?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
To prepare immunomodulation peptides from rice protein hydrolysates (RPHs), trypsin was employed for enzymatic hydrolysis, and the immunomodulating activities of RPHs were studied using mouse peritoneal macrophage proliferation assay. The peptide fractions with the highest activity were further purified using macroporous adsorption resin, strong cation-exchange chromatography, gel filtration chromatography and reversed-phase high-performance liquid chromatography. The peptide sequence was identified as Tyr-Gly-Ile-Tyr-Pro-Arg (YGIYPR) by high-performance liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometer. Evaluating its immunomodulatory activity showed that YGIYPR enhanced the proliferation of macrophage RAW 264.7 cells in the range of 12.5–100 μg/mL, and even a minimum dose achieved good proliferation. The results demonstrated that the RPHs prepared by trypsin could serve as a source of immunomodulating peptides and the immunomodulatory peptide YGIYPR is a potential natural ingredient for manufacture of functional foods or health care products.
Introduction
The immune system is an autonomous defense system comprising many biological structures and processes within an organism that prevent and control various infectious diseases. However, many immunomodulatory pharmaceuticals are inappropriate for chronic or preventive use, resulting in increasing demand for natural and non-pharmaceutical immunomodulators.
Food-derived immunoactive peptides have a variety of regulatory functions in the human immune system. They are generally derived from cheese (Jiehui et al., Citation2014), shellfish (He, Cao, Pan, Yang, & Zhang, Citation2015; Suleria, Addepalli, Masci, Gobe, & Osborne, Citation2017), wheat (Rodríguez-Carrio, Fernández, Riera, & Suárez, Citation2014; Wu et al., Citation2017), rice (Hartati, Widjanarko, Widyaningsih, & Rifa’i, Citation2017) and soybean .(Chen, Suetsuna, & Yamauchi, Citation1995) These immunodulatory peptides can enhance immune cell function, cytokine activity (Yang et al., Citation2009), antimicrobial activity (Nelson, Katayama, Mine, Duarte, & Matar, Citation2007) and antitumor activity (Wang et al., Citation2010). Moreover, some immunodulating peptides have been further purified and identified, including Asn-Gly-Met-Thr-Tyr (Hou, Fan, Li, Xue, & Yu, Citation2012), Pro-His-Thr-Cys (He et al., Citation2015) and Glu-Cys-Phe-Ser-Thr-Ala (Wu et al., Citation2017).
Among these immunodulatory peptides, the enzymatic peptides from rice protein (RP) have been reported to exhibit strong immune activities in animal experiments as well as in clinical research (Takahashi et al., Citation1996; Takahashi, Moriguchi, Yoshikawa, & Sasaki, Citation1994; Wen et al., Citation2016). In addition, rice is one of the most important staple foods for more than half of the world’s population, and provides approximately 60% of the food intake in some Asian countries (Cao et al., Citation2010; Gross & Zhao, Citation2014). The RP is rich in essential and balanced amino acids and has higher biological and protein values than other cereal proteins (Ochiai, Tanaka, Tanaka, & Taniguchi, Citation2016; Zhang & Wang, Citation2013). In our previous study, we showed that RP hydrolysates (RPHs) had strong immune enhancing activity (Wen et al., Citation2016); however, the sequence of the immunomodulatory peptides remained unknown.
Herein, one new peptide with high mouse macrophage proliferation activities was separated using macroporous adsorption resin (DA201-C), ion exchange chromatography (001 × 7), gel filtration chromatography (Sephadex G-10) and reverse phase high-performance liquid chromatography (RP-HPLC) (C18) (). Furthermore, the amino acid sequence (Tyr-Gly-Ile-Tyr-Pro-Arg, YGIYPR) of the peptide was identified using a HPLC electrospray ionization quadrupole time-of-flight (HPLC-ESI-Q-TOF) mass spectrometer. Its immunomodulating activity in vitro was confirmed to verify the potential use of the peptide, which is important, especially when a therapeutic effect is expected.
2. Materials and methods
2.1. Materials
Rice was purchased from Jinjian Cereals Industry Co. Ltd (Changde, China) and trypsin from Hefei Bomei Biotechnology Co. Ltd (Anhui, China). Styrene-based macroporous adsorption resin (MAR) DA201-C and styrene-based gel-type strong acid cation-exchange resin 001 × 7 were obtained from Suqing Water Treatment Engineering Group Co. Ltd (Jiangsu, China). Sephadex G-10 was purchased from GE Healthcare (Bio-Sciences AB, Fairfield, CT, USA). Acetonitrile and trifluoroacetic acid (TFA) were of HPLC grade, and all other chemicals and reagents used were of analytical grade. The murine macrophage RAW264.7 cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA); dimethyl sulfoxide (DMSO) and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) were purchased from Sangon (Shanghai, China).
2.2. Enzymatic hydrolysis
The RP was prepared according to the method as below. Rice flour was dispersed in 0.05 mol/L NaOH solution (1:10, w/v), and the resultant dispersion was gently stirred for 2.0 h at room temperature, then centrifuged at 3150 × g for 30.0 min. The pellet was discarded, and the supernatant was adjusted to pH 4.8 with 2.0 mol/L HCl and then centrifuged at 3150 × g for 10 min. The obtained precipitate was re-dispersed in deionized water and centrifuged three times at 3150 × g for 10 min. The final precipitate was subsequently freeze-dried to produce RP powder. The RP powder was dissolved in distilled water (5% w/w, protein basis) and stirred until complete dissolution; then the solution was pre-incubated at enzymatic temperature for 30 min, and was hydrolyzed subsequently with commercial trypsin (2940 U/g) at pH 7.5 and 41°C for 1.9 h, at constant temperature and pH. The pH was maintained by continuous addition of 1.0 M NaOH according to the pH-stat technique of Adler-Nissen (Adler-Nissen, Citation1986). The reaction was terminated by heating at 85°C for 10 min, then cooled with an ice bath and centrifuged at 3150 × g for 20 min. The supernatant containing the RPHs was collected, lyophilized and stored at −20°C before use.
2.3. Macroporous resin purification
The fraction of RPHs was lyophilized, and the powder obtained was prepared at 20 mg/mL and loaded into a DA 201-C macroporous resin column at a flow rate of 0.5 BV/h. After loading, the column was washed with deionized water at a flow rate of 1.0 BV/h until the eluent had the same conductivity as deionized water. Then, five fractions were obtained from step-elution using 20, 40, 60 and 80% aqueous ethanol solution at a flow rate of 1.0 BV/h. The eluting solvent was changed when the absorbance of the eluent at UV 220 nm remained stable at a low value (Zhang, Yokoyama, & Zhang, Citation2012). All fractions were concentrated by rotary evaporation, lyophilized and stored at −20°C. The murine macrophage proliferation ability of each fraction was determined. The fraction with the highest immunomodulatory activity was used for further purification.
2.4. Strong acid cation-exchange resin purification
That fraction which had shown the highest murine macrophage proliferative activity after MAR DA201-C was further isolated on a strong acid cation-exchange resin 001 × 7 column (2.6 cm × 40 cm). Fractionation parameters with this resin were determined by test tube experiment, with optimal parameters as follows. The column was initially equilibrated with citric acid–disodium hydrogen phosphate buffer (pH 3, 0.2 M). Then the sample was suspended in the same buffer and loaded into the column. The column was eluted stepwise with citric acid–disodium hydrogen phosphate buffer solution of pH 3 (0.2 M), phosphate buffer solutions of pH 5 and pH 7 (0.2 M), carbonate–bicarbonate buffer solution of pH 10 (0.2 M) and ammonia solution buffer of pH 12 (0.2 M) at a flow rate of 3 mL/min. Absorbance of the eluent was monitored at 220 nm. All fractions were collected and lyophilized for the immunomodulating assay. The fraction with the highest immunomodulatory activity was stored at −20°C for further purification.
2.5. Gel filtration chromatography purification
The purification method was modified according to a previously published method (Xing et al., Citation2016). The higher immunomodulating fraction (0.12 g) obtained from strong acid cation-exchange resin 001 × 7 was suspended in 4 mL of ultrapure water and then loaded into a Sephadex G-10 gel filtration column (1.6 cm × 100 cm) which had been equilibrated with water. The column was then eluted with the same solution, and the fractions were collected at a flow rate of 15.0 mL/h. Fractions were detected at 220 nm and analyzed for murine macrophage proliferation ability. The dried fractions were stored at −20°C for further separation.
2.6. RP-HPLC purification
The fraction exhibiting the highest proliferative activity from gel filtration chromatography was further purified according to the previous methods (Amrouche, Boutin, & Fliss, Citation2006; Cheng, Wang, Hsu, & Hwang, Citation2015). This fraction was purified using RP-HPLC on a SunFireTM C18 column (5 µm, 4.6 mm × 250 mm, Waters, USA). The fraction was eluted with a linear gradient of 5–40% acetonitrile containing 0.05% TFA at a flow rate of 1.0 mL/min and the elution was monitored at 220 nm. The sample injection volume was 100 µL. The highest active fraction was collected and subjected to re-chromatography under different linear gradient conditions. The purification procedures were repeated until enough samples were collected for the activity analysis and sequence identification.
2.7. Analysis of amino acid composition
The macroporous adsorption resin-purified hydrolysates were digested in 5.0 mL of 6.0 M HCl under a nitrogen atmosphere for 24 h at 105°C. Then, the digested samples in 50-mL volumetric flasks were washed using distilled water three times and made up to volume of 50 mL. Aliquots of 3.0 mL were placed in an oven at 60°C for drying. The dried samples were analyzed by HPLC on a PICO-TAG column (Hitachi, Tokyo, Japan) after being dissolved in 1.0 mL of sample buffer and filtered through a 0.22-μm membrane (Ren, Wu, Li, Lai, & Xiao, Citation2014). Amino acid composition was reported as gram of amino acid per 100 g protein.
2.8. Evaluation of hydrophobicity
According to Ney’s method (Ney, Citation1979) the average hydrophobic value was calculated as follows:(1)
(1) where AAi is content of each amino acid in 100 g protein (g), Mi is molar mass of each amino acid (g/mol), ∑AAi/Mi was the total moles of amino acid in 100 g of protein (mol), Δfti is hydrophobic value of side chain of amino acid (kJ/mol) and Q is hydrophobic value of the protein (kJ/mol).
2.9. Molecular weight distribution
To estimate the molecular weight (MW) range, the peptide was subjected to high-performance size exclusion chromatography using a Waters 600 HPLC (Waters Corp., USA) equipped with a TSKgel G2000 SWXL column (7.8 mm × 300 mm) (Tosoh Corp. Japan). Aliquots of 10-µL samples in 45% aqueous acetonitrile with 0.1% TFA were injected into the column, and elution performed isocratically in the same TFA–acetonitrile buffer at 30°C with a flow rate of 0.5 mL/min over 30 min. The UV detector was selected at 220 nm, and the molecular mass was measured approximately by comparing the elution time against those of MW markers (Sigma-Aldrich, USA) including cytochrome C (12,500 Da), bacitracin (1450 Da), glycine-glycine-tyrosine-arginine (451 Da) and triglycine (189 Da), which yielded a linear log MW vs elution time regression line (R = 0.9934). The relative content of each peptide fraction was expressed as percentage area of its chromatogram peak.
2.10. Determination of amino acid sequence of the purified peptide
Molecular mass and amino acid sequence of the purified peptide from RPHs was determined with a HPLC-ESI-Q-TOF mass spectrometer (Waters MALDI SYNAPT Q-TOF MS, Waters, Milford, MA, USA) coupled with an ESI source according to Himaya, Ngo, Ryu, and Kim (Himaya, Ngo, Ryu, & Kim, Citation2012). The purified peptide dissolved in methanol/water (1:1, v/v) was infused into the ESI source, and molecular mass determined by the doubly charged (M + 2H)2+ state in the mass spectrum. After molecular mass determination, the peptide was automatically selected for fragmentation, and sequence information was obtained by tandem MS analysis.
2.11. In vitro mouse macrophage proliferation assay
Immunomodulating effect of RPHs and peptide fractions isolated by MAR DA201-C, strong acid cation-exchange resin 001 × 7 and gel filtration chromatography were evaluated in vitro by measuring their impact on mouse macrophage proliferation. The MTT colorimetric assay was used to determine multiplication of the sample on macrophage. Pre-cultured RAW264.7 cells (1.5 × 104 cells/well) were plated on a 96-well microplate in Dulbecco’s Modified Eagle Medium (DMEM) high glucose containing 10% fetal bovine serum that was supplemented with Pen Strep (10,000 units/mL penicillin and 10,000 units/mL streptomycin). Different concentrations of the sample were prepared with serial dilutions and sterile water was used as the control group. After 24 h of incubation, 20 µL of MTT (5 mg/mL) was added to each well and the plate incubated for 4 h at 37°C, 5% CO2. The medium was then discarded, and 150 µL of DMSO was added into each well to solubilize the formazan crystals (Wu, Hsu, Huang, & Chou, Citation2007). Absorbance was read at 490 nm using a microplate reader. The immunocompetence function was expressed as the stimulation index (SI) and calculated as follows:(2)
(2) where OD1 is OD value of sample-treated culture and OD2 is OD of culture without sample. This indicated that the sample enhanced murine macrophage proliferation when SI > 1, but inhibited it for SI < 1.
2.12. Statistical analysis
All experiments were carried out at least three times, and data were expressed as means with standard deviation (SD). Significant differences (P < 0.05) between means were identified by Tukey’s procedures using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
3.1. Purification of peptides by macroporous resin
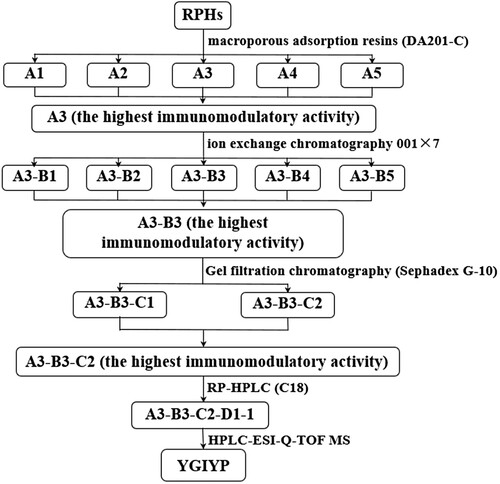
Because bioactivity of peptides may be associated with hydrophobicity, macroporous resins (styrene/divinyl resins) were used to fractionate RPHs. Ethanol of different concentrations can elute peptide fractions with different hydrophobic properties. The elution chromatogram of RPHs on DA201-C showed good separation results ((A)). Elution with 0, 20, 40, 60 and 80% (v/v) ethanol resulted in desorption of A1, A2, A3, A4 and A5, respectively. The average hydrophobicity of amino acids is an important indicator of immune activity (Tossavainen, Outinen, Harju, & Makinen-Kiljunen, Citation1996). Average hydrophobicity was calculated using the hydrophobic values for each amino acid and is shown in (D) and Table 1S. The average hydrophobicity of fractions A3-A5 was relatively high. The amount of hydrophobic amino acids (Ile, Pro, Phe, Leu, Tyr and Val, hydrophobicity in order of strong to weak) was higher in the fraction eluted with the higher ethanol concentration. The in vitro macrophage proliferation assay showed that the fraction corresponding to elution conditions of 40% ethanol (fraction A3), had the highest immunomodulatory activity ((B)), and the yield of A3 was the highest. Consequently, A3 was chosen for further separation.
3.2. Purification of peptides by strong acid cation-exchange resin
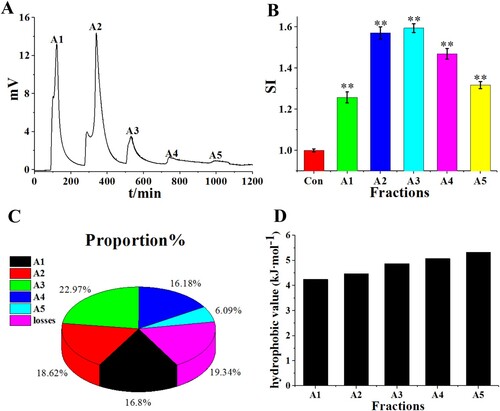
A strong acid cation-exchange resin can separate samples according to their charges. The 001 × 7 (styrene-divinyl benzene copolymer) was a strong acid cation exchanger and is widely utilized for separating bioactive peptides. When using buffer solution with different pH values as the eluent, fractions with different charges will be eluted one by one. In this procedure, A3 was separated into five fractions: A3-B1, A3-B2, A3-B3, A3-B4 and A3-B5 ((A). The in vitro macrophage RAW 264.7 proliferation activity of the five fractions was investigated ((B). Fraction A3-B3 showed the highest immunomodulatory activity, with the highest SI value. Thus, A3-B3 was selected for further purification.
3.3. Separation of immunomodulating fractions using gel filtration chromatography
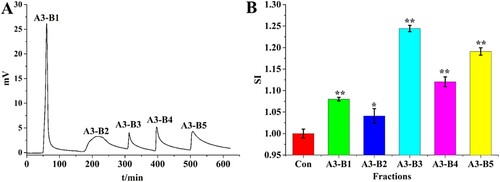
Peptide length is considered to be closely related to biological activity (Cao et al., Citation2010; Gross & Zhao, Citation2014). Different peptide length-fractions of particular size ranges were fractionated by gel filtration chromatography, as a type of size exclusion chromatography. In order to choose the suitable gel of small fractionation rang for the gel filtration chromatography, TSKgel G2000 SWXL column, a larger fractionation range (up to 150,000 Da) was run to identify A3-B3 MW distributions ((C)). Components of MW < 1 kDa comprised 86.5% of total peptides ((D)), indicating that hydrolysis and isolation resulted in many short-chain peptides. Based on this MW distribution, we chose the A3-B3 fraction for purification by gel filtration with the Sephadex G-10 medium (down to 700 Da). Fraction A3-B3 was separated into two fractions: A3-B3-C1 and A3-B3-C2 ((A)). The first eluted sub-fraction of A3-B3-C1 represented the large-size peptide fragments, and sub-fraction A3-B3-C2 corresponded to small-sized peptide fragments. The in vitro murine macrophage RAW 264.7 proliferation activity of sub-fractions separated by gel filtration chromatography was determined. The immunomodulatory analysis ((B)) indicated that the immunomodulatory activity of sub-fractions A3-B3-C1 and A3-B3-C2 increased with decreasing MW of peptides. In addition, fraction A3-B3-C2 showed significantly higher (P < 0.05) immunomodulatory activity than A3-B3.
Figure 4 (A) Chromatography of fraction A3-B3 on a Sephadex G-10 column. (B) Murine macrophage proliferation activity of each elution fraction. (C) and (D) Fraction A3-B3 molar mass distribution.
Additionally, many studies have revealed that immunopeptides derived from food proteins are usually of low MW. Jolles et al. (Jolles et al., Citation1981) obtained a peptide (Val-Glu-Pro-Ile-Pro-Tyr) from human casein protein hydrolysates, which could enhance the phagocytic ability of macrophages. Yoshikawa et al. (Yoshikawa et al., Citation1993) reported that a peptide (Gln-Arg-Pro-Arg) from soybean protein hydrolysate exhibited good immune activity. Liu et al. (Liu, Wang, Qi, Wang, & Song, Citation1998) also extracted a peptide fraction containing 12 amino acid residues from buckwheat pollen hydrolysate that exhibited an immunostimulatory effect on lymphocytes. Thus, we selected A3-B3-C2 for RP-HPLC purification.
3.4. Purification of immunomodulating peptides using RP-HPLC
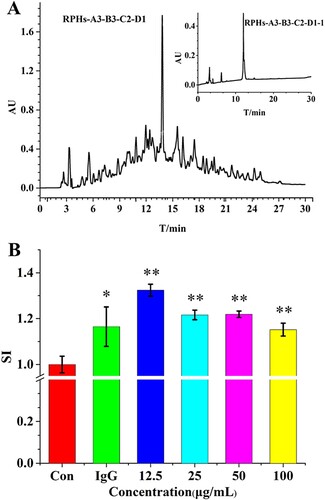
Fraction A3-B3-C2 was further purified on a SunFireTM C18 column (5 µm, 4.6 mm × 250 mm, Waters, USA) in an analytical RP-HPLC system. After the first step separation, A3-B3-C2-D1 was the main fraction ((A)); we collected A3-B3-C2-D1, then evaporated and re-chromatographed it on an analytical RP-HPLC column. The RP-HPLC showed a major peak (A3-B3-C2-D1-1) ((A) inset). The effect of fraction A3-B3-C2-D1-1, isolated from enzymatic digests of RP, on proliferation of murine macrophage RAW264.7 is shown in (B). Again, proliferation was evaluated in non-stimulated cells, and preliminary assays established the optimal concentration of 12.5 µg/mL for fraction A3-B3-C2-D1-1. At this concentration, there was a significant stimulating effect (P < 0.05) for fraction A3-B3-C2-D1-1 with a SI = 1.324. For comparison, the SI value of IgG was determined and was less active than fraction A3-B3-C2-D1-1 at a concentration of 50 µg/mL. These results suggested that fraction A3-B3-C2-D1-1 showed strong murine macrophage RAW264.7 proliferation activity. Peptides in the A3-B3-C2-D1-1 fraction were further analyzed by HPLC-ESI-Q-TOF mass spectrometer.
Figure 5 (A) Chromatography of the fraction A3-B3-C2 on a C18 column. The inset shows chromatography of fraction A3-B3-C2-D1 on a C18 column. (B) The murine macrophage proliferation activity of control (without fraction A3-B3-C2-D1), IgG (50 µg/mL) and different concentrations of fraction A3-B3-C2-D1.
3.5. Identification of amino acid sequence of immunomodulating peptides
The secondary MS spectra result () showed that the MW of the peptide was 767 Da, and the amino acid sequence was Tyr-Gly-Ile-Tyr-Pro-Arg (YGIYPR). This new peptide had not been previously reported, although many immunomodulating peptides have been reported in recent years (). The hydrophobic value is reported to correlate with immunomodulatory activity (Wu et al., Citation2016), which may increase the interaction of the peptide and cytomembrane and so improve immunomodulation (Mercier, Gauthier, & Filiss, Citation2004). In addition, being rich in basic amino acids or hydrophobic amino acids in terminals is also related to immunomodulatory activity (Chen, Muramoto, & Yamauchi, Citation1995). In our study, YGIYPR had three hydrophobic amino acids (Ile, Gly and Pro) and Arg was a basic amino acid in the carboxyl terminal – these features may contribute to its immunomodulatory activity.
Figure 6. Identification of molecular mass and amino acid sequences of purified peptides (fraction A3-B3-C2-D1-1) by HPLC-ESI-Q-TOF MS system.
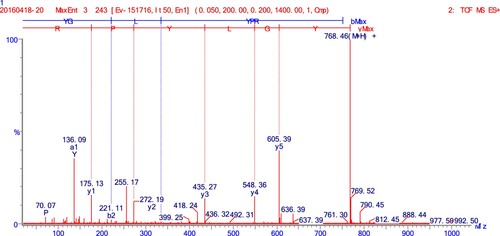
Table 1. Reported immunomodulating peptides.
4. Conclusion
We obtained RPHs through trypsin digestion and demonstrated their in vitro macrophage RAW264.7 proliferation activities. After purification by consecutive chromatographic methods, a single hexapeptide with high macrophage proliferation activity was separated and sequenced as YGIYPR using HPLC-ESI-Q-TOF mass spectrometer. The SI value of YGIYPR was 1.324 in presence of 12.5 μg/mL purified peptide. Our study indicated that YGIYPR was an immunomodulatory peptide. It may be a promising compound in functional foods. Future research efforts will be directed to explore the underlying regulatory mechanism of the peptide and potential effects on other immune indices.
Supplemental Material
Download ()Acknowledgements
This work was supported by the [National Science Foundation of China #1] under Grant [No. 31171627]; [National Science Foundation of China #2] under Grant [No. 31771901] and the Grain-oil Process and Quality Control 2011 Collaborative and Innovative Grant from Hunan Province #3 under Grant No. 2013448.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adler-Nissen, J. (1986). In enzymatic hydrolysis of food proteins. Canadian Medical Association Journal, 172(8), 1783–1785.
- Amrouche, T., Boutin, Y., & Fliss, I. (2006). Effects of bifidobacterial cytoplasm peptide and protein fractions on mouse lymphocyte proliferation and cytokine production. Food & Agricultural Immunology, 17(1), 29–42. doi: 10.1080/09540100600565895
- Cao, L.-Y., Zhan, X.-D., Chen, S.-G., Feng, Y., Wu, W.-M., Shen, X.-H., & Cheng, S.-H. (2010). Breeding methodology and practice of super rice in China. Rice Science, 17(2), 87–93. doi: 10.1016/S1672-6308(08)60109-2
- Chen, H. M., Muramoto, K., & Yamauchi, F. (1995). Structural analysis of antioxidative peptides from soybean.beta.-Conglycinin. Journal of Agricultural and Food Chemistry, 43(3), 574–578. doi: 10.1021/jf00051a004
- Chen, J. R., Suetsuna, K., & Yamauchi, F. (1995). Isolation and characterization of immunostimulative peptides from soybean. The Journal of Nutritional Biochemistry, 6(6), 310–313. doi: 10.1016/0955-2863(95)00022-R
- Cheng, M. L., Wang, H. C., Hsu, K. C., & Hwang, J. S. (2015). Anti-inflammatory peptides from enzymatic hydrolysates of tuna cooking juice. Food & Agricultural Immunology, 26(6), 770–781. doi: 10.1080/09540105.2015.1036352
- Gross, B. L., & Zhao, Z. J. (2014). Archaeological and genetic insights into the origins of domesticated rice. Proceedings Of the National Academy Of Sciences Of the United States Of America, 111(17), 6190–6197. doi: 10.1073/pnas.1308942110
- Hartati, F. K., Widjanarko, S. B., Widyaningsih, T. D., & Rifa’i, M. (2017). Anti-Inflammatory evaluation of black rice extract inhibits TNF-α, IFN-γ and IL-6 cytokines produced by immunocompetent cells. Food & Agricultural Immunology, 28(6), 1116–1125. doi: 10.1080/09540105.2017.1332006
- He, X. Q., Cao, W. H., Pan, G. K., Yang, L., & Zhang, C. H. (2015). Enzymatic hydrolysis optimization of Paphia undulata and lymphocyte proliferation activity of the isolated peptide fractions. Journal of the Science of Food & Agriculture, 95(7), 1544–1553. doi: 10.1002/jsfa.6859
- Himaya, S. W. A., Ngo, D.-H., Ryu, B., & Kim, S.-K. (2012). An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chemistry, 132(4), 1872–1882. doi: 10.1016/j.foodchem.2011.12.020
- Hou, H., Fan, Y., Li, B., Xue, C., & Yu, G. (2012). Preparation of immunomodulatory hydrolysates from Alaska Pollock frame. Journal of the Science of Food and Agriculture, 92(15), 3029–3038. doi: 10.1002/jsfa.5719
- Jiehui, Z., Liuliu, M., Haihong, X., Yang, G., Yingkai, J., Lun, Z., … Shaohui, Z. (2014). Immunomodulating effects of casein-derived peptides QEPVL and QEPV on lymphocytes in vitro and in vivo. Food & Function, 5(9), 2061–2069. doi: 10.1039/C3FO60657K
- Jolles, P., Parker, F., Floc’h, F., Migliore, D., Alliel, P., Zerial, A., & Werner, G. H. (1981). Immunostimulating substances from human casein. Journal of Immunopharmacology, 3(3–4), 363–370. doi: 10.3109/08923978109031067
- Liu, J., Wang, S., Qi, J., Wang, X., & Song, Y. (1998). The immunostimulatory effect of bio-active peptide from pollen on murine and human lymphocytes. Mechanisms of Ageing and Development, 104(2), 125–132. doi: 10.1016/S0047-6374(98)00063-3
- Mercier, A., Gauthier, S. F., & Filiss, I. (2004). Immunomodulating effects of whey proteins and their enzymatic digests. International Dairy Journal, 14(3), 175–183. doi: 10.1016/j.idairyj.2003.08.003
- Nelson, R., Katayama, S., Mine, Y., Duarte, J., & Matar, C. (2007). Immunomodulating effects of egg yolk low lipid peptic digests in a murine model. Food & Agricultural Immunology, 18(1), 1–15. doi: 10.1080/09540100601178623
- Ney, K. H. (1979). Bitterness of peptides: Amino acid composition and chain length. Food Taste Chemistry, 115, 149–173. doi: 10.1021/bk-1979-0115.ch006
- Ochiai, A., Tanaka, S., Tanaka, T., & Taniguchi, M. (2016). Rice bran protein as a potent source of antimelanogenic peptides with Tyrosinase inhibitory activity. Journal of Natural Products, 79(10), 2545–2551. doi: 10.1021/acs.jnatprod.6b00449
- Ren, Y., Wu, H., Li, X., Lai, F., & Xiao, X. (2014). Purification and characterization of high antioxidant peptides from duck egg white protein hydrolysates. Biochemical And Biophysical Research Communications, 452(4), 888–894. doi: 10.1016/j.bbrc.2014.08.116
- Rodríguez-Carrio, J., Fernández, A., Riera, F. A., & Suárez, A. (2014). Immunomodulatory activities of whey β-lactoglobulin tryptic-digested fractions. International Dairy Journal, 34(1), 65–73. doi: 10.1016/j.idairyj.2013.07.004
- Suleria, H. A. R., Addepalli, R., Masci, P., Gobe, G., & Osborne, S. A. (2017). In vitro anti-inflammatory activities of blacklip abalone (Haliotis rubra) in RAW 264.7 macrophages. Food & Agricultural Immunology, 28(4), 711–724. doi: 10.1080/09540105.2017.1310186
- Takahashi, M., Moriguchi, S., Ikeno, M., Kono, S., Ohata, K., Usui, H., … Yoshikawa, M. (1996). Studies on the ileum-contracting mechanisms and identification as a complement C3a receptor agonist of oryzatensin, a bioactive peptide derived from rice albumin. Peptides, 17(1), 5–12. doi: 10.1016/0196-9781(95)02059-4
- Takahashi, M., Moriguchi, S., Yoshikawa, M., & Sasaki, R. (1994). Isolation and characterization of oryzatensin: A novel bioactive peptide with ileum-contracting and immunomodulating activities derived from rice albumin. Biochemistry and Molecular Biology International, 33(6), 1151–1158.
- Tossavainen, O., Outinen, M., Harju, M., & Makinen-Kiljunen, S. (1996). Removal of β-lactoglobulin residues from an enzymatic whey protein hydrolysate. Milchwissenschaft-milk Science International, 51(11), 628–632.
- Wang, Y.-K., He, H.-L., Wang, G.-F., Wu, H., Zhou, B.-C., Chen, X.-L., & Zhang, Y.-Z. (2010). Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Marine Drugs, 8(2), 255–268. doi: 10.3390/md8020255
- Wen, L., Chen, Y., Zhang, L., Yu, H., Xu, Z., You, H., & Cheng, Y. (2016). Rice protein hydrolysates (RPHs) inhibit the LPS-stimulated inflammatory response and phagocytosis in RAW264.7 macrophages by regulating the NF-κB signaling pathway. Rsc Advances, 6(75), 71295–71304. doi: 10.1039/C6RA08927E
- Wu, T. F., Hsu, C. Y., Huang, H. S., & Chou, S. P. (2007). Proteomic analysis of pycnogenol effects in RAW 264.7 macrophage reveals induction of cathepsin D expression and enhancement of phagocytosis. Journal of Agricultural and Food Chemistry, 55(24), 9784–9791. doi: 10.1021/jf070453o
- Wu, W., Zhang, M., Ren, Y., Cai, X., Yin, Z., Zhang, X., … Wu, H. (2017). Characterization and immunomodulatory activity of a novel peptide, ECFSTA, from wheat germ globulin. Journal of Agricultural & Food Chemistry, 65(27), 5561–5569. doi: 10.1021/acs.jafc.7b01360
- Wu, W., Zhang, M., Sun, C., Brennan, M., Li, H., Wang, G., … Wu, H. (2016). Enzymatic preparation of immunomodulatory hydrolysates from defatted wheat germ (Triticum Vulgare) globulin. International Journal Of Food Science And Technology, 51(12), 2556–2566. doi: 10.1111/ijfs.13238
- Xing, L.-j., Hu, Y.-y., Hu, H.-y., Ge, Q.-f., Zhou, G.-h., & Zhang, W.-g. (2016). Purification and identification of antioxidative peptides from dry-cured Xuanwei ham. Food Chemistry, 194, 951–958. doi: 10.1016/j.foodchem.2015.08.101
- Yang, R., Zhang, Z., Pei, X., Han, X., Wang, J., Wang, L., … Li, Y. (2009). Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chemistry, 113(2), 464–470. doi: 10.1016/j.foodchem.2008.07.086
- Yoshikawa, M., Kishi, K., Takahashi, M., Watanabe, A., Miyamura, T., Yamazaki, M., & Chiba, H. (1993). Immunostimulating peptide derived from soybean protein. Annals of the New York Academy of Sciences, 685, 375–376. doi: 10.1111/j.1749-6632.1993.tb35892.x
- Zhang, H. J., & Wang, J. (2013). Study on in vitro hypocholesterolemic effects of white rice protein, brown rice protein, soybean protein and their hydrolysates. Journal of Chinese Institute of Food Science & Technology, 13(12), 28–33.
- Zhang, H. J., Yokoyama, W. H., & Zhang, H. (2012). Concentration-dependent displacement of cholesterol in micelles by hydrophobic rice bran protein hydrolysates. Journal Of the Science Of Food And Agriculture, 92(7), 1395–1401. doi: 10.1002/jsfa.4713