Abstract
We developed a sensitive lateral flow immunoassay (LFI) for the detection of total malachite green (MG) residues in fish tissues. LFI was based on a colloidal gold nanoparticle-labeled monoclonal antibody against MG , which was generated by fast immunization of carboxymalachite green-bovine serum albumin. The half-maximum inhibition concentration (IC50) of MG monoclonal antibody (MG mAb) was 0.057 ng/ml with a linear range of 0.020–0.162 ng/ml. Following the optimization of LFI, a qualitatively visual limit of detection of 1 ng/ml and a semi-quantitatively limit of detection of 0.418 ng/ml (IC50 of the calibration curve) were obtained for total MG residues in 8 min. Cross-reactivity with most MG analogs was negligible, expect with crystal violet (CV; 78.6% cross-reactivity). LFI is a novel and sensitive assay based on MG mAb that has applications for the rapid detection of total MG and CV in fish products.
Introduction
Malachite green (MG, ), a dye with a triphenylmethane core, is used in textile and aquaculture industries (Singh et al., Citation2011). MG, which is relatively inexpensive and commercially available, is an effective therapeutic agent for the prevention of fungal, parasitic, and protozoan infections in fish (Bilandzic, Varenina, Kolanovic, Oraic, & Zrncic, Citation2012; Cho et al., Citation2003; Pierrard et al., Citation2012; Wan, Weng, Liang, Lu, & He, Citation2011).
However, MG is pH-sensitive and is rapidly metabolized into a colorless leuco form, leucomalachite green (LMG). LMG tends to accumulate in fish muscle and fat tissues due to its lipophilic nature (Stead et al., Citation2010). Studies have reported that both MG and LMG have potential carcinogenic, tumorigenic, mutagenic, and teratogenic effects in humans (Culp et al., Citation2002, Citation2006; Mittelstaedt et al., Citation2004). Therefore, MG and LMG have been banned in aquaculture industries in several countries, including the USA, Europe, and China. In accordance with the minimum required performance limit (MRPL) of 2 µg/kg established by the European Union in the Commission Decision 2004/25/EC, simple and sensitive detection methods are crucial for the protection of human health.
Quantitative MG analytical methods include liquid chromatography-tandem mass spectrometry (LC-MS; Abro, Mahesar, Iqbal, & Perveen, Citation2014), LC-MS/MS (Ascari et al., Citation2012; Hashimoto et al., Citation2012; Hurtaud-Pessel, Couedor, Verdon, & Dowell, Citation2013; Kinsella, Citation2014), high-performance liquid chromatography (HPLC; Maleki, Farhadi, & Nikkhahi, Citation2012; Zou et al., Citation2014), and HPLC-MS/MS (Nebot et al., Citation2013). Immunoassays (Oplatowska, Connolly, Stevenson, Stead, & Elliott, Citation2011; Shen et al., Citation2011; Xu et al., Citation2013; Zhang et al., Citation2015) and similar applications (Dong et al., Citation2014; Guo, Gai, Hao, Duan, & Wang, Citation2011; Neng et al., Citation2010) have been developed for the rapid and sensitive on-site detection of total MG.
Lateral flow immunoassay (LFI) is a simple and time-saving method that does not require complicated instruments or trained personnel (Guo et al., Citation2015). However, no studies have focused on immunochromatographic assays for MG detection in fish tissues. In this study, a LFI was developed based on a monoclonal antibody against MG (MG mAb). The results revealed that LFI is a rapid and sensitive method for the detection of total MG in fish tissues.
Materials and methods
Materials and reagents
MG immunogen, coating antigen, MG mAb, and gold nanoparticle (GNP) were developed in our laboratory. MG, crystal violet (CV), LMG, leucocrystal violet (LCV), methylene blue (MB), and pararosaniline (PA) were obtained from Aladdin Reagent Co. (Shanghai, China); 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) was purchased from Sigma-Aldrich Co. LLC. (Shanghai, China).
Strip materials, including sample pad (glass fiber membrane), conjugatedpad (Ahlstrom 8964), absorbance pad (H5076 filter paper), nitrocellulose membrane (NC membrane), and backing pad (polyethylene adhesive card), were obtained from JieYi Biotech (Shanghai, China). All other reagents were of analytical grade; instruments including glassware were washed seven times with Millipore ultrapure water produced by Saifei Co. (Naijing, China).
Determination of MG monoclonal antibody
The optimal concentrations of MG coating antigen and mAb were assessed by titration (Chen et al., Citation2014; Peng et al., Citation2014; Xu et al., Citation2011). The titer was tested by indirectly competitive enzyme-linked immunosorbent assay (ic-ELISA; Li et al., Citation2013; Sun, Liu, Kuang, & Xu, Citation2013; Zhang et al., Citation2013). The antibodies were diluted from 1/1000 to 1/27,000 and added to the plate coated with 0.1–0.9 µg MG coating antigen in 0.05 M carbonate buffer (pH 9.6) per well. HRP-labeled goat anti-mouse antibody and subsequent TMB solution were added to the plate following incubation and washing steps. Finally, stop buffer (2 M H2SO4) was added. Results were measured at 450 nm in a Multiskan Mks microplate reader (Thermo Labsystems Co., Beijing, China).
Certain modifications were performed to assess MG mAb specificity (Liu, Kuang, Peng, Wang, & Xu, Citation2014; Xing, Hao, Liu, Xu, & Kuang, Citation2014). The volume of antibody was replaced by a similar amount of antibody and standard analyte. The standard curves between logarithmic concentrations of standard analytes and B/B0 values were generated, where B0 is the optical density (OD) value without the standard while B is the OD value with the standard. The half inhibition concentration (IC50 value) was defined as the concentration resulting in a 50% reduction of OD value.
Conjugation of MG mAb to GNP
MG mAb was conjugated to GNP by the method established in our lab (Xing et al., Citation2015), with slight modifications. Briefly, MG mAb was diluted to 0.1 mg/ml in borate buffer, followed by the dropwise addition of GNP. The pH value was adjusted to 7.0 with 0.1 M K2CO3. The solution was mixed, incubated at room temperature for 4 h, and blocked to eliminate nonspecific coupling. Following a 2 h incubation, the solution was centrifuged at 12,000 × g for 10 min to remove excess reagents. This process was repeated three times. The gold-labeled MG mAb conjugate (MG mAb-GNP) was re-suspended in 100 μl of 0.01 M phosphate buffer solution (PBS) containing 2% (w/v) bovine serum albumin (BSA), 2% (w/v) sucrose, and 0.02% (w/v) sodium azide.
Generation of ICT strips
The ICT strips were consisted of sample pad, conjugated pad, absorbance pad, NC membrane, and backing pad (Wang et al., Citation2011). The conjugated pad containing MG mAb-GNP was incubated at 37°C for 2 h. MG-coated antigen and the secondary antibody (goat anti-mouse IgG antibody, GAM Ab) were added to the lateral side of the NC membrane to develop the test and control zones. The generated card was vacuum-desiccated overnight, cut into 4 mm wide strips, and stored.
MG measurement in fish tissues
MG-negative fish were purchased from a local aquafarm in Wuxi (Jiangsu, China). Samples were mixed with different amounts of MG or MG analogs and analyzed by LFI at room temperature. During pretreatment, leuco-analytes, such as LMG and LCV, were oxidized with 100 µg DDQ (Oplatowska et al., Citation2011; Xu et al., Citation2013). The prepared strips were placed vertically into the plate wells for 3 min and retrieved horizontally (Chen, Liu, Kuang, Song, & Xu, Citation2013; Xing, Kuang, et al., Citation2014). The color intensity of the test zone was recorded in 5 min. In addition, the intensity deviations of the strip test line were quantitatively analyzed by a BioDot TSR3000 Membrane Strip reader (Gene Co. Ltd., Shanghai, China). The assay was performed three times.
Results and discussion
Sensitivity and specificity of MG mAb
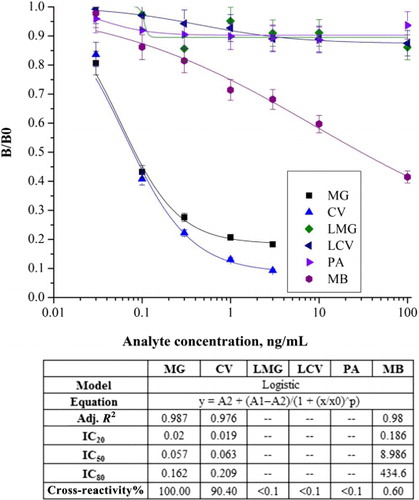
MG mAb was screened by cell fusion and characterized by ic-ELISA in PBS. As depicted in , MG mAb had an IC50 value of 0.057 ng/mL and a linear range (IC80–IC20) of 0.02–0.162 ng/ml. To estimate the specificity of mAb, its cross-reactivity to MG analogs was assessed. The results revealed that cross-reactivity was <0.10% to LMG, LCV, and PA and 0.60% to MB. A high cross-reactivity (90.40%) to CV was obtained because of similar triphenylmethane cores. Therefore, MG mAb was used for the detection of chromatic forms of triphenylmethane dyes and leuco forms following oxidation by DDQ or lead oxide.
Optimization of LFI strips
LFI strip performance conditions, e.g., concentrations of MG coating antigen, MG mAb, GAM Ab, and MG mAb-GNP, were optimized to improve the sensitivity of the assay.
GNP was synthesized with an average diameter of 30 nm. Coloring density in the test and control zones was affected by MG mAb concentrations. MG is a pH-responsive molecule; the neutral form of MG can be converted into a carbocationic form under acidic conditions. However, an irreversible aggregation of MG mAb was observed at high pH values, while there was insufficient coupling between MG mAb and GNP at low pH values. The stability of MG and mAb was investigated to establish an optimum working range.
Optimum LFI strip performance was obtained under following conditions, 4.5 mg/ml MG coating antigen, 0.3 mg/ml GAM Ab, 0.2 mg/ml MG mAb, and 5 nmol/L MG mAb-GNP.
Assessment of LFI strips
The LFI strip is a membrane-based immunochromatographic assay that has broad applications. The MG detection principle of the LFI strip, which is similar to that of ic-ELISA, is based on MG antigen immobilized on the test zone of the LFI strip and MG standard solution bound to MG mAb-GNP on the conjugated pad.
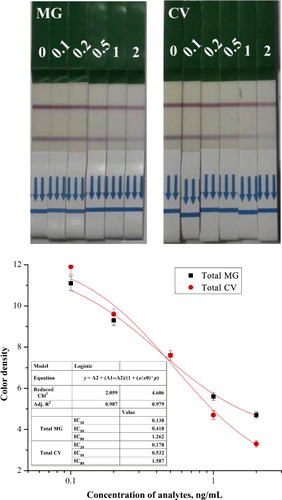
In this study, sample (80 µl) flowed laterally to the conjugated pad (MG mAb-GNP). The results were obtained in 5 min. GAM Ab was immobilized in the control line. If there is no MG in the sample, MG mAb-GNP binds to the immobilized antigen on the test zone, resulting in red test and control lines (). If there is MG in the sample, the red color intensity in the test line decreases. However, the color intensity did not disappear because MG antigen was blue on the test line.
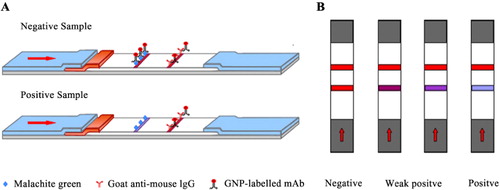
Qualitatively, the visual limit of detection was the concentration of MG standard at which the intensity in the test line significantly decreased. Semi-quantitatively, sensitivity, or limit of detection was defined as the concentration of MG standard that contributed to IC50 on the calibration curve.
Validation of LFI strips in fish tissue
MG is rapidly metabolized into colorless LMG in fish muscle and fat tissues. The majority of MG in fish tissue is converted into LMG. Therefore, LMG in fish tissues has to be oxidized before the validation of total MG residues.
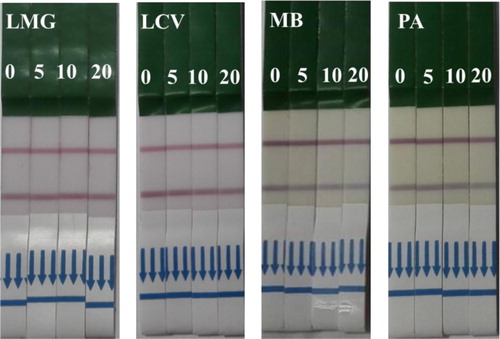
Samples were spiked with a gradient dilution of MG standard (0, 0.1, 0.2, 0.5, 1, and 2 ng/ml) in 0.01 M PBS and oxidized with 100 µg DDQ. Four MG analogs (LMG, LCV, PA, and MB) were spiked with 0, 5, 10, and 20 ng/mL. The results were observed by the naked eye (). The color intensity of MG on the test line decreased at 1 ng/ml, while the visual limit of detection of CV strips was 1 ng/ml. The limit of detection of total MG in the calibration curve was 0.418 ng/ml with a linear range of 0.138–1.262 ng/ml, while the limit of detection of total CV was 0.532 ng/ml with a linear range of 0.178–1.587 ng/ml. The results revealed that LFI can be used in the detection of total MG residues in fish tissues in accordance with the MRPL. Furthermore, we developed a simple and sensitive GNP-labeled immunochromatographic assay for the detection of total MG and CV residues in fish tissues.
The specific coupling of antigen and antibody depended on the molecular structure and configuration, particularly the antigenic epitopes. High cross-reactivity between MG and CV was attributed to the presence of a similar triphenylmethane core. However, there was a reduction in color density in the MG analogs (LMG, LCV, PA, and MB), indicating negligible cross-reactivity (). Similar results were obtained by ic-ELISA.
Conclusion
MG residues in fish could potentially increase carcinogenesis, teratogenesis, and mutagenesis in consumers. In this study, we developed an ultrasensitive mAb against MG by immunization of BSA-coupled antigen. A novel, simple, and sensitive immunochromatographic assay was established for the rapid detection of MG in fish tissue. The visual limit of detection of LFI strip was 1 ng/ml for qualitative detection and 0.418 ng/ml for semi-quantitative detection. LFI has promising applications in the detection of triphenylmethane dyes in fish products.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work is financially supported by the Key Programs from MOST [grant number 2012AA06A303], [grant number 2012BAK08B01]; Natural Science Foundation of Jiangsu Province, MOF and MOE [grant number BE2013613], [grant number BE2013611], [grant number 201310135].
References
- Abro, K., Mahesar, S. A., Iqbal, S., & Perveen, S. (2014). Quantification of malachite green in fish feed utilising liquid chromatography-tandem mass spectrometry with a monolithic column. Food Additives and Contaminants: Part A , 31, 827–832. doi:10.1080/19440049.2014.893398
- Ascari, J., Dracz, S., Santos, F. A., Lima, J. A., Diniz, M. H. G., & Vargas, E. A. (2012). Validation of an LC-MS/MS method for malachite green (MG), leucomalachite green (LMG), crystal violet (CV) and leucocrystal violet (LCV) residues in fish and shrimp. Food Additives and Contaminants: Part A , 29, 602–608. doi:10.1080/19440049.2011.653695
- Bilandzic, N., Varenina, I., Kolanovic, B. S., Oraic, D., & Zrncic, S. (2012). Malachite green residues in farmed fish in Croatia. Food Control, 26, 393–396. doi:10.1016/j.foodcont.2012.02.001
- Chen, X. J., Liu, L. Q., Kuang, H., Song, S. S., & Xu, C. L. (2013). A strip-based immunoassay for rapid determination of fenpropathrin. Analytical Methods, 5, 6234–6239. doi:10.1039/c3ay41030g
- Chen, X. J., Xu, L. G., Ma, W., Liu, L. Q., Kuang, H., Wang, L. B., & Xu, C. L. (2014). General immunoassay for pyrethroids based on a monoclonal antibody. Food and Agricultural Immunology, 25, 341–349. doi:10.1080/09540105.2013.794328
- Cho, B. P., Yang, T. L., Blankenship, L. R., Moody, J. D., Churchwell, M., Beland, F. A., & Culp, S. J. (2003). Synthesis and characterization of N-demethylated metabolites of malachite green and leucomalachite green. Chemical Research in Toxicology, 16, 285–294. doi:10.1021/tx0256679
- Culp, S. J., Beland, F. A., Heflich, R. H., Benson, R. W., Blankenship, L. R., Webb, P. J., … Manjanatha, M. G. (2002). Mutagenicity and carcinogenicity in relation to DNA adduct formation in rats fed leucomalachite green. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis, 506–507, 55–63. doi:10.1016/S0027-5107(02)00152-5
- Culp, S. J., Mellick, P. W., Trotter, R. W., Greenlees, K. J., Kodell, R. L., & Beland, F. A. (2006). Carcinogenicity of malachite green chloride and leucomalachite green in B6C3F(1) mice and F344 rats. Food and Chemical Toxicology, 44, 1204–1212. doi:10.1016/j.fct.2006.01.016
- Dong, J.-X., Xu, C., Wang, H., Xiao, Z.-L., Gee, S. J., Li, Z.-F., … Hammock, B. D. (2014). Enhanced sensitive immunoassay: Noncompetitive phage anti-immune complex assay for the determination of malachite green and leucomalachite green. Journal of Agricultural and Food Chemistry, 62, 8752–8758. doi:10.1021/jf5019824
- Guo, J. N., Liu, L. Q., Xue, F., Xing, C. R., Song, S. S., Kuang, H., & Xu, C. L. (2015). Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food and Agricultural Immunology, 26, 282–292. doi:10.1080/09540105.2014.907242
- Guo, Z. Y., Gai, P. P., Hao, T. T., Duan, J., & Wang, S. (2011). Determination of malachite green residues in fish using a highly sensitive electrochemiluminescence method combined with molecularly imprinted solid phase extraction. Journal of Agricultural and Food Chemistry , 59, 5257–5262. doi:10.1021/jf2008502
- Hashimoto, J. C., Paschoal, J. A. R., Queiroz, S. C. N., Ferracini, V. L., Assalin, M. R., & Reyes, F. G. R. (2012). A simple method for the determination of malachite green and leucomalachite green residues in fish by a modified QuEChERS extraction and LC/MS/MS. Journal of AOAC International, 95, 913–922. doi:10.5740/jaoacint.11-140
- Hurtaud-Pessel, D., Couedor, P., Verdon, E., & Dowell, D. (2013). Determination of residues of three triphenylmethane dyes and their metabolites (malachite green, leuco malachite green, crystal violet, leuco crystal violet, and brilliant green) in aquaculture products by LC/MS/MS: First action 2012.25. Journal of AOAC International, 96, 1152–1157. doi:10.5740/jaoacint.13-142
- Kinsella, B. (2014). A simple SPE method for the determination of malachite green, crystal violet, and other synthetic dyes in seafood using LC-MS-MS. LC GC Europe, 27, 161.
- Li, Z. K., Song, S. S., Xu, L. G., Kuang, H., Guo, S. D., & Xu, C. L. (2013). Development of an ultrasensitive immunoassay for detecting tartrazine. Sensors, 13, 8155–8169. doi:10.3390/s130708155
- Liu, L. Q., Kuang, H., Peng, C. F., Wang, L. B., & Xu, C. L. (2014). Fragment-based hapten design and screening of a highly sensitive and specific monoclonal antibody for ractopamine. Analytical Methods, 6, 229–234. doi:10.1039/C3AY41827H
- Maleki, R., Farhadi, K., & Nikkhahi, Y. (2012). Trace determination of malachite green in water samples using dispersive liquid–liquid microextraction coupled with high-performance liquid chromatography-diode array detection. International Journal of Environmental Analytical Chemistry, 92, 1026–1035. doi:10.1080/03067319.2010.536227
- Mittelstaedt, R. A., Mei, N., Webb, P. J., Shaddock, J. G., Dobrovolsky, V. N., McGarrity, L. J., … Heflich, R. H. (2004). Genotoxicity of malachite green and leucomalachite green in female Big Blue B6C3F(1) mice. Mutation Research-Genetic Toxicology and Environmental Mutagenesis, 561(1–2), 127–138. doi:10.1016/j.mrgentox.2004.04.003
- Nebot, C., Iglesias, A., Barreiro, R., Miranda, J. M., Vazquez, B., Franco, C. M., & Cepeda, A. (2013). A simple and rapid method for the identification and quantification of malachite green and its metabolite in hake by HPLC–MS/MS. Food Control, 31(1), 102–107. doi:10.1016/j.foodcont.2012.09.020
- Neng, J., Harpster, M. H., Zhang, H., Mecham, J. O., Wilson, W. C., & Johnson, P. A. (2010). A versatile SERS-based immunoassay for immunoglobulin detection using antigen-coated gold nanoparticles and malachite green-conjugated protein A/G. Biosensors & Bioelectronics, 26, 1009–1015. doi:10.1016/j.bios.2010.08.015
- Oplatowska, M., Connolly, L., Stevenson, P., Stead, S., & Elliott, C. T. (2011). Development and validation of a fast monoclonal based disequilibrium enzyme-linked immunosorbent assay for the detection of triphenylmethane dyes and their metabolites in fish. Analytica Chimica Acta, 698(1–2), 51–60. doi:10.1016/j.aca.2011.04.047
- Peng, J., Meng, X., Deng, X. F., Zhu, J. P., Kuang, H., & Xu, C. L. (2014). Development of a monoclonal antibody-based sandwich ELISA for the detection of ovalbumin in foods. Food and Agricultural Immunology, 25(1), 1–8. doi:10.1080/09540105.2012.716398
- Pierrard, M.-A., Kestemont, P., Delaive, E., Dieu, M., Raes, M., & Silvestre, F. (2012). Malachite green toxicity assessed on Asian catfish primary cultures of peripheral blood mononuclear cells by a proteomic analysis. Aquatic Toxicology, 114–115, 142–152. doi:10.1016/j.aquatox.2012.02.020
- Shen, Y.-D., Deng, X.-F., Xu, Z.-L., Wang, Y., Lei, H.-T., Wang, H., … Sun, Y.-M. (2011). Simultaneous determination of malachite green, brilliant green and crystal violet in grass carp tissues by a broad-specificity indirect competitive enzyme-linked immunosorbent assay. Analytica Chimica Acta, 707, 148–154. doi:10.1016/j.aca.2011.09.006
- Singh, G., Koerner, T., Gelinas, J. M., Abbott, M., Brady, B., Huet, A. C., … Godefroy, S. B. (2011). Design and characterization of a direct ELISA for the detection and quantification of leucomalachite green. Food Additives and Contaminants: Part A, 28, 731–739. doi:10.1080/19440049.2011.567360
- Stead, S. L., Ashwin, H., Johnston, B., Tarbin, J. A., Sharman, M., Kay, J., & Keely, B. J. (2010). An RNA-aptamer-based assay for the detection and analysis of malachite green and leucomalachite green residues in fish tissue. Analytical Chemistry, 82, 2652–2660. doi:10.1021/ac902226v
- Sun, F. X., Liu, L. Q., Kuang, H., & Xu, C. L. (2013). Development of ELISA for melamine detection in milk powder. Food and Agricultural Immunology, 24(1), 79–86. doi:10.1080/09540105.2011.641170
- Wan, H., Weng, S., Liang, L., Lu, Q., & He, J. (2011). Evaluation of the developmental toxicity of leucomalachite green administered orally to rats. Food and Chemical Toxicology, 49, 3031–3037. doi:10.1016/j.fct.2011.10.003
- Wang, L. B., Ma, W. W., Chen, W., Liu, L. Q., Ma, W., Zhu, Y. Y., … Xu, C. L. (2011). An aptamer-based chromatographic strip assay for sensitive toxin semi-quantitative detection. Biosensors & Bioelectronics, 26, 3059–3062. doi:10.1016/j.bios.2010.11.040
- Xing, C. R., Hao, C. L., Liu, L. Q., Xu, C. L., & Kuang, H. (2014). A highly sensitive enzyme-linked immunosorbent assay for copper(II) determination in drinking water. Food and Agricultural Immunology, 25, 432–442. doi:10.1080/09540105.2013.821600
- Xing, C. R., Kuang, H., Hao, C. L., Liu, L. Q., Wang, L. B., & Xu, C. L. (2014). A silver enhanced and sensitive strip sensor for Cadmium detection. Food and Agricultural Immunology, 25, 287–300. doi:10.1080/09540105.2013.781140
- Xing, C. R., Liu, L. Q., Song, S. S., Feng, M., Kuang, H., & Xu, C. L. (2015). Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosensors & Bioelectronics, 66, 445–453. doi:10.1016/j.bios.2014.12.004
- Xu, H. Y., Chen, X. L., Guo, L., Zhang, J. W., Lai, W. H., Aguilar, Z. P., … Xiong, Y. H. (2013). Monoclonal antibody-based enzyme-linked immunosorbent assay for detection of total malachite green and crystal violet residues in fishery products. International Journal of Environmental Analytical Chemistry, 93, 959–969. doi:10.1080/03067319.2012.672982
- Xu, N. F., Qu, C. L., Ma, W., Xu, L. G., Liu, L. Q., Kuang, H., & Xu, C. L. (2011). Development and application of one-step ELISA for the detection of neomycin in milk. Food and Agricultural Immunology, 22, 259–269. doi:10.1080/09540105.2011.569882
- Zhang, X., Feng, M., Liu, L. Q., Xing, C. R., Kuang, H., Peng, C. F., … Xu, C. L. (2013). Detection of aflatoxins in tea samples based on a class-specific monoclonal antibody. International Journal of Food Science and Technology, 48, 1269–1274. doi:10.1111/ijfs.12086
- Zhang, Y., Yang, J. Y., Lei, H. T., Wang, H., Xu, Z. L., Shen, Y. D., … Sun, Y. M. (2015). Development of chemiluminescent enzyme immunoassay for the determination of malachite green in seafood. Food and Agricultural Immunology, 26, 204–217. doi:10.1080/09540105.2014.884056
- Zou, Y. M., Zhang, Z., Shao, X. L., Chen, Y., Wu, X. Y., Yang, L. Q., … Zhang, D. M. (2014). Application of three-phase hollow fiber LPME using an ionic liquid as supported phase for preconcentration of malachite green from water samples with HPLC detection. Bulletin of the Korean Chemical Society, 35, 371–376. doi:10.5012/bkcs.2014.35.2.371