ABSTRACT
Interest in proso millet as a functional food is growing. However, studies on the chemical composition, antioxidant, and antiproliferative activities’ differences of proso millet cultivars in China are limited. Nine Chinese proso millet varieties (five waxy varieties – S, D, N2, N4, N8 and four non-waxy varieties – J1, J3, J7, J9) were investigated. Results showed that the non-waxy varieties were significantly higher than waxy varieties in the content of amylase and resistant starch (22.95%, 0.23%, 2.02%, and 0.78%, respectively; P < .05). Lys was the first limit amino acid (AAS: 16.08%). Linolenic acid (61.74%) and oleic acid (22.16%) were the dominant fatty acids. Overall, the antioxidant content and antioxidant activities of J1 and J3 were higher than other cultivars. And, J7, J3, and J1 possessed better antiproliferative effects than other proso millet varieties. These results are anticipated to provide useful information on the development of proso millet-based functional food.
1. Introduction
Proso millet (Panicum miliaceum L.) is a kind of the oldest cultivated millet crop and consumed as a staple food among the majority of people who live in arid and semiarid tropics of the world, such as Asia, Africa, and parts of Europe (Lu et al., Citation2009). Several reports have shown that millet is superior to other major cereals in nutritional value (Pathak, Srivastava, & Grover, Citation2000). Starch, which is the most abundant nutrient in proso millet, is nearly 70%. However, proso millet-based products own a lower glycemic index (GI) than corn, which means proso millet appears to be a good ingredient for producing low-GI products (McSweeney, Seetharaman, Ramdath, & Duizer, Citation2017). Studies have also shown that protein accounts for 12% and the essential amino acid index (EAAI) of proso millet was higher (51%) compared to wheat (Kalinova & Moudry, Citation2006). Besides, proso millet also contains fat, crude fibre, minerals, vitamins, and other phytochemicals, such as phenolic, flavonoid, proanthocyanidin, and phytic acid (Shahidi & Chandrasekara, Citation2013).
Recently, proso millet has attracted attention for its considerable health benefits and functional components, particularly with respect to the antioxidant, anti-diabetic, anti-cancer, anti-liver injury, and antiproliferative effects. A large number of studies have shown that the aqueous/ethanol/methanol extracts of proso millet possess antioxidant properties (Chandrasekara & Shahidi, Citation2011a, Citation2011b; Chandrasekara & Shahidi, Citation2012; Ragaee, Abdel-Aal, & Noaman, Citation2006). Park, Ito, Nagasawa, Choi, and Nishizawa (Citation2008) suggest that proso millet protein concentrate may have potential for therapeutic intervention in type 2 diabetes. Nishizawa et al. (Citation2002) suggested that proso millet is considered to be another preventive food for liver injury by examining the effects of dietary protein from proso millet on liver injury induced by D-galactosamine in rats.
In spite of good characteristics, proso millets are still underutilized, and there is little information on the differences of Chinese proso millet varieties. Therefore, the present study was carried out to (1) compare the chemical compositions, particularly with respect to amino acid and fatty acid components, (2) evaluate the antioxidant activities and antiproliferative effect on MDA-MB-231-Breast Cell Lines of extracts from nine Chinese proso millet varieties and (3) investigate correlations between the antioxidant activities and antiproliferative effect.
2. Materials and methods
2.1. Proso millet materials
Nine proso millet varieties, including five waxy proso millet varieties (S, D, N2, N4, and N8) and four non-waxy proso millet varieties (J1, J3, J7, and J9), were collected. S and D were procured from Millet Research Institute, Shanxi Academy of Agricultural Sciences, China. The others were procured from Gansu Academy of Agricultural Science, China. All samples were dried at 40°C, dehusked in a centrifugal sheller, ground in a laboratory mill (Landert-Motoren AG, Buelach, Switzerland), and passed through a Ф 0.178 mm screen sieve successively to obtain the whole proso millet flour.
2.2. Chemicals
Mixed amino acid standard H was produced by Wako Pure Chemical Industries (Osaka, Japan). Fetal bovine serum (FBS), penicillin, streptomycin, and gentamicin were purchased from Life Technologies (Grand Island, NY). Standards of tryptophan, oleanolic acid, gallic acid, rutin, Folin Ciocalteu’s reagent, 6-hydroxy-2,5,7,8-tetramethylcrroman-2-carboxylic acid (Trolox), DPPH (2,2-diphenyl-1-picrylhydrazyl), phosphate-buffered saline (PBS), and minimum essential medium (MEM) were all obtained from Sigma-Aldrich (Shanghai, China). Total antioxidant capacity assay kits (the ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)method and ferric reducing ability of plasma (FRAP) method) were obtained from TIANDZ (Beijing, China). Megazyme assay kits were purchased from Ireland Ltd (Megazyme Int; Wicklow, Ireland). All other reagents and solvents used were of analytical and HPLC grade.
2.3. Nutritional composition analyses
Crude starch, amylose, and resistant starch were measured using the Megazyme assay kits. Protein and fat contents were determined according to the methods of the Association of Official Analytical Chemists (Citation2005) 979.09, 996.11.
Amino acids were determined by an amino acid analyzer (L-8800; Hitachi, Japan) after sample pretreatment (Qin et al., Citation2014). Besides, the tryptophan was analysed alone after alkaline hydrolysis (Guo et al., Citation2017). EAAI was calculated as a geometric average of amino acid score (AAS) of all essential amino acids. AAS = 100× the amino acid content in tested protein/content of the amino acid in standard protein according to FAO/WHO.
Fatty acid analyses were performed according to the method of Miao et al. (Citation2010), using gas chromatography (Agilent Agilent Technologies, Palo Alto, CA) equipped with a flame ionisation detector.
2.4. Preparation of proso millet extracts
Extracts for the determination of antioxidant compounds, antioxidant activities, and antiproliferation activity were prepared according to the method of Shi, Yao, Zhu, and Ren (Citation2017) and some modifications were made. In brief, two grams of proso millet flours were mixed with 20 mL of 70% methanol in a 50-mL centrifuge tube. Then, the mixture was placed in an ultrasonic bath (Powe sonic 420, Hwashin co., Korea) and sonicated at 50°C for 45 min. Subsequently, the mixture was cooled. Then, the mixture was centrifuged at 5000 rpm/min for 10 min. The supernatants were transferred into culture tubes and stored at −4°C for the determination of antioxidant content and antioxidant activities, and the remaining supernatants were dried by a stream of nitrogen for the measurement of cytotoxicity and antiproliferative experiments.
2.5. Determination of antioxidant compounds
The measurement of the total saponin content (TSC) was done following the method of Wu, Lin, and Chau (Citation2001) using oleanolic acid as a standard. The results were expressed as mg/g of extracts. Total phenolic content (TPC) measurement was based on the Folin–Ciocalteu method of Dewanto, Wu, Adom, and Liu (Citation2002) using gallic acid as a standard. The results were expressed as mg/g of extracts. Total flavonoid content (TFC) detection was based on aluminium chloride calorimetric assay of Kim, Jeong, and Lee (Citation2003) using rutin as a standard. The results were expressed as mg/g of extracts.
2.6. Determination of antioxidant activity
The DPPH radical scavenging activity was estimated according to the method of Cheung, Cheung, and Ooi (Citation2003) and calculated based on a calibration curve using Trolox and expressed as μM/g of extracts.
The scavenging capacity of 2,2’-azino-di-[3-ethylbenzthiazoline sulphonate] (ABTS•+ radical cations) was measured using a total antioxidant capacity assay kit (the ABTS method). Briefly, 200-μL ABTS+ solution and 10 μL of the supernate (distilled water or solution of Trolox standard) were added to a 96-well microplate. The mixture was mixed and left for 2–6 min at room temperature under dim light, and then the absorbance was measured at 734 nm using a microplate reader. The results were reflected in μM/g of extracts.
The FRAP assay was determined using a total antioxidant capacity assay kit (the FRAP method). Briefly, 180-μL FRAP solution and 5 μL of the supernate (distilled water or solution of FeSO4 standard) were added to a 96-well microplate. The mixture was mixed and left for 3–5 min at room temperature under dim light, and then the absorbance was measured at 593 nm using a microplate reader. The results were expressed as the concentration of extracts (μM/g of extracts) having a ferric reducing ability equivalent to that of 1 mmol/L FeSO4.
2.7. Cell culture
MDA-MB-231 human breast cells were purchased from American Type Culture Collection Stock (ATCC, MD, USA). The cells were cultured in MEM, containing 10% FBS, 1 mmol/L Hepes, 0.01 mg/mL insulin, 50 units/mL penicillin, 50 μg/mL streptomycin, and 100 μg/mL gentamicin (Wang, Xie, Wang, Liu, & Ju, Citation2014) as described previously. All cultures were incubated at 37°C in humidified atmosphere with 5% CO2.
2.8. Measurement of cytotoxicity by methylene blue assay
The cytotoxicity was determined using the method reported by Wang et al. (Citation2014) and Yoon and Liu (Citation2008). Briefly, MDA-MB-231 cells were seeded into a 96-well microplate (4.0 × 104 cells/well) in 100 μL of growth medium/well and were incubated for 24 h. Then, the cells were treated with 100 μL of medium with different concentrations (31.25, 62.5, 125, 250, 500 μg/mL) of proso millet extracts; the wells that received medium without proso millet extract served as the control. After 24 h of incubation, the cells were stained using 50-μL methylene blue coloured liquid (98% HBSS, 0.7% glutaraldehyde and 0.6% methylene blue). After 1 h of incubation, the cells were washed six times in deionised water, and 100 μL of elution buffer (49% PBS, 50% ethanol, and 1% acetic acid) was added. The absorbance was measured at 570 nm in a microplate reader (Bio Rad, IMAX, Hercules, USA) after shaking for 20 min on a tablet oscillator. The cytotoxicity was determined as percentage compared to the control. If a certain concentration of proso millet extract reduced cell viability compared to the control, then that concentration was considered to be cytotoxic (Felice, Sun, & Liu, Citation2009).
2.9. Measurement of antiproliferative activity by methylene blue assay
The antiproliferative activities of proso millet extracts were assessed using the method of Wang et al. (Citation2014) and Yoon and Liu (Citation2008). Briefly, MDA-MB-231 cells were seeded into the central 96-well microplate (2.5 × 104 cells/well), and cell-free medium was added to the peripheral wells of the 96-well microplate at 100 μL of growth medium/well. After 8 h of incubation, the medium was removed and 100 μL of medium with different concentrations (31.25, 62.5, 125, 250, 500 μg/mL) of proso millet extracts was added; the wells that received medium without proso millet extract served as the control. Following 96 h of incubation, the staining solution was removed and the 96-well microplates were washed six times in deionised water. Then, 100 μL of elution buffer (49% PBS, 50% ethanol, 1% acetic acid) was added to each well. The 96-well microplates were transferred to a tablet oscillator for 20 min. Absorbance was measured at 570 nm using a microplate reader (Bio Rad, IMAX, Hercules, USA). The antiproliferative activities were evaluated by the EC50 (Effective concentration of 50% cell proliferation)values, which were expressed as μg of proso millet extracts/mL.
2.10. Statistical analysis
All determinations were made in triplicate. The experimental data were expressed as the mean ± SD. One-way analysis of variance and Dunnett’s multiple range tests (SPSS 17.0) were used to determine the significant differences between a group means at P < .05.Correlations between DPPH, ABTS+, FRAP, and EC50 values of antiproliferative activity were identified using Spearman’s correlation (SPSS 17.0). Correlations were considered highly significant at P < .01.
3. Results and discussion
3.1. Total starch, amylose, and resistant starch
The total starch content of these 9 proso millet varieties ranged from 65.82% to 78.59% (). The average amylase content of these 4 non-waxy proso millets (22.95%) were significantly higher (P < .05) than that of these 5 waxy proso millets (0.23%), which were in agreement with the earlier reports of Ragaee et al.(Citation2006). The RS content of these 9 proso millet varieties ranged from 0.64% (S) to 2.21% (J7), and non-waxy proso millet varieties were significantly richer (P < .05) than waxy proso millet varieties, which were in agreement with the report of Ragaee et al. (Citation2006). RS is a fraction of starch that resists digestion within the small intestine, reaching the large intestine intact. RS-rich foods may be particularly useful for managing diabetes and improving gut microbiota composition (Matsumoto et al., Citation2016; Nielsen, Theil, Purup, NØrskov, & Knudsen, Citation2015). Thus, the non-waxy proso millets, especially the variety of J7, may be a good ingredientfor the prevention of diabetes and improvement of intestinal flora.
Table 1. The contents (%) of total starch, amylose, and resistant starch in nine Chinese proso millet cultivars.
3.2. Total protein and amino acid profile
The contents of protein and 18 kinds of amino acids in 9 Chinese proso millet varieties are shown in . The average protein content of waxy proso millet varieties was higher than non-waxy proso millet varieties (13.38% and 12.05%, respectively). All varieties of proso millet were particularly rich in Glu, Leu, and Ala (averaging 32.55 mg/g, 16.19 mg/g, and 12.25 mg/g, respectively). Besides, the AAS, which is based on the theory of balance of essential amino acids, was used to judge the limit amino acid (). It can be found that Lys was the first limit amino acid for all these nine proso millet cultivars and sulphurous amino acid (Met and Cys) was the second limit amino acid (AAS: 16.08% and 62.00%, respectively), which were in agreement with the earlier reports of Kalinova and Moudry (Citation2006). The average EAAI of waxy proso millet varieties (80.64%) was higher than non-waxy proso millet varieties (77.57%), and the highest value of protein (EAAI) was found in J7(85.22%). The results were higher than that of wheat and the report of Kalinova and Moudry (Citation2006), and this is possibly due to the difference of varieties and the conditions for growth.
Table 2. Protein (%) and amino acid content in nine Chinese proso millet cultivars (mg/g).
Table 3. Amino acid content (AA, mg/g protein), amino acid score (AAS, %), and essential amino acid index (EAAI, %) of nine Chinese proso millet cultivars.
3.3. Total fat and fatty acid profile
The fat and fatty acid compositions of nine Chinese proso millet varieties are presented in . The fat contents ranged from 1.54% (J3) to 3.77% (D), which were lower than that in brown grains but in agreement with the range of 1.2% to 3.8% in polished and whole grains reported by Devisetti, Yadahally, and Bhattacharya (Citation2014). There were clear differences in the fatty acid contents of the 9 Chinese proso millet cultivars. Linolenic acid and oleic acid were the two dominant fatty acids in the nine proso millet varieties, and their average contents were 61.74% and 22.16%, respectively. These results were consistent with the report (65.5% and 20.0%, respectively) of Annor, Marcone, Corredig, Bertoft, and Seetharaman (Citation2015). D and N8 had the highest linolenic acid (64.23%) and linolenic acid (2.51%) among all proso millet cultivars, respectively. Linolenic acid and linolenic acids are essential fatty acid and must be supplied via the diet (Kim, Nam, Kim, Hayes, & Lee, Citation2014; Malcicka, Visser, & Ellers, Citation2017). It is reported that linolenic acid has many benefits for human health, such as cardiovascular-protective, anti-cancer, neuroprotective, and anti-inflammatory effects (Kim et al., Citation2014). Therefore, proso millet may be beneficial as a healthy food ingredient.
Table 4. Contents of crude fat and fatty acid (%) in nine Chinese proso millet cultivars.
3.4. The phytochemical compounds and antioxidant activities of methanol extracts
It has been reported that methanol is an effective solvent in extracting phenolics and other polar substances in grains (Suma & Urooj, Citation2012). In this study, 70% methanol extracts from proso millets were used for the determination of phytochemical compounds, antioxidant, and antiproliferative activities. The TSC, TPC, and TFC ranged from 100.93 to 162.11 mg oleanolic acid equivalents, 9.28–19.05 mg gallic acid equivalents, and 3.51–7.33 mg rutin equivalents per gram of extracts, respectively (). Non-waxy proso millets were significantly higher than waxy proso millet in TSC (136.12 mg/g and 108.51 mg/g, respectively; P < .05). J1 had the highest TSC and TPC, while S possessed the lowest. The results were in agreement with the studies of Choi, Jeong, and Lee (Citation2007) and higher than wheat, barley, and rice (Kim, Hyun, & Kim, Citation2010).
Table 5. The content of total saponin (TSC), total phenolic (TPC), total flavonoid (TFC), and antioxidant activities of proso millet extracts.
The antioxidant properties of proso millets were evaluated on the basis of measuring scavenging for DPPH radicals, ABTS radical cations, and FRAP by proso millet methanol extracts. The average proso millet extracts’ values were 35.60 μmol TE/g, 75.76 μmol TE/g, and 86.36 μmol FeSO4/g as determined by the DPPH, ABTS, and FRAP assays, respectively (). As a whole, non-waxy proso millet possessed a higher antioxidant activity than waxy proso millet. Among all these nine Chinese proso millet varieties, N2 displayed the highest DPPH radical scavenging activity (41.28 μM/g), J3 had the highest ABTS radical scavenging activity and FRAP value (104.16 μM/g and 117.54 μM/g, respectively), whereas S showed the lowest antioxidant activity. The differences between DPPH, ABTS+ radical scavenging activity, and FRAP among the varieties may be due to the content and composition of phenolics and flavonoids of the extracts (Chandrasekara and Shahidi, 2011).
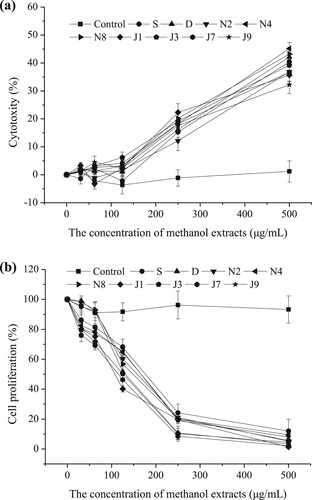
3.5. Cytotoxicity and antiproliferative effects on MDA-MB-231-Breast Cell Lines
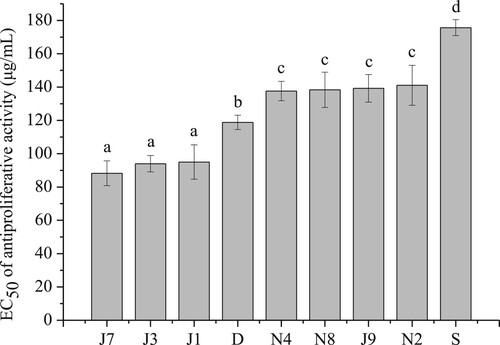
The cytotoxic effects and inhibition of MDA-MB-231-Breast Cell Lines’ proliferation by the proso millet extracts are presented in . As shown in (a), all of the extracts had no cytotoxic effect (<10% reduction in the absorbance reading at 570 nm compared to the control) on MDA-MB-231-Breast Cell Lines in a low dosage range (0–125 μg/mL). (b) shows that all extracts inhibited MDA-MB-231-Breast Cell Lines’ proliferation at doses of 31.25, 62.5, 125, 250, 500 μg/mL, and the inhibition was dose-dependent. For the different cultivars of proso millet, the EC50 values of antiproliferative activities are shown in . The EC50 of J7 (88.26 μg/mL), J3 (93.98 μg/mL), and J1 (94.98 μg/mL) were significantly lower than all other cultivars (P < .05). Therefore, the methanol extracts of J7, J3, and J1 possessed better antiproliferative effects than other proso millet varieties. The result suggested that the inhibitory effect was not attributed to a cytotoxic effect but to the anti-cancer effects of the extracts in a certain extract range. Zhang, Liu, and Wei (Citation2014) also reported that both free and bound phenolic extracts of edible proso millet showed antiproliferative activities towards MDA cells. However, further researches are needed to elucidate which function compounds are responsible for the antiproliferative activity of proso millet.
3.6. Correlations
The correlations between antioxidant activity (DPPH, ABTS, and FRAP value) and antiproliferative activity (EC50 value) are shown in . The results showed that antiproliferative activity significantly negatively correlates with ABTS (P < .01) and FRAP (P < .05). The negative correlation indicates that the higher ABTS values resulted in a higher inhibition of cancer cell proliferation. However, there was no significant linear relationship between the DPPH value and EC50 value of anti-cancer activity of MDA-MB-231-Breast Cell Lines (R 2 = −0.587, P < .05).
Table 6. The correlation of antioxidant activity and antiproliferative activity.
4. Conclusion
Although all the nine kinds of Chinese proso millet had good nutritional characteristics of antioxidant and antiproliferative activities, there were still significant differences among the evaluated proso varieties. The non-waxy varieties were significantly higher than waxy varieties in the content of amylase and resistant starch (P < .01). All of the proso millets showed high content of protein and suitable amino acid constitution (EAAI = 79.27%). Oleic and linolenic acids were the dominant fatty acids. In general, non-waxy proso millet, especially J3 and J7, possessed a higher content of phytochemical compounds, antioxidant, and antiproliferative activity than waxy proso millet. Besides, significant negative correlations (P < .05) of antiproliferative activity with ABTS and FRAP were observed. The nutritional and biological activities data suggest that the proso millets, particularly non-waxy proso millets, hold promise as healthy food ingredients. These results are anticipated to provide useful information on the selection of daily diets or the development of proso millet-based functional food.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Annor, G., Marcone, M., Corredig, M., Bertoft, E., & Seetharaman, K. (2015). Effects of the amount and type of fatty acids present in millets on their in vitro starch digestibility and expected glycemic index (eGI). Journal of Cereal Science, 64(11), 76–81. doi: https://doi.org/10.1016/j.jcs.2015.05.004
- AOAC. (2005). Official methods of analysis (18th ed.). Arlington, VA: Association of OfficialAnalytical Chemists.
- Chandrasekara, A., & Shahidi, F. (2011a). Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. Journal of Functional Foods, 3(3), 144–158. doi: https://doi.org/10.1016/j.jff.2011.03.007
- Chandrasekara, A., & Shahidi, F. (2011b). Inhibitory activities of soluble and bound millet seed phenolics on free radicals and reactive oxygen species. Journal of Agricultural & Food Chemistry, 59(1), 428–436. doi: https://doi.org/10.1021/jf103896z
- Chandrasekara, A., & Shahidi, F. (2012). Antioxidant phenolics of millet control lipid peroxidation in human LDL cholesterol and food systems. Journal of the American Oil Chemists Society, 89(2), 275–285. doi: https://doi.org/10.1007/s11746-011-1918-5
- Chao, G., Gao, J., Liu, R., Wang, L., Li, C., Wang, Y., … Feng, B. (2014). Starch physicochemical properties of waxy proso millet (Panicum Miliaceum L.). Starch – Stärke, 66(11–12), 1005–1012. doi: https://doi.org/10.1002/star.201400018
- Cheung, L., Cheung, P., & Ooi, V. (2003). Antioxidant activity and total phenolics of edible mushroom extracts. Food Chemistry, 81(2), 249–255. doi: https://doi.org/10.1016/S0308-8146(02)00419-3
- Choi, Y., Jeong, H., & Lee, J. (2007). Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chemistry, 103(1), 130–138. doi: https://doi.org/10.1016/j.foodchem.2006.08.004
- Devisetti, R., Yadahally, S., & Bhattacharya, S. (2014). Nutrients and antinutrients in foxtail and proso millet milled fractions: Evaluation of their flour functionality. LWT – Food Science and Technology, 59(2), 889–895. doi: https://doi.org/10.1016/j.lwt.2014.07.003
- Dewanto, V., Wu, X., Adom, K., & Liu, R. (2002). Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Food Science & Technology, 50(10), 3010–3014.
- Felice, D., Sun, J., & Liu, R. (2009). A modified methylene blue assay for accurate cell counting. Journal of Functional Foods, 1(1), 109–118. doi: https://doi.org/10.1016/j.jff.2008.09.014
- Guo, H., Yang, X., Zhou, H., Luo, X., Qin, P., Li, J., & Ren, G. (2017). Comparison of nutritional composition, aroma compounds, and biological activities of two kinds of Tartary Buckwheat Tea. Journal of Food Science, 82(7), 1735. doi: https://doi.org/10.1111/1750-3841.13772
- Kalinova, J., & Moudry, J. (2006). Content and quality of protein in proso millet (Panicum miliaceum L.) varieties. Plant Foods for Human Nutrition, 61(1), 45–49. doi: https://doi.org/10.1007/s11130-006-0013-9
- Kim, J., Hyun, T., & Kim, M. (2010). Anti-oxidative activities of sorghum, foxtail millet and proso millet extracts. African Journal of Biotechnology, 9(9), 2683–2690.
- Kim, D., Jeong, S., & Lee, C. (2003). Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry, 81(3), 321–326. doi: https://doi.org/10.1016/S0308-8146(02)00423-5
- Kim, K. B., Nam, Y. A., Kim, H. S., Hayes, A. W., & Lee, B. M. (2014). α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food and Chemical Toxicology, 70, 163–178. doi: https://doi.org/10.1016/j.fct.2014.05.009
- Lu, H., Zhang, J., Liu, K., Wu, N., Li, Y., Zhou, K., … Li, Q. (2009). Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proceedings of the National Academy of Sciences of the United States of America, 106(18), 7367–7372.
- Malcicka, M., Visser, B., & Ellers, J. (2017). An evolutionary perspective on linoleic acid synthesis in animals. Evolutionary Biology, 1–12.
- Matsumoto, K., Yasuyoshi, E., Nishi, K., Honda, Y., Nakaya, M., & Kitamura, S. (2016). Resistant starch-rich wx/ae, brown rice prevents insulin resistance and hypertriglyceridaemia in type 2 diabetic NSY mice. Journal of Functional Foods, 23, 556–564. doi: https://doi.org/10.1016/j.jff.2016.01.046
- McSweeney, M. B., Seetharaman, K., Ramdath, D. D., & Duizer, L. M. (2017). Chemical and physical characteristics of proso millet (Panicum miliaceum)-based products. Cereal Chemistry Journal, 94(2), 357–362. doi: https://doi.org/10.1094/CCHEM-07-16-0185-R
- Miao, X., Zhu, M., Xu, W., Shen, H., Du, S., Pei, Y., … Hu, G. (2010). Rapid determination on fatty acids content by gas chromatography in soybean. Soybean Science, 23(11), 1037–1044.
- Nielsen, T., Theil, P., Purup, S., Nørskov, N., & Knudsen, K. (2015). Effects of resistant starch and arabinoxylan on parameters related to large intestinal and metabolic health in pigs fed fat-rich diets. Journal of Agricultural & Food Chemistry, 63(48), 10418–10430. doi: https://doi.org/10.1021/acs.jafc.5b03372
- Nishizawa, N., Sato, D., Ito, Y., Nagasawa, T., Hatakeyama, Y., Choi, M., … Wei, Y. (2002). Effects of dietary protein of proso millet on liver injury induced by D-galactosamine in rats. Bioscience Biotechnology & Biochemistry, 66(1), 92–96. doi: https://doi.org/10.1271/bbb.66.92
- Park, K., Ito, Y., Nagasawa, T., Choi, M., & Nishizawa, N. (2008). Effects of dietary Korean proso-millet protein on plasma adiponectin, HDL cholesterol, insulin levels, and gene expression in obese type 2 diabetic mice. Bioscience Biotechnology & Biochemistry, 72(11), 2918–2925. doi: https://doi.org/10.1271/bbb.80395
- Pathak, P., Srivastava, S., & Grover, S. (2000). Development of food products based on millets, legumes and fenugreek seeds and their suitability in the diabetic diet. International Journal of Food Sciences & Nutrition, 51(5), 409–414. doi: https://doi.org/10.1080/096374800427019
- Qin, P., Song, W., Yang, X., Sun, S., Zhou, X., Yang, R., … Ren, G. (2014). Regional distribution of protein and oil compositions of soybean cultivars in China. Crop Science, 54(3), 1139–1146. doi: https://doi.org/10.2135/cropsci2013.05.0314
- Ragaee, S., Abdel-Aal, E., & Noaman, M. (2006). Antioxidant activity and nutrient composition of selected cereals for food use. Food Chemistry, 98(1), 32–38. doi: https://doi.org/10.1016/j.foodchem.2005.04.039
- Shahidi, F., & Chandrasekara, A. (2013). Millet grain phenolics and their role in disease risk reduction and health promotion: A review. Journal of Functional Foods, 5(2), 570–581. doi: https://doi.org/10.1016/j.jff.2013.02.004
- Shi, Z., Yao, Y., Zhu, Y., & Ren, G. (2017). Nutritional composition and biological activities of 17 Chinese adzuki bean (Vigna angularis) varieties. Food and Agricultural Immunology, 28(1), 78–89. doi: https://doi.org/10.1080/09540105.2016.1208152
- Suma, P., & Urooj, A. (2012). Antioxidant activity of extracts from foxtail millet (Setaria italica). Journal of Food Science & Technology, 49(4), 500–504. doi: https://doi.org/10.1007/s13197-011-0300-9
- Wang, L., Xie, H., Wang, Y., Liu, R., & Ju, X. (2014). Anti-proliferative effects in human breast cancer MDA. MCF-7 Cells & Human Breast Epithelial MCF-10a Cells and Western Blot Analysis from Adlay (Coix Lacryma-Jobi L.) Varieties Phenolic Extracts. Journal of Food & Nutrition Research, 2(11), 792–799.
- Wu, J., Lin, L., & Chau, F. (2001). Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrasonics Sonochemistry, 8(4), 347–352. doi: https://doi.org/10.1016/S1350-4177(01)00066-9
- Yoon, H., & Liu, R. (2008). Effect of 2α-hydroxyursolic acid on NF-κB activation induced by TNF-α in human breast cancer MCF-7 cells. Journal of Agricultural and Food Chemistry, 56(18), 8412–8417. doi: https://doi.org/10.1021/jf8012844
- Zhang, L., Liu, R., & Wei, N. (2014). Phytochemical and antiproliferative activity of proso millet. Plos One, 9(8), e104058. doi: https://doi.org/10.1371/journal.pone.0104058