ABSTRACT
Flunixin meglumine (FM) is a novel nonsteroidal anti-inflammatory drug for animals, which has antipyretic, analgesic, and anti-inflammatory effects. The drug, which was originally used to relieve inflammation in horses, musculoskeletal disorders, and pain, has been approved for use in endotoxemia, infectious diseases in swine, etc. A sensitive anti-FM monoclonal antibody 2H4 was prepared and used to develop an indirect competitive enzyme-linked immunosorbent assay and immunochromatographic strip assay for the detection of FM in milk. The complete antigen and coating antigen were conjugated with bovine serum albumin and ovalbumin, respectively. The monoclonal antibody 2H4, with a half inhibition concentration of 0.29 ng/mL, had a limit of detection of 0.432 ng/mL and a linear range of detection of 0.08664–0.97226 ng/mL. A sensitive and convenient immunochromatographic strip assay was developed with an FM cutoff value of 0.29 ng/mL. The developed methods were suitable for the detection of FM in milk.
Introduction
Flunixin meglumine (FM) is a novel nonsteroidal anti-inflammatory drug that was approved for veterinary uses by the Ministry of Agriculture in China in June 2007 (Choi et al., Citation2016; Coetzee, Citation2011; Johnson & Myers, Citation2015; Miciletta, Cuniberti, Barbero, & Re, Citation2014; Stafford & Mellor, Citation2011). In October 2004, FM was approved for use in lactating dairy cows by the FDA and has been widely used in the United States, France, Switzerland, Germany, and Britain. FM impacts prostaglandin synthesis by inhibiting cyclooxygenase (COX) and reduces inflammation and pain within 15 min of administration. Compared with FM, antipyrine, butazodine, and antondin pyrazolone are more frequently used in animals. Its long-term use causes aplastic anemia, neutropenia, thrombocytopenia, proteinuria, interstitial nephritis, and liver damage. However, in the treatment of claudication and swelling of joints, FM is four times more effective than phenylbutazone on a weight basis.
FM is easily metabolized and eliminated in vivo. The main FM metabolites are 4-hydroxy flunixin, 5-hydroxy flunixin, and 2-methylhydroxy. After 24 h of administration, approximately 90% is excreted via urine and feces.
FM, a nicotinic acid derivative, inhibits COX, which transfers arachidonic acid to prostanoids (Donalisio et al., Citation2013; Lee & Maxwell, Citation2014; Milovanović, Vučković, Prostran, Trailović, & Jovanović, Citation2016; Yilmaz et al., Citation2014). In China, FM has been used in cattle (Cetin et al., Citation2014; Cooke, Cappellozza, Guarnieri, & Bohnert, Citation2013; Currah, Hendrick, & Stookey, Citation2009; Huber, Arnholdt, Mostl, Gelfert, & Drillich, Citation2013; Newby et al., Citation2017), pigs (Whalin, Pairis-Garcia, Proudfoot, Stalder, & Johnson, Citation2016), horses (Duz, Parkin, Cullander, & Marshall, Citation2015; Keegan, Messer, Reed, Wilson, & Kramer, Citation2008), cats, dogs, and sheep (Marini et al., Citation2016; Paull, Lee, Colditz, Atkinson, & Fisher, Citation2007) to treat acute inflammation (Yang et al., Citation2013), musculoskeletal diseases, bellyache, pain, sow endometritis, mastitis, agalactia syndrome, and canine endotoxemia. The European Agency for the Evaluation of Medicinal Products set the maximum permissible limit (MPL) of FM in bovine muscle, fat, liver, and kidney tissues at 20, 30, 300, and 100 µg/kg, respectively. In April 2002, the FDA established that the MPL of FM in bovine liver and muscle is 125 and 25 µg/kg, respectively. Additionally, the MPL in porcine muscle, skin and fat, liver, and kidney is 50, 10, 200, and 30 µg/kg, respectively. China has not established the corresponding MPLs of FM. To prevent the abuse and misuse of veterinary drugs, monitor residue levels in meat products, and ensure food safety, it is necessary to develop an effective FM detection method.
Currently, FM detection methods include high performance liquid chromatography (Belal, Abd El-Razeq, Fouad, & Fouad, Citation2015) and chromatography (Howard et al., Citation2014; Lee & Maxwell, Citation2014). Even though these methods are highly accurate and sensitive, they are costly and require complex sample pretreatment steps and trained personnel. Immunoassays include radioimmunoassay, enzyme immunoassay, fluorescence immunoassay, immunoaffinity chromatography, and enzyme-linked immunosorbent assay (ELISA). In this study, we developed an immunochromatographic strip assay and indirect competitive ELISA (IC-ELISA) for FM detection in milk.
Materials and methods
Chemicals and instruments
FM was purchased from J&K Scientific Ltd. (Beijing, China). Bovine serum albumin (BSA), ovalbumin (OVA), N-Hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), N,N-Dimethylformamide (DMF), 2-morpholino-ethanesulfonic acid (MES), Freund’s complete adjuvant (FCA), Freund’s incomplete adjuvant (FIA), enzyme immunoassay-grade horseradish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin, gelatin, 3,3′,5,5′-tetramethyl-benzidine (TMB), Tween-20, and HRP were obtained from Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 cell culture medium, hypoxanthine–thymidine supplement, hypoxanthine–aminopterin–thymidine supplement, and fetal calf serum were acquired from Gibco BRL (Paisley, UK). All other chemicals and reagents were supplied by the National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China).
The instruments used in this study consisted of a UV/VIS scanner (Bokin Instruments, Tsushima, Japan), a Multiskan MKS microplate reader (Thermo LabSystems Inc., Beijing, China), a vortex machine (Shanghai Huxi Analysis Instrument Factory Co. Ltd, Shanghai, China), a membrane dispenser (Xinqidian Gene-Technology Co. Ltd, Beijing, China), and a water bath (Shanghai Instrument Group Co. Ltd., Supply & Sales Co., Shanghai, China).
Solutions
We prepared the following solutions: boracic acid buffer (BB, pH 8.8), coating buffer (CB; 0.05 M carbonate bicarbonate, pH 9.6), blocking buffer (0.2% gelatin in CB), washing buffer (PBST; 0.05% Tween-20 (v/v) in 0.01 M PBS), and stop solution (2 M sulfuric acid). The substrate buffer consisted of a mixture of solutions A (0.3% H2O2, v/v in phosphate buffer) and B (0.01% TMB, w/v in glycol) at a 5:1 (v/v) ratio.
Antigen preparation
Complete antigen (FM-EDC-BSA) was prepared by conjugating hapten with carrier protein using the carbodiimide (EDC) method and identified by UV/VIS spectroscopy (Gu, Liu, Song, Kuang, & Xu, Citation2016; Kong, Liu, Song, Kuang, & Xu, Citation2016; Sun, Liu, Song, Kuang, & Xu, Citation2016). FM hapten (2.2 mg) was dissolved in 400 μL of DMF, and 1.6 mg of NHS and 2.6 mg of EDC were successively added to the mixture. The mixture was stirred at room temperature (RT) for 8 h and added to 10 mg of BSA in 4 mL of BB. The mixture was stirred at RT for 18 h, dialyzed against 0.01 M PBS for 3 d with six changes of dialysate, and stored at −20°C.
FM was conjugated to OVA (FM-EDC-OVA) by the EDC method and was used as coating antigen. FM hapten (1.6 mg) was dissolved in 300 μL of DMF, and 1.2 mg of NHS and 1.9 mg of EDC were successively added to the mixture. The mixture was stirred at RT for 8 h and added to 5 mg OVA in 2 mL of BB. The mixture was stirred overnight at RT, dialyzed against 0.01 M PBS for 3 d, and stored at −20°C. All conjugates were identified by UV/VIS spectroscopy.
FM monoclonal antibody preparation
Female BALB/c mice (6–8 weeks of age) were immunized with complete antigen (FM-EDC-BSA) in FCA (80 μg per mice) by subcutaneous injection for the first immunization. Booster immunizations were performed 3 weeks after the first immunization. Each mouse was injected with FIA mixed with isometric complete antigen (40 μg per mice). Tail blood sampling was carried out after the third immunization; serum was analyzed by IC-ELISA (Suryoprabowo, Liu, Peng, Kuang, & Xu, Citation2014). The mouse with the highest inhibitory rate and titer was intraperitoneally injected with complete antigen (20 μg). After 3 weeks, spleen cells from the immunized mouse were fused with Sp2/0 myeloma cells to produce hybridoma cells (Peng, Liu, Kuang, Cui, & Xu, Citation2017), which were screened by IC-ELISA. The limited dilution method was performed to subclone positive clones. After three subcloning procedures, we obtained a hybridoma cell line capable of producing specific monoclonal antibodies (mAbs). The selected hybridoma cell line was cultured and intraperitoneally injected into mice for ascite production (Wang et al., Citation2017). Antibodies were purified by octanoic acid–ammonium sulfate precipitation (Li et al., Citation2014; Yan, Liu, Xu, Kuang, & Xu, Citation2015). After dialysis against PBS at 4°C for 3 d, antibodies (2 mg/mL) were stored at −20°C.
Development of IC-ELISA
We measured FM mAb titer by IC-ELISA and determined the optimal working concentration of coating antigen and FM mAb by square matrix titrimetry. FM-EDC-OVA (1 µg/mL, coating antigen) was diluted three times with CB by gradient dilution; 100 µL was transferred to 96-well microtiter ELISA plates. The plates were dried at 37°C for 2 h and washed three times with PBST. Blocking reagent (200 µL per well) was added, and the plate was incubated at 37°C for 2 h. The blocked plates were washed three times with washing buffer, dried, and stored at 4°C.
FM standard was diluted with 0.01 M PBS buffer solution containing potassium salt, and FM antibody was diluted with antibody dilution (PBS containing 0.1% gelatin). FM standard (50 µL) and antibody (50 µL) were successively added into each well, and the plates were incubated at 37°C for 30 min. After washing the wells three times, HRP-labeled goat anti-mouse IgG was diluted 3000-fold with antibody dilution and added into each well. The plates were incubated at 37°C for 30 min. Following four washes, substrate buffer mixed with solutions A and B at 5:1 (v/v) ratio was added into each well and incubated at 37°C for 15 min. Following the addition of stop solution, absorbance was measured at 450 nm in a microplate reader. A standard curve of mAb 2H4 against FM concentration was drawn. The 50% inhibitory concentration (IC50) of FM mAb, which was the half maximal inhibitory concentration.
Labeling FM mAb with colloidal gold particles
Colloidal gold-labeled FM mAb was prepared as previously reported with slight modifications (Feng et al., Citation2015). First, the pH of colloidal gold solution was adjusted to ∼8.8 with 0.1 M K2CO3. Second, 1 mL of gold nanoparticle and 4 µL purified FM mAb 2H4 were continuously stirred at RT for 50 min. BSA (10% w/v, 40 µL) was added, and the mixture was incubated at RT for 2 h. Following centrifugation at 8000 rpm for 20 min for three times, the resulting pellet was washed with gold-labeled resuspension (20 mmol tris containing 0.1% polyethylene glycol (PEG), 0.1% tween, 5% sucrose, 5% trehalose, 0.2% BSA, and 5% polyvinyl pyrrolidone (PVP), pH 8.2). Colloidal gold-labeled FM mAb was resuspended in PVP and stored at 4°C.
Preparation of the immunochromatographic strip
For the preparation of the immunochromatographic strip, polyvinyl chloride (PVC) backing card, sample pad, absorption pad, and nitrocellulose (NC) membrane were assembled in layers. The NC membrane contained the control line and test line. Using a membrane dispenser, coating antigen (FM-EDC-BSA) was applied to form the test line and goat anti-mouse antibody was applied to form the control line (1 µL/cm). The strip was dried at 37°C and stored in a desiccator.
Principle of the immunochromatographic strip assay
Standard FM (150 µL) was mixed with 50 µL colloidal gold-labeled FM mAb in a microtiter well. Following incubation at RT for 5 min, the mixture was added onto the sample pad, where it migrated to the absorption pad by capillary action. The results were visualized by the naked eye within 5 min. In FM-negative samples, colloidal gold-labeled FM mAb migrates and is captured by FM-EDC-BSA, leading to a deep color. In FM-positive samples, colloidal gold-labeled FM mAb is combined with FM in the sample; therefore, less colloidal gold-labeled FM mAb is able to combine with FM-EDC-BSA on the test line, leading to a light color on the test line.
The principle of the immunochromatographic strip assay is based on the competition between FM present in the sample and the coating antigen present on the test line for colloidal gold-labeled FM mAb. FM-EDC-BSA was fixed on the test line of the NC membrane. In FM-positive samples, FM competes with the coating antigen fixed on the test line and conjugated with colloidal gold-labeled FM mAb. With increasing FM concentration in the sample, the color intensity of the test line becomes weaker until it finally disappears. Accordingly, there is an inverse proportional relationship between the color intensity of the test line and the concentration of FM in the sample.
Analysis of milk samples
Milk samples were purchased from local supermarkets. The samples were spiked with FM and measured by IC-ELISA.
Results and discussion
Identification of complete antigen and coating antigen
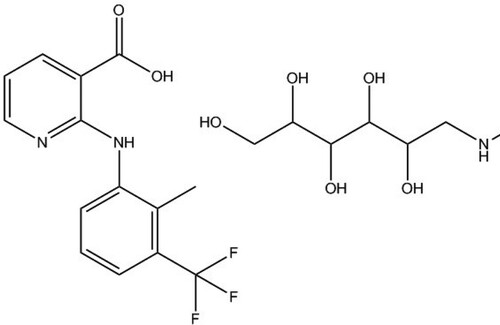
The carboxyl groups on FM are crucial for the conjugation reaction to carrier proteins (). BSA and OVA conjugated to FM by EDC were used as complete antigen and coating antigen, respectively.
Figure 1. The molecular structural formula of FM. A composite salt of flunixin with meglumine (1:1).
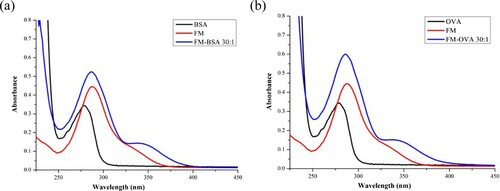
The complete antigen and coating antigen were identified by UV/VIS spectroscopy (). The characteristic absorption peaks of FM were obtained at 325–350 nm and 285 nm. BSA and OVA had an absorption peak at 278 nm, and FM-EDC-BSA and FM-EDC-OVA had an absorption peak at 325–350 nm, which was higher than that of FM within the same range. The primary peak of the conjugates was between the absorption peaks of BSA/OVA and FM. These results revealed that FM was successfully conjugated to carrier proteins.
Characteristic of mAb 2H4
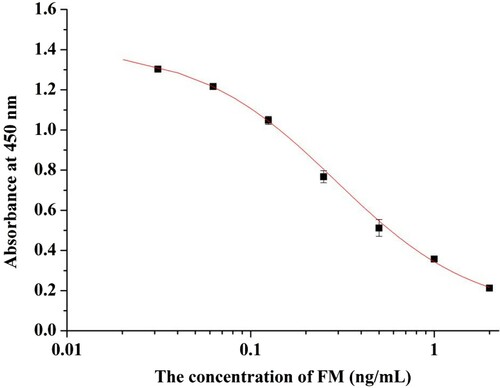
FM-EDC-BSA was used as immunogen in mice, and FM-EDC-OVA was used as coating antigen in IC-ELISA. Following cell fusion, ascites were purified by octanoic acid–ammonium sulfate precipitation, and the antibodies were identified by IC-ELISA. MAbs 1H5, 2C10, 2F10, and 2H4 were obtained. MAb 2H4 was selected as the most sensitive antibody among all mAbs. A standard curve generated between mAb 2H4 and FM concentration (y = 0.0857 + 1.324/[1+ (x/0.290)1.145]) had a linear regression correlation coefficient (R2) of 0.99928. IC50 of mAb 2H4 was 0.29 ng/mL, and limit of detection (LOD) (IC10) was 0.0432 ng/mL with a linear range (IC20 to IC80) (Li et al., Citation2017) of 0.08664–0.97226 ng/mL ().
Characteristics of the immunochromatographic strip for FM in milk
Optimization of coating antigen
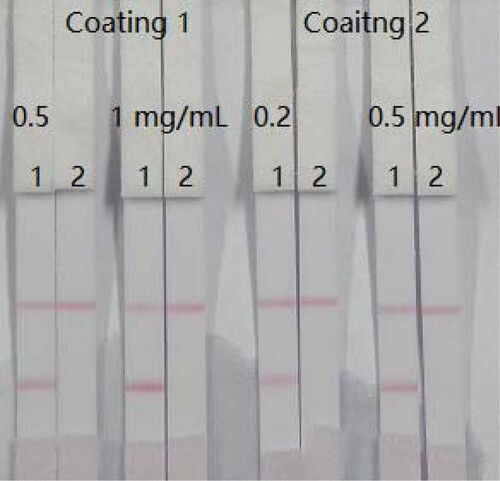
Two types of coating (coating 1: FM-EDC-BSA; coating 2: FM-EDC-OVA) were evaluated. Different coating antigen concentrations were added to FM standard. shows that pad 1 and pad 2 were added 0 and 5 ng/mL FM standard, respectively. The optimum conditions consisted of 1 mL of gold nanoparticle with 4 µL K2CO3, 8 µg/mL antibody, and 0.5 mg/mL coating 1. We selected FM-EDC-BSA as the coating antigen for subsequent experiments.
Figure 4. Optimization of two kinds of coating and two kinds of concentration. Coating concentration is 0.5 or 1 mg/mL (coating 1, FM-EDC-BSA) and 0.2 or 0.5 mg/mL (coating 2, FM-EDC-OVA). Strip 1: FM-negative sample (0 ng/mL); Strip 2: FM-positive sample (5 ng/mL). Coating 1 is carried out with concentration was 0.5 mg/mL.
Analysis of milk samples
Four FM-negative milk samples were spiked with FM (0 or 5 ng/mL) without any pretreatment steps. After 5 min, the test line of the spiked sample became colorless (). Whole milk was used in subsequent experiments.
Figure 5. Optimization of four kinds of milk samples. Coating concentration is 1 mg/mL (coating 1, FM-EDC-BSA). (a) FM standard was spiked into whole milk; (b) FM standard was spiked into infant milk; (c) FM standard was spiked into goat milk; (d) FM standard was spiked into liquid milk. Strip 1: FM-negative sample (0 ng/mL); Strip 2: FM-positive sample (5 ng/mL).
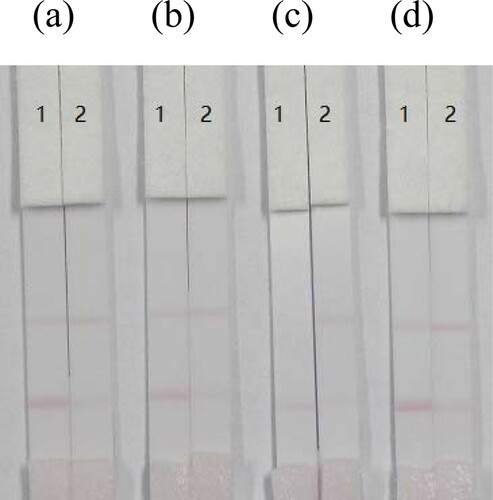
Optimization of resuspensions
Twelve types of resuspensions (PVP, PEG, BSA, Casein, sucrose, trehalose, sorbitol, mannitol, Tween-20, Brij-35, Triton X-100, and On-870) were used to optimize the assay. shows that the cutoff value was 5 ng/mL with PVP. Accordingly, resuspension 1 (PVP) was used in subsequent experiments.
Figure 6. Optimization of resuspension solution for sample pad: 20 Mm pH 8.2 tris + 0.1% PEG + 0.1% tween + 5% sucrose + 5% trehalose + 0.2% BSA + 1% or 5% 14 kinds of resuspensions. 1 = PVP, 2 = PEG, 4 = BSA, 5 = Casein, 6 = sucrose, 7 = trehalose, 8 = sorbitol, 9 = mannitol, 11 = tween-20, 12 = Brij-35, 13 = triton X-100, and 14 = On-870. Strip 1: FM-negative sample (0 ng/mL); Strip 2: FM-positive sample (5 ng/mL).

Different FM standard concentrations (0, 0.1, 0.25, 0.5, 1, 2.5, and 5 ng/mL) were added to whole milk. Spiked whole milk (100 μL) was added to the sample pad (). The color of the test line was weak at 0.1 ng/mL FM and disappeared at 5 ng/mL FM. LOD was 0.1 ng/mL with a cutoff value of 5 ng/mL.
Figure 7. FM image detection by immunochromatographic strip in whole milk. Coating concentration is 0.5 mg/mL (coating 1, FM-EDC-BSA). A series of whole milk samples spiked FM were used for the immunochromatographic strip test. 1 = 0 ng/mL, 2 = 0.1 ng/mL, 3 = 0.25 ng/mL, 4 = 0.5 ng/mL, 5 = 1 ng/mL, 6 = 2.5 ng/mL, and 7 = 5 ng/mL. The cutoff value is 5 ng/mL.

Conclusions
In this study, we developed a highly sensitive mAb 2H4 for the determination of FM, with an IC50 of 0.29 ng/mL, an LOD (IC10) of 0.1 ng/mL, and a linear range of 0.08664–0.97226 ng/mL. Furthermore, through the optimization of coating antigen and resuspensions, an effective and portable immunochromatographic strip assay was developed with a cutoff value of 0.29 ng/mL FM in milk. The immunochromatographic strip assay is a simple, fast, portable, and sensitive method for the detection of FM in milk.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Lu Lin
Lu Lin got her bachelor from Dalian Ocean University, Dalian, China in 2015 and then she began to study in Jiangnan University (Wuxi, China) for her Master Degree in food science. Her research interests are immunoassay applications in food.
Wei Jiang
Wei Jiang got her bachelor from Yangzhou University, Yangzhou, China in 2015 and then she began to study in Jiangnan University (Wuxi, China) for her Master Degree in food science. Her research interest includes immunoassay development for food safety.
Liguang Xu
Liguang Xu earned his Ph.D. in food science in 2012 from Jiangnan University, Wuxi, China and then became a faculty in college of food science and technology of Jiangnan University. His research is focused on the synthesis and controllable assembly of nanoparticles, especially noble metal nanoparticles.
Liqiang Liu
Liqiang Liu got his Ph.D. in food science in 2014 from Jiangnan University, Wuxi, China and then became a faculty in college of food science and technology of Jiangnan University. His research interests are immunochromatographic strip design and application.
Shanshan Song
Shanshan Song got her Master degree in food science in 2012 from Jiangnan University, Wuxi, China and then became a research assistant in college of Food science and technology of Jiangnan University. Her research interests are monoclonal antibody development.
Hua Kuang
Hua Kuang got her Ph.D. from China Agricultural University in 2009 and then began to work as a faculty in college of food science and technology of Jiangnan University. She is currently a full professor in food safety. Her research interests are biosensor development.
References
- Belal, F. F., Abd El-Razeq, S. A., Fouad, M. M., & Fouad, F. A. (2015). Micellar high performance liquid chromatographic determination of flunixin meglumine in bulk, pharmaceutical dosage forms, bovine liver and kidney. Analytical Chemistry Research, 3, 63–69. doi: https://doi.org/10.1016/j.ancr.2014.12.003
- Cetin, Y., Kocamuftuoglu, M., Ozyurtlu, N., Kucukaslan, I., Sendag, S., & Wehrend, A. (2014). Effect of flunixin meglumine or prostaglandin E2 treatment 15 days after breeding on fertility in Saanen does. Theriogenology, 81(3), 424–427. doi: https://doi.org/10.1016/j.theriogenology.2013.10.015
- Choi, H. J., Do, K. H., Park, J.-H., Kim, J., Yu, M., Park, S.-H., & Moon, Y. (2016). Early epithelial restitution by nonsteroidal anti-inflammatory drug–activated gene 1 counteracts intestinal ulcerative injuries. The Journal of Immunology, 197(4), 1415–1424. doi: https://doi.org/10.4049/jimmunol.1501784
- Coetzee, J. F. (2011). A review of pain assessment techniques and pharmacological approaches to pain relief after bovine castration: Practical implications for cattle production within the United States. Applied Animal Behaviour Science, 135(3), 192–213. doi: https://doi.org/10.1016/j.applanim.2011.10.016
- Cooke, R. F., Cappellozza, B. I., Guarnieri, T. A., & Bohnert, D. W. (2013). Effects of flunixin meglumine administration on physiological and performance responses of transported feeder cattle. Journal of Animal Science, 91(11), 5500–5506. doi: https://doi.org/10.2527/jas.2013-6336
- Currah, J. M., Hendrick, S. H., & Stookey, J. M. (2009). The behavioral assessment and alleviation of pain associated with castration in beef calves treated with flunixin meglumine and caudal lidocaine epidural anesthesia with epinephrine. Canadian Veterinary Journal – La Revue Veterinaire Canadienne, 50(4), 375–382.
- Donalisio, C., Barbero, R., Cuniberti, B., Vercelli, C., Casalone, M., & Re, G. (2013). Effects of flunixin meglumine and ketoprofen on mediator production in ex vivo and in vitro models of inflammation in healthy dairy cows. Journal of Veterinary Pharmacology and Therapeutics, 36(2), 130–139. doi: https://doi.org/10.1111/j.1365-2885.2012.01396.x
- Duz, M., Parkin, T. D., Cullander, R. M., & Marshall, J. F. (2015). Effect of flunixin meglumine and firocoxib on ex vivo cyclooxygenase activity in horses undergoing elective surgery. American Journal of Veterinary Research, 76(3), 208–215. doi: https://doi.org/10.2460/ajvr.76.3.208
- Feng, M., Kong, D. Z., Wang, W. B., Liu, L. Q., Song, S. S., & Xu, C. L. (2015). Development of an immunochromatographic strip for rapid detection of Pantoea stewartii subsp. stewartii. Sensors, 15(2), 4291–4301. doi: https://doi.org/10.3390/s150204291
- Gu, H., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of an immunochromatographic strip assay for ractopamine detection using an ultrasensitive monoclonal antibody. Food and Agricultural Immunology, 27(4), 471–483. doi: https://doi.org/10.1080/09540105.2015.1126808
- Howard, J. T., Baynes, R. E., Brooks, J. D., Yeatts, J. L., Bellis, B., Ashwell, M. S., … Maltecca, C. (2014). The effect of breed and sex on sulfamethazine, enrofloxacin, fenbendazole and flunixin meglumine pharmacokinetic parameters in swine. Journal of Veterinary Pharmacology and Therapeutics, 37(6), 531–541. doi: https://doi.org/10.1111/jvp.12128
- Huber, J., Arnholdt, T., Mostl, E., Gelfert, C. C., & Drillich, M. (2013). Pain management with flunixin meglumine at dehorning of calves. Journal of Dairy Science, 96(1), 132–140. doi: https://doi.org/10.3168/jds.2012-5483
- Johnson, A., & Myers, M. (2015). NSAIDs inhibit NFkβ activation to prevent inflammation (IRC4P.602). The Journal of Immunology, 194(1 Supplement), 57.19–57.19.
- Keegan, K. G., Messer, N. T., Reed, S. K., Wilson, D. A., & Kramer, J. (2008). Effectiveness of administration of phenylbutazone alone or concurrent administration of phenylbutazone and flunixin meglumine to alleviate lameness in horses. American Journal of Veterinary Research, 69(2), 167–173. doi: https://doi.org/10.2460/ajvr.69.2.167
- Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of IC-ELISA and lateral-flow immunochromatographic assay strip for the detection of folic acid in energy drinks and milk samples. Food and Agricultural Immunology, 27(6), 841–854. doi: https://doi.org/10.1080/09540105.2016.1183600
- Lee, C. D., & Maxwell, L. K. (2014). Effect of body weight on the pharmacokinetics of flunixin meglumine in miniature horses and quarter horses. Journal of Veterinary Pharmacology and Therapeutics, 37(1), 35–42. doi: https://doi.org/10.1111/jvp.12056
- Li, Z., Liu, L., Song, S., Guo, S., Kuang, H., & Xu, C. (2014). Development of an enzyme-linked immunosorbent assay for octylphenol. Food and Agricultural Immunology, 25(3), 397–410. doi: https://doi.org/10.1080/09540105.2013.821597
- Li, C., Zhang, Y., Eremin, S. A., Yakup, O., Yao, G., & Zhang, X. (2017). Detection of kanamycin and gentamicin residues in animal-derived food using IgY antibody based ic-ELISA and FPIA. Food Chemistry, 227, 48–54. doi: https://doi.org/10.1016/j.foodchem.2017.01.058
- Marini, D., Pippia, J., Colditz, I. G., Hinch, G. N., Petherick, C. J., & Lee, C. (2016). Palatability and pharmacokinetics of flunixin when administered to sheep through feed. PeerJ, 4, e1800. doi: https://doi.org/10.7717/peerj.1800
- Miciletta, M., Cuniberti, B., Barbero, R., & Re, G. (2014). In vitro enantioselective pharmacodynamics of carprofen and flunixin-meglumine in feedlot cattle. Journal of Veterinary Pharmacology and Therapeutics, 37(1), 43–52. doi: https://doi.org/10.1111/jvp.12052
- Milovanovic, M., Vuckovic, S., Prostran, M., Trailovic, S., & Jovanovic, M. (2016). L-arginine-NO system participates in the analgesic effect of flunixin meglumine in the rat. Acta Veterinaria, 66(1), 103–114. doi: https://doi.org/10.1515/acve-2016-0008
- Newby, N. C., Leslie, K. E., Dingwell, H. D., Kelton, D. F., Weary, D. M., Neuder, L., … Duffield, T. F. (2017). The effects of periparturient administration of flunixin meglumine on the health and production of dairy cattle. Journal of Dairy Science, 100(1), 582–587. doi: https://doi.org/10.3168/jds.2016-11747
- Paull, D. R., Lee, C., Colditz, I. G., Atkinson, S. J., & Fisher, A. D. (2007). The effect of a topical anaesthetic formulation, systemic flunixin and carprofen, singly or in combination, on cortisol and behavioural responses of Merino lambs to mulesing. Australian Veterinary Journal, 85(3), 98–106. doi: https://doi.org/10.1111/j.1751-0813.2007.00115.x
- Peng, J., Liu, L., Kuang, H., Cui, G., & Xu, C. (2017). Development of an icELISA and immunochromatographic strip for detection of norfloxacin and its analogs in milk. Food and Agricultural Immunology, 28(2), 288–298. doi: https://doi.org/10.1080/09540105.2016.1263987
- Stafford, K. J., & Mellor, D. J. (2011). Addressing the pain associated with disbudding and dehorning in cattle. Applied Animal Behaviour Science, 135(3), 226–231. doi: https://doi.org/10.1016/j.applanim.2011.10.018
- Sun, C., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of a highly sensitive ELISA and immunochromatographic strip to detect pentachlorophenol. Food and Agricultural Immunology, 27(5), 689–699. doi: https://doi.org/10.1080/09540105.2016.1148668
- Suryoprabowo, S., Liu, L. Q., Peng, J., Kuang, H., & Xu, C. L. (2014). Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Analytical Methods, 7(10), 2163–2168. doi: https://doi.org/10.1007/s12161-014-9863-1
- Wang, Z., Xie, Z., Cui, G., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of an indirect competitive enzyme-linked immunosorbent assay and immunochromatographic assay for hydrocortisone residues in milk. Food and Agricultural Immunology, 28(3), 476–488. doi: https://doi.org/10.1080/09540105.2017.1297779
- Whalin, L., Pairis-Garcia, M., Proudfoot, K., Stalder, K., & Johnson, A. (2016). Validating behavioral sampling techniques for lame sows administered flunixin meglumine and meloxicam. Livestock Science, 191, 103–107. doi: https://doi.org/10.1016/j.livsci.2016.07.017
- Yang, F., Li, G. H., Meng, X. B., Wang, L. Q., Huang, X. H., Shan, Q., … Zeng, Z. L. (2013). Pharmacokinetic interactions of flunixin meglumine and doxycycline in broiler chickens. Journal of Veterinary Pharmacology and Therapeutics, 36(1), 85–88. doi: https://doi.org/10.1111/j.1365-2885.2012.01382.x
- Yan, H., Liu, L., Xu, N., Kuang, H., & Xu, C. (2015). Development of an immunoassay for carbendazim based on a class-selective monoclonal antibody. Food and Agricultural Immunology, 26(5), 659–670. doi: https://doi.org/10.1080/09540105.2015.1007446
- Yilmaz, O., Korkmaz, M., Jaroszewski, J. J., Yazici, E., Ulutas, E., & Saritas, Z. K. (2014). Comparison of flunixin meglumine and meloxicam influence on postoperative and oxidative stress in ovariohysterectomized bitches. Polish Journal of Veterinary Sciences, 17(3), 493–499. doi: https://doi.org/10.2478/pjvs-2014-0071