ABSTRACT
The deficient functional polarization of macrophages is implicated in the disease progression of autoimmune hepatitis (AIH). This study aims to evaluate the impact of Magnesium isoglycyrrhizinate (MgIG) on concanavalin A (Con A)-induced hepatitis in a mouse model, thereby clarifying the molecular mechanisms with which it is associated. MgIG was periodically administered to C57BL/6 mice before one intravenous injection of Con A (20 mg/kg). The MgIG treatment demonstrated a protective function in mice for Con A-induced AIH, the expression of proinflammatory cytokines, and the serum levels of alanine aminotransferase and aspartate aminotransferase. In addition, the MgIG pre-treatment had a significant effect on the number of F4/80+ cells entering the liver. MgIG efficiently facilitated macrophage polarization toward an M2 phenotype. The results indicate that a relationship may exist between the protective impacts of MgIG with respect to Con A-induced liver injury and the capability of the hepatoprotective agent to regulate macrophage polarization.
1. Introduction
As a progressive inflammatory liver disease involving the diminishment of self-tolerance, ultimately resulting in the emergence of autoantibodies paired with lymphoplasmacytic inflammatory infiltrates in the portal tracts, autoimmune hepatitis (AIH) is also characterized by the appearance of the fragmentary necrosis of periportal hepatocytes (McFarlane, Citation1999). The underlying causal mechanisms that give rise to AIH are not yet understood. Although it is now known that autoreactivity to certain hepatocyte components is crucial in the course of the disease's development, the molecular mechanisms that contribute to the impairment of immune tolerance in AIH are not understood (Heneghan, Yeoman, Verma, Smith, & Longhi, Citation2013). The disease is characterized by the difficulties healthcare practitioners have when attempting to control its progression. This is especially the case among patients who are refractory to (or intolerant of) conventional therapy, which involves prednisone and azathioprine (Rubin & Te, Citation2016). Hence, the exploration of novel therapeutic agents and treatment modalities is a priority in the literature.
Concanavalin A (Con A)-induced liver injury refers to a frequently used mouse model that can identify immune cell-mediated acute hepatitis and is comparable to the pathology of human AIH in several ways (Wahl, Wegenka, Leithauser, Schirmbeck, & Reimann, Citation2009). One of the crucial contributors to the Con A-induced breakdown of liver immune homeostasis is the macrophage, which is implicated in the production of proinflammatory cytokines (Assis et al., Citation2014). Macrophages are defined as highly plastic cells that change in view of the surrounding tissue microenvironment (Peng & Tian, Citation2016; Tacke & Zimmermann, Citation2014; Wynn & Vannella, Citation2016). These cells engage in various functions in the body, such as immune responses, tissue repair, and homeostasis (Wynn, Chawla, & Pollard, Citation2013). Usually, resident macrophages in the liver, classified as Kupffer cells, were considered as being neither diverse nor dynamic. Lloyd et al. showed F4/80+, CD11b or CD68 could be used to identify Kupffer cells (Beyazit et al., Citation2015). Meanwhile, nitric oxide may be a potential mediator of hepatic inflammation and fibrogenesis in AIH. The serum nitric oxide metabolites like inducible nitric oxide synthase (iNOS) and nitric oxide activity can reflect oxidant stress in the liver and detect the inflammation and disease progression (Lloyd, Phillips, Cooper, & Dunbar, Citation2008; Yang, Zhang, Han, Jin, & Yang, Citation2017). In addition, various surface markers and cytokines are expressed and secreted, respectively, by the classical activation (M1) and the alternative activation phenotypes (M2), both of which have variable impacts in the context of immunoregulation (Ivashkiv, Citation2013; Ka, Daumas, Textoris, & Mege, Citation2014; Ying, Cheruku, Bazer, Safe, & Zhou, Citation2013). In recent years, the literature has made it clear that macrophage imbalances are a fundamental contributor to the pathogenesis of autoimmune diseases (Alleva, Pavlovich, Grant, Kaser, & Beller, Citation2000; Cosin-Roger et al., Citation2013; Espinoza-Jimenez, Peon, & Terrazas, Citation2012; Parsa et al., Citation2012; Zigmond et al., Citation2014).
Magnesium isoglycyrrhizinate (MgIG) is a magnesium salt of the 18-alpha-glycyrrhizic acid stereoisomer and is classified as a new molecular compound identified in the roots of the Glycyrrhiza glabra (licorice) tree (Wu et al., Citation2018). The compound is notable due to its anti-inflammatory and antioxidant effects and has been used to treat liver conditions (Ming & Yin, Citation2013; van Rossum, Vulto, de Man, Brouwer, & Schalm, Citation1998). Nevertheless, the impact of MgIG on Con A-induced AIH is not well understood. Therefore, this study investigates the preventive value of the compound with respect to liver injury using a mouse model of hepatitis (induced via intravenous Con A injection) and its underlying molecular mechanisms.
2. Materials and methods
2.1. Chemicals and reagents
The MgIG used in this study was procured from Jiangsu Chia-Tai Tianqing Pharmaceutical Co., Ltd. (Nanjing, Jiangsu, China). The Con A and lipopolysaccharide (LPS) used were procured from Sigma-Aldrich (St. Louis, MO, United States). Unless otherwise stated, the remaining chemicals used were also purchased from Sigma-Aldrich (St. Louis, MO, United States).
2.2. Animals and treatment
The Experimental Animal Centre at the Tianjin Medical University (TMU, Tianjin, China) was used to purchase male C57BL/6 mice aged 6–8 weeks. All of the mice were kept at 25 ± 2°C and 50 ± 10% relative humidity. This was paired with a 12-hour light/dark cycle. The animals were fed using a standard pellet diet with drinking water. The research proceedings were authorized by the ethical committee at the TMU and all of the procedures followed the Care and Use of Laboratory Animals regulations.
The mice were separated into the following categories: control mice (n = 7), mice that received Con A treatment (n = 7), mice that received both Con A treatment and 50 mg/kg MgIG (n = 7), and mice that received both Con A treatment and 100 mg/kg MgIG (n = 7). To prepare the Con A-induced liver injury mouse model, the mice received 20 mg/kg Con A via intravenous injection. The MgIG-treated mice were administered 50 or 100 mg/kg MGL (diluted using saline) 2 h before Con A challenging. Before sacrifice, the mice in the control group received an equal volume of saline. All of the mice were sacrificed 24 h after Con A injection for blood and liver tissue analysis.
2.3. Cell culture and treatment
Murine macrophage-like RAW264.7 cells were acquired from Sigma Chemical, Co., Ltd. (St. Louis, MO, United States) and were pre-cultured using the DMEM medium (Gibco BRI, Grand Island, NY, United States). The DMEM was combined with 10% fetal bovine serum. The cells were then randomly allocated into one of the following groups: a control group (comprised of cells that received phosphate-buffered saline [PBS] treatment), an LPS group (treated with 100 ng/mL LPS), an MgIG intervention + LPS group (treated with 50 μM MgIG + 100 ng/mL LPS), and an MgIG control group. For the MgIG intervention + LPS group, the cells were exposed to 100 ng/mL LPS and MgIG for 24 more hours. Following treatment, all of the cells underwent washing and harvesting (the latter by centrifugation), thereby facilitating RNA harvesting.
2.4. Evaluation of liver histopathology
Each liver sample was fixed in 4% paraformaldehyde, embedded in paraffin, and then cut into 5-μm sections. The sections were then stained using hematoxylin and eosin (H&E). Every sample underwent histological assessment by drawing on a pair of professional pathologists, which involved the use of the Knodell scoring system (KSS) to assess the extent of liver damage (Knodell et al., Citation1981). The KSS assesses liver damage based on the following criteria: (1) absence of inflammation or spotty necrosis, (2) fragmentary or confluent necrosis (less than 10%), (3) confluent necrosis (10–50%), and (4) significant confluent necrosis (more than 50%) accompanied by or not accompanied by bridging necrosis.
2.5. Biochemical analysis
After the mice from each group were sacrificed at the end of the experiment, blood was extracted from the vena cava. In turn, using the Automatic Chemical Analyzer 7600-100 (Hitachi, Ltd., Tokyo, Japan), the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serum levels were examined.
2.6. RNA preparation and analysis
Frozen liver tissues were cut to small sections and washed with PBS buffers. 5–10 mg of tissue was added to 700 μl lysis buffer and homogenised with a TissueLyser II (Qiagen). Then Trizol reagent (Invitrogen, Carlsbad, CA, United States) was used to extract total RNA from frozen liver tissue and cultured cells according to the manufacturer's guidelines. The RNA was reverse transcribed with random hexamers and avian myeloblastosis virus reverse transcriptase using a commercial kit (Perfect Real Time, SYBRR PrimeScriPTM TaKaRa, Japan). In addition, the gene expression analysis involved the use of qRT-PCR with SYBR Premix EX TaqTM II, facilitated by the ABI PRISM 7900 sequence detector (Applied Biosystems, Foster City, CA, United States). Sangon Biotech Co., Ltd. (Shanghai, China) was the source of the primer sequences for the target genes (). The standard curve, paired with 2-ΔΔCt methods, was used to identify quantification.
Table 1. Mouse primer sequences used for qRT-PCR.
2.7. Immunofluorescence analysis
Deparaffinization of the paraffin-embedded liver sections was conducted using xylene. Furthermore, the sections were rehydrated and incubated using a blocking solution (5% bovine serum albumin in PBS). In turn, fluorescein isothiocyanate conjugated F4/80 antibodies were incubated (BD Biosciences, San Diego, CA, United States) overnight at 4°C. After washing the PBS three times, coverslips were applied to the sections and observations were made using a confocal microscope (Olympus Inc., Center Valley, PA, United States).
2.8. Statistical analysis
The results are expressed as mean ± SD. The data were examined using Student's t-test and one-way analysis of variance. To facilitate the statistical analysis, GraphPad Prism 5.0 was used (GraphPad Software, San Diego, CA, United States). Statistical significance was determined at the 5% level.
3. Results
3.1. MgIG preconditioning positively affects con A-induced liver damage
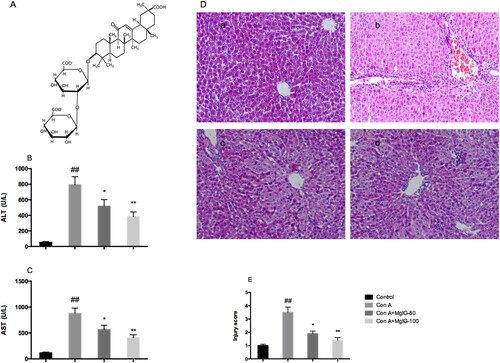
MgIG was administered intraperitoneally (depending on the mouse group, 50 or 100 mg/kg), as outlined in the previous section. This facilitated the study's attempt to identify the degree of MgIG's protective role in Con A-induced hepatitis ((A)). When comparatively examined against the saline control groups, the serum ALT and AST levels evidently increased as a consequence of the Con A treatment. Furthermore, in a dose-dependent way, MgIG pre-treatment reduced ALT and AST activity (P < 0.05), thereby indicating the protective role of MgIG in the context of Con A-induced hepatitis ((B and C)). As indicated in (D), morphological examination provided additional evidence suggesting the protective impact of MgIG. Administering Con A induced significant liver damage (in particular, hepatocyte necrosis accompanied by sinusoidal hyperemia associated with hemorrhage). However, after MgIG was administered, hepatocyte necrotic injury improved considerably and the number of infiltrating inflammatory cells in the necrotic area decreased. In addition, when comparatively examining the MgIG pre-treated mice to the Con A-treated mice ((E)), the liver injury score was favorable for the former.
Figure 1. Impacts of pre-treatment using MgIG (intraperitoneal administration of 50 or 100 mg/kg) for Con A-induced liver damage. (A) MgIG's chemical structure; (B) Serum ALT levels; (C) Serum AST levels; (D) H&E staining of liver tissue; (E) Liver injury score. The data are expressed as mean ± SD (n = 7, ##P < 0.01 versus control; *P < 0.05, **P < 0.01 versus Con A). a: Control; b: Con A; c: Con A + MgIG-50; d: Con A + MgIG-100.
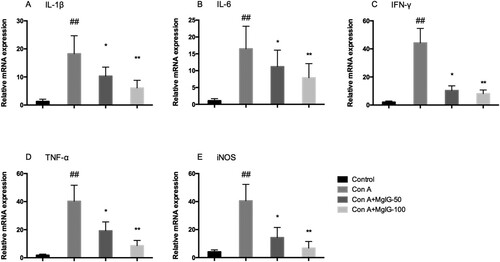
3.2. MgIG preconditioning inhibits the secretion of proinflammatory cytokines for con A-treated mice
Given that the secretion of large quantities of inflammatory cytokines performs an important function in the disease progression of Con A-induced AIH, one of the aims of this study is to determine the extent to which MgIG pre-treatment affects the expression of inflammatory cytokines in mice. As outlined in , the mRNA levels of pro-inflammatory cytokines (IL-1β, IL-6, IFN-γ, TNF-α, and inducible nitric oxide synthase [iNOS]) in the liver were considerably greater in response to Con A exposure than those of the control mice (P < 0.01). Nevertheless, MgIG administration rehabilitated this increasing trend among the Con A-induced mice in a dose-dependent manner (P < 0.05).
3.3. Preconditioning with MgIG-Impaired macrophage infiltration of the liver for con A-treated mice
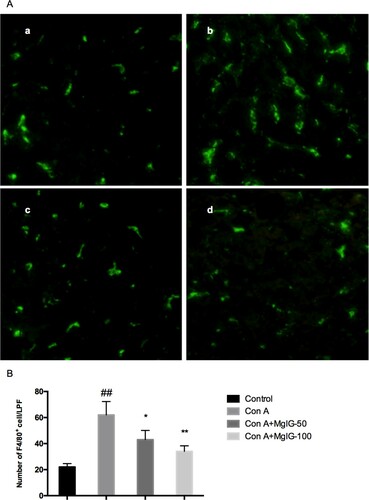
Immunofluorescence assays were used to illuminate the impact of MgIG on the infiltration of F4/80+ macrophages in the livers of the Con A-induced mice. As indicated in , the F4/80+ macrophage numbers increased considerably following Con A challenging (P < 0.01). However, it was possible to move them back to basal levels after MgIG treatment (P < 0.05 or P < 0.01).
Figure 3. Impact of MgIG on F4/80+ macrophage infiltration in the liver samples of Con A-induced mice. (A) Immunofluorescence assays were used to identify F4/80+ macrophage infiltration; (B) Quantification of F4/80+ macrophages in the mice livers took place for each group. The data are expressed as mean ± SD (n = 7, ##P < 0.01 versus control; *P < 0.05, **P < 0.01 versus Con A). a: Control; b: Con A; c: Con A + MgIG-50; d: Con A + MgIG-100.
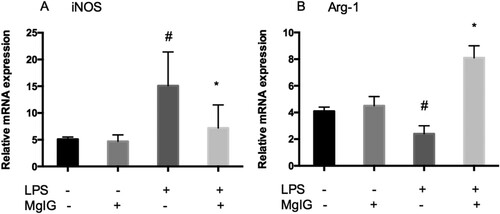
3.4. MgIG regulates M1 and M2 inflammatory macrophage polarization in LPS-Stimulated RAW264.7 cells
Finally, to examine the impact of MgIG on the polarization of macrophages within the RAW264.7 cells, the degree to which MgIG could convert macrophages into an anti-inflammatory M2 phenotype was assessed. iNOS, the M1 phenotype marker, increased considerably due to LPS stimulation in the RAW264.7 cells and LPS-induced iNOS generation was inhibited following MgIG treatment. In contrast, the M2 marker arginase-1 (Arg-1) expression in the LPS-induced RAW264.7 cells was markedly upregulated by MgIG (). Thus, MgIG facilitated M1 phenotype switching to the M2 macrophage.
4. Discussion
Licorice has a long history as a medicinal agent. Appearing in an early materia medica manuscript, “Shennong's Classic Materia Medica,” it has since been applied across China in many contexts as a result of its characteristics (Yang et al., Citation2016). MgIG, a magnesium salt of the 18-alpha-glycyrrhizic acid stereoisomer and classified as a new molecular compound identified in the roots of the Glycyrrhiza glabra (licorice) tree (Lu, Xu, et al., Citation2017), is associated with anti-inflammatory and antioxidant properties and hepatic protection and liver function enhancement (He et al., Citation2010; Sun et al., Citation2007). For the inhibition of ethanol-induced lipid peroxidation, MgIG has also been used as a pharmaceutical for liver protection (Xie et al., Citation2015). Other liver-protective properties stem from how the drug prevents ischemia/reperfusion-induced liver injury (Huang, Qin, & Lu, Citation2014) and free fatty acid-induced hepatic lipotoxicity (Cheng, Zhang, Shang, & Zhang, Citation2009). Nevertheless, with respect to AIH, the literature on MgIG's anti-inflammatory and immunoregulatory impacts is not extensive. Con A-induced liver damage is a common mouse model that has been used to identify immune cell-mediated acute hepatitis and is similar to the pathology of human AIH in various respects (Wahl et al., Citation2009). In this study, the mice suffering from Con A-induced hepatitis had elevated ALT and AST serum levels accompanied by hepatic damage. Nevertheless, following MgIG pre-treatment, the inflammatory pathway was inhibited, thus reducing the pathological damage of liver cells and enhancing liver cell function.
This study also seeks to identify the underlying molecular mechanism through which MgIG gains its protective properties for mice suffering from Con A-induced hepatitis. Evidently, the breakdown of immune tolerance is important in AIH pathogenesis and macrophages perform critical functions in innate immune defense and homeostasis (Lavin et al., Citation2014). The literature is replete with publications showing how macrophages are similarly implicated in the Con A-induced hepatitis model (Higashimoto et al., Citation2013; Orsolic, Jazvinscak Jembrek, & Terzic, Citation2017). Hence, the inhibition of macrophage infiltration constitutes the therapeutic target for Con A-induced hepatitis. In this study, the number of infiltrating F4/80+ cells in the livers of the Con A-induced hepatitis mice increased considerably. However, this could be inhibited by MgIG pre-treatment. Macrophages, which are key factors in promoting innate immunity, are not a cell population characterized by homogeneity and when exposed to certain signals, these cells can undergo classical M1 or alternative M2 activation (Lu, Chen, Ren, Yang, & Zhao, Citation2017; Udalova, Mantovani, & Feldmann, Citation2016). The cells are defined by how they express cell surface markers, how they secrete cytokines and chemokines, and their transcription and epigenetic pathways (Zhou et al., Citation2014). The findings cumulatively suggest that M1 macrophage activation plays an important role in liver inflammation and fibrosis. In particular, M1 macrophages are responsible for secreting a range of pro-inflammatory mediators, such as TGF-β1, TNF-α, IL-1β, and IL-6, which result in the inflammatory cascade (Beljaars et al., Citation2014; Sica, Invernizzi, & Mantovani, Citation2014). Contrastingly, several studies have demonstrated that M2 macrophages, typified by conventional M2 markers, such as Arg-1, YM1, FIZZ1, and MGL2 (Sica et al., Citation2008), can inhibit hepatic inflammation and are also important in solving fibrosis (Labonte, Sung, Jennelle, Dandekar, & Hahn, Citation2017; Tosello-Trampont et al., Citation2016). The phenotype alteration informs the part they play in liver immunoregulation, the implication of which is that M1/M2 macrophage polarization manipulation may yield effective therapeutic interventions (Tacke, Citation2017; Xu et al., Citation2015). This study indicates that MgIG treatment constitutes an alternative to M2 macrophage polarization, as it induces Arg-1 expression and lowers the production of M1 inflammatory markers. In view of this, this study proposes that the anti-inflammatory impact of MgIG stems from altering macrophage phenotype.
Taken together, this study's findings show that MgIG constitutes a protective agent against Con A-induced hepatitis. Furthermore, it is clear that its positive action in this respect can be accounted, as it facilitates the regulation of macrophage polarization. These findings may allow for the development of a new pharmacological treatment for AIH in future research.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81600509).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alleva, D. G., Pavlovich, R. P., Grant, C., Kaser, S. B., & Beller, D. I. (2000). Aberrant macrophage cytokine production is a conserved feature among autoimmune-prone mouse strains: Elevated interleukin (IL)-12 and an imbalance in tumor necrosis factor-alpha and IL-10 define a unique cytokine profile in macrophages from young nonobese diabetic mice. Diabetes, 49(7), 1106–1115. doi: https://doi.org/10.2337/diabetes.49.7.1106
- Assis, D. N., Leng, L., Du, X., Zhang, C. K., Grieb, G., Merk, M., … Bucala, R. (2014). The role of macrophage migration inhibitory factor in autoimmune liver disease. Hepatology, 59(2), 580–591. doi: https://doi.org/10.1002/hep.26664
- Beljaars, L., Schippers, M., Reker-Smit, C., Martinez, F. O., Helming, L., Poelstra, K., & Melgert, B. N. (2014). Hepatic localization of macrophage phenotypes during fibrogenesis and resolution of fibrosis in mice and humans. Frontiers in Immunology, 5, 430. doi: https://doi.org/10.3389/fimmu.2014.00430
- Beyazit, Y., Efe, C., Tanoglu, A., Purnak, T., Sayilir, A., Taskıran, I., … Wahlin, S. (2015). Nitric oxide is a potential mediator of hepatic inflammation and fibrogenesis in autoimmune hepatitis. Scandinavian Journal of Gastroenterology, 50(2), 204–210. doi: https://doi.org/10.3109/00365521.2014.974203
- Cheng, Y., Zhang, J., Shang, J., & Zhang, L. (2009). Prevention of free fatty acid-induced hepatic lipotoxicity in HepG2 cells by magnesium isoglycyrrhizinate in vitro. Pharmacology, 84(3), 183–190. doi: https://doi.org/10.1159/000235873
- Cosin-Roger, J., Ortiz-Masiá, D., Calatayud, S., Hernández, C., Álvarez, A., Hinojosa, J., … Mizoguchi, E. (2013). M2 macrophages activate WNT signaling pathway in epithelial cells: Relevance in ulcerative colitis. PLoS One, 8(10), e78128. doi: https://doi.org/10.1371/journal.pone.0078128
- Espinoza-Jimenez, A., Peon, A. N., & Terrazas, L. I. (2012). Alternatively activated macrophages in types 1 and 2 diabetes. Mediators of Inflammation, 2012, 1–10. doi: https://doi.org/10.1155/2012/815953
- He, Y., Zeng, F., Liu, Q., Ju, W., Fu, H., Hao, H., … Xie, Y. (2010). Protective effect of magnesium isoglycyrrhizinate on ethanol-induced testicular injuries in mice. Journal of Biomedical Research, 24(2), 153–160. doi: https://doi.org/10.1016/S1674-8301(10)60024-3
- Heneghan, M. A., Yeoman, A. D., Verma, S., Smith, A. D., & Longhi, M. S. (2013). Autoimmune hepatitis. Lancet, 382(9902), 1433–1444. doi: https://doi.org/10.1016/S0140-6736(12)62163-1
- Higashimoto, M., Sakai, Y., Takamura, M., Usui, S., Nasti, A., Yoshida, K., … Kaneko, S. (2013). Adipose tissue derived stromal stem cell therapy in murine ConA-derived hepatitis is dependent on myeloid-lineage and CD4+ T-cell suppression. European Journal of Immunology, 43(11), 2956–2968. doi: https://doi.org/10.1002/eji.201343531
- Huang, X., Qin, J., & Lu, S. (2014). Magnesium isoglycyrrhizinate protects hepatic L02 cells from ischemia/reperfusion induced injury. International Journal of Clinical and Experimental Pathology, 7(8), 4755–4764.
- Ivashkiv, L. B. (2013). Epigenetic regulation of macrophage polarization and function. Trends in Immunology, 34(5), 216–223. doi: https://doi.org/10.1016/j.it.2012.11.001
- Ka, M. B., Daumas, A., Textoris, J., & Mege, J. -L. (2014). Phenotypic diversity and emerging new tools to study macrophage activation in bacterial infectious diseases. Frontiers in Immunology, 5, 500. doi: https://doi.org/10.3389/fimmu.2014.00500
- Knodell, R. G., Ishak, K. G., Black, W. C., Chen, T. S., Craig, R., Kaplowitz, N., … Wollman, J. (1981). Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology, 1(5), 431–435. doi: https://doi.org/10.1002/hep.1840010511
- Labonte, A. C., Sung, S.- S. J., Jennelle, L. T., Dandekar, A. P., & Hahn, Y. S. (2017). Expression of scavenger receptor-AI promotes alternative activation of murine macrophages to limit hepatic inflammation and fibrosis. Hepatology, 65(1), 32–43. doi: https://doi.org/10.1002/hep.28873
- Lavin, Y., Blecher-Gonen, R., David E., Keren-Shaul H., Merad M., … Amit I. (2014). Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell, 159(6), 1312–1326. doi: https://doi.org/10.1016/j.cell.2014.11.018
- Lloyd, C. M., Phillips, A. R. J., Cooper, G. J.S., & Dunbar, P. R. (2008). Three-colour fluorescence immunohistochemistry reveals the diversity of cells staining for macrophage markers in murine spleen and liver. Journal of Immunological Methods, 334(1-2), 70–81. doi: https://doi.org/10.1016/j.jim.2008.02.005
- Lu, Y. L., Chen, J., Ren, D., Yang, X., & Zhao, Y. (2017). Hepatoprotective effects of phloretin against CCl4-induced liver injury in mice. Food and Agricultural Immunology, 28(2), 211–222. doi: https://doi.org/10.1080/09540105.2016.1258546
- Lu, C., Xu, W., Shao, J., Zhang, F., Chen, A., & Zheng, S. (2017). Blockade of hedgehog pathway is required for the protective effects of magnesium isoglycyrrhizinate against ethanol-induced hepatocyte steatosis and apoptosis. IUBMB Life, 69(7), 540–552. doi: https://doi.org/10.1002/iub.1639
- McFarlane, I. G. (1999). Pathogenesis of autoimmune hepatitis. Biomedicine & Pharmacotherapy, 53(5-6), 255–263. doi: https://doi.org/10.1016/S0753-3322(99)80096-1
- Ming, L. J., & Yin, A. C. (2013). Therapeutic effects of glycyrrhizic acid. Natural Product Communications, 8(3), 415–418.
- Orsolic, N., Jazvinscak Jembrek, M., & Terzic, S. (2017). Honey and quercetin reduce ochratoxin A-induced DNA damage in the liver and the kidney through the modulation of intestinal microflora. Food and Agricultural Immunology, 28(5), 812–833. doi: https://doi.org/10.1080/09540105.2017.1313819
- Parsa, R., Andresen, P., Gillett, A., Mia, S., Zhang, X.-M., Mayans, S., … Harris, R. A. (2012). Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes, 61(11), 2881–2892. doi: https://doi.org/10.2337/db11-1635
- Peng, H., & Tian, Z. G. (2016). Tissue-resident natural killer cells in the livers. Science China-Life Sciences, 59(12), 1218–1223. doi: https://doi.org/10.1007/s11427-016-0334-2
- Rubin, J. N., & Te, H. S. (2016). Refractory autoimmune hepatitis: Beyond standard therapy. Digestive Diseases and Sciences, 61(6), 1757–1762. doi: https://doi.org/10.1007/s10620-015-4022-0
- Sica, A., Invernizzi, P., & Mantovani, A. (2014). Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology, 59(5), 2034–2042. doi: https://doi.org/10.1002/hep.26754
- Sica, A., Larghi, P., Mancino, A., Rubino, L., Porta, C., Totaro, M. G., … Mantovani, A. (2008). Macrophage polarization in tumour progression. Seminars in Cancer Biology, 18(5), 349–355. doi: https://doi.org/10.1016/j.semcancer.2008.03.004
- Sun, L., Shen, M. J., Pang, M. X., Lu, L., Mao, Y., & Zeng, M. (2007). Phase I safety and pharmacokinetic study of magnesium isoglycyrrhizinate after single and multiple intravenous doses in Chinese healthy volunteers. The Journal of Clinical Pharmacology, 47(6), 767–773. doi: https://doi.org/10.1177/0091270007299757
- Tacke, F. (2017). Targeting hepatic macrophages to treat liver diseases. Journal of Hepatology, 66(6), 1300–1312. doi: https://doi.org/10.1016/j.jhep.2017.02.026
- Tacke, F., & Zimmermann, H. W. (2014). Macrophage heterogeneity in liver injury and fibrosis. Journal of Hepatology, 60(5), 1090–1096. doi: https://doi.org/10.1016/j.jhep.2013.12.025
- Tosello-Trampont, A. C., Krueger, P., Narayanan, S., Landes, S. G., Leitinger, N., & Hahn, Y. S. (2016). NKp46(+) natural killer cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation in mice. Hepatology, 63(3), 799–812. doi: https://doi.org/10.1002/hep.28389
- Udalova, I. A., Mantovani, A., & Feldmann, M. (2016). Macrophage heterogeneity in the context of rheumatoid arthritis. Nature Reviews Rheumatology, 12(8), 472–485. doi: https://doi.org/10.1038/nrrheum.2016.91
- van Rossum, T. G., Vulto, A. G., de Man, R. A., Brouwer, J. T., & Schalm, S. W. (1998). Review article: Glycyrrhizin as a potential treatment for chronic hepatitis C. Alimentary Pharmacology & Therapeutics, 12(3), 199–205. doi: https://doi.org/10.1046/j.1365-2036.1998.00309.x
- Wahl, C., Wegenka, U. M., Leithauser, F., Schirmbeck, R., & Reimann, J. (2009). IL-22-dependent attenuation of T cell-dependent (ConA) hepatitis in herpes virus entry mediator deficiency. The Journal of Immunology, 182(8), 4521–4528. doi: https://doi.org/10.4049/jimmunol.0802810
- Wu, Z., Zhang, Y., Song, T., Song, Q., Zhang, Y., Zhang, X., … Chu, L. (2018). Magnesium isoglycyrrhizinate ameliorates doxorubicin-induced acute cardiac and hepatic toxicity via anti-oxidant and anti-apoptotic mechanisms in mice. Experimental and Therapeutic Medicine, 15(1), 1005–1012.
- Wynn, T. A., Chawla, A., & Pollard, J. W. (2013). Macrophage biology in development, homeostasis and disease. Nature, 496(7446), 445–455. doi: https://doi.org/10.1038/nature12034
- Wynn, T. A., & Vannella, K. M. (2016). Macrophages in tissue repair, regeneration, and fibrosis. Immunity, 44(3), 450–462. doi: https://doi.org/10.1016/j.immuni.2016.02.015
- Xie, C., Li, X., Wu, J., Liang, Z., Deng, F., Xie, W., … Zhong, C. (2015). Anti-inflammatory activity of magnesium isoglycyrrhizinate through inhibition of phospholipase A2/arachidonic acid pathway. Inflammation, 38(4), 1639–1648. doi: https://doi.org/10.1007/s10753-015-0140-2
- Xu, J., Chi, F., Guo, T., Punj, V., Lee, W. N. P., French, S. W., & Tsukamoto, H. (2015). NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. Journal of Clinical Investigation, 125(4), 1579–1590. doi: https://doi.org/10.1172/JCI76468
- Yang, Q., Cao, H., Liu, N., Xu, K., Ding, M., & Mao, L. -J (2016).Amelioration of concanavalin A-induced autoimmune hepatitis by magnesium isoglycyrrhizinate through inhibition of CD4(+)CD25(-)CD69(+) subset proliferation. Drug Design, Development and Therapy, 10, 443–453. doi: https://doi.org/10.2147/DDDT.S115121
- Yang, D. W., Zhang, J., & Han, D., Jin, E., & Yang, Z. (2017). The role of apparent diffusion coefficient values in characterization of solid focal liver lesions: A prospective and comparative clinical study. Science China-Life Sciences, 60(1), 16–22. doi: https://doi.org/10.1007/s11427-016-0387-4
- Ying, W., Cheruku, P. S., Bazer, F. W., Safe, S. H., & Zhou, B. (2013). Investigation of macrophage polarization using bone marrow derived macrophages. Journal of Visualized Experiments, 76, e50323. doi:https://doi.org/10.3791/50323
- Zhou, D., Huang, C., Lin, Z., Zhan, S., Kong, L., Fang, C., & Li, J. (2014). Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cellular Signalling, 26(2), 192–197. doi: https://doi.org/10.1016/j.cellsig.2013.11.004
- Zigmond, E., Bernshtein, B., Friedlander, G., Walker, C. R., Yona, S., Kim, K. -W., … Jung, S. (2014). Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity, 40(5), 720–733. doi: https://doi.org/10.1016/j.immuni.2014.03.012