ABSTRACT
The aim of the study was to analyse the effect of spirulina supplementation on blood levels of selected cytokines in athletes exposed to strenuous physical exercise, with emphasis on Th1/Th2 balance. The double-blind study included 19 members of the Polish Rowing Team. The subjects were randomly assigned to the supplemented group, receiving 1500 mg of spirulina extract for 6 weeks, or to the placebo group. After the supplementation, athletes from the placebo group showed a significant post-recovery reduction of IL-2 level, which contributed to a significant decrease in IL-2/IL-4 and IL-2/IL-10 ratios at the end of a 24-h recovery period. Although this study did not demonstrate significant post-supplementation changes in Th1 and Th2 cytokine levels, the lack of a shift in Th1/Th2 balance toward Th2-type response in the supplemented group implies that some constituents of spirulina may exert an indirect beneficial effect on athletes’ immunity.
Introduction
An antagonism between cellular and humoral mechanisms constitutes a basis for the phenomenon referred to as polarization of the immune response. Stimulated by various antigens and cytokines, T-helper (Th) lymphocytes can differentiate into either Th1 cells triggering cellular immune response or Th2 cells that elicit humoral immune response. Therefore, activation of the cellular response is associated with attenuation of the humoral response and vice versa. The predominance of either humoral or cellular response is associated with their different effectiveness against various pathogens. Th1 cells undergo activation if the infection is caused by an intracellular etiological factor (e.g. virus), whereas Th2-type response predominates in the case of infection with an extracellular pathogen that can be effectively neutralized by complement system and antibodies. Thus, appropriate polarization of the immune response is a key determinant of one's immunity and ability to control various infections (Chamorro, Salazar, Favila, & Bourges, Citation1996; Kaiko, Horvat, Beagley, & Hansbro, Citation2008).
A disruption of Th1/Th2 balance can be detected by the measurement of Th1 (e.g. IL-2 and INF-gamma) and Th2 cytokine (e.g. IL-4 and IL-10) concentrations (Chamorro et al., Citation1996; Franca et al., Citation2010; Kaiko et al., Citation2008; Romagnani, Citation1996). The Th1/Th2 imbalance has been implicated in many pathological conditions; predominance of Th1-type response was reported inter alia in multiple sclerosis, type 1 diabetes mellitus and rheumatoid arthritis, and predominance of Th2-type response in neoplastic diseases, allergies and systemic lupus erythematosus (SLE) (Franca et al., Citation2010). Also strenuous physical exercise may contribute to a shift in Th1/Th2 balance toward Th2-type response. Due to resultant cellular immunosuppression, athletes not only become more susceptible to various infections, but may also present with excessive inflammatory response and overtraining symptoms (Lancaster et al., Citation2004;Teixeira Guimarães, Terra, & Lourenço Dutra, Citation2017; Xiang, Marshall, & Rehm, Citation2014).
Supplementation with spirulina (cyanobacterium Spirulina platensis) (SPR) may be a form of mild and relatively safe intervention, contributing to normalization of Th1/Th2 balance. SPR is a nutraceutical food supplement with a wide spectrum of action. Previous studies, both in humans and in animal models, demonstrated that spirulina-based supplements can exert a beneficial effect not only on prooxidant–antioxidant balance (Wu et al., Citation2016), but also on immune function (Capelli & Cysewski, Citation2010). Moreover, athletes supplemented with spirulina were shown to be able to exercise longer and to be less prone to post-exercise muscle damage (Kalafati et al., Citation2010). Available evidence suggests that the anti-inflammatory effect of SPR is associated with antioxidant activity of this supplement (Wu et al., Citation2016).
The safety of SPR as a dietary supplement was confirmed both by the results of toxicology studies and based on the lack of reported adverse effects (Chamorro, Gutiérrez-Salmeán, Fabila-Castillo, & Chamorro-Cevallos, Citation2015; Wu et al., 1996b25). Aside from high content of protein, vitamins, minerals and unsaturated fatty acids (gamma-linoleic acid), spirulina contains also other compounds, such as phycocyanin and lipopolysaccharides. The latter two substances, important components of the cellular wall in Gram-negative bacteria, were shown to be potent triggers of host's immune response (Tornabene, Bourne, Raziuddin, & Ben-Amotz, Citation1985). Published evidence suggests that supplementation with SPR may promote cellular immune response, inter alia through stimulation of IL-12, INF-gamma and IL-2 production and inhibition of IL-4 synthesis (Hirahashi et al., Citation2002; Løbner, Walsted, Larsen, Bendtzen, & Nielsen, Citation2008; Maerten et al., Citation2005).
Supplementation with immunomodulatory compounds may be also a form of mild and safe intervention to normalize disrupted immune balance. The fact that SPR promotes Th1-type immune response justifies a research on immunomodulatory function of this supplement in athletes undertaking strenuous physical exercise, in whom large training loads may contribute to a shift toward Th2-type immune response.
Materials and methods
Study population
The study included 19 men, members of the Polish Rowing Team (15 heavy-weight and 4 light-weight rowers). Basic characteristics and sport classes of the athletes are presented in . The study was conducted between March and May, during a 6-week training camp, scheduled between the preparatory and competitive phase of the yearly training cycle. Basic characteristics of training profile, such as its intensity, volume (in minutes) and type (specific, i.e. rowing: endurance, technical, speed, etc., and non-specific: jogging, strength) were recorded on a daily basis. The intensity of the training was classified based on the lactic acid (LA) threshold (4 mmol/L), as extensive (below the LA threshold), highly intensive (above the LA threshold), and extremely intensive (control tests) ().
Table 1. Basic characteristics of the study groups (mean ± standard deviation).
Food intake
Throughout the study period, the athletes were accommodated at one of the Olympic Training Centers, whereby they had all their meals. Their regular menu consisted of a mixed diet, providing them with the recommended dietary allowance (RDA) of carbohydrates, proteins, fats and micronutrients (vitamins and minerals), in line with the Polish Nutrition Society guidelines (Jarosz, Citation2012). Daily intakes of food, calories, fruits and vegetables were the same throughout the study period.
The study subjects declared that they had ceased all drugs, medications and dietary supplements at least two weeks prior to the study and did not use them throughout the entire study period.
Experimental procedure
Athletes who were randomized to the supplemented group (n = 10) received capsules with S. platensis extract, manufactured by GAL (Poznan, Poland). A single 596-mg capsule was made of 500 mg S. platensis containing 5.9 mg chlorophyll, 0.093 mg vitamin B6, 6.5 µg vitamin K, 0.8 µg vitamin B12, 3.5 µg selenium and 9.0 µg iodine, coated with 96 mg gelatin. The subjects were asked to take one capsule before each of their three main meals during the day, for a period of six weeks, which corresponded to 1500 mg of spirulina extract per day. Athletes randomized to the placebo group (n = 9) received visually identical capsules with calcium gluconate (500 mg per capsule).
All subjects received information about the nature of the investigation and provided their written informed consent to participate in the study. Protocol of the study was approved by the Local Ethics Committee at the Poznan University of Medical Sciences.
Training programme
Training volumes (expressed in minutes per day) during a week preceding the 1st and the 2nd examination are shown in , separately for extensive rowing, intensive rowing, kilometres and extensive non-specific training. During the load training phase (before the 1st examination), the training volume amounted to 1130 min/wk, including approximately 49.6% of extensive rowing, 26.5% of non-specific training (e.g. power training) and 23.9% of intensive rowing. Total training volume before the 2nd examination was 940 min/wk, and included approximately 66.9% of extensive rowing, 10.7% of intensive rowing (with 5.2% of maximum-intensity control tests) and 11.7% of land training.
Rowing performance test
The athletes performed a controlled 2000-m time test on the first day (prior to the supplementation) and at the end of the training camp (after the supplementation). Each subject had to cover the distance on a rowing ergometer (Concept II, USA) in a short time as possible. Because the results of both tests were taken into consideration during selection to the championship team, the athletes were well motivated to perform both tests at a maximal effort. Prior to each test, the subjects performed a 5-min individual warm-up.
Sample treatment
Blood samples were obtained before the 2000-m test (in the morning, after overnight fasting), 1 min after completing the test, and after a 24-h recovery. Blood samples were collected to tubes without anticoagulant and centrifuged (20 min at 4000 rpm). The sera were stored at −80°C until the analysis. Additionally, capillary blood samples were obtained from the earlobe before and after each exercise test to assess the athletes’ lactic acid levels.
Measurements
Serum concentrations of interleukins (in pg/ml) were measured using commercially available enzyme immunoassays (ELISA; Abcam, Cambridge, UK), with assay ranges amounting to 1.87–60 pg/ml for IL-2, 0.31–10 pg/ml for IL-4, and 1.56–50 pg/ml for IL-10. Serum concentrations of interferon gamma (IFN-gamma, in pg/ml) were quantified using a commercially available enzyme immunoassay (ELISA; Quantikine HS, R&D Systems, Minneapolis, USA) with an assay range of 15.6–1000 pg /ml.
Total antioxidant capacity (TAC), a marker of plasma antioxidant capacity, was assessed with a commercially available kit (LDN Labor Diagnostika Nordhorn, Germany) with an assay range of 0.375–3 mmol/L; the results were expressed in mmol/L. Concentration of lactic acid (LA) in capillary blood was determined immediately after sampling, using a commercially available kit (Dr Lange, Germany); lactic acid concentrations were expressed in mmol/L. Coefficients of variation for all assays were <13%.
Statistical analysis
Statistical analysis was carried out with STATISTICA v. 10.0 software package (StatSoft, Cracow, Poland). All parameters were compared using 2 (group: supplemented vs. placebo) × 3 (sampling time: prior to the test vs. after the test vs. after a 24-h recovery) repeated measures analysis of variance (ANOVA). Normal distribution of the study variables was verified with Shapiro–Wilk test. If significant changes were demonstrated on ANOVA, Fisher post hoc tests were conducted to identify the source of variance. Anthropometric parameters of the study groups were compared with unpaired Student t-test. Except for the rowing time, the results of the 2000-m tests performed prior to and after the supplementation were subjected to intragroup comparisons with paired Student t-test, and unpaired Student t-test was used for intergroup comparisons. The results of the 2000-m simulated rowing test were subjected to one-way ANOVA. Statistical characteristics of the study variables are presented as means ± standard deviations (SD). The threshold of statistical significance for all the tests was set at P < 0.05.
Results
Athletes form the supplemented group did not differ significantly from the controls in terms of their mean age, body height, body weight, and years of training ().
No significant intragroup differences were found in mean power output and total run time during the 2000-m test performed at the beginning and at the end of the training camp (1st and 2nd examination, respectively). Furthermore, no significant intragroup differences in the pre- and post-supplementation blood lactate levels were documented ().
Table 2. Changes in 2000-m rowing ergometer performance prior to and after supplementation.
During the 1st examination, a significant post-exercise increase in IL-2 level was observed in both study groups ((A)). During the 2nd examination, the post-recovery level of IL-2 in the controls was significantly lower than the level of this cytokine immediately after the exercise test. Spirulina supplementation did not exert a significant effect on the IL-2 level (ANOVA, main effect, P = .901).
Figure 1. Changes in IL-2 (A), IL-4 (B), IL-10 (C) and IFN-gamma (D) levels during exercise tests performed prior to and after the supplementation (mean ± SD).
Note. IL: interleukins; INF γ: interferon gamma; • SUPPL: supplemented group; □ – PLA: placebo group; B: baseline; Ex: post-exercise; R: after a 1-day recovery; †: significantly different compared to the baseline level; #: significantly different compared to the post-exercise level.
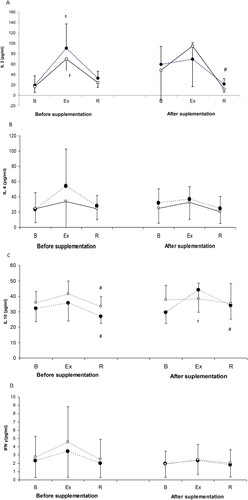
Changes in IL-10 levels in the study athletes are presented in (C). During the 1st examination, the post-recovery levels of IL-10 in both study groups were significantly lower than immediately after the exercise test. Although supplementation with spirulina did not exert a significant effect on IL-10 level (main effect, P = .203), during the 2nd examination, statistically significant changes, namely a post-exercise increase with normalization after a 24-h recovery, were observed solely in the supplemented group.
ANOVA demonstrated that physical exercise exerted significant effects on the values of IL-2/IL-4 and IL-2/IL-10 ratios (main effect, P = .001 and P = .001, respectively; (A and B)). During the 2nd examination, statistically significant changes in both ratios were observed solely in the controls; after a 24-h recovery, the values of IL-2/IL-4 and IL-2/IL-10 ratios in this group were significantly lower than immediately after the exercise test. Supplementation with spirulina did not exert statistically significant effects on IL-2/IL-4 and IL-2/IL-10 ratios (main effect, P = .803 and P = .218, respectively).
Figure 2. IL-2/IL-4 (A), IL-2/IL-10 (B), IFN-gamma/IL-4 (C) and IFN-gamma/IL-10 (D) ratios during exercise tests performed before and after the supplementation (mean ± SD).
Note. IL: interleukins; INF γ: interferon gamma; • SUPPL: supplemented group; □ – PLA: placebo group; B: baseline; Ex: post-exercise; R: after a 1-day recovery; †: significantly different compared to the baseline level; #: significantly different compared to the post-exercise level.
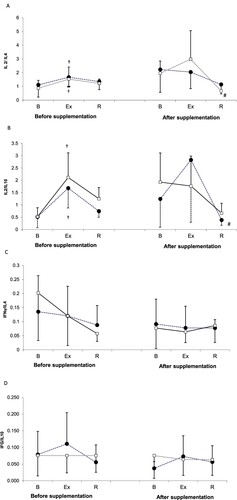
IL-4 and IFN-gamma levels and IFN-gamma/IL-4 and IFN-gamma/IL-10 ratios at various study time points are presented in (C and D) and (C and D). Neither spirulina supplementation nor physical exercise exerted statistically significant effects on these parameters.
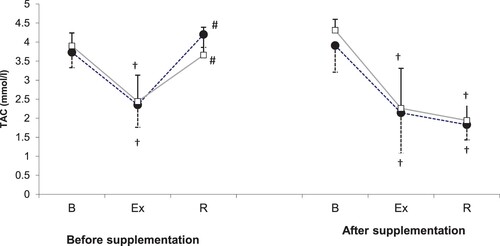
Statistical characteristics of total antioxidant capacity (TAC) during the 1st and the 2nd examination are shown in . Statistical analysis demonstrated that TAC was modulated solely by physical exercise (P < .001). Prior to the supplementation (1st examination), a significant post-exercise decrease in serum TAC with subsequent normalization after a 24-h recovery was observed in both study groups. During the 2nd examination, both post-exercise and post-recovery levels of TAC in either the supplemented group or the controls were significantly lower than at the baseline.
Figure 3. TAC levels during exercise tests performed prior to and after the supplementation (mean ± SD).
Note. TAC: total antioxidants status; • – SUPPL: supplemented group; □: PLA = placebo group; B = baseline; Ex = post-exercise; R = after a 1-day recovery; † – significantly different compared to the baseline level; # - significantly different compared to the post-exercise level.
Discussion
The results of previous studies analysing a relationship between physical exercise and cytokine synthesis are inconclusive. However, researchers agree that exercise (acting as a physical stressor), may influence human immune function. The immunomodulatory effects of physical activity seem to be primarily related to the duration and intensity of the exercise (Neto, Lira, de Mello, & Santos, Citation2011). While moderate exercise is associated with polarization of the immune response toward the Th1 phenotype, strenuous exercise contributes to a shift toward Th2-type response which may result in cellular immunosuppression and greater susceptibility of athletes to upper respiratory tract infections (Gleeson & Pyne, Citation2016; Zhao, Zhou, Davie, & Su, Citation2012).
In this study, we analysed the effects of SPR supplementation on the production of selected Th1 (IL-2 and INF-gamma) and Th2 cytokines (IL-10 and IL-4) in athletes exposed to intensive training loads. Moreover, we verified whether supplementation with SPR and intensive physical exercise contributed to a polarization of the immune response, manifesting as changes in the levels of these cytokines.
Th1 cells promote phagocytosis, a key component of cellular immune response. Our study included two Th1 cytokines, INF-gamma and IL-2. We did not demonstrate a significant effect of physical exercise and spirulina supplementation on INF-gamma levels ((D)). Regarding IL-2, while during the 1st examination (prior to the supplementation) a significant post-exercise increase in the level of this cytokine was observed in both study groups, during the 2nd examination, statistically significant changes, namely a post-recovery decrease was demonstrated solely in the controls ((A)). Although no statistically significant effect of SPR administration on IL-2 level was documented on ANOVA, during the 2nd examination the exercise test did not induce any changes in this cytokine concentration solely in the supplemented group.
Cytokines synthesized by Th2 cells, inter alia IL-4 and IL-10, prevent activation of macrophages by microbial antigens, and stimulate the production of extracellular proteins involved in tissue repair. Consequently, activation of Th2 cells is associated with suppression of Th1-type immune response. During the 1st examination, prior to the supplementation, the post-recovery levels of IL-10 in both study groups were significantly lower than immediately after the exercise test. After the supplementation, however, statistically significant changes, namely a post-exercise increase with normalization after a 24-h recovery, were observed solely in the supplemented group ((C)).
To accurately analyse the Th1/Th2 balance, concentrations of Th1 and Th2 cytokines need to be analysed together, rather than separately. Frequently, upregulation of Th1 cytokines is associated with a compensatory downregulation of Th2 cytokines, and vice versa (Kaiko et al., Citation2008; Romagnani, Citation1996). Thus, analysis of exercise-induced changes in Th1/Th2 balance on the basis of INF-gamma/IL-4, INF-gamma/IL10, IL-2/IL-4 and IL-2/IL-10 ratios provides more accurate data than the analysis of individual cytokine levels.
IFN-gamma/IL-4 and IFN-gamma/IL-10 ratios are important markers, providing information about a predominant phenotype of immune response. IFN-gamma acts antagonistically to IL-4 and IL-10. IL-4 promotes differentiation of Th lymphocytes to Th2 cells and resultant activation of humoral immune response, at the same time inhibiting differentiation of Th lymphocytes to Th1 cells. Also IL-10 promotes humoral response via downregulation of IL-2 and IFN-gamma. In contrast, IFN-gamma inhibits the differentiation of Th lymphocytes to Th2 cells and hence, promotes cellular response (Kicielińska & Pajtasz-Piasecka, Citation2014; Kidd, Citation2003; Viallard et al., Citation1999).
Also IL-2/IL-4 and IL-2/IL-10 ratios, reflecting antagonistic effects of IL-4 and IL-10 in relation to IL-2, found application in clinical research, inter alia as markers of inflammation and tissue injury during SLE flares or as indicators of cytokine imbalance in vitiligo (Wang & Huang, Citation2005). We did not observe statistically significant effects of spirulina supplementation on the values of these ratios in our athletes ((A and B)). However, during the 2nd examination, statistically significant exercise-induced changes in IL-2/IL-4 and IL-2/IL-10 ratios were found in the controls; after a 24-h recovery, IL-2/IL-4 and IL-2/IL-10 ratios in this group were significantly lower than immediately after the exercise test ((A and B)). The post-exercise predominance of Th2-type immune response over Th1-type response seems to support the hypothesis that strenuous exercise contributes to a shift in Th1/Th2 balance toward Th2 (Teixeira Guimarães et al., Citation2017). In turn, the lack of statistically significant changes in IL-2 levels and all cytokine ratios in the supplemented group might correspond to a stabilizing role of spirulina which maintained pre-exercise values of all these parameters up to 24 h after the test.
Wang and Huang (Citation2005) showed that intensive physical exercise may decrease concentration of glutathione (GSH) in lymphocytes, inducing oxidative stress. The deficiency of intracellular glutathione may contribute to a shift from Th1- to Th2-type immune response. Macrophages containing oxidized glutathione were shown to undergo Th2 polarization, which suggests that Th1/Th2 balance is at least in part determined by redox status of immune cells (Kidd, Citation2003). This might be a mechanism through which strenuous physical exercise, associated with severe oxidative stress, contributes to Th2 polarization of the immune response (Franca et al., Citation2010; Powers & Jackson, Citation2008).
According to Wu et al. (Citation2016) spirulina shows its antioxidant activity solely in the presence of a prooxidant factor. Strenuous physical exercise is an established disruptor of prooxidant–antioxidant balance; exercise-induced oxidative stress usually results from enhanced synthesis of free radicals, a decrease in the number of free radical scavengers and/or lesser activity of enzymatic systems involved in the elimination of reactive oxygen species. During the 2nd examination, both post-exercise and post-recovery levels of TAC in either the supplemented group or the controls were significantly lower than at the baseline (), which might correspond to inadequate antioxidant potential and/or high severity of oxidative stress. Analysis of training programmes () showed that our athletes were exposed to large training loads, and it was probably a reason behind the post-exercise decrease in their TAC. Franca et al. (Citation2010) did not observe a significant effect of spirulina (supplemented for 4 weeks at 7.5 g per day) on prooxidant–antioxidant balance parameters in cyclists exposed to strenuous physical exercise, which is consistent with the results of our present study. However, beneficial effects of spirulina supplementation on post-exercise changes in prooxidant–antioxidant balance were documented in persons who undertook physical activity solely on a recreational basis (Kalafati et al., Citation2010; Lu, Hsieh, Hsu, Yang, & Chou, Citation2006).
Table 3. Training schedule for the week preceding blood sampling during the 1st and the 2nd examination.
Lu et al. (Citation2006) and Kalafati et al. (Citation2010) demonstrated that supplementation with spirulina contributed to lesser muscle damage and better exercise performance. However, in our present study, we did not observe a beneficial effect of spirulina supplementation on athletes’ performance, expressed by mean power output and total run time during the 2000-m test ().
Conclusions
Although this study did not demonstrate significant post-supplementation changes in Th1 and Th2 cytokine levels, the fact that a shift in Th1/Th2 balance toward Th2-type response was observed solely in the controls, implies that some constituents of spirulina may exert a beneficial effect on athletes’ immunity. The notable finding of the present study is that during the 2nd examination, aside from a statistically significant reduction of IL-2 level, athletes from the placebo group showed also a post-restitution decrease in IL-2/IL-10 and IL-2/IL-4 ratios. However, all these parameters remained unchanged in the supplemented group, which implies that spirulina may maintain the functional balance of IL-2, IL-4 and IL-10 up to 24 h post-exercise. The lack of a shift toward Th2-type response, observed in the supplemented group but not in the controls, might reflect SPR's potential to counterbalance some immune disorders in athletes. This justifies further research on this supplement with a larger panel of cytokines. The beneficial effect of SPR on the immune response to strenuous physical exercise was not associated with antioxidant potential of this supplement.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Capelli, B., & Cysewski, G. (2010). Potential health benefits of spirulina microalgae pass. Nutrafoods, 9(2), 19–26. doi: 10.1007/BF03223332
- Chamorro, G., Salazar, M., Favila, L., & Bourges, H. (1996). Pharmacology and toxicology of spirulina alga. Revista De Investigacion Clinica; Organo Del Hospital De Enfermedades De La Nutricion, 48(5), 389–399.
- Franca, G. A. M., Silva, A. S., Costa, M. J. C., Junior, J. S. M., Nébrega, T. K. S., Gonçalves, M. C. R., & Asciutti, I. S. R. (2010). Spirulina does Not decrease muscle damage nor oxidative stress in Cycling athletes with Adequate Nutritional status. Biology of Sport, 27(4), 249–253. doi: 10.5604/20831862.927489
- Gleeson, M., & Pyne, D. B. (2016). Respiratory inflammation and infections in high-performance athletes. Immunology and Cell Biology, 94(2), 124–131. doi: 10.1038/icb.2015.100
- Gutiérrez-Salmeán, G., Fabila-Castillo, L., & Chamorro-Cevallos, G. (2015). Nutritional and Toxicological Aspects of spirulina (Arthrospira). Nutricion Hospitalaria, 32(1), 34–40.
- Hirahashi, T., Matsumoto, M., Hazeki, K., Saeki, Y., Ui, M., & Seya, T. (2002). Activation of the human innate immune system by spirulina: Augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. International Immunopharmacology, 2(4), 423–434. doi: 10.1016/S1567-5769(01)00166-7
- Jarosz, M. (2012). Nutrition standards for the Polish population – amendment. Warszawa.
- Kaiko, G. E., Horvat, J. C., Beagley, K. W., & Hansbro, P. M. (2008). Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology, 123(3), 326–338. doi: 10.1111/j.1365-2567.2007.02719.x
- Kalafati, M., Jamurtas, A. Z., Nikolaidis, M. G., Paschalis, V., Theodorou, A. A., Sakellariou, G. K., … Kouretas, D. (2010). Ergogenic and antioxidant effects of spirulina supplementation in humans. Medicine and Science in Sports and Exercise, 42(1), 142–151. doi: 10.1249/MSS.0b013e3181ac7a45
- Kicielińska, J., & Pajtasz-Piasecka, E. (2014). The role of IL-10 in the modulation of the immune response in normal conditions and the tumor environment. Postępy Higieny i Medycyny Doświadczalnej, 68, 879–892. doi: 10.5604/17322693.1111123
- Kidd, P. (2003). Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Alternative Medicine Review: A Journal of Clinical Therapeutic, 8(3), 223–246.
- Lancaster, G. I., Halson, S. L., Khan, Q., Drysdale, P., Wallace, F., Jeukendrup, A. E., … Gleeson, M. (2004). Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exercise Immunology Review, 10, 91–106.
- Løbner, M., Walsted, A., Larsen, R., Bendtzen, K., & Nielsen, C. H. (2008). Enhancement of human Adaptive immune Responses by administration of a high-Molecular-weight Polysaccharide extract from the cyanobacterium Arthrospira platensis. Journal of Medicinal Food, 11(2), 313–322. doi: 10.1089/jmf.2007.564
- Lu, H.-K., Hsieh, C.-C., Hsu, J.-J., Yang, Y.-K., & Chou, H.-N. (2006). Preventive effects of Spirulina platensis on skeletal muscle damage under exercise-induced oxidative stress. European Journal Of Applied Physiology, 98(2), 220–226. doi: 10.1007/s00421-006-0263-0
- Maerten, P., Shen, C., Bullens, D. M. A., Van Assche, G., Van Gool, S., Geboes, K., … Ceuppens, J. L. (2005). Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. Journal Of Autoimmunity, 25(2), 112–120. doi: 10.1016/j.jaut.2005.04.001
- Neto, J. C. R., Lira, F. S., de Mello, M. T., & Santos, R. V. T. (2011). Importance of exercise immunology in health promotion. Amino Acids, 41(5), 1165–1172. doi: 10.1007/s00726-010-0786-x
- Powers, S. K., & Jackson, M. J. (2008). Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiological Reviews, 88(4), 1243–1276. doi: 10.1152/physrev.00031.2007
- Romagnani, S. (1996). The Th1-Th2 paradigm in disease ( Molecular biology intelligence unit). Austin, TX: R.G. Landes Company.
- Teixeira Guimarães, T., Terra, R., & Lourenço Dutra, P. M. (2017). Chronic effects of exhausting exercise and overtraining on the immune response: Th1 and Th2 profile. Motricidade, 13(3), 69–78. doi: 10.6063/motricidade.10049
- Tornabene, T. G., Bourne, T. F., Raziuddin, S., & Ben-Amotz, A. (1985). Lipid and lipopolysaccharide constituents of cyanobacterium Spirulina platensis (Cyanophyceae, Nostocales). Marine Ecology Progress Series, 22, 121–125. doi: 10.3354/meps022121
- Viallard, J. F., Pellegrin, J. L., Ranchin, V., Schaeverbeke, T., Dehais, J., Longy-Boursier, M., … Moreau, J. F. (1999). Th1 (IL-2, interferon-gamma (IFN-gamma)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE). Clinical And Experimental Immunology, 115(1), 189–195. doi: 10.1046/j.1365-2249.1999.00766.x
- Wang, J.-S., & Huang, Y.-H. (2005). Effects of exercise intensity on lymphocyte apoptosis induced by oxidative stress in men. European Journal Of Applied Physiology, 95(4), 290–297. doi: 10.1007/s00421-005-0005-8
- Wu, Q., Liu, L., Miron, A., Klímová, B., Wan, D., & Kuča, K. (2016). The antioxidant, immunomodulatory, and anti-inflammatory activities of spirulina: An overview. Archives of Toxicology, 90(8), 1817–1840. doi: 10.1007/s00204-016-1744-5
- Xiang, L., Marshall, G. D., & Rehm, K. E. (2014). Effects of strenuous exercise on Th1/Th2 gene expression from human peripheral blood mononuclear cells of marathon participants. Molecular Immunology, 60, 129–134. doi: 10.1016/j.molimm.2014.03.004
- Zhao, G., Zhou, S., Davie, A., & Su, Q. (2012). Effects of moderate and high intensity exercise on T1/T2 balance. Exercise Immunology Review, 18, 98–114.
