Bu, G., Luo, Y., Zheng, Z., & Zheng, H. (2009). Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food and Agricultural Immunology, 20(3), 195–206.
- Eggesbo, M., Botten, G., Halvorsen, R., & Magnus, P. (2001). The prevalence of allergy to egg: A population-based study in young children. Allergy, 56, 403–411.
- Eigenmann, P.A. (2003). Future therapeutic options in food allergy. Allergy, 58(12), 1217–1223.
- Fu, T.J., Maks, N., & Banaszewski, K. (2010). Effect of heat treatment on the quantitative detection of egg protein residues by commercial enzyme-linked immunosorbent assay test kits. Journal of Agricultural and Food Chemistry, 58, 4831–4838.
- Fuller, H.R., Goodwin, P.R., & Morris, G.E. (2007). An enzyme-linked immunosorbent assay (ELISA) for the major crustacean allergen, tropomyosin, in food. Food and Agricultural Immunology, 17(1), 43–52.
- Hird, H., Lloyd, J., Goodier, R., Brown, J., & Reece, P. (2003). Detection of peanut using real-time polymerase chain reaction. European Food Research and Technology, 217(3), 265–268.
- Hoffman, D.R. (1983). Immunochemical identification of the allergens in egg white. Journal of Allergy and Clinical Immunology, 71, 481–486.
- Li, Y., Song, C., Zhang, K., Wang, M., Yang, K., Yang, A., et al. (2008). Establishment of a highly sensitive sandwich enzyme-linked immunosorbent assay specific for ovomucoid from hen's egg white. Journal of Agricultural and Food Chemistry, 56, 337–342.
- Mariager, B., Solve, M., Eriksen, H., & Brogren, C.H. (1994). Bovine β-lactoglobulin in hypoallergenic and ordinary infant formulas measured by an indirect competitive ELISA using monoclonal and polyclonal antibodies. Food and Agricultural Immunology, 6(1), 73–83.
- Matthias, B. (2001). Determination of allergens in foods. Trends in Analytical Chemistry, 20(11), 662–672.
- Mine, Y., & Rupa, P. (2004). Immunological and biochemical properties of egg allergens. World's Poultry Science Journal, 60, 321–330.
- Mine, Y., & Yang, M. (2008). Recent advances in the understanding of egg allergens: Basic, industrial, and clinical perspectives. Journal of Agricultural and Food Chemistry, 56, 4874–4900.
- Mine, Y., & Zhang, J.W. (2002). Comparative studies on antigenicity and allergenicity of native and denatured egg white proteins. Journal of Agricultural and Food Chemistry, 50, 2679–2683.
- Monaci, L., & Visconti, A. (2009). Mass spectrometry-based proteomics methods for analysis of food allergens. Trends in Analytical Chemistry, 28(5), 581–591.
- Poms, R.E., Klein, C.L., & Anklam, E. (2004). Methods for allergen analysis in food: A review. Food additives and contaminants, 21(1), 1–31.
- Sampson, H.A. (2004). Update on food allergy. Journal of Allergy and Clinical Immunology, 113, 805–819.
- Sletten, G.B., Lovberg, K.E., Moen, L.H., Skarpeid, H.J., & Egaas, E. (2005). A comparison of time-resolved fluoroimmunoassay and ELISA in the detection of casein in foodstuffs. Food and Agricultural Immunology, 16(3), 235–243.
- Vidal, M.L., Gautron, J., & Nys, Y. (2005). Development of an ELISA for quantifying lysozyme in hen egg white. Journal of Agricultural and Food Chemistry, 53, 2379–2385.
- Xu, X., Ye, Z.Z., Wu, J., & Ying, Y.B. (2010). Application and research development of surface plasmon resonance-based immunosensors for protein detection. Chinese Journal of Analytical Chemistry, 38(7), 1052–1059.