ABSTRACT
Portulaca oleracea L. is a traditional oriental medicine known for its pharmacological effects. The present study aimed to investigate the immunostimulatory effects of PPCE on cyclophosphamide-stimulated rat model and in isolated spleen cells. The result showed that PPCE exerted an immunostimulatory effect in vivo and in vitro. Specifically, PPCE significantly enhanced splenocyte proliferation and upregulated inflammatory cytokines (IL-2, IL-12, TNF-α, and IFN-γ) and NK cell activity in vitro. The immunostimulatory effect was further investigated through various immunological assays. Oral administration of PPCE increased the swimming time and number of immune cells in rats. Histological analysis revealed that PPCE administration exerted splenic recovery. Thus, P. oleracea L. based complex extracts may be used as an immunostimulatory agent in medicine or functional foods.
Introduction
Portulaca oleracea L. has been used as a folk medicine in many countries (Mohanapriya, Senthilkumar, Sivakumar, Dineshkumar, & Subbhuraam, Citation2006; Rasheed, Afifi, Shaedah, & Taha, Citation2004), and it is a rich source of bioactive compounds such as flavonoids, monoterpene glycosides, coumarins, and alkaloids (Michalak & Mohammad, Citation2004). P. oleracea L. is known to exhibit pharmacological effects, including skeletal muscle relaxant (Ellard & Parry, Citation1993), analgesic (Chan et al., Citation2000), wound healing (Rashed, Afifi, & Disi, Citation2003), and antioxidant (Erkan, Citation2012) activities. However, no studies have investigated immune-enhancing effects of P. oleracea L.
The immune system comprises various organs such as lymph nodes, thymus, bone marrow, and spleen. The body is prone to infections caused by invading organisms when the immune system is compromised. Therefore, immune enhancement is important for protection against pathogenic infections. Recently, natural plants have been assessed for the development of potent immunomodulators that can be used as components of functional foods (Haddad, Azar, Groom, & Boivin, Citation2005)
Cancer is one of the leading causes of mortality among humans worldwide. Chemotherapy and cancer therapeutics are known to damage the immune system and cause other side effects. Furthermore, most anti-carcinogens are known to be immunosuppressive. Although various treatment strategies, including chemotherapy, are available for cancer treatment, high systemic toxicity to normal tissues and drug resistance of the cancer cells have reduced the success of treatment outcomes (Saltiel & McGuire, Citation1983). New therapeutic strategies for cancer treatment have been developed to improve immune function without damaging the host (Kim et al., Citation2010; Wasser, Citation2002). In particular, immune cell activation, including natural killer cells, lymphocytes, and macrophages, is important for host defense against tumour cell growth (Yamamura & Azuma, Citation1983).
Cyclophosphamide (Cy) is a commonly used alkylating agent in chemotherapy for various cancers and autoimmune disorders (Pass et al., Citation2005). However, Cy often results in cytotoxic effects and immunosuppression (Singh, Gupta, Shau, & Ray, Citation1993; Tripathi & Jena, Citation2009). Cy administration results in a sudden change in Th1 / Th2 balance, resulting in immunosuppression (Adams & Hamilton, Citation1984). Immunological effects of Cy reportedly include a reduction in the absolute number of T cells and circulating B cells (Yu et al., Citation2015), IgG synthesis (Yu et al., Citation2014), lymphocyte proliferation, cytokine production by Th1 cells (IL-2, IL-12, TNF-α, and IFN-γ) and Th2 cells (IL-4) (Zhou et al., Citation2018).
In recent years, numerous efforts have been directed to identify novel natural compounds with immune-enhancing effects. In addition, many carbohydrates isolated from various medicinal plants have been described to exhibit immunostimulatory effects by virtue of their immunomodulatory effects (Schepetkin & Quinn, Citation2006). The role of activated immune cells in defending against cancer cells has been extensively studied. Moreover, evidence that activated immune cells recognize and eliminate cancer cells, including drug-resistant cancer cells, has been reported (Hume, Citation2006; Klimp, de Vries, Scherphof, & Daemen, Citation2002). The present study aimed to investigate the immune-enhancing effects of P. oleracea L.-based complex extracts through in vitro and in vivo analyses.
Materials and methods
Preparation of P. oleracea L.-based complex extracts
P. oleracea L. and Perilla frutescens seed complex extracts (PPCE) was manufactured by MnFkorea, Ltd. (Sancheong-Gun, Gyeongnam, Korea). The dry aerial parts of P. oleracea L. and Perilla frutescens seed were harvested from Hansalim Contract Farming complex in Sancheong, Gyeongnam, Republic of Korea, dried, and powdered. A voucher specimen (IV-RC-17-1606-09) was deposited at the Jecheon Traditional Korean Medicine Farming Association, Korea. The powder of P. oleracea L.(150 kg) and Perilla frutescens seed (150 kg) were extracted with a supercritical CO2 extractor (Woo HC, Cho, Koo, Kim, & Han, Citation2011) and 75 L of extracted oil was obtained. The sample was stored at 50°C and 250 bars for 10 min. Extraction was then performed by passing CO2 (99.9%) through the column at a flow rate of 2.0 ml/min to obtain the oil. The extraction oil was supplied from MnF Korea, Ltd. (Gyeongnam, Korea).
Animals
Five-week-old male Sprague Dawley (SD) (n = 36) rats were purchased from Samtaco Inc. (Osan, Gyeonggi-do, Korea) and adapted to the following conditions for 7 d: 12 h light/12 h dark cycle; temperature, 23 ± 1°C; humidity, 50 ± 5%; and illumination, 150–300 lux. The animals had ad libitum access to food (Purina diet; Purina Korea, Seongnam, Gyeonggi-do, Korea) and water. Splenocytes were harvested from one rat for the study. The remaining 35 rats were randomly assigned to five groups (seven rats per group). The protocols used for these animal studies were approved by the Committee on Care and Use of Laboratory Animals of Wonkwang University (Iksan, Jeollabuk-do, Korea; approval no. WKU12-47).
Splenocyte viability assay
Cell viability assays were performed using a WST-1 assay kit (ITSBio, Seoul, Korea) in accordance with the manufacturer’s instructions. Briefly, splenocytes (2 × 105 cells/well) were seeded into 96-well plates and incubated for a minimum of 4 h at 37°C to allow for cell stabilization. Thereafter, the cells were treated with PPCE (0, 3, 3, 5, 10, 30, 50, 100, 300, 500, or 1000 μg/mL) or PPCE (0, 1, 3, 5, 10, 30, 50, 100, or 300 μg/mL) and cyclophosphamide (Cy) (1,600 μg/mL) for 24 h in a 5% CO2 incubator. Each experiment was performed in triplicates. Splenocyte proliferation rate was assessed using a WST-1 assay kit and the absorbance was measured with a 96-well microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Determination of cytokine levels in splenocytes
Splenocytes (2 × 105 cells/well) were seeded into 96-well plates with RPMI-1640 containing 10% FBS and 1% antibiotics (growth media), after which PPCE (0, 5, 10, 30, 50, 100, 300 μg/ml) and Cy (1,600 μg/mL) were added to the wells. The cells were then incubated for 24 h in a 5% CO2 incubator. The levels of interleukin (IL)-2, IL-12, tumour necrosis factor (TNF)-α, and interferon (IFN)-γ in the culture medium from each well were then measured using Cytokine Activation Analysis Kits (R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturer’s instructions. The results were measured using an ELISA reader. Each experiment was performed in triplicate.
NK cell activity assay
YAC-1 were used as target cells for NK cell activity assay, Splenocytes were co-cultured with YAC-1 cells in 96-well plates at a ratio of effector cells to target cells (20:1) and cultured in a 5% CO2 incubator at 37°C for 24 h. YAC-1 viability rate was measured by LDH Assay Kit. The NK cell activity was calculated as the survival rate of YAC-1 compared to that of the control group.
Modified porsolt forced swim test
The modified Porsolt forced swim test was performed with reference to our previous study (Kim et al., Citation2013). For the forced swim test, a Plexglass cylinder (150 cm [height] × 80 cm [diameter]) was constructed with nontransparent materials to prevent the animals from seeing each other. The test was performed after 1-h treatment with PPCE on day 28, the final treatment day. Animal experiments were performed using five groups as follows: the normal group, cyclophosphamide (1,600 μg/mL) group, and PPCE (30, 100, or 300 mg kg−1 day−1) groups. As the animals stopped moving, they were placed in the device and the time up to 10 s was checked and recorded.
CBC analysis
In this experiment, SD rats were orally administered saline or PPCE (0, 30, 100, or 300 mg/kg) for 28 d. Rats administered saline constituted the control group. After final administration of various drugs, rats were weighed and anesthetized via an intraperitoneal injection of 2,2,2-tribromoethanol (Sigma-Aldrich). Whole blood was collected from the abdominal vena cava into ethylenediaminetetraacetic acid (EDTA) microtubes. Thereafter, the rats were euthanized via a brief exposure to 100% CO2, followed by cervical dislocation. The numbers of WBCs, lymphocytes, and neutrophils in each whole blood sample were measured using Hemavet 950 system (Drew Scientific Group, Dallas, TX, USA).
Histological analysis
After euthanasia, the spleen was dissected out, weighed, and fixed in 10% neutral buffered formalin. Spleen was then processed for embedding in paraffin, after which they were sectioned into 4–7-μm-thick slices, using a microtome (Thermo Scientific, Waltham, MA, USA). The sectioned tissues were then stained with hematoxylin and eosin (HE). Tissue damage was assessed using an optical microscope (Olympus, Fukuoka, Japan).
Statistical analysis
All variables are presented as mean ± standard error of the mean (SEM) values. Results were analyzed using one-way analysis of variance, followed by Duncan’s multiple range tests using SAS software (version 9.3; SAS Institute Inc., Cary, NC, USA). A value of P < 0.05 was considered statistically significant.
Results and discussion
PPCE enhanced the viability of Cy-treated splenocytes
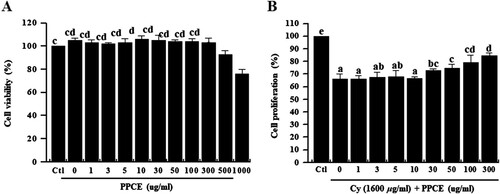
Splenocytes comprise various cell types, including macrophages, dendritic cells, and T and B lymphocytes, with different immune functions (Klimp et al., Citation2002). Lymphocyte and macrophage proliferation is an important event in the activation phase of cellular and humoral immune responses. An increase in splenocyte viability ultimately leads to an increase in the release of cytokines, a process that potentially explains the immune-enhancing and antitumor activity of medicinal plants (Conniot et al., Citation2014; Zeng, Ju, Shen, Zhou, & Huang, Citation2013). However, Cy exhibits a cytotoxic effect on immune cells (Dumontet et al., Citation2001). Therefore, recovery of splenocyte viability is an indication of immune activation. In this experiment, we assessed the cytotoxicity and PPCE and its effect on cell viability. First, to investigate the cytotoxicity of PPCE on splenocytes, the cells were incubated with PPCE (0, 1, 3, 5, 10, 30, 50, 100, 300, 500, or 1000 μg/mL) for 24 h. No significant change in cell viability was observed at concentrations bellow 30 μg/mL of PPCE ((A)). Therefore, subsequent studies were conducted at concentrations below 30, the optimal non-toxic level of PPCE. Thereafter, to assess splenocyte viability, splenocytes were incubated with cyclophosphamide (1600 μg/mL) and PPCE (0, 1, 3, 10, 30, 50, 100, or 300 mg/mL) for 24 h. Notably, PPCE treatment increased splenocyte viability in a dose-dependent manner ((B)). This result indicates that P. oleracea greatly restores proliferation reduced by cyclophosphamide-induced immunosuppression.
Figure 1. Effect of PPCE on toxicity and cyclophosphamide-stimulated splenocyte viability. Splenocytes were seeded into a 96-well plate with PPCE (0, 1, 3, 5, 10, 30, 50, 100, 300, 500, or 1000 μg/mL) or PPCE (0, 1, 3, 5, 10, 30, 50, 100, or 300 μg/ml) and cyclophosphamide (1,600 μg/ml) for 24 h in a 5% CO2 incubator and their viability rates were measured. Bars labeled with different superscripts indicate significant differences (P < 0.05 versus control). Data are presented as means ± standard errors (n = 3).
PPCE increased Cy-induced cytokines expression in splenocytes
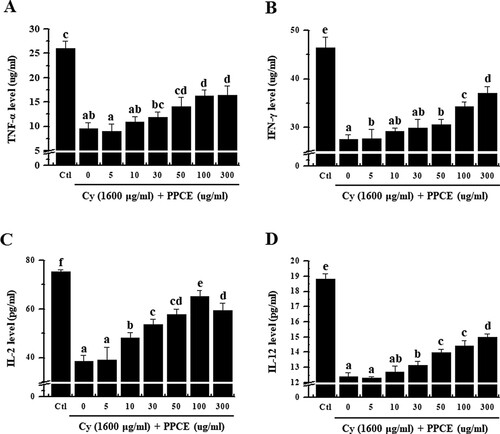
T cells play an important role and are classified as T helper (Th) cells, T cell cytotoxic cells, and T cell suppressor cells, each of which plays a different role. Currently, it is now well known that type 1 helper T cell (Th1) cells induces a cell-mediated immune response and type 2 helper T cells (Th2) cells promote humoral or allergic responses (Constant & Bottomly, Citation1997). Among these, helper T cells are important in increasing immunity. Furthermore, induction of the immune response of Th cells depends on cytokines such as IL-2, IL-12, TNF-α, IFN-γ, and GM-CSF produced by Th1 cells (Mosmann & Coffman, Citation1989). Among these, IL-2 plays a major role in T cell growth (Liao, Lin, & Leonard, Citation2011). IL-2 promotes T cell responses by regulating homeostasis and the differentiation of T cells into the memory T cells, effector T cells, and regulatory T cells (Zhang et al., Citation2013). Furthermore, IL-12 plays an essential role in the differentiation of naive T cells into Th1 cells and activation of natural killer cells (Hsieh et al., Citation1993). IL-12 is produced by various immune cells including macrophages, neutrophils, dendritic cells, and B-lymphoblastic cells (Dorman & Holland, Citation2000), and promotes the production of TNF-α and IFN-γ by stimulating T cells and natural killer cells (Watford, Moriguchi, Morinobu, & O’Shea, Citation2003). Concurrently, our results showed that PPCE recovered the levels of IL-2, IL-12, TNF-α, IFN-γ in the Cy-treated splenocytes (). Thus, this result support the hypothesis that administration of P. oleracea L.-based complex extracts can stimulate the original adaptive immunity by promoting the production of immune-related cytokines.
Figure 2. Effect of PPCE on cytokine levels in splenocytes. Cells were seeded into 96-well plates, followed by treatment with PPCE (0, 5, 10, 30, 50, 100, 300 μg/ml) and cyclophosphamide (Cy; 1,600 μg/ml) and incubated for 24 h in a 5% CO2 incubator. Levels of TNF-α, IFN-γ, IL-2, and IL-12 secretion into the culture medium were analyzed using ELISA kits. Bars labeled with different superscripts have significantly different values (P < 0.05 vs. control). Data are presented as means ± standard errors (n = 3).
Effect of PPCE on NK cell activity assay
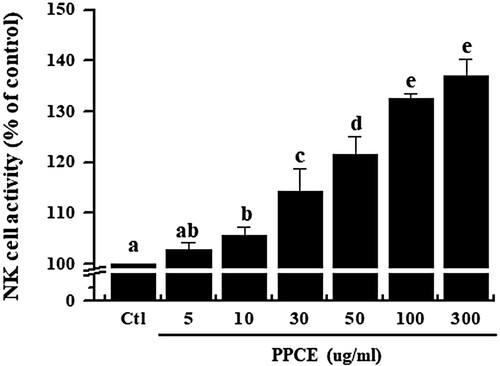
NK cells have been shown to be activated by various cytokines and play an important role in the regulation of tumour growth and metastasis (Krug et al., Citation2004). NK cells are a major population of cytotoxic T cell and are known to play an important role in defense against viruses and cancer (Medzhitov & Janeway, Citation1997; Moretta, Bottino, Cantoni, Mingari, & Moretta, Citation2001). NK cell activity assays have been used to assess the effect of non-specific cell-mediated immunity on functional foods. Therefore, we investigated the effects on NK cell activity by PPCE. In , we confirmed that PPCE was significantly increased the NK cell activity. The results suggested that the PPCE can regulate the innate immune response against cancer cell.
Figure 3. Effect of PPCE on NK cell activity. Splenocytes were co-cultured with target cells (YAC-1) in 96-well plates, followed by treatment with PPCE (0, 5, 10, 30, 50, 100, and 300 μg/ml) and incubated for 24 h in a 5% CO2 incubator with a ratio of effector to target cells of 20:1. The NK cell activity was calculated as the survival rate of YAC-1 compared to that of the control group. Bars labeled with different superscripts have significantly different values (P < 0.05 vs. control). Data are presented as means ± standard errors (n = 3).
PPCE increased the swimming time and number of immune cells in immunosuppressed rats
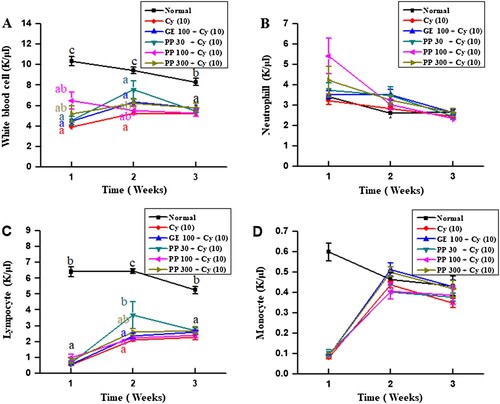
Immunosuppression is an indication of a weakened immune system. With aging, the immune system becomes weaker. For instance, immune tissues shrink and the number of WBCs decreases (Connor, Brewer, Kelly, & Harkin, Citation2005). To assess physiological changes following immune capacity modulation in rats, the modified Porsolt swim test was performed. Rats were administered PPCE without or with cyclophosphamide for four weeks and their swimming durations were measured. We confirmed that cyclophosphamide treatment decreased the swimming time. However, treatment with PPCE resulted in a significant increase in the swimming time (). As shown in , PPCE improved the number of immune cells (WBC, neutrophils, lymphocytes, monocytes, eosinophils, and basophils). The immune response triggered by various immune cells plays a critical role in immune modulation, including regulation of inflammation, lymphocyte differentiation, host defense against bacterial infection, cell survival, cell death, and immune responses (Aggarwal, Citation2003; Boyman & Sprent, Citation2012; Lin, Plessner, Voitenok, & Flynn, Citation2007). Therefore, the increase in the number of immune cells in rats indicates an immunostimulatory effect of PPCE.
Figure 4. The effect of PPCE on the swimming time. The test was carried out an hour after the treatment with PPCE on day 28, the final day of treatment. The animal experiment was performed in six groups [the normal group, Normal (saline) and Control (saline), GE (100 mg/kg, positive control), PPCE (30, 100, or 300 mg/kg) + Cy (10 mg/kg) ]. When the animal stopped moving, they were placed in the device and were assessed for up to 10 s. Bars labeled with different superscripts indicate significant differences values (P < 0.05 versus control). Data are presented as means ± standard errors (n = 7).
![Figure 4. The effect of PPCE on the swimming time. The test was carried out an hour after the treatment with PPCE on day 28, the final day of treatment. The animal experiment was performed in six groups [the normal group, Normal (saline) and Control (saline), GE (100 mg/kg, positive control), PPCE (30, 100, or 300 mg/kg) + Cy (10 mg/kg) ]. When the animal stopped moving, they were placed in the device and were assessed for up to 10 s. Bars labeled with different superscripts indicate significant differences values (P < 0.05 versus control). Data are presented as means ± standard errors (n = 7).](/cms/asset/1710c1f3-0660-4fa1-9c7e-d1e1e588754c/cfai_a_1540552_f0004_ob.jpg)
Figure 5. Effects of PPCE on the absolute number of leukocytes, neutrophils, lymphocytes, monocytes, eosinophils, and basophils in whole blood of rats. Seven male rats per group were orally administered saline, cyclophosphamide (Cy; 10 mg/kg), GE (100 mg/kg, positive control) + Cy (10 mg/kg), and PPCE (30, 100, or 300 mg/kg) + Cy (10 mg/kg) once daily for 28 days. Whole blood was collected from the abdominal vena cava in ethylenediaminetetraacetic acid (EDTA) microtubes and leukocytes, neutrophils, lymphocytes, monocytes, eosinophils, and basophils were enumerated with Hemavet950 (Drew Scientific Group, Dallas, TX, USA). Bars labeled with different superscripts indicate significant differences values (P < 0.05 versus control). Data are presented as means ± standard errors (n = 7).
PPCE protected against spleen damage in immunosuppressed rats
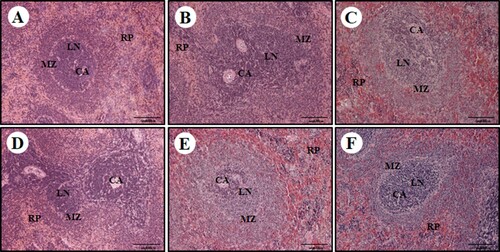
To investigate the effects of PPCE on organs of the immune system, morphological changes in the spleen were examined via HE staining (). Spleen tissue of the normal group was uniformly distributed in the white vein around the central vein (CA), and the lymph node (LN) was surrounded by the marginal zone (MZ); these were clearly distinguished from each other ((A)). However, in the control group treated with Cy, the boundary zone was not clear and prominent destruction of the white pulp area was confirmed. Condensed cells were observed in the red pulp area and the arrangement also appeared very irregular ((B)). However, in the group treated with PPCE at a high concentration, a clear boundary region between white pulp and red pulp was identified and condensed cells were not observed ((F)). In the positive control group (GE 100), the marginal zone was relatively clear, and decay of the white pulp area was somewhat favourable compared to the control group; however, cell condensation in the red pulp area was not alleviated ((C)). These results suggest that P. oleracea L.-based complex extracts increases immunity by improving the histopathological characteristics of CY-induced spleen damage.
Figure 6. Effect of PPCE on cyclophosphamide (Cy)-associated spleen damage in Sprague Dawley (SD) rats. SD rats were oral administrated with the normal (saline), Cy (10 mg/kg), GE (100 mg/kg, positive control) + Cy (10 mg/kg), and PPCE (30, 100, or 300 mg/kg) + Cy (10 mg/kg) after which spleen damage was analyzed histologically. Representative images of the sectioned spleens of (a) normal rats (saline treatment), (b) control rats (treated with only Cy), (c) PPCE 30 mg/kg + Cy, (d) PPCE 100 mg/kg + Cy), (e) PPCE 300 mg/kg + cy), and (F) positive control (GE 100 mg/kg and Cy). Scale bar = 200 μm. CV, central vein; LN, lymph nodule; MZ, marginal zone; RP, red pulp.
Conclusion
Many studies are being actively conducted to identify immunomodulators in natural products (Fang et al., Citation2015; Lee, Choi, Sohng, Pandey, & Park, Citation2016; Song et al., Citation2017; Wang et al., Citation2015). Herein, we report that PPCE enhanced the immunity in vitro and in vivo analyses. PPCE increased the production of IL-2 and IL-12 cytokine in Cy-treated splenocytes. Moreover, PPCE increased the viability of Cy-treated splenocytes and increased the swimming time, immune cell numbers, recovery effects of rats with the splenocyte damage due to CY-induced immunosuppression. The present results indicate that P. oleracea L.-based complex extracts has the potential to be used as a novel immunostimulatory agent in functional foods or medicine.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams, D. O., & Hamilton, T. A. (1984). The cell biology of macrophage activation. Annual Review of Immunology, 2, 283–318. doi: 10.1146/annurev.iy.02.040184.001435
- Aggarwal, B. B. (2003). Signalling pathways of the TNF superfamily: A double-edged sword. Nature Reviews Immunology, 3(9), 745–756. doi: 10.1038/nri1184
- Boyman, O., & Sprent, J. (2012). The role of interleukin-2 during homeostasis and activation of the immune system. Nature Reviews Immunology, 12(3), 180–190. doi: 10.1038/nri3156
- Chan, K., Islam, M. W., Kamil, M., Radhakrishnan, R., Zakaria, M. N., Habibullah, M., & Attas, A. (2000). The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. Sativa (Haw.) Celak. Journal of Ethnopharmacology, 73(3), 445–451. doi: 10.1016/S0378-8741(00)00318-4
- Conniot, J., Silva, J. M., Fernandes, J. G., Silva, L. C., Gaspar, R., Brocchini, S., … Barata, T. S. (2014). Cancer immunotherapy: Nanodelivery approaches for immune cell targeting and tracking. Frontiers in Chemistry, 2, 105. doi: 10.3389/fchem.2014.00105
- Connor, T. J., Brewer, C., Kelly, J. P., & Harkin, A. (2005). Acute stress suppresses pro-inflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. Journal of Neuroimmunology, 159(1–2), 119–128. doi: 10.1016/j.jneuroim.2004.10.016
- Constant, S. L., & Bottomly, K. (1997). Induction of Th1 and Th2 CD4+ T cell responses: The alternative approaches. Annual Review of Immunology, 15, 297–322. doi: 10.1146/annurev.immunol.15.1.297
- Dorman, S. E., & Holland, S. M. (2000). Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine & Growth Factor Reviews, 11(4), 321–333. doi: 10.1016/S1359-6101(00)00010-1
- Dumontet, C., Drai, J., Thieblemont, C., Hequet, O., Espinouse, D., Bouafia, F., … Coiffier, B. (2001). The superoxide dismutase content in erythrocytes predicts short-term toxicity of high-dose cyclophosphamide. British Journal of Haematology, 112(2), 405–409. doi: 10.1046/j.1365-2141.2001.02595.x
- Ellard, S., & Parry, J. M. (1993). A comparative study of the use of primary Chinese hamster liver cultures and genetically engineered immortal V79 Chinese hamster cell lines expressing rat liver CYP1A1, 1A2 and 2B1 cDNAs in micronucleus assays. Toxicology, 82(1–3), 131–149. doi: 10.1016/0300-483X(93)02608-J
- Erkan, N. (2012). Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chemistry, 133(3), 775–781. doi: 10.1016/j.foodchem.2012.01.091
- Fang, Q., Wang, J. F., Zha, X. Q., Cui, S. H., Cao, L., & Luo, J. P. (2015). Immunomodulatory activity on macrophage of a purified polysaccharide extracted from Laminaria japonica. Carbohydrate Polymers, 134, 66–73. doi: 10.1016/j.carbpol.2015.07.070
- Haddad, P. S., Azar, G. A., Groom, S., & Boivin, M. (2005). Natural health products, modulation of immune function and prevention of chronic diseases. Evidence-Based Complementary and Alternative Medicine, 2(4), 513–520. doi: 10.1093/ecam/neh125
- Hsieh, C. S., Macatonia, S. E., Tripp, C. S., Wolf, S. F., O’Garra, A., & Murphy, K. M. (1993). Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science, 260(5107), 547–549. doi: 10.1126/science.8097338
- Hume, D. A. (2006). The mononuclear phagocyte system. Current Opinion in Immunology, 18(1), 49–53. doi: 10.1016/j.coi.2005.11.008
- Kim, H. S., Kim, J. Y., Kang, J. S., Kim, H. M., Kim, Y. O., Hong, I. P., … Han, S. B. (2010). Cordlan polysaccharide isolated from mushroom Cordyceps militaris induces dendritic cell maturation through toll-like receptor 4 signalings. Food and Chemical Toxicology, 48(7), 1926–1933. doi: 10.1016/j.fct.2010.04.036
- Kim, J. H., Shin, E. H., Lee, H. Y., Lee, B. G., Park, S. H., Moon, D. I., … Oh, H. G. (2013). Immunostimulating effects of extract of Acanthopanax sessiliflorus. Experimental Animals, 62(3), 247–253. doi: 10.1538/expanim.62.247
- Klimp, A. H., de Vries, E. G., Scherphof, G. L., & Daemen, T. (2002). A potential role of macrophage activation in the treatment of cancer. Critical Reviews in Oncology/Hematology, 44(2), 143–161. doi: 10.1016/S1040-8428(01)00203-7
- Krug, A., French, A. R., Barchet, W., Fischer, J. A., Dzionek, A., Pingel, J. T., … Colonna, M. (2004). TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity, 21(1), 107–119. doi: 10.1016/j.immuni.2004.06.007
- Lee, J., Choi, J. W., Sohng, J. K., Pandey, R. P., & Park, Y. I. (2016). The immunostimulating activity of quercetin 3-O-xyloside in murine macrophages via activation of the ASK1/MAPK/NF-kappaB signaling pathway. International Immunopharmacology, 31, 88–97. doi: 10.1016/j.intimp.2015.12.008
- Liao, W., Lin, J. X., & Leonard, W. J. (2011). IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Current Opinion in Immunology, 23(5), 598–604. doi: 10.1016/j.coi.2011.08.003
- Lin, P. L., Plessner, H. L., Voitenok, N. N., & Flynn, J. L. (2007). Tumor necrosis factor and tuberculosis. Journal of Investigative Dermatology Symposium Proceedings, 12(1), 22–25. doi: 10.1038/sj.jidsymp.5650027
- Medzhitov, R., & Janeway, C. A., Jr. (1997). Innate immunity: Impact on the adaptive immune response. Current Opinion in Immunology, 9(1), 4–9. doi: 10.1016/S0952-7915(97)80152-5
- Michalak, R., & Mohammad, H. A. (2004). 51V-NMR and (51)T(1)(T) of charge ordering and spin gap in alpha'-NaV(2)O(5). Solid State Nuclear Magnetic Resonance, 26(3–4), 187–196. doi: 10.1016/j.ssnmr.2004.04.003
- Mohanapriya, S., Senthilkumar, P., Sivakumar, S., Dineshkumar, M., & Subbhuraam, C. V. (2006). Effects of copper sulfate and copper nitrate in aquatic medium on the restoration potential and accumulation of copper in stem cuttings of the terrestrial medicinal plant, Portulaca oleracea linn. Environmental Monitoring and Assessment, 121(1–3), 233–244. doi: 10.1007/s10661-005-9117-1
- Moretta, L., Bottino, C., Cantoni, C., Mingari, M. C., & Moretta, A. (2001). Human natural killer cell function and receptors. Current Opinion in Pharmacology, 1(4), 387–391. doi: 10.1016/S1471-4892(01)00067-4
- Mosmann, T. R., & Coffman, R. L. (1989). TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annual Review of Immunology, 7, 145–173. doi: 10.1146/annurev.iy.07.040189.001045
- Pass, G. J., Carrie, D., Boylan, M., Lorimore, S., Wright, E., Houston, B., … Wolf, C. R. (2005). Role of hepatic cytochrome p450s in the pharmacokinetics and toxicity of cyclophosphamide: Studies with the hepatic cytochrome p450 reductase null mouse. Cancer Research, 65(10), 4211–4217. doi: 10.1158/0008-5472.CAN-04-4103
- Rashed, A. N., Afifi, F. U., & Disi, A. M. (2003). Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. Journal of Ethnopharmacology, 88(2–3), 131–136. doi: 10.1016/S0378-8741(03)00194-6
- Rasheed, A. N., Afifi, F. U., Shaedah, M., & Taha, M. O. (2004). Investigation of the active constituents of Portulaca oleraceae L. (Portulacaceae) growing in Jordan. Pakistan Journal of Pharmaceutical Sciences, 17(1), 37–45.
- Saltiel, E., & McGuire, W. (1983). Doxorubicin (adriamycin) cardiomyopathy. Western Journal of Medicine, 139(3), 332–341.
- Schepetkin, I. A., & Quinn, M. T. (2006). Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. International Immunopharmacology, 6(3), 317–333. doi: 10.1016/j.intimp.2005.10.005
- Singh, K. P., Gupta, R. K., Shau, H., & Ray, P. K. (1993). Effect of ASTA-Z 7575 (INN Maphosphamide) on human lymphokine-activated killer cell induction. Immunopharmacology and Immunotoxicology, 15(5), 525–538. doi: 10.3109/08923979309019729
- Song, X., Ren, T., Zheng, Z., Lu, T., Wang, Z., Du, F., & Tong, H. (2017). Anti-tumor and immunomodulatory activities induced by an alkali-extracted polysaccharide BCAP-1 from Bupleurum chinense via NF-kappaB signaling pathway. International Journal of Biological Macromolecules, 95, 357–362. doi: 10.1016/j.ijbiomac.2016.10.112
- Tripathi, D. N., & Jena, G. B. (2009). Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: A study in mice. Chemico-Biological Interactions, 180(3), 398–406. doi: 10.1016/j.cbi.2009.03.017
- Wang, H., Gao, T., Du, Y., Yang, H., Wei, L., Bi, H., & Ni, W. (2015). Anticancer and immunostimulating activities of a novel homogalacturonan from Hippophae rhamnoides L. berry. Carbohydrate Polymers, 131, 288–296. doi: 10.1016/j.carbpol.2015.06.021
- Wasser, S. P. (2002). Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology, 60(3), 258–274. doi: 10.1007/s00253-002-1076-7
- Watford, W. T., Moriguchi, M., Morinobu, A., & O’Shea, J. J. (2003). The biology of IL-12: Coordinating innate and adaptive immune responses. Cytokine & Growth Factor Reviews, 14(5), 361–368. doi: 10.1016/S1359-6101(03)00043-1
- Woo HC, S. B., Cho, I., Koo, H., Kim, M., & Han, J. (2011). Bioactive materials : Anti-obesity effect of carbon dioxide supercritical fluid extracts of Panax ginseng C. A. Meyer. Journal of the Korean Society for Applied Biological Chemistry, 54, 538–543.
- Yamamura, Y., & Azuma, I. (1983). Immunostimulation in cancer patients. Advances in Experimental Medicine and Biology, 166, 1–13. doi: 10.1007/978-1-4757-1410-4_1
- Yu, Q., Nie, S. P., Wang, J. Q., Huang, D. F., Li, W. J., & Xie, M. Y. (2015). Signaling pathway involved in the immunomodulatory effect of Ganoderma atrum polysaccharide in spleen lymphocytes. Journal of Agricultural and Food Chemistry, 63(10), 2734–2740. doi: 10.1021/acs.jafc.5b00028
- Yu, Q., Nie, S. P., Wang, J. Q., Liu, X. Z., Yin, P. F., Huang, D. F., … Xie, M. Y. (2014). Chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. International Journal of Biological Macromolecules, 64, 395–401. doi: 10.1016/j.ijbiomac.2013.12.029
- Zeng, G., Ju, Y., Shen, H., Zhou, N., & Huang, L. (2013). Immunopontentiating activities of the purified polysaccharide from evening primrose in H22 tumor-bearing mice. International Journal of Biological Macromolecules, 52, 280–285. doi: 10.1016/j.ijbiomac.2012.10.005
- Zhang, P., Tey, S. K., Koyama, M., Kuns, R. D., Olver, S. D., Lineburg, K. E., … Hill, G. R. (2013). Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo. The Journal of Immunology, 191(10), 5291–5303. doi: 10.4049/jimmunol.1301181
- Zhou, Y., Chen, X., Yi, R., Li, G., Sun, P., Qian, Y., & Zhao, X. (2018). Immunomodulatory effect of Tremella Polysaccharides against Cyclophosphamide-induced immunosuppression in Mice. Molecules, 23(2), doi: 10.3390/molecules23020239
