ABSTRACT
The roles of differentiation and cytokine production of helper T and killer T cells by antigen stimulation were investigated to clarify the mechanisms of CHS exacerbation by naturally oxidized olive oil. Mice were orally administered naturally oxidized olive oil once every 2 days for 1 week after sensitization. IL-18 expression in the ear auricle and AP-1 and caspase-1 activity in the splenocytes were measured. The splenic T cell subpopulation and IL-4 and IFN-γ production in each subpopulation were also analysed. IL-18 expression and AP-1 and caspase-1 activity increased in the oxidized olive oil-administered group. CD3+CD4+ and CD3+CD8+ cells in splenocytes of OXA-sensitized mice increased from oxidized olive oil administration, and IFN-γ, a Th1 cytokine produced by antigen-stimulated CD3+CD4+ cells, was increased. These results suggest that excessive consumption of oxidized oils exacerbates the allergic reaction by promoting antigen-specific T cell differentiation and increasing production of inflammatory cytokines.
Introduction
Lifestyle choices and simplified dietary habits in industrialized countries have resulted in an increased intake of heated and processed dietary fats. Increasing intake of cooking oils oxidized by frying induces oxidative stress in individuals by the oxidation of unsaturated fatty acids and the generation of free radicals. Oxidative stress is a state of imbalance between oxidation and anti-oxidation and is caused by excess exposure to oxidants. Oxidative stress may be associated with various allergic diseases such as bronchitis, asthma, and dermatitis (Bao et al., Citation2017; Bowler & Crapo, Citation2002; Kang et al., Citation2016). Therefore, increased oxidative stress from the ingestion of oxidized cooking oils may increase allergic symptoms.
Skin is a common region in humans for allergic diseases, and an animal model with contact hypersensitivity (CHS) is a useful tool to investigate the inflammatory effects of different oxidants. CHS is a T cell-mediated contact dermatitis induced by exposure of the skin to low molecular weight chemicals called haptens including oxazolone (OXA) (Usatine & Riojas, Citation2010). Hapten-primed CD8+ cells and CD4+ cells, especially type-1 helper T (Th1) cells, are involved in the inflammatory response of CHS, and cytokines, such as interleukin (IL)-1β, tumour necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-17A, regulate recruitment and inflammation (Engeman, Gorbachev, Kish, & Fairchild, Citation2004; Kish, Li, & Fairchild, Citation2009; Martin et al., Citation2011). IL-18 is a member of the IL-1 cytokine superfamily and induces IFN-γ (Okamura et al., Citation1995). IL-18 is a potent activator of Th1 cell polarization for IFN-γ production and lymphocyte proliferation and is an important regulator of innate and acquired immunity (Dinarello, Novick, Kim, & Kaplanski, Citation2013; Lebel-Binay et al., Citation2000). Activated immune cells, T cells, B cells, natural killer cells, macrophages, dendritic cells, and neutrophils first produce IL-18 as an inactive precursor pro-IL-18, which is cleaved by caspase-1 into the active form inside the cell and then secreted extracellularly (Gu et al., Citation1997). IL-18 expression is regulated through the activation of nuclear factor-kappa B (NF-κB) and activator protein 1 (AP-1) (Venkatesan et al., Citation2010). IL-18 secretion may be regulated not only by post-transcriptional modification by caspase-1 but also by NF-κB or AP-1 transcriptional activation.
We have previously shown that administration of naturally oxidized olive oil increases the allergic reaction using a CHS mouse model (Ogino, Sakazaki, Okuno, Arakawa, & Ueno, Citation2015). We found that hydroperoxides, which are oxidants contained in oxidized olive oil, increase the allergic reaction in the skin. Furthermore, we discovered that naturally oxidized olive oil increased the expression levels of IL-18, but not IL-12, in skin tissue in the sensitizing phase of CHS, which was followed by an increase of IFN-γ expression after elicitation (Ogino, Koichi, Okuno, Arakawa, & Hitoshi, Citation2018). Because IL-18 promotes the differentiation of Th1 in cooperation with IL-12, Th1 cells may be highly abundant in oxidized olive oil-administered CHS mice. However, changes in T cell subpopulations and in the T cell subpopulation promoting IFN-γ production in oxidized olive oil-administered CHS mice are not clear. We investigated IL-18 production in lymph nodes, serum, and skin tissue to understand the mechanism of CHS exacerbation by oxidized olive oil. In addition, we examined whether the T cell distribution fluctuated from elevated IL-18 levels and measured Th1 and type-2 helper T (Th2) cytokine production in each subpopulation.
Methods
Preparation of oxidaized olive oil
Oxidized olive oil was autoxidized by keeping it at room temperature for 2–4 years, and it was adjusted to 50 mEq/kg (± 10% variation) as peroxide value (POV) by mixing it with fresh olive oil. The acid value (AV) and thiobarbituric acid reactive substance (TBARS) of oxidized olive oil were similar to those of fresh olive oil.
Chemical analyses
The POV was determined by the American Oil Chemists’ Society official method Ja 8-87(Official method and recommended practices Citation2010) using 0.01 mol/L sodium thiosulfate after dissolving oils in an acetic acid-chloroform (3:2) solution. The POV (mEq/kg) was calculated with the added amount of sodium thiosulfate.
Animals
Animal protocols met the Animal Experiment Guidelines of Setsunan University that were established by revising the guidelines of the Japanese Society for Pharmacology. This study was approved by the Committee for the Ethical Use of Experimental Animals at Setsunan University. All efforts were made to minimize animal suffering, reduce the number of animals used, and use alternatives to in vivo techniques. Female BALB/c mice (5–6 weeks old) were purchased from Japan SLC, Inc., Shizuoka, Japan and were acclimated in a specific pathogen-free room at 23 ± 1°C and 47–67% humidity under a 12 h light/dark cycle (lights on at 7:00 am) for at least one week before the start of experiments.
OXA sensitization
Seven-week-old mice were sensitized by the topical application of 50 μL 3% OXA (Sigma-Aldrich Inc., St. Louis, MO, USA) in a 3:1 (v/v) mixture of ethanol and acetone on the dorsal skin and were orally administered 100 μL of the test oil once every 2 days for 7 days.
IL-18 analysis
Ear auricle and axillary lymph nodes were collected in 0.1% Tween 20 and crushed using Micro Smash (TOMY SEIKO Co., Ltd., Tokyo, Japan). After centrifugation (12,000g for 20 min at 4°C), the protein content of the soluble fraction was determined by the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Collected whole blood was allowed to clot by leaving it undisturbed at room temperature for 30 min. The clot was removed by centrifugation at 12,000g for 10 min at 4°C, and the supernatant (serum) was collected. The amount of mature IL-18 in these samples was measured with Mouse IL-18 Platinum ELISA (Thermo Fisher Scientific, Inc.).
AP-1 and caspase-1 activity assay
Spleens were harvested under sterile conditions and single-cell suspensions were prepared by passage through a nylon mesh filter. After the destruction of red blood cells using an ammonium-chloride-potassium buffer, splenocytes were crushed and the soluble fraction was collected. AP-1 activity was measured using c-Jun (pS73) + Total c-Jun SimpleStep ELISA Kit (Abcam plc., Cambridge, UK). Caspase-1 activity was measured using the Caspase-Glo 1 Inflammasome Assay (Promega Corporation, Madison, WI, USA).
Flow cytometry and cell sorting
Splenocytes were Fc blocked with TruStain fcX (BioLegend, Inc., San Diego, CA, USA) and then stained using combinations of fluorescently labelled anti-mouse antibodies: APC-CD3, PE/Cy7-CD4, and FITC-CD8 (BioLegend, Inc.). Stained cells were analysed on BD FACSAria Fusion (BD Biosciences, San Diego, CA, USA), and CD3+CD4+ and CD3+CD8+ cells (each >98% purity) were isolated based on the analysis results.
APC preparation
Mouse peritoneal macrophages were prepared as described by Koike et al. and used as APC (Koike et al., Citation2016). In brief, cells harvested by peritoneal lavage were disrupted by osmotic shock using one-third-isotonic saline. Cells were seeded onto a cell culture dish in the culture medium and incubated at 37°C for 1 h. After removal of non-adherent cells, adherent cells were obtained as macrophages. More than 95% of the cells were macrophages as identified by their phagocytic activity toward zymosan.
T cell and APC co-culture
Sorted CD3+CD4+ cells were cultured in flat 96-well plates with peritoneal macrophages. Cells were co-cultured at a 5:1 T cell/APC ratio (500,000–100,000). Cells were stimulated by 20 ng/mL of 12-O-tetradecanoylphorbol 13-acetate (PMA) + 1 μg/mL of ionomycin (Io) or 10 μg/mL OXA. After culturing for 48 h, the culture supernatants were collected, and the amount of IL-4 and IFN-γ production was measured using Mouse Uncoated ELISA Kit (Thermo Fisher Scientific, Inc.).
Reverse transcription-polymerase chain reaction (RT–PCR)
Isolated CD3+CD4+ cells were immersed in Sepasol RNA I Super G (Nacalai Tesque, Inc., Kyoto, Japan). RNA extraction was performed according to the manufacturer’s protocol. An aliquot (2 μg) of total RNA sample was reverse transcribed using a Reverse Transcription Kit (Thermo Fisher Scientific, Inc.), and 1 μL of the cDNA solution was used for PCR using SYBR Green I Master (Roche Diagnostics, GmbH, Mannheim, Germany) and LightCycler 480 System II (Roche Diagnostics, GmbH). Primer sets for IFN-γ, IL-4, T-box expressed in T cells (T-bet), GATA-binding protein 3 (GATA3), and Ribosomal protein S18 (Rps18, an internal control) were obtained (TaKaRa Bio, Inc., Shiga, Japan). The mRNA levels were calculated as ratios relative to the corresponding Rps18 mRNA levels.
Statistical analysis
Results were statistically analysed with a Student’s t-test or one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests using LightStone Origin Pro (Tokyo, Japan). P-values (p < .05) were considered significant. The data represent the mean ± standard deviation (SD).
Results
Contribution of AP-1 and caspase-1 activity to IL-18 upregulation
To investigate the relationship between IL-18 expression in local skin and lymphoid organs at the sensitizing phase and exacerbation of CHS by oxidized olive oil administration, we measured IL-18 content in ear auricles, lymph nodes, and serum 1 week after OXA sensitization. Mice were sensitized by the application of 3% OXA to the dorsal skin and were administered oxidized olive oil on alternate days for one week. IL-18 content in the ear auricles, lymph nodes, and serum were increased approximately 2-fold in the oxidized olive oil-administered group compared with that of the fresh olive oil-administered group ().
Figure 1. IL-18 content 1 week after sensitization. Mice were orally administered fresh olive oil (POV = 0.92 ± 0.21 mEq/kg) and oxidized olive oil (POV = 50.5 ± 1.51 mEq/kg) 7 days from OXA-sensitization. Fresh olive oil (
 ). The values are mean ± SD (n = 5). **p < .01 vs. the fresh olive oil group.
). The values are mean ± SD (n = 5). **p < .01 vs. the fresh olive oil group.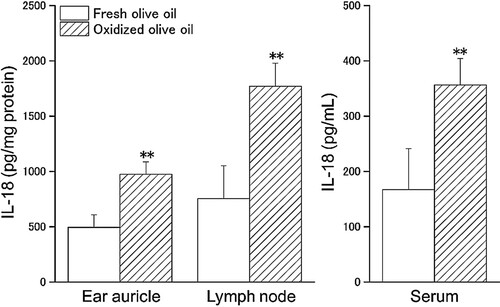
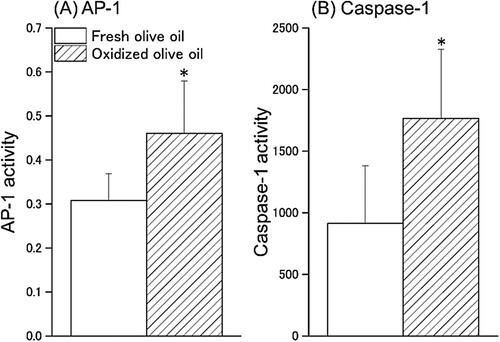
The activity of AP-1, a transcription factor of IL-18, and caspase-1, the activator of IL-18, in splenocytes were measured. AP-1 activity in the oxidized olive oil group was significantly increased compared with that of the fresh olive oil group ((A)). The activity of caspase-1 in the oxidized olive oil group was approximately 2-fold higher than that in the fresh olive oil group ((B)).
T cell subpopulation ratio and cytokine production under in vitro OXA-stimulation
To investigate whether the administration of oxidized olive oil affects T cell subpopulations in OXA-sensitized mice, we measured the ratio of CD3+CD4+ and CD3+CD8+ cells in splenocytes, a peripheral lymphoid tissue. The relative frequency of the T cell subsets in splenocytes isolated from OXA-sensitized mice was defined by CD3, CD4, and CD8 fluorescent antibody staining and analyzed by flow cytometry. Percentages of CD3+CD4+ double-positive and CD3+CD8+ double-positive cells were gated and quantified as shown in . Both the percentages of CD3+CD4+ and CD3+CD8+ cells in the oxidized olive oil group were significantly higher than those in the fresh olive oil group ().
Figure 3. CD3+CD4+ and CD3+CD8+ cell subpopulation. Mice were orally administered fresh olive oil (POV = 1.52 ± 1.31 mEq/kg) and oxidized olive oil (POV = 51.8 ± 0.09 mEq/kg) 7 days from OXA-sensitization. Seven days after sensitization, splenocytes were analyzed by flow cytometry. Shown are representative dot plots of CD3 vs. CD4 and CD8 staining.
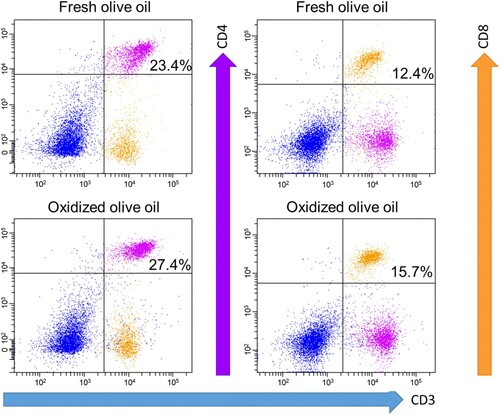
Table 1. T cell subpopulation rate.
We examined the mRNA expression of IFN-γ, IL-4, T-bet, and GATA3 in CD3+CD4+ cells sorted from splenocytes. Administration of oxidized olive oil significantly enhanced the expression of Th1 cytokine, IFN-γ, and the Th1 master regulator T-bet compared with the expression in mice administered fresh olive oil (). Although the expression of Th2 cytokine, IL-4, and the Th2 master regulator GATA3 were not significantly different between the fresh and oxidized olive oil group, expression tended to decrease in the oxidized olive oil group.
Figure 4. mRNA expression in CD3+CD4+ cells. CD3+CD4+ cells were sorted from splenocytes by FACS. POV of fresh and oxidized olive oil were 6.04 ± 1.01 and 48.8 ± 0.54 mEq/kg. The mRNA expression levels of target genes were normalized by Rps18. The relative expression levels of the fresh olive oil group were designated as 100%. Fresh olive oil (
 ). The values are mean ± SD (n = 4). *p < .05, **p < .01 vs. the fresh olive oil group.
). The values are mean ± SD (n = 4). *p < .05, **p < .01 vs. the fresh olive oil group.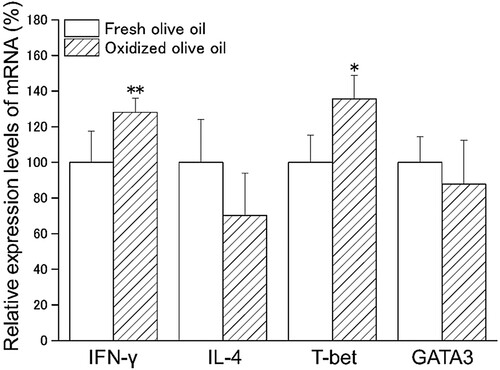
To investigate the antigen responsiveness of sensitized T cells, splenocytes or sorted T cells were stimulated with OXA in a co-culture with APC, and the amount of IL-4 and IFN-γ production in the culture supernatant was measured. IL-4 production was observed by OXA-stimulation and markedly increased by PMA/Io stimulation in a monoculture of splenocytes ((A)). In the co-culture of splenocytes with APC, although IL-4 production was increased by OXA-stimulation and was low in oxidized olive oil-administered mice, there was no significant difference between the amount of IL-4 in the fresh olive oil group and the oxidized olive oil group ((B)). The amount of IL-4 produced by CD3 + CD4+ cells from OXA-stimulation tended to decrease in the oxidized olive oil group ((C)). CD3 + CD8+ cells did not produce IL-4 regardless of the presence or absence of OXA-stimulation ((D)). Significant IFN-γ production was not observed in a monoculture of splenocytes with OXA-stimulation, but marked secretion was observed by PMA/Io stimulation ((A)). In a co-culture of splenocytes with APC, a significant increase in IFN-γ production by OXA-stimulation was observed in the oxidized olive oil-administered group compared with that in the fresh olive oil-administered group ((B)). IFN-γ production of CD3+CD4+ cells by OXA-stimulation was markedly increased by oxidized olive oil-administration ((C)). No difference was observed between the amount of IFN-γ produced by CD3+CD8+ cells in the fresh olive oil group and the oxidized olive oil group, but a large amount of IFN-γ was produced by OXA-stimulation ((D)).
Figure 5. IL-4 production by splenocytes and sorted T cells. IL-4 in culture supernatant 24 h after stimulation was measured by ELISA. (A) Monoculture of splenocytes. (B) Co-culture of splenocytes with APC. (C) Co-culture of CD3+CD4+ cells with APC. (D) Co-culture of CD3+CD8+ cells with APC. POV of fresh and oxidized olive oil were 7.20 ± 2.18 and 53.1 ± 1.07 mEq/kg. Fresh olive oil (
 ). The values are mean ± SD (n = 5).
). The values are mean ± SD (n = 5).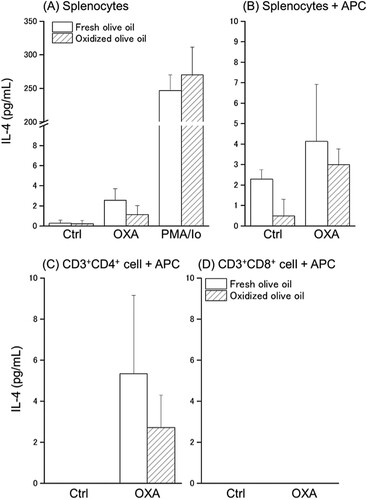
Figure 6. IFN-γ production by splenocytes and sorted T cells. IFN-γ in culture supernatant 24 h after stimulation was measured by ELISA. (A) Monoculture of splenocytes. (B) Co-culture of splenocytes with APC. (C) Co-culture of CD3+CD4+ cells with APC. (D) Co-culture of CD3+CD8+ cells with APC. POV of fresh and oxidized olive oil were 7.20 ± 2.18 and 53.1 ± 1.07 mEq/kg. Fresh olive oil (
 ). The values are mean ± SD (n = 5). *p < .05, **p < .01 vs. the fresh olive oil group.
). The values are mean ± SD (n = 5). *p < .05, **p < .01 vs. the fresh olive oil group.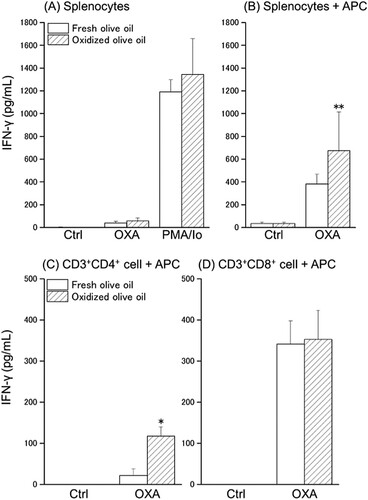
Discussion
There is concern that some allergic diseases may be exacerbated by an increase in oxidative stress from the ingestion of oxidized fats and oils. Consumption of oxidized vegetable oils increases the expression of oxidative stress regulation systems, such as glutathione peroxidase (GPx) and superoxide dismutase (SOD), via activation of nuclear factor erythroid 2-related factor 2 (Nrf2) and NF-κB in animal experiments (Liu et al., Citation2014; Varady, Eder, & Ringseis, Citation2010). Consumption of 4-hydroxy-2-hexenal (4-HHE), an oxidized polyunsaturated fatty acid end-product, causes accumulation of fatty acid peroxide in blood, which increases oxidative stress and inflammation (Awada et al., Citation2012). Sivaranjani, Rao, and Rajeev (Citation2013) showed that increased lipid peroxidation exacerbates atopic dermatitis. Our previous study suggested that hydroperoxides in oxidized olive oil exacerbate CHS (Ogino et al., Citation2015). These reports indicate that enhancement of oxidative stress by oxidized oil intake may potentiate allergic reactions. However, little is known about the relationship between oxidized oils and inflammatory cytokines such as IL-18 and IFN-γ.
We demonstrated that oxidized olive oil enhances IL-18 expression in OXA-sensitized mice. Although IL-18 strongly promotes IFN-γ production of Th1, the cytokine does not induce Th1 differentiation by itself (Dinarello et al., Citation2013; Lebel-Binay et al., Citation2000). IL-18 strongly promotes Th1 development by IL-12 as a costimulatory factor (Robinson et al., Citation1997). Our previous study showed that oxidized olive oil enhanced CHS by increased IL-18 but not IL-12 in the sensitization phase followed by increasing IFN-γ in the elicitation phase. IL-18 is produced as pro-IL-18 by a transcription factor, such as AP-1, and is secreted upon activation by caspase-1. Transcription factors, such as AP-1 and NF-κB, are activated by oxidative stress, which increases in the expression of inflammatory substances (Mazière, Conte, Degonville, Ali, & Mazière, Citation1999). Furthermore, oxidative stress promotes caspase-1 activity via Nrf2 (Garstkiewicz et al., Citation2017). In this study, we found that the administration of oxidized olive oil increased the amount of IL-18 and the activity of AP-1 and caspase-1. These results suggest that activation of AP-1 and caspase-1 from excess oxidative stress induced by oxidized olive oil increases IL-18 in the sensitizing phase and exacerbates CHS.
CHS is mediated by CD8+ T cells that expand in lymphoid organs and are recruited to the elicited skin, which has cytotoxic T lymphocyte (CTL) activity (Kolesaric, Stingl, & Elbe-Bürger, Citation1997; Saint-Mezard et al., Citation2003). CD4+ T cells are important for CHS development and are effector cells in CHS (Kondo et al., Citation1996; Wang et al., Citation2000). We demonstrated that the administration of oxidized olive oil increased CD3+CD4+ and CD3+CD8+ cells in splenocytes, which are peripheral lymphoid tissues in OXA-sensitized mice. In the oxidized olive oil-administered group, the mRNA expression levels of IFN-γ and T-bet, which are Th1-related factors, were increased, but the mRNA expression levels of IL-4 and GATA3, which are Th2-related factors, were decreased. IFN-γ determines the direction of Th1 differentiation, whereas IL-4 has an important role in Th2 differentiation. Both cytokines induce the expression of T-bet or GATA3, which are transcription factors that regulate the direction of Th1 or Th2 differentiation, respectively (Murphy et al., Citation2000; Murphy & Reiner, Citation2002). IFN-γ produced by Th1 cells suppresses the differentiation of Th2 cells, and IL-4 produced from Th2 cells acts on macrophages and T cells to suppress the differentiation of Th1 cells (Inoue & Kubo, Citation2004; Kubo, Hanada, & Yoshimura, Citation2003). Our results suggest that oxidized olive oil increased CD3+CD4+ and CD3+CD8+ cells by altering the Th1/Th2 balance toward Th1 by OXA-sensitization. Although IL-4 production by antigen-specific helper T cells isolated from the sensitized mice was decreased by oxidized olive oil-administration, IFN-γ production was increased. On the other hand, no significant difference between the fresh and oxidized olive oil groups was observed in the amount of IL-4 or IFN-γ produced by PMA/Io, which is a non-specific antigen. These results suggest that ingestion of oxidized olive oil increases Th1 differentiation and proliferation by increasing IL-18 expression in the CHS sensitizing phase, and increases antigen-specific T cells. This leads to an increase in antigen-specific IFN-γ production in the elicitation phase and exacerbates the inflammatory reaction of CHS. Taken together, we proposed a model showing the mechanism of CHS exacerbation by oxidized olive oil-administration ().
Figure 7. Schematic of the proposed mechanism of CHS exacerbation by oxidized olive oil-administration. Hydroperoxide contained in the administered oxidized olive oil increases the activity of caspase-1 via Nrf2 and increases pro-IL-18 production via AP-1 followed by an increase of mature IL-18. Secreted IL-18 promotes the differentiation of naïve T cells to Th1 by stimulation of APC. CHS is exacerbated by promoting antigen-specific IFN-γ production by increased antigen-specific Th1 cells.
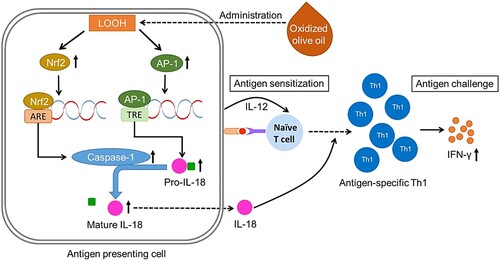
In summary, our study revealed that administration of oxidized olive oil increased the expression level of IL-18 by promoting the activity of AP-1 and caspase-1. Our study also showed that oxidized olive oil increases antigen-specific IFN-γ production by CD3+CD4+ cells upon CHS elicitation in vitro. These results suggest that excessive consumption of oxidized fats and oils may exacerbate CHS by promoting Th1 differentiation through increased production of IL-18.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Ogino, H., upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Awada, M., Soulage, C. O., Meynier, A., Debard, C., Plaisancié, P., Benoit, B., … Michalski, M. C. (2012). Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. Journal of Lipid Research, 53(10), 2069–2080. doi: 10.1194/jlr.M026179
- Bao, A., Yang, H., Ji, J., Chen, Y., Bao, W., Li, F., … Ben, S. (2017). Involvements of p38 MAPK and oxidative stress in the ozone-induced enhancement of AHR and pulmonary inflammation in an allergic asthma model. Respiratory Research, 18, 216. doi: 10.1186/s12931-017-0697-4
- Bowler, R. P., & Crapo, J. D. (2002). Oxidative stress in allergic respiratory diseases. Journal of Allergy and Clinical Immunology, 110, 349–356. doi: 10.1067/mai.2002.126780
- Dinarello, C. A., Novick, D., Kim, S., & Kaplanski, G. (2013). Interleukin-18 and IL-18 binding protein. Frontiers in Immunology, 4, 289.
- Engeman, T., Gorbachev, A. V., Kish, D. D., & Fairchild, R. L. (2004). The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenged sites. Journal of Leukocyte Biology, 76(5), 941–949. doi: 10.1189/jlb.0304193
- Garstkiewicz, M., Strittmatter, G. E., Grossi, S., Sand, J., Fenini, G., Werner, S., … Beer, H. D. (2017). Opposing effects of Nrf2 and Nrf2-activating compounds on the NLRP3 inflammasome independent of Nrf2-mediated gene expression. European Journal of Immunology, 47(5), 806–817. doi: 10.1002/eji.201646665
- Gu, Y., Kuida, K., Tsutsui, H., Ku, G., Hsiao, K., Fleming, M. A., … Kunimoto, M. (1997). Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science, 275(5297), 206–209. doi: 10.1126/science.275.5297.206
- Inoue, H., & Kubo, M. (2004). SOCS proteins in T helper cell differentiation: Implications for allergic disorders? Expert Reviews in Molecular Medicine, 6(22), 1–11. doi: 10.1017/S1462399404008348
- Kang, J., Song, J., Shen, S., Li, B., Yang, X., & Chen, M. (2016). Diisononyl phthalate aggravates allergic dermatitis by activation of NF-kB. Oncotarget, 7, 85472–85482.
- Kish, D. D., Li, X., & Fairchild, R. L. (2009). CD8 T cells producing IL-17 and IFN-gamma initiate the innate immune response required for responses to antigen skin challenge. Journal of Immunology, 182(10), 5949–5959. doi: 10.4049/jimmunol.0802830
- Koike, A., Shibano, M., Mori, H., Kohama, K., Fujimori, K., & Amano, F. (2016). Simultaneous addition of shikonin and its derivatives with lipopolysaccharide induces rapid macrophage death. Biological & Pharmaceutical Bulletin, 39(6), 969–976. doi: 10.1248/bpb.b15-00948
- Kolesaric, A., Stingl, G., & Elbe-Bürger, A. (1997). MHC class I+/II- dendritic cells induce hapten-specific immune responses in vitro and in vivo. Journal of Investigative Dermatology, 109(4), 580–585. doi: 10.1111/1523-1747.ep12337508
- Kondo, S., Beissert, S., Wang, B., Fujisawa, H., Kooshesh, F., Stratigos, A., … Sauder, D. N. (1996). Hyporesponsiveness in contact hypersensitivity and irritant contact dermatitis in CD4 gene targeted mouse. Journal of Investigative Dermatology, 106(5), 993–1000. doi: 10.1111/1523-1747.ep12338505
- Kubo, M., Hanada, T., & Yoshimura, A. (2003). Suppressors of cytokine signaling and immunity. Nature Immunology, 4(12), 1169–1176. doi: 10.1038/ni1012
- Lebel-Binay, S., Berger, A., Zinzindohoué, F., Cugnenc, P., Thiounn, N., Fridman, W. H., & Pagès, F. (2000). Interleukin-18: Biological properties and clinical implications. European Cytokine Network, 11(1), 15–26.
- Liu, P., Kerr, B. J., Weber, T. E., Chen, C., Johnson, L. J., & Shurson, G. C. (2014). Influence of thermally oxidized vegetable oils and animal fats on intestinal barrier function and immune variables in young pigs. Journal of Animal Science, 92(7), 2971–2979. doi: 10.2527/jas.2012-5710
- Martin, S. F., Esser, P. R., Weber, F. C., Jakob, T., Freudenberg, M. A., Schmidt, M., & Goebeler, M. (2011). Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy, 66(9), 1152–1163. doi: 10.1111/j.1398-9995.2011.02652.x
- Mazière, C., Conte, M. A., Degonville, J., Ali, D., & Mazière, J. C. (1999). Cellular enrichment with polyunsaturated fatty acids induces an oxidative stress and activates the transcription factors AP1 and NFkappaB. Biochemical Biophysical Research Commununications, 265(1), 116–122. doi: 10.1006/bbrc.1999.1644
- Murphy, K. M., Ouyang, W., Farrar, J. D., Yang, J., Ranganath, S., Asnagli, H., … Murphy, T. L. (2000). Signaling and transcription in T helper development. Annual Review of Immunology, 18, 451–494. doi: 10.1146/annurev.immunol.18.1.451
- Murphy, K. M., & Reiner, S. L. (2002). The lineage decisions of helper T cells. Nature Reviews Immunology, 2(12), 933–944. doi: 10.1038/nri954
- Official method and recommended practices of the American chemist’ society (2010). (6th ed.). Champaign: AOCS Press, Method Ja 8-87.
- Ogino, H., Koichi, M., Okuno, T., Arakawa, T., & Hitoshi, U. (2018). IL-18 and IFN-gamma expression enhances contact hypersensitivity after oral administration of naturally oxidized olive oil to mice. Food and Agricultural Immunology, 29(1), 886–897. doi: 10.1080/09540105.2018.1472222
- Ogino, H., Sakazaki, F., Okuno, T., Arakawa, T., & Ueno, H. (2015). Oxidized dietary oils enhance immediate- and/or delayed-type allergic reactions in BALB/c mice. Allergology International, 64(1), 66–72. doi: 10.1016/j.alit.2014.07.004
- Okamura, H., Tsutsui, H., Komatsu, T., Yutsudo, M., Hakura, A., Tanimoto, T., … Kurimoto, M. (1995). Cloning of new cytokine that induces IFN-γ production by T cells. Nature, 378(6552), 88–91. doi: 10.1038/378088a0
- Robinson, D., Shibuya, K., Mui, A., Zonin, F., Murphy, E., Sana, T., … O’Garra, A. (1997). IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activated IRAK and NFkappaB. Immunity, 7(4), 571–581. doi: 10.1016/S1074-7613(00)80378-7
- Saint-Mezard, P., Krasteva, M., Chavagnac, C., Bosset, S., Akiba, H., Kehren, J., … Berard, F. (2003). Afferent and efferent phases of allergic contact dermatitis (ACD) can be induced after a single skin contact with haptens: Evidence using a mouse model of primary ACD. Journal of Investigative Dermatology, 120(4), 641–647. doi: 10.1046/j.1523-1747.2003.12093.x
- Sivaranjani, N., Rao, S. V., & Rajeev, G. (2013). Role of reactive oxygen species and antioxidants in atopic dermatitis. Journal of Clinical and Diagnostic Research, 7(12), 2683–2685.
- Usatine, R. P., & Riojas, M. (2010). Diagnosis and management of contact dermatitis. American Family Physician, 82(3), 249–255.
- Varady, J., Eder, K., & Ringseis, R. (2010). Dietary oxidized fat activates the oxidative stress-responsive transcription factors NF-κB and Nrf2 in intestinal mucosa of mice. European Journal of Nutrition, 50(8), 601–609. doi: 10.1007/s00394-011-0181-8
- Venkatesan, B., Valente, A. J., Prabhu, S. D., Shanmugam, P., Delafontaine, P., & Chandrasekar, B. (2010). EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3 K/Akt/IKK/NF-kappaB and MKK7/JNK/AP-1 signaling. Journal of Molecular and Cellular Cardiology, 49(4), 655–663. doi: 10.1016/j.yjmcc.2010.05.007
- Wang, B., Fujisawa, H., Zhuang, L., Freed, I., Howell, B. G., Shivji, G. M., … Sauder, D. N. (2000). CD4+ th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. Journal of Immunology, 165(12), 6783–6790. doi: 10.4049/jimmunol.165.12.6783