ABSTRACT
In this study, we developed an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) for determination of thiamethoxam residues. We prepared a specific highly sensitive monoclonal antibody (mAb) based on a thiamethoxam-derived hapten. After mouse immunisation and cell fusion, we obtained three monoclonal cell lines: 3B2, 3G6, and 4D2. The antibodies produced by cell line 4D2 had the highest affinity and sensitivity. The half-maximal inhibitory concentration was 0.87 ng/mL, and the cross-reactivity was <1%. We optimised the ic-ELISA method and the optimal conditions for ic-ELISA consisted of pH 7.4, 0.8% NaCl, and 0% methanol in PBS buffer. Using this mAb, an ic-ELISA and immunochromatographic strip (ICS) assay were used to detect thiamethoxam residues in cucumbers. The working range of ic-ELISA was 0.5–1.7 ng/mL, and the cut-off vaule of the ICS was 10 ng/mL. The recovery of thiamethoxam in cucumber samples ranged from 89% to 97%. Both methods can serve as rapid tools for the detection of thiamethoxam in vegetables.
Introduction
Thiamethoxam or 3-(2-chlorothiazol-5-ylmethyl)-5-methyl-4-nitroimino-1,3,5-oxadiazinane, a second-generation neonicotinoid, belongs to the thionicotinyl sub-class. This compound may contaminate water and soil via wind drift and leaching. Thiamethoxam is toxic to aquatic species, such as mysid shrimp and rainbow trout embryos, as well as to honey bees and parasitic wasps. The detrimental effects of 67 µg/L−1 thiamethoxam on honey bees have been documented (Henry et al., Citation2012), thus leading to restrictions on its agricultural use across the European Union. When mixed with fungicides such as mancozeb, metalaxyl, and folpet, thiamethoxam may lose activity or become phytotoxic in the formulation known as Actara25-WG.
Current methods used in the detection of thiamethoxam residues include high-performance liquid chromatography (HPLC) together with some extraction methods (Karmakar, Singh, & Kulshrestha, Citation2012), gas chromatography (Morais, Rodrigues, Queiroz, Neves, & Morais, Citation2014), and ultraperformance liquid chromatography tandem mass spectrometry (Liu et al., Citation2010). Both HPLC-MS and HPLC-MS/MS (Chen, Shangguan, Fu, & University, Citation2016) are more sensitive methods than conventional HPLC. However, these methods are costly and time-consuming, and they require complex sample preparation, thus limiting on-site applications. The enzyme-linked immunosorbent assay (ELISA), which allows for rapid detection, is inexpensive and simple to perform. Several ELISAs for thiamethoxam have been developed. When handling liquid samples, such as water, juice, or biological samples, no extraction procedure is required (Abad, Manclús, Moreno, & Montoya, Citation2001; Watanabe, Baba, Eun, & Miyake, Citation2007). Various sensitive ELISAs have been used to detect residual nicotinoids in agricultural samples (Castle, Byrne, Bi, & Toscano, Citation2005; Watanabe et al., Citation2004; Xu, Jacobsen, Cho, Hara, & Li, Citation2006). Kim et al. have generated a monoclonal antibody (mAb) specific to thiamethoxam and have measured the concentrations of thiamethoxam in stream and tap water, and in potato, cucumber, and apple samples with competitive ELISA (Kim, Shelver, Hwang, Xu, & Li, Citation2006). Subsequently, Huixin Ma et al. used the ELISA to measure thiamethoxam in honey samples (Ma et al., Citation2009). In this study, we designed a hapten containing a thiamethoxam fragment and produced a highly sensitive and specific mAb against thiamethoxam. The developed mAb was used in an immunochromatographic strip (ICS) assay and an indirect competitive ELISA (ic-ELISA) for the detection of thiamethoxam residues in cucumber samples.
Materials and methods
Chemicals and materials
Thiamethoxam, clothianidin, thiacloprid, imidacloprid, acetamiprid, and nitenpyram were purchased from J&K Scientific Ltd. (Beijing, China). Keyhole limpet hemocyanin (KLH), ovalbumin, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide, Freund’s complete adjuvant, Freund’s incomplete adjuvant, 3,3′,5,5′-tetramethylbenzidine, and gold chloride trihydrate were obtained from Sigma-Aldrich (St Louis, MO, USA). RPMI-1640 cell culture medium, hypoxanthine-aminopterin-thymidine supplement, hypoxanthine-thymidine supplement, polyethylene glycol 1500, and fetal calf serum were acquired from Gibco BRL (Paisley, UK). All reagents and solvents were of analytical grade or higher.
The hapten and antigen were characterised with a UV/vis scanner (Bokin instruments, Tsushima, Japan). All buffer solutions were prepared with ultrapure water generated by a Waters Maldi Synapt Q-Tof MS (Waters, Shanghai, China). The results obtained from ic-ELISA were measured with a Multiskan MKS microplate reader (Thermo Labsystems Company, Beijing, China). Other instruments included a membrane dispenser (Xinqidian Gene-Technology Co. Ltd, Beijing, China), vortex machine (Shanghai Huxi Analysis Instrument Factory Co., Ltd, Shanghai, China), and water bath (Shanghai Instrument Group Co., Ltd, Supply & Sales Co., Shanghai, China).
Hapten synthesis
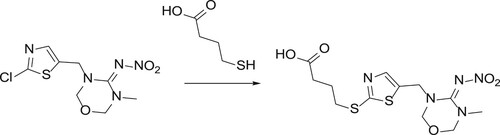
The structure of the hapten synthesised in this study is shown in . A solution containing thiamethoxam (2.4 g, 8.2 mmol), 4-mercaptobutanoic acid (1.0 g, 8.2 mmol), and potassium hydroxide (0.94 g, 16.4 mmol) in DMSO (20 mL) was stirred for 72 h at 75°C. Water and aqueous HCl were added. After extraction with n-butanol, the residue was purified by preparation. Finally, HPLC was performed, thus yielding compound 2 (150 mg) as a red oil (5% yield).
Antigen preparation
To synthesise the immunogen TMX-EDC-KLH, TMX must be covalently linked to KLH with EDC (Gu, Liu, Song, Kuang, & Xu, Citation2016). Briefly, TMX (6.5 mg), EDC (10 mg), and N-hydroxysuccinimide (6 mg) were dissolved in 300 μL anhydrous DMF (solution A) and stirred at room temperature for 5 h. KLH (3 mL, 5 mg/mL) was mixed with an equal volume of borate buffer solution, thus resulting in solution B. Finally, solutions A and B were mixed and allowed to react overnight at room temperature. The product of this reaction consisted of the immunogen TMX-EDC-KLH. The coating antigen TMX-EDC-OVA was prepared in a similar manner.
Production of mAb against TMX
Ten 6-week-old female BALB/c mice were subcutaneously injected (Dong et al., Citation2018; Hao et al., Citation2009; Xu, Xu, Ma, Kuang, & Xu, Citation2015) with the antigen TMX-EDC-KLH. The first immunisation consisted of 100 μg antigen as an emulsion of PBS and Freund’s complete adjuvant. Four sequential boosters were administered at three-week intervals with 50 μg of immunogen emulsified in Freund’s incomplete adjuvant. After each booster, the antibody specificity of the serum collected from the tail vein of each mouse was assessed with ic-ELISA. The mouse with the highest titre and the best specificity to TMX was selected for a subsequent intraperitoneal immunisation with 25 μg immunogen dissolved in 100 μL normal saline.
Hybridomas for secreting anti-TMX antibodies were generated as previously described (Chen et al., Citation2015; Kong et al., Citation2015). Briefly, splenocytes isolated from the target mice were fused with SP2/0 cells by using polyethylene glycol 1500, and the fused cells were distributed into 96-well culture plates. The supernatants from the plates were analysed with ic-ELISA after one week. Selected cells were subcloned through the limiting dilution method. Five 10-week-old BALB/c mice were prepared to produce ascites, and mAbs were purified with the caprylic acid-ammonium sulfate precipitation method (Liu, Hung, Lu, Chou, & Yu, Citation2014), dialysed for 3 d, and stored at −20°C.
Development of ic-ELISA for thiamethoxam detection
In this experiment, ic-ELISA was developed as previously reported (Guo, Song, et al., Citation2015). The most appropriate coating antigen and antibody in ic-ELISA was determined by using a checkerboard assay. TMX-EDC-OVA (coating antigen) was diluted with coating buffer to 0.01, 0.03, 0.1, and 0.3 μg/mL, and the mAb was diluted with antibody diluent to 0.03, 0.1, 0.3, and 1 μg/mL. To improve both the stability and sensitivity of the assay, we evaluated the effects of different variables on the experimental results, including the pH (5.0, 6.0, 7.4, 8.6, and 9.6), NaCl concentration (0, 0.4, 0.8, 1.6, and 3.2%), and methanol concentration (0, 5, 10, and 20%, v/v). A standard mAb curve was generated by plotting OD450 values on the y-axis against thiamethoxamine concentration (log10) on the x-axis. The antibody sensitivity was determined according to the half-maximal inhibitory concentration (IC50) (Chen et al., Citation2014).
Cross-reactivity
To assess cross-reactivity (CR), we analysed thiamethoxam, clothianidin, thiacloprid, imidacloprid, acetamiprid, and nitenpyram with ic-ELISA. CR was calculated according to the following equation: CR (%) = [IC50 (thiamethoxam)/IC50 (analogs)] × 100%.
Development of an ICS assay for thiamethoxam detection
Preparation of gold nanoparticles
Gold nanoparticles were prepared by reducing gold chloride with sodium citrate, as previously reported (Li et al., Citation2016; Wu et al., Citation2013; Xu et al., Citation2016; Yan et al., Citation2012). First, 100 mL of AuCl3·HCl·4H2O solution (0.01%, w/v) was boiled under constant stirring. Subsequently, 2 mL of freshly prepared trisodium citrate (1%, w/v) was added under constant stirring. After 1 min, the solution, which turned dark red, was boiled for 15 min, and its volume was adjusted to 100 mL with ultrapure water. Finally, the solution was cooled to room temperature and stored at 4°C. The gold nanoparticles were characterised with an UV-vis spectrophotometry and transmission electron microscopy. The wavelength at the UV maximum absorption peak was 520 nm, and the average diameter of the gold nanoparticles was 18 ± 2 nm.
Preparation of gold-labelled anti-TMX mAb
In this experiment, 1 mL of colloidal gold solution was added to an optimal amount of 0.1 M K2CO3. An adequate amount of anti-TMX mAb was added to the colloidal gold solution and mixed for 2 h at room temperature. BSA solution (10%, 100 mL) in ultrapure water was added to block unbound sites on the closed colloidal gold, to avoid non-specific adsorption of the gold nanoparticles and to ensure the stability of the colloidal gold. After 2 h, centrifugation was performed at 8000 rpm for 25 min, and the supernatant was discarded. The precipitate was dissolved in 1 mL of ultrapure water and centrifuged. Subsequently, gold-labelled anti-TMX mAb was resuspended in borate buffer (0.002 M BB, 1% sucrose, and 0.01% Tween-20, pH 7.2, w/v) and stored at 4°C (Guo, Liu, et al., Citation2015; Sun et al., Citation2012).
ICS preparation
The test strip consisted of four parts: a conjugate pad, an NC membrane, a sample pad, and an absorbent pad. Goat anti-mouse IgG antibody and the appropriate concentration of coating antigen were sprayed onto the NC membrane using a dispensing platform (BioDot Inc., Irvine, CA, USA), thus resulting in a test band (T band) and control band (C band). The sample pad immersed in the treatment liquid and the absorbent pad were attached to the dried NC membrane near the end of the T line and C line respectively and dried at 37°C for 4 h. The absorbent pad was attached to the dried NC membrane near the end of the C line. Finally, the pad was cut longitudinally into 3.8-mm strips (Chen, Liu, Kuang, Song, & Xu, Citation2013).
ICS assay
The sample and gold-labeled mAbs were mixed and allowed to react for 2–3 min, and the assembled test strip was inserted to the mixture. The liquid moved from the sample pad to the absorbent pad through capillary action. After approximately 2 min, if both the control and test lines appeared dark red, the sample was considered to be negative. If the control line was dark and the test line was light or devoid of colour, the sample was considered to be positive.
Sample pre-treatment and spiked sample analyses
Chopped cucumber samples (10 g) were placed in 50-mL centrifuge tubes, spiked with different amounts of thiamethoxam (0, 5, 10, 25, 50, and 100 ng), and mixed with 20 mL acetonitrile. The spiked samples were subjected to ultrasonic extraction for 15 min and centrifuged at 2400 × g for 10 min. An aliquot of the resulting supernatant (1 mL) was dried with nitrogen, adjusted to 5 mL with PBS, and analysed by ic-ELISA and ICS (Yao, Liu, Song, Kuang, & Xu, Citation2017).
Results and discussion
Synthesis of hapten and artificial antigen
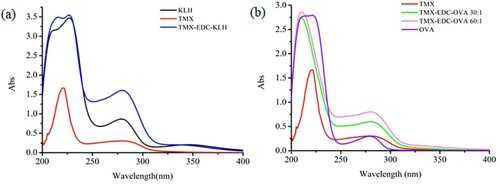
Thiamethoxam has no reactive groups such as carboxyl or amino groups that can be directly coupled to proteins; therefore, it is necessary to modify small molecules to introduce special active groups, i.e. to synthesise haptens. In this study, we used mercaptopropionic acid. After a series of reactions, we obtained the hapten TMX that contained a carboxyl group with three carbon chains. We used EDC to couple TMX with KLH and OVA to synthesise the immunogen and coating antigen, respectively. The immunogen TMX-EDC-KLH and the coating antigen CARB-EDC-OVA were verified by UV-vis spectroscopy (). TMX and KLH had absorption peaks at 285 nm and 280–350 nm, respectively; TMX-EDC-KLH had an absorption peak at 275–282 nm. The synthetic immunogen had an absorption peak at 350 nm. TMX-EDC-OVA also yielded the same results. Therefore, the immunogen and coating antigen were successfully synthesised.
Determination of antibody sensitivity in ic-ELISA
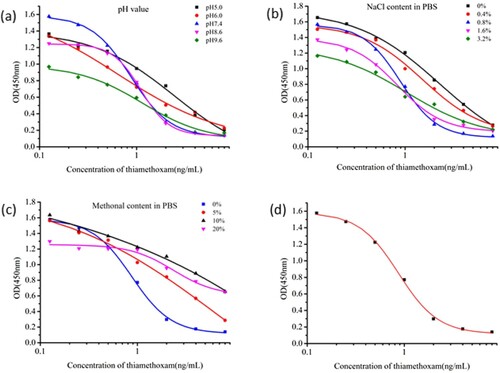
We obtained 3B2, 3G6, and 4D2 cell lines. Of these three cell lines, mAb 4D2 was selected for subsequent optimisation experiments, because 4D2 had the lowest IC50 value (0.87 ng/mL). The optimal concentrations of the coating and antibody were 0.03 and 0.3 μg/mL, respectively. The antibody had the lowest IC50 value and the highest absorbance value (Absmax) at pH 7.4 ((a)). NaCl (0–3.2%) had no significant effects on absorption, and the IC50 value was the lowest at 0.8% NaCl ((b)). With increasing methanol concentration, IC50 increased ((c)); the lowest IC50 value was obtained with no methanol. Therefore, the optimal conditions for ic-ELISA consisted of pH 7.4, 0.8% NaCl, and 0% methanol in PBS buffer. At these conditions, IC50 had a minimum value of 0.87 ng/mL ((d)).
Antibody specificity
Antibody specificity was determined by measurement of CR. The IC50 values and CR of the analogs used in this study are shown in . The IC50 values of analogs were >1000 ng/mL, whereas CR was <0.087%. Therefore, the antibody was specific to thiamethoxam.
Table 1. Cross-reactivity of mAb to thiamethoxam and analogs.
Thiamethoxam recovery tests
Owing to the high volatility of acetonitrile, we dried 1 mL of the extraction solution with nitrogen and adjusted its volume to 5 mL with PBS. In this way, we converted the acetonitrile extraction solution to a PBS system and diluted the extraction solution five-fold, thus resulting in different concentrations of thiamethoxam. In addition, dilution minimised matrix interference. The amount of thiamethoxam recovered from cucumber samples was calculated () and ranged from 89% to 97%, with a standard deviation of 0.056–0.407 and a coefficient of variation of 2.72–11.57%. Therefore, the sample pretreatment steps were suitable.
Table 2. Accuracy evaluation of immunoassay methods for the sample.
Thiamethoxam detection by ICS
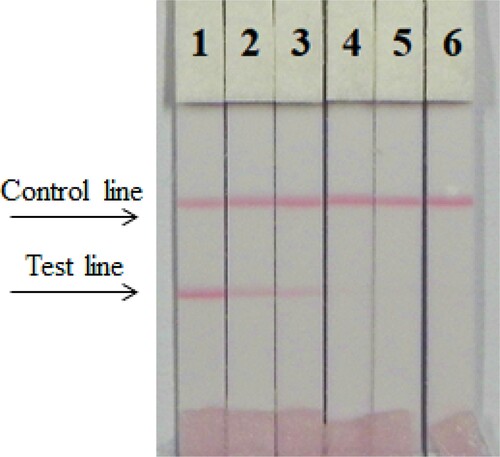
ICS assays were performed with a wet method. We optimised three different antibodies (4D2, 3B2, and 3G6) and coating antigens (TMX-EDC-OVA-30 and TMX-EDC-OVA-60); under optimal conditions, 1 mL of gold nanoparticles was used with 4 µL K2CO3 and 8 µg/mL antibody (). First, 7 µL of gold nanoparticle mix was diluted with 43 µL of resuspends #4, #9 pad and was mixed with 150 µL PBS (0 ppb). The concentration of the coating was 0.5 mg/mL. The thiamethoxam standard concentrations were 0 and 10 ng/mL. According to our findings, antibody 4D2 and coating antigen TMX-EDC-OVA-60 were the most suitable.
Figure 4. Optimisation of different antibodies and coating antigens. Antibody 4D2 and coating 2 were better. 1 = 0 ng/mL, 2 = 10 ng/mL.
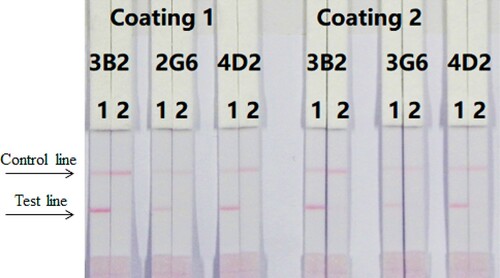
The concentration of the coating antigen was 0.5 mg/mL (TMX-EDC-OVA-60). Different concentrations of thiamethoxam (0–10 ng/mL) were dissolved in PBS. shows that the strip cut-off value was 10 ng/mL.
Figure 5. The sensitivity of the immunochromatographic assay in PBS for thiamethoxam. 1 = 0 ng/mL, 2 = 0.5 ng/mL, 3 = 1 ng/mL b, 4 = 2.5 ng/mL, 5 = 5 ng/mL, 6 = 10 ng/mL. Cut off at 10 ng/mL.

The extract solution of cucumber samples used for ICS was similar to that used for ic-ELISA. As shown in and , the test strip performed adequately. In agreement with the ic-ELISA results, the cutoff value for cucumber samples was 10 ng/mL.
Conclusion
We produced a highly sensitive and specific mAb against thiamethoxam with an IC50 value of 0.8 ng/mL. Additionally, we developed ic-ELISA and ICS for the rapid detection of thiamethoxam in cucumbers. The working range of ic-ELISA was 0.5–1.7 ng/mL. The cut-off value of thiamethoxam was 10 ng/mL in cucumbers. The two immunoassays generated similar results. The ICS assay, which is quick, convenient, simple, and sensitive, can thus be used to detect thiamethoxam residues in vegetables.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abad, A., Manclús, J. J., Moreno, M. J., & Montoya, A. (2001). Determination of thiabendazole in fruit juices by a new monoclonal enzyme immunoassay. Journal of Aoac International, 84(1), 156–161.
- Castle, S. J., Byrne, F. J., Bi, J. L., & Toscano, N. C. (2005). Spatial and temporal distribution of imidacloprid and thiamethoxam in citrus and impact on Homalodisca coagulata populations. Pest Management Science, 61(1), 75–84. doi: https://doi.org/10.1002/ps.949
- Chen, L., Shangguan, L., Fu, F., & University, F. (2016). Simultaneous determination of seven carbamates, neonicotinoids and diafenthiuron pesticide residues in tea by QuEChERS-HPLC-MS/MS. Scientia Sinica.
- Chen, X., Liu, L., Kuang, H., Song, S., & Xu, C. (2013). A strip-based immunoassay for rapid determination of fenpropathrin. Analytical Methods, 5(21), 6234–6239. doi: https://doi.org/10.1039/c3ay41030g
- Chen, X., Xu, L., Ma, W., Liu, L., Kuang, H., Wang, L., & Xu, C. (2014). General immunoassay for pyrethroids based on a monoclonal antibody. Food and Agricultural Immunology, 25(3), 341–349. doi: https://doi.org/10.1080/09540105.2013.794328
- Chen, Y., Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Analytical Methods, 9(4), 1–10.
- Dong, G., Pan, Y., Wang, Y., Ahmed, S., Liu, Z., Peng, D., & Yuan, Z. (2018). Preparation of a broad-spectrum anti-zearalenone and its primary analogues antibody and its application in an indirect competitive enzyme-linked immunosorbent assay. Food Chemistry, 247, 8–15. doi: https://doi.org/10.1016/j.foodchem.2017.12.016
- Gu, H., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of an immunochromatographic strip assay for ractopamine detection using an ultrasensitive monoclonal antibody. Food and Agricultural Immunology, 27(4), 471–483. doi: https://doi.org/10.1080/09540105.2015.1126808
- Guo, J., Liu, L., Xue, F., Xing, C., Song, S., Kuang, H., & Xu, C. (2015). Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food and Agricultural Immunology, 26(2), 282–292. doi: https://doi.org/10.1080/09540105.2014.907242
- Guo, L., Song, S., Liu, L., Peng, J., Kuang, H., & Xu, C. (2015). Comparsion of an immunochromatographic strip with ELISA for simultaneous detection of thiamphenicol, florfenicol and chloramphenicol in food samples. Biomedical Chromatography, 29(9), 1432–1439. doi: https://doi.org/10.1002/bmc.3442
- Hao, X. L., Kuang, H., Li, Y. L., Yuan, Y., Peng, C. F., Chen, W., … Xu, C. L. (2009). Development of an enzyme-linked immunosorbent assay for the α-cyano pyrethroids multiresidue in Tai lake water. Journal of Agricultural and Food Chemistry, 57(8), 3033–3039. doi: https://doi.org/10.1021/jf803807b
- Henry, M., Beguin, M., Requier, F., Rollin, O., Odoux, J.-F., Aupinel, P., … Decourtye, A. (2012). A common pesticide decreases foraging success and survival in honey bees. Science, 336(6079), 348–350. doi: https://doi.org/10.1126/science.1215039
- Karmakar, R., Singh, S. B., & Kulshrestha, G. (2012). Water based microwave assisted extraction of thiamethoxam residues from vegetables and soil for determination by HPLC. Bulletin of Environmental Contamination and Toxicology, 88(2), 119–123. doi: https://doi.org/10.1007/s00128-011-0444-3
- Kim, H. J., Shelver, W. L., Hwang, E. C., Xu, T., & Li, Q. X. (2006). Automated flow fluorescent immunoassay for part per trillion detection of the neonicotinoid insecticide thiamethoxam. Analytica Chimica Acta, 571(1), 66–73. doi: https://doi.org/10.1016/j.aca.2006.04.084
- Kong, N., Song, S., Peng, J., Liu, L., Kuang, H., & Xu, C. (2015). Sensitive, fast, and specific immunoassays for methyltestosterone detection. Sensors, 15(5), 10059–10073. doi: https://doi.org/10.3390/s150510059
- Li, S., Xu, L., Ma, W., Wu, X., Sun, M., Kuang, H., … Xu, C. (2016). Dual-mode ultrasensitive quantification of microRNA in living cells by chiroplasmonic nanopyramids self-assembled from gold and upconversion nanoparticles. Journal of the American Chemical Society, 138(1), 306–312. doi: https://doi.org/10.1021/jacs.5b10309
- Liu, B. H., Hung, C. T., Lu, C. C., Chou, H. N., & Yu, F. Y. (2014). Production of monoclonal antibody for okadaic acid and its utilization in an ultrasensitive enzyme-linked immunosorbent assay and one-step immunochromatographic strip. Journal of Agricultural and Food Chemistry, 62(6), 1254–1260. doi: https://doi.org/10.1021/jf404827s
- Liu, S., Zheng, Z., Wei, F., Ren, Y., Gui, W., Wu, H., & Zhu, G. (2010). Simultaneous determination of seven neonicotinoid pesticide residues in food by ultraperformance liquid chromatography tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 58(6), 3271–3278. doi: https://doi.org/10.1021/jf904045j
- Ma, H., Xu, Y., Li, Q. X., Xu, T., Wang, X., & Li, J. (2009). Application of enzyme-linked immunosorbent assay for quantification of the insecticides imidacloprid and thiamethoxam in honey samples. Food Additives & Contaminants, 26(5), 713–718. doi: https://doi.org/10.1080/02652030802672638
- Morais, E. H. D. C., Rodrigues, A. A. Z., Queiroz, M. E. L. R. D., Neves, A. A., & Morais, P. H. D. (2014). Determination of thiamethoxam, triadimenol and deltamethrin in pineapple using SLE-LTP extraction and gas chromatography. Food Control, 42(2), 9–17. doi: https://doi.org/10.1016/j.foodcont.2014.01.024
- Sun, F., Liu, L., Ma, W., Xu, C., Wang, L., & Kuang, H. (2012). Rapid on-site determination of melamine in raw milk by an immunochromatographic strip. International Journal of Food Science & Technology, 47(7), 1505–1510. doi: https://doi.org/10.1111/j.1365-2621.2012.02998.x
- Watanabe, E., Baba, K., Eun, H., & Miyake, S. (2007). Application of a commercial immunoassay to the direct determination of insecticide imidacloprid in fruit juices. Food Chemistry, 102(3), 745–750. doi: https://doi.org/10.1016/j.foodchem.2006.06.031
- Watanabe, E., Eun, H., Baba, K., Arao, T., Ishii, Y., Endo, S., & Ueji, M. (2004). Rapid and simple screening analysis for residual imidacloprid in agricultural products with commercially available ELISA. Analytica Chimica Acta, 521(1), 45–51. doi: https://doi.org/10.1016/j.aca.2004.05.056
- Wu, X., Xu, L., Liu, L., Ma, W., Yin, H., Kuang, H., … Kotov, N. A. (2013). Unexpected chirality of nanoparticle dimers and ultrasensitive chiroplasmonic bioanalysis. Journal of the American Chemical Society, 135(49), 18629–18636. doi: https://doi.org/10.1021/ja4095445
- Xu, L., Zhao, S., Ma, W., Wu, X., Li, S., Kuang, H., … Xu, C. (2016). Multigaps embedded nanoassemblies enhance in situ Raman spectroscopy for intracellular telomerase activity sensing. Advanced Functional Materials, 26(10), 1602–1608. doi: https://doi.org/10.1002/adfm.201504587
- Xu, N., Xu, L., Ma, W., Kuang, H., & Xu, C. (2015). Development and characterisation of an ultrasensitive monoclonal antibody for chloramphenicol. Food and Agricultural Immunology, 26(3), 440–450. doi: https://doi.org/10.1080/09540105.2014.950201
- Xu, T., Jacobsen, C. M., Cho, I. K., Hara, A. H., & Li, Q. X. (2006). Application of an enzyme-linked immunosorbent assay for the analysis of imidacloprid in wiliwili tree, erythrina sandwicensis O. Deg, for control of the wasp quadrastichus erythrinae. Journal of Agricultural and Food Chemistry, 54(22), 8444–8449. doi: https://doi.org/10.1021/jf062004e
- Yan, W., Xu, L., Xu, C., Ma, W., Kuang, H., Wang, L., & Kotov, N. A. (2012). Self-assembly of chiral nanoparticle pyramids with strong R/S optical activity. Journal of the American Chemical Society, 134(36), 15114–15121. doi: https://doi.org/10.1021/ja3066336
- Yao, L., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of indirect competitive enzyme-linked immunosorbent and immunochromatographic strip assays for carbofuran detection in fruits and vegetables. Food & Agricultural Immunology, 28(4), 639–651. doi: https://doi.org/10.1080/09540105.2017.1309359