ABSTRACT
In this work, a goldmag-based enzyme-linked immunosorbent assay was developed for determination of α-lactalbumin (α-LA) in milk. The magnetic nanoparticle functionalized with polyetherimide was synthesized by one-pot method and coated with two layers of gold nanoparticles on the surface to synthesize goldmag nanoparticles. Anti-α-LA monoclonal antibody, prepared by hybridoma cell lines via cell fusion, was then bound to this goldmag nanoparticle to develop a capture nanoprobe. The results showed that this developed immunoassay had a good linear range of 2.33–127.1 ng/mL with IC50 of 17.2 ng/mL. Besides, recovery rates for α-LA in four commercial milks were from 86.7% to 109.8%. The coefficients of variation in intra-assay and inter-assay were 3.9–6.8% and 5.5–9.8%, separately, which could meet the requirements to quantify α-LA content in milk. This goldmag-based immunoassay might have considerable potentials in the detection of food allergies.
Introduction
Food allergy has become a serious problem throughout the world. Recently, with the development of scientific and technological progress, great changes in people’s living environment and food types have taken place, and the number of people suffered from food allergic disease has extensively increased (Ponce, Diesner, Szepfalusi, & Eiwegger, Citation2016). Dairy products are widely utilized in food industries due to their high protein content and balanced nutrition. However, it was said that the incidence of disease induced by cow milk allergy (CMA) was 0.3–7.5% in the population all over the world. According to epidemiological survey, more than 8% of the minors and 2% of adults suffered from food allergies in the developed countries (Ebisawa, Ikematsu, Imai, & Tachimoto, Citation2003; Woods et al., Citation2002). Especially in the United States, about 5.3% of adults (Vierk, Koehler, Fein, & Street, Citation2007) and about 6% of infants and children suffered food allergy (Sampson, Citation2004). Common kinds of allergic food, including soy, wheat, milk, peanuts, eggs, nuts, fish, and crustaceans, accounted for about 90% of all food allergies (Schafer et al., Citation2001). Casein (CN), β-lactoglobulin (β-LG), and α-lactalbumin (α-LA) were recognized as the main allergens in cow milk, and the high cross-reactivity existed between milk allergens from different mammalian species (Carroccio, Cavataio, & Iacono, Citation1999). Nevertheless, α-LA was highlighted as a major cow’s milk allergen, and Adams’s studies indicated that 75% of the CMA serum contained α-LA specific antibodies (Adams et al., Citation1991).
At present, the detection of milk allergens was done by in vivo and in vitro methods. The allergens in vivo were detected by skin test and histamine release test (Nordlee & Taylor, Citation1995), but it made patients very miserable. In vitro detecting technology can be classified into three categories: immunological detection technology, the allergen analysis technology based on DNA, and instrumental analysis testing technology. Immunological methods were enzyme-linked immunosorbent assay (ELISA) (Fuller, Goodwin, & Morris, Citation2006; Zhou et al., Citation2013), rocket immunoelectrophoresis (Moen et al., Citation2005), and immunochromatographic strip tests (Puerta, Diez-Masa, & de Frutos, Citation2006; Puerta, Jaulmes, De Frutos, Diez-Masa, & Vidal-Madjar, Citation2002). Assays based on DNA analysis were mainly polymerase reaction (PCR) and fluorescence quantitative PCR (Galan, Brohee, Silva, van Hengel, & Chassaigne, Citation2011). Although PCR was highly sensitive and stable, it was not applicable for all allergens detection because of a relatively high false-positive. Instrumental methods mainly included chromatography (Wang, Zhang, Wang, & Li, Citation2009) and mass spectrometry (Mamone et al., Citation2003), but they were complicated, expensive, and professional, and not convenient to normal operation. Moreover, these analytical methods, such as capillary electrophoresis method (Mayer, Heidler, & Rockenbauer, Citation1997), required pre-treated samples and long analysis time, which led to high cost.
Nanomaterials had been widely applied in biochemical detection with development of ultrasensitive signal tags. Magnetic bead based on immunoassay was first established in the 1980s because of the main advantages of increasing the surface area for immobilization of antibody or antigen, reducing the incubation time from hours to minutes, and improving sensitivity of the assay (Biagini et al., Citation2004; Moscoso, Kiefer, Kutlar, & Garver, Citation1988). Magnetic bead-based immunoassay was comprehensively applied in detection of macromolecular substances, such as β-bungarotoxin (Mu et al., Citation2014), β-casein allergen (Li et al., Citation2015; Song et al., Citation2014), enterobacter cloacae (Zhang, Zhou, Zhang, Zhang, & Su, Citation2016), pathogens, and cancer biomarkers (Baniukevic et al., Citation2013; Chen et al., Citation2016; Shukla, Lee, Song, Park, & Kim, Citation2016). However, the application of goldmag-based immunoassay in the quantification of α-LA content in milk has not been studied.
In this study, goldmag-based ELISA was developed for determination of α-LA in milk. The goldmag nanoparticles were immobilized with anti-α-LA monoclonal antibody (McAb) as the capture probe. In the probe, magnetic nanobeads were used as carriers for McAb, allowing us to improve the kinetic of the antibody–antigen immunoreaction. Horseradish peroxidase (HRP) labelled antigen was a biological macromolecule with double functional groups, which could participate in a highly specific immunoresponse and amplify biocatalysis effect. The analyte in commercial milks was directly detected by the amplified colorful conjugation based on goldmag-labelled ELISA.
Materials and methods
Animals
BALB/c mice were purchased from SLAC Laboratory Animal (Shanghai, China). All mice were females and kept in conventional conditions under 12 h light cycles. Animal experiments were approved by the committee on animal experimentation at Shanghai Normal University.
Reagents
The regents of CN, α-LA, β-LG, dimethyl sulfoxide, hypoxanthine aminopterin thymidine (HAT) medium, hypoxanthine thymidine medium, Freund’s complete adjuvant, Freund’s incomplete adjuvant, and polyethylene glycol 1500 (PEG1500) were purchased from Sigma. IMDM culture medium, fetal bovine serum (FBS), liquid paraffin were purchased from Shanghai Chuzhi Biological Technology Co., Ltd.; mouse myeloma SP2/0 cells were obtained from the Chinese Academy of Sciences. Chloroauric acid (HAuCl4) and trisodium citrate were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Bovine serum albumin (BSA), ovalbumin (OVA), and goat anti-mouse immunoglobulin horseradish peroxidase (IgG-HRP) were purchased from Solarbio.
The blocking solution was 0.01 mol/L (M) PBS buffer (pH7.4 PBS) containing 1% (w/v) gelatin. The washing solution was 0.01 M PBS buffer (pH 7.4) containing 1% (w/v) Tween 20. The solution of 0.01 M PBS (pH 7.4) was used as the dilution buffer for serum and proteins samples. 3,3′,5,5′-tetramethylbenzidine (TMB) solution was prepared by using 0.01% (w/v) TMB, 0.005% (v/v) H2O2 and 50 mM sodium citrate buffer (pH 5.0). The stop buffer was 2 M H2SO4. All other reagents were of analytical grade. Deionized water was used throughout the study.
Equipment
Magnetic stirring instrument was purchased from IKA. Full wavelength UV spectrophotometer was purchased from Pgeneral. Pipettes were purchased from Eppendorf. Microplate reader and Infrared spectrometer were purchased from Thermo Electron Corp. Water treatment systems were purchased from Siemens. Analytical balance was purchased from Sartorius.
Preparation and characterization of monoclonal
Immunization
Eight-week-old female BALB/c mouse was immunized preliminarily with α-LA of 200 μg and was injected five times in booster immunization with 100 μg α-LA every two weeks. The first immunization was performed using complete Freund’s adjuvant. Incomplete Freund’s adjuvant was used for subsequent immunizations. Three days before cell fusion, blood was collected and antibody titer was determined by indirect competitive ELISA. The mouse with the highest titer was injected intravenously with 100 μg α-LA without adjuvant (Hadavi et al., Citation2010; Wu, Yu, & Kang, Citation2015).
Fusing, screening, and cloning of α-LA cells
The McAb against α-LA was generated according to the procedures in our previous work (Kong, Liu, Xing, Kuang, & Xu, Citation2015; Xu et al., Citation2015). Mouse myeloma SP2/0 cells, used as fusion partners, were cultured and propagated in IMDM culture medium and 10% FBS. Three days after the last immunization, splenocytes were isolated from the immunized mouse and mixed with the SP2/0 cells at a ratio of 6:1. The mixture was washed twice with pre-warmed IMDM (37°C). Then, 0.8 mL pre-warmed PEG1500 was used for fusion. Selective HAT medium was used for selection of hybridoma cells. The reactivity of culture supernatants against α-LA was then tested by indirect competitive ELISA using α-LA. Briefly, the plates were coated with 5 μg/mL of α-LA. The culture supernatant of hybridoma cells was added as the primary antibody. The HRP conjugated goat anti-mouse IgG antibody served as the secondary, whose HRP activity was further detected at A450 nm using TMB as substrate. Finally, positive hybridomas were subcloned to single clones by limiting dilution process (Loirat, Gourbil, Frioux, Midler, & Blanchard, Citation1992).
Antibody production and purification
Cells of desired single clones in density of 106 cells/0.5 mL PBS were injected intraperitoneally into each mouse, which had been previously injected with 0.5 mL liquid paraffin before one week. Their ascites were harvested about 10 days later when their abdomens were completely enlarged. The related supernatants were collected after centrifugation of the ascites and their McAbs were purified from ascites using the method of caprylic acid–ammonium sulfate precipitation (Xiang et al., Citation2010).
Subtyping, affinity assay of monoclonal antibody
The subtype of anti-α-LA McAb was identified by an ELISA commercial kit “mouse monoclonal antibody isotyping identified reagents” (Shanghai Shifeng Biology Technology Co., Ltd).
To verify the specificity against α-LA monoclonal antibodies (McAbs), the purified α-LA McAbs were tested by Western-blot using α-LA as antigen. Briefly, α-LA was diluted to 100 μg/mL with PBS and applied on a 10% SDS-PAGE gel. After electrophoresis, resolved proteins were transferred onto PVDF membrane (Millipore) at 4°C and 400 mA for 60 min. The membrane was blocked with 5% gelatin in PBS for 2 h at 37°C. α-LA monoclonal antibody was added as the primary antibody. The HRP conjugated goat anti-mouse IgG antibody served as the secondary, whose HRP activity was detected by ECL Chemiluminescence Detection System (GE Healthcare).
The affinity of monoclonal antibody against α-LA was determined according to the procedure described as follows. After coated with different concentration of antigen α-LA (3, 2, 1.5, 1, 0.5, 0.25, 0 μg/mL), the microplate was added with anti-α-LA McAb with serious dilutions, and then the goat anti-mouse IgG-HRP and substrate were added subsequently. The absorbance value of each well was measured at 450 nm by microplate reader after the reaction was stopped by 2 M H2SO4. A curve diagram was made to show the relationship between the concentration of antibodies as the abscissa and the value of absorption as the ordinate. Relative affinity of anti-α-LA McAb was measured by determining the 50% inhibition of control values (IC50). The affinity constant (Ka) was calculated using the reported method (Beatty, Beatty, & Vlahos, Citation1987; Loomans et al., Citation1995).
Preparation and characterization of gold magnetic probes
Synthesis of goldmag conjugation
The goldmag conjugation of Fe3O4/Au composite was prepared according to previous work (Wang, Xu, Zhang, & Wei, Citation2013). Briefly, we synthesized the magnetic nanoparticle (MNP) functionalized with PEI (Fe3O4–PEI); 5 nm colloidal gold particles were first produced from the NaBH4 (0.075%) reduction of 1% HAuCl4 in 38.8 mM sodium citrate. The Fe3O4–PEI–Au–PEI composite was synthesized by embedding the Fe3O4–PEI–Au with a 5 mg/mL PEI solution at 60°C for 2 h, and the products were rinsed for three times with an external magnet. The last step of the process was to construct a Fe3O4–PEI–Au–PEI–Au structure. The prepared composite was dissolved in a three-necked flask in the presence of 50 mL colloidal gold, which was obtained in the way as mentioned above. The mixture was stirred mechanically at room temperature for 2 h, while the Au NPs were deposited onto the surfaces of the Fe3O4–PEI–Au–PEI complexes. The final Fe3O4/Au composite was dried in vacuum and was then dispersed in water at 1 mg/mL.
Conjugation procedures
Coupling principle (Song et al., Citation2014) was illustrated as follows ((A)). A large amount of gold nanoparticles were connected with on Fe3O4/Au nanoparticle composites surface. Au nanoparticles were negatively charged, and antibody protein was positively charged. They could form a solid combination by electrostatic adsorption.
Figure 1. Scheme of goldmag-based ELISA for quantification of α-LA (A: synthesis of capture and detection probes, B: detection procedure).
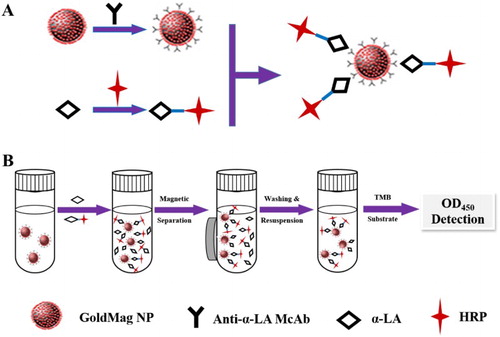
Briefly, Fe3O4/Au nanoparticle composites (5 mg) were washed three times with 2 mL PBS buffer (0.01 M, pH = 7.4) and were mixed with 500 μL McAbs (optimized dosage). The mixture was incubated in the shaking table at 37°C for 60 min. Fe3O4/Au composites coupled with McAbs were magnetically separated and blocked with 2% gelatin of PBS buffer with slight stirring for 2 h in order to block without combining sites. Finally, the goldmag probes were resuspended in PBS buffer (0.02 M, pH 7.4) and stored at 4°C for further use.
Preparation of α-LA-HRP
The α-LA was conjugated with HRP ((A)) according to the indirect sodium periodate oxidation method. The carbohydrate chain on the surface of HRP could be oxidized to aldehyde group by sodium periodate, which made it bond directly to the amino of coating antigen of α-LA. Then adding NaHB4 could be formed into a stable combination.
Establishment of gold magnetic enzyme-linked immunoassay
Goldmag based ELISA was launched by competitive combination of α-LA antigen and HRP-α-LA to the goldmag immunoprobe. The analyte was directly detected by the amplified colorful products produced by the catalyzing oxidation of HRP and TMB. The absorbance value of the colorful products was inversely proportional to the concentration of the target. The more the content of α-LA, the less the absorbance value of the antigen.
Briefly, 100 μL of magnetic α-LA-capturing probe was added to each tube, in which 100 μL of superparamagnetic Fe3O4 nanoparticles and magnetic α-LA-capturing probe were used as negative and blank control. Then 50 μL of α-LA standard solution with different concentrations and 50 μL of HRP-α-LA (1:1000 dilution) were added except the negative and blank control sample. And incubated at 37°C for 1 h, and the tubes were then washed with PBST for three times by magnetic separation. Each tube was added into 100 μL of TMB, incubated at 37°C for 45 min. Then the reaction was terminated by adding 50 μL of 2M H2SO4. The supernatant was detected at 450 nm. The whole process is depicted in (B).
The α-LA standard was dissolved in 0.01 M, pH 7.4 PBS with concentrations of 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1024 ng/mL. Each concentration was tested in six parallels. The standard curve was obtained with inhibition rate (%) as the ordinate and logarithm of α-LA concentration as the abscissa, respectively.
Evaluation of detection system
Selectivity of the developed assay
Other proteins, including CN, β-LG, BSA, and OVA were tested for selectivity study. Each protein solution (500 μL) at concentrations of 0, 1, 2, 4, 8, 16, 32, 64, 128, 256, 512 ng/mL was investigated, respectively.
Stability
The stability of the goldmag immunoprobe was detected by using the same procedures described above, using α-LA standard solution (1 mg/mL) every three days in one month.
Coefficient of variation
The accuracy of the established method was assessed through calculating intra-assay and inter-assay coefficients of variation. Simply, each concentration (10, 80, and 160 ng/mL of α-LA standard solution) was tested by repeating the testing six times, calculating the value of coefficient of variation (CV), which constitutes the intra-assay. Repeating six times at different times, calculating the value of CV, which constitutes the inter-assay. The same procedures were described above.where SD is the standard deviation and X0 is the average of determination values.
Detection of α-LA in commercial milk samples
To validate the performance of the new developed immunoassay, bovine milk samples of four different brands were purchased from local supermarket. One milliliter of milk sample was centrifuged for 10 min (12000 r/min, 4°C) to remove the fat. Then l0 μL of the milk sample was diluted 100 times with PBS buffer. The concentration of CN was measured by the developed assay simultaneously, and the results were compared.
Results and discussion
Preparation and characterization of McAbs against α-LA
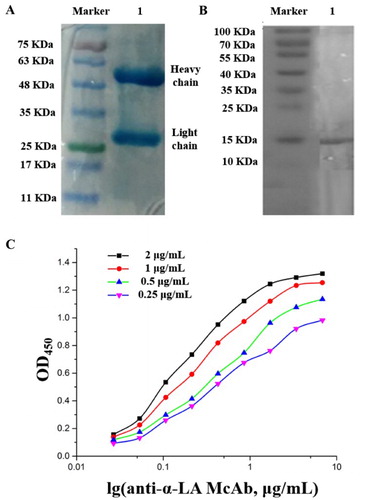
The concentration of anti-α-LA McAb named 11G10 was determined as 8.6 mg/mL by the classic dying method with Coomassie Brilliant Blue G250 (Sedmak & Grossberg, Citation1977). Results in SDS-PAGE and Western-blot showed that this purified McAb 11G10 was specific to identify α-LA antigen ((A,B)). The titer of this 11G10 McAb against α-LA was calculated to be 1:1,024,000. The affinity was analyzed by non-completed ELISA in (C). Different concentrations (2, 1, 0.5, 0.25 μg/mL) of coating antigen (α-LA) were used to determine the affinity constant. The average affinity for 11G10 McAb was calculated to be about 1.37 × 108 L/mol according to the method of Elma E.M.G. Loomans and Ni Jin (Jin, Ling, Yang, & Wang, Citation2014; Loomans et al., Citation1995; Sedmak & Grossberg, Citation1977). Thus, this obtained antibody was believed to match McAbs with high specificity, titer, and affinity. The isotype of 11G10 was also detected to be IgG1 using a commercial mouse sub-isotyping kit.
Characterization of goldmag conjugation
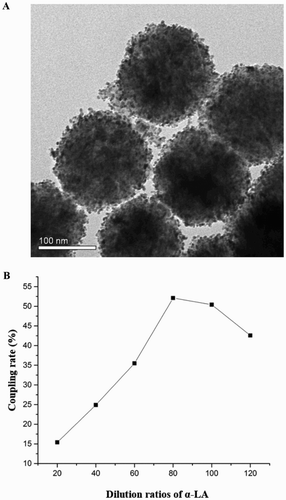
The goldmag conjugation of Fe3O4/Au-McAbs composite (the capture probe) was synthesized by covalent binding of Fe3O4/Au composite and anti-α-LA McAb, and was characterized by the transmission electron microscopy (TEM) in (A). The diameters of Fe3O4/Au nanoparticles were about 165–180 nm. After coupled with McAb, the average diameter of the composite microspheres slightly increased from 170 to 185 nm. In order to achieve the goal of best immune response and saving costs, the amount of McAb immobilized on the MNPs should be optimized. As shown in (B), the coupling rate increased with the higher amount of McAb immobilized on the surface of goldmag conjugation. The best coupling rate was obtained when the McAb solution was diluted to 80 times but could not increase at higher amount. Therefore, the diluted 100 times was selected as the optimal amount.
Characterization of α-LA-HRP
The concentration of α-LA-HRP was also determined by traditionally dying method with Coomassie brilliant blue G250. According to the standard curve for the detection of protein concentration, the concentration of HRP-α-LA was 2.816 mg/mL. TMB substrate solution was added to 100 μL of the HRP-α-LA (concentration of 1 and 10 ng/mL) at 37°C for 15 min (the same concentration of HRP solution was set as a control group). And then, 50 μL the stop buffer (2 mol/L H2SO4) was added to the mixture and the value of optical density (OD) was tested at 450 nm. The loss of enzyme activity was calculated according to the following equation:Based on the OD value of different concentrations of HPR and HRP-α-LA, the loss ratio of enzyme activity was 7.63%.
Establishment of the standard curve
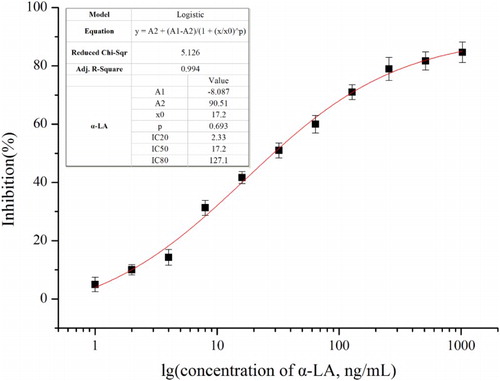
As shown in (A), the standard curve of this established goldmag-based immunoassay was performed at concentration of 1–1024 ng/mL with α-LA standard substance. When the concentration of α-LA was in the range of 2.33–127.1 ng/mL, a considerable linear correlation was obtained between the logarithm of α-LA concentration and the inhibition efficiency. When the developed immunoassay was performed to detect α-LA, the sensitivity (IC50) was 50% of inhibition. According to the developed standard curve and the regression equation, IC50 was 17.2 ng/mL. This sensitivity might confirm the signal-amplification effect of goldmag probe, as to the developed detection method for α-LA, a colloidal gold immunochromatographic strip assay for simple and fast detection of human α-LA had a detection limit of 10 μg/mL. In contrast, goldmag-based ELISA for detecting α-LA content in bovine milk in this study improved the detection sensitivity by almost 500 folds. Goldmag probe and HRP-α-LA had great signal-amplification effect, and MNPs were rapidly separated; it was promising to the problem of nonspecific interference and low-sensitivity for analysis of complex samples.
Evaluation of goldmag-based detection system
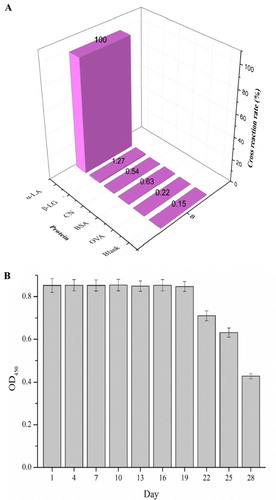
The selectivity of the developed immunoassay with β-LG, CN, BSA, and OVA was estimated. The result was shown in (A), and cross-reactivity was less than 2% to the proteins of β-LG, CN, BSA, and OVA. Therefore, the developed technique could be used for selective detection of α-LA in bovine milk samples.
The stability of this goldmag immunoprobe was detected and the result was shown in (B). It is depicted that the value of OD450 started to decrease at the 19th day so that this goldmag nanoprobe could kept stable in 4°C in 20 days.
The CV in each concentration (10, 80 and 160 ng/mL of α-LA standard solution) was tested by repeating six times. The results of intra-assay and inter-assay were shown in and the intra-CV was between 3.9% and 6.8% while the inter-CV was 5.5–9.8%. The intra-CV and inter-CV of the established goldmag-based enzyme-linked immunosorbent method were less than 10%, which suggested that this developed immunoassay had a good precision in detection of α-LA content in commercial milks.
Table 1. Intra- and inter-coefficients of variation of the developed immunoassay (n = 6).
Detection of α-LA in commercial milk samples
To validate the performance of the developed immunoassay, bovine milk samples of four different brands were purchased from local supermarket. The concentrations of α-LA were measured by the developed assay and the results were shown in . The recovery rates of the commercial milks were between 86.7% and 109.8%. It indicated that the developed immunoassay was credible for detecting α-LA in bovine milks.
Table 2. Detection of α-LA in commercial milks by the developed immunoassay (n = 6).
Conclusions
The developed goldmag ELISA was characterized and successfully applied to detect α-LA content in milk. Based on anti-α-LA monoclonal antibody, the goldmag-based ELISA showed a linear detection range of 2.33–127.1 ng/mL, and IC50 was 17.2 ng/mL, with negligible cross-reactivity (<10%) to main proteins in bovine milk. The recovery rate by this method for α-LA detection in commercial samples was 86.7–109.8%. Thus, it was prospective that this established goldmag immunoassay was sensitive and specific for α-LA detection in bovine milk.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Naifeng Xu completed thesis work related to rapid detection methods of main chemical hazards in aquatic products and received his PhD degree from School of Food Science and Technology in Jiangnan University (2015). Dr. Xu is now a teacher-candidate postdoctor at Shanghai Normal University (SHNU) and his primary research interest is quantitative detection and quality control of allergens in the manufacturing process.
Li Pan focused on a goldmag nanoparticle based immnunoassay for quantification of milk allergen and got her master degree at Shanghai Normal University in 2016.
Chao Yu worked on the synthesis and characterization of nanomaterials and nanoprobes and got her master degree at Shanghai Normal University in 2016.
Dr. Xinlin Wei got his PhD degree in 2004 from Jiangnan University and worked in the field of food safety and food engineering in plant extract. He became a professor at SHNU in 2007. His current research interests focus on innovative “green” process and technology for health-promoting plant ingredients.
Dr. Yuanfeng Wang obtained her PhD degree in 2005 from Jiangnan University and worked more than 10 years of research experience in the field of food safety and extraction, isolation, activity and structure of plant extract. She is now an associate professor at SHNU and her current research interests focus on innovative “green” process and technology for health-promoting plant ingredients.
Additional information
Funding
References
- Adams, S. L., Barnett, D., Walsh, B. J., Pearce, R. J., Hill, D. J., & Howden, M. E. (1991). Human IgE-binding synthetic peptides of bovine beta-lactoglobulin and alpha-lactalbumin. In vitro cross-reactivity of the allergens. Immunology and Cell Biology, 69(3), 191–197. doi: 10.1038/icb.1991.28
- Baniukevic, J., Boyaci, I. H., Bozkurt, A. G., Tamer, U., Ramanavicius, A., & Ramanaviciene, A. (2013). Magnetic gold nanoparticles in SERS-based sandwich immunoassay for antigen detection by well oriented antibodies. Biosensors and Bioelectronics, 43, 281–288. doi: 10.1016/j.bios.2012.12.014
- Beatty, J. D., Beatty, B. G., & Vlahos, W. G. (1987). Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. Journal of Immunological Methods, 100(1–2), 173–179. doi: 10.1016/0022-1759(87)90187-6
- Biagini, R. E., Smith, J. P., Sammons, D. L., MacKenzie, B. A., Striley, C. A., Robertson, S. K., & Snawder, J. E. (2004). Development of a sensitivity enhanced multiplexed fluorescence covalent microbead immunosorbent assay (FCMIA) for the measurement of glyphosate, atrazine and metolachlor mercapturate in water and urine. Analytical and Bioanalytical Chemistry, 379(3), 368–374. doi: 10.1007/s00216-004-2628-8
- Carroccio, A., Cavataio, F., & Iacono, G. (1999). Cross-reactivity between milk proteins of different animals. Clinical & Experimental Allergy, 29(8), 1014–1016. doi: 10.1046/j.1365-2222.1999.00620.x
- Chen, Y. P., Xianyu, Y. L., Sun, J. S., Niu, Y. J., Wang, Y., & Jiang, X. Y. (2016). One-step detection of pathogens and cancer biomarkers by the naked eye based on aggregation of immunomagnetic beads. Nanoscale, 8(2), 1100–1107. doi: 10.1039/C5NR07044A
- Ebisawa, M., Ikematsu, K., Imai, T., & Tachimoto, H. (2003). Food allergy in Japan. Allergy & Clinical Immunology International – Journal of the World Allergy Organization, 15(5), 214–217. doi: 10.1027/0838-1925.15.5.214
- Fuller, H. R., Goodwin, P. R., & Morris, G. E. (2006). An enzyme-linked immunosorbent assay (ELISA) for the major crustacean allergen, tropomyosin, in food. Food and Agricultural Immunology, 17(1), 43–52. doi: 10.1080/09540100600572651
- Galan, A. M. G., Brohee, M., Silva, E. D., van Hengel, A. J., & Chassaigne, H. (2011). Development of a real-time PCR method for the simultaneous detection of soya and lupin mitochondrial DNA as markers for the presence of allergens in processed food. Food Chemistry, 127(2), 834–841. doi: 10.1016/j.foodchem.2011.01.019
- Hadavi, R., Zarnani, A. H., Ahmadvand, N., Mahmoudi, A. R., Bayat, A. A., Mahmoudian, J., … Rabbani, H. (2010). Production of monoclonal antibody against human nestin. Avicenna Journal of Medical Biotechnology, 2(2), 69–77.
- Jin, N., Ling, S., Yang, C., & Wang, S. (2014). Preparation and identification of monoclonal antibody against Citreoviridin and development of detection by Ic-ELISA. Toxicon, 90, 226–236. doi: 10.1016/j.toxicon.2014.08.057
- Kong, D. Z., Liu, L. Q., Xing, C. R., Kuang, H., & Xu, C. L. (2015). Sensitive and highly specific detection of Cronobacter sakazakii based on monoclonal sandwich ELISA. Food and Agricultural Immunology, 26(4), 566–576. doi: 10.1080/09540105.2014.998634
- Li, Y. S., Meng, X. Y., Zhou, Y., Zhang, Y. Y., Meng, X. M., Yang, L., … Wang, X. R. (2015). Magnetic bead and gold nanoparticle probes based immunoassay for beta-casein detection in bovine milk samples. Biosensors and Bioelectronics, 66, 559–564. doi: 10.1016/j.bios.2014.12.025
- Loirat, M. J., Gourbil, A., Frioux, Y., Midler, J. Y., & Blanchard, D. (1992). A murine monoclonal antibody directed against the Gerbich 3 blood group antigen. Vox Sanguinis, 62(1), 45–48. doi: 10.1111/j.1423-0410.1992.tb01166.x
- Loomans, E. E., Roelen, A. J., Van Damme, H. S., Bloemers, H. P., Gribnau, T. C., & Schielen, W. J. (1995). Assessment of the functional affinity constant of monoclonal antibodies using an improved enzyme-linked immunosorbent assay. Journal of Immunological Methods, 184(2), 207–217. doi: 10.1016/0022-1759(95)00089-S
- Mamone, G., Caira, S., Garro, G., Nicolai, A., Ferranti, P., Picariello, G., … Addeo, F. (2003). Casein phosphoproteome: Identification of phosphoproteins by combined mass spectrometry and two-dimensional gel electrophoresis. Electrophoresis, 24(16), 2824–2837. doi: 10.1002/elps.200305545
- Mayer, H., Heidler, D., & Rockenbauer, C. (1997). Determination of the percentages of cows’, ewes’ and goats’ milk in cheese by isoelectric focusing and cation-exchange HPLC of γ-and para-κ-caseins. International Dairy Journal, 7(10), 619–628. doi: 10.1016/S0958-6946(97)00064-2
- Moen, L., Sletten, G., Miller, I., Plassen, C., Gutleb, A., & Egaas, E. (2005). Rocket immunoelectrophoresis and ELISA as complementary methods for the detection of casein in foods? Food and Agricultural Immunology, 16(2), 83–90. doi: 10.1080/09540100400029928
- Moscoso, H., Kiefer, C., Kutlar, A., & Garver, F. (1988). Quantification of hemoglobins S, C, and F by a magnetic affinity immunoassay. Clinical Chemistry, 34(5), 902–905.
- Mu, X. H., Tong, Z. Y., Huang, Q. B., Liu, B., Liu, Z. W., Hao, L. Q., & Zhang, J. P. (2014). Magnetic affinity immunoassay based enzyme-labeled phage displayed antibody. Chinese Journal of Analytical Chemistry, 42(6), 785–790. doi: 10.1016/S1872-2040(13)60736-7
- Nordlee, J. A., & Taylor, S. L. (1995). Immunological analysis of food allergens and other food proteins. Food Technology (USA). Retrieved from http://aims.fao.org/aos/agrovoc/c_3807
- Ponce, M., Diesner, S. C., Szepfalusi, Z., & Eiwegger, T. (2016). Markers of tolerance development to food allergens. Allergy, 71(10), 1393–1404. doi: 10.1111/all.12953
- Puerta, A., Diez-Masa, J. C., & de Frutos, M. (2006). Immunochromatographic determination of beta-lactoglobulin and its antigenic peptides in hypoallergenic formulas. International Dairy Journal, 16(5), 406–414. doi: 10.1016/j.idairyj.2005.05.006
- Puerta, A., Jaulmes, A., De Frutos, M., Diez-Masa, J. C., & Vidal-Madjar, C. (2002). Adsorption kinetics of beta-lactoglobulin on a polyclonal immunochromatographic support. Journal of Chromatography A, 953(1–2), 17–30. doi: 10.1016/S0021-9673(02)00124-3
- Sampson, H. A. (2004). Update on food allergy. Journal of Allergy and Clinical Immunology, 113(5), 805–819. doi: 10.1016/j.jaci.2004.03.014
- Schafer, T., Bohler, E., Ruhdorfer, S., Weigl, L., Wessner, D., Heinrich, J., … Ring, J. (2001). Epidemiology of food allergy/food intolerance in adults: Associations with other manifestations of atopy. Allergy, 56(12), 1172–1179. doi: 10.1034/j.1398-9995.2001.00196.x
- Sedmak, J. J., & Grossberg, S. E. (1977). A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Analytical Biochemistry, 79(1–2), 544–552. doi: 10.1016/0003-2697(77)90428-6
- Shukla, S., Lee, G., Song, X., Park, S., & Kim, M. (2016). Immunoliposome-based immunomagnetic concentration and separation assay for rapid detection of Cronobacter sakazakii. Biosensors and Bioelectronics, 77, 986–994. doi: 10.1016/j.bios.2015.10.077
- Song, F., Zhou, Y., Li, Y. S., Meng, X. M., Meng, X. Y., Liu, J. Q., … Zhang, J. H. (2014). A rapid immunomagnetic beads-based immunoassay for the detection of beta-casein in bovine milk. Food Chemistry, 158, 445–448. doi: 10.1016/j.foodchem.2014.02.150
- Vierk, K. A., Koehler, K. M., Fein, S. B., & Street, D. A. (2007). Prevalence of self-reported food allergy in American adults and use of food labels. Journal of Allergy and Clinical Immunology, 119(6), 1504–1510. doi: 10.1016/j.jaci.2007.03.011
- Wang, Y. F., Xu, F., Zhang, L., & Wei, X. L. (2013). One-pot solvothermal synthesis of Fe3O4-PEI composite and its further modification with Au nanoparticles. Journal of Nanoparticle Research, 15(1), 1338–1348. doi: 10.1007/s11051-012-1338-y
- Wang, J., Zhang, Q. H., Wang, Z. H., & Li, H. M. (2009). Determination of major bovine milk proteins by reversed phase high performance liquid chromatography. Chinese Journal of Analytical Chemistry, 37(11), 1667–1670. doi: 10.1016/S1872-2040(08)60146-2
- Woods, R. K., Stoney, R. M., Raven, J., Walters, E. H., Abramson, M., & Thien, F. C. (2002). Reported adverse food reactions overestimate true food allergy in the community. European Journal of Clinical Nutrition, 56(1), 31–36. doi: 10.1038/sj.ejcn.1601306
- Wu, X. L., Yu, S. J., & Kang, K. R. (2015). Development of a monoclonal antibody-based indirect competitive immunosorbent assay for 4(5)-Methylimidazole detection in caramels. Food Chemistry, 170, 354–359. doi: 10.1016/j.foodchem.2014.07.148
- Xiang, J. J., Zhai, Y. F., Tang, Y., Wang, H., Liu, B., & Guo, C. W. (2010). A competitive indirect enzyme-linked immunoassay for lead ion measurement using mAbs against the lead-DTPA complex. Environmental Pollution, 158(5), 1376–1380. doi: 10.1016/j.envpol.2010.01.002
- Xu, N. F., Xu, L. G., Ma, W., Liu, L. Q., Kuang, H., & Xu, C. L. (2015). An ultrasensitive immunochromatographic assay for non-pretreatment monitoring of chloramphenicol in raw milk. Food and Agricultural Immunology, 26(5), 635–644. doi: 10.1080/09540105.2014.998640
- Zhang, X., Zhou, J., Zhang, C. D., Zhang, D. J., & Su, X. R. (2016). Rapid detection of Enterobacter cloacae by immunomagnetic separation and a colloidal gold-based immunochromatographic assay. RSC Advances, 6(2), 1279–1287. doi: 10.1039/C5RA23533B
- Zhou, Y., Song, F., Li, Y. S., Liu, J. Q., Lu, S. Y., Ren, H. L., … Wang, X. R. (2013). Double-antibody based immunoassay for the detection of beta-casein in bovine milk samples. Food Chemistry, 141(1), 167–173. doi: 10.1016/j.foodchem.2013.03.010