 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to investigate the effects of probiotic Lactobacillus rhamnosus MLGA and its cell wall components on the induction of avian β-defensin 9 (AvBD9) expression in intestinal epithelial cells (IECs) from chicken embryos and to elucidate the underlying mechanism. Chicken IECs were stimulated with L. rhamnosus MLGA or cell wall components. Quantitative RT-PCR analysis was used to determine the expressions of target genes. L. rhamnosus MLGA and its cellular components significantly induced AvBD9 and TLR2 expression, with the whole peptidoglycan (WPG) being the most potent inducer. Pretreatment of chicken IECs with a TLR2 neutralizing antibody diminished AvBD9 induction by L. rhamnosus MLGA and WPG. The inhibition tests showed that induction of AvBD9 in IECs by L. rhamnosus MLGA and WPG was dependent on NF-κB or JNK pathways. These results demonstrate that L. rhamnosus MLGA and WPG induce AvBD9 expression in chicken IECs via a TLR2-mediated NF-κB/JNK pathway.
Introduction
Defensins, which are gene-encoded cationic antimicrobial peptides and important multifunctional effector molecules of the innate immune system in vertebrates, are widely distributed in various tissues, especially in the epithelium of the skin, digestive, respiratory, and urogenital tracts (Cuperus, Coorens, van Dijk, & Haagsman, Citation2013; Zasloff, Citation2002). In addition to their direct antimicrobial activities, defensins play an important role in the integration of the innate and acquired immune responses (Lai & Gallo, Citation2009; Yuan et al., Citation2015). The defensin family can be divided into α, β, and θ-defensin subgroups based on the spacing pattern of cysteines, which form three conserved disulfide bridges (Avila, Citation2017). Currently, only β-defensins (avian β-defensins, AvBDs) are found in avian species (Cuperus et al., Citation2013). The chicken genome encodes a total of 14 AvBDs (AvBD1–14), which are densely clustered on chicken chromosome 3q and expressed in a wide range of tissues (Lynn et al., Citation2004; Xiao et al., Citation2004).
AvBD9 is extensively expressed in various organs and tissues of chickens, with strong expression in the trachea, liver, bursa, muscle, and kidney (Lynn et al., Citation2004; van Dijk, Veldhuizen, & Haagsman, Citation2008; Xiao et al., Citation2004), moderate expression in female and male reproductive tracts, and low to moderate expression in the small intestine and lymphoid organs (Cuperus et al., Citation2013; Lynn et al., Citation2004; van Dijk et al., Citation2007; Xiao et al., Citation2004). AvBD9 displays broad-spectrum antibacterial activities against gram-positive and gram-negative strains, in addition to prominent fungicidal activity against both unicellular and multicellular fungi (van Dijk et al., Citation2007; Yacoub et al., Citation2016). In addition to the potent antimicrobial activity of AvBD9, this peptide exhibits low hemolytic activity and low toxicity against animal cells (Yacoub et al., Citation2016) and also has multiple functions in the host defense against infection (Sunkara et al., Citation2011), suggesting that AvBD9 plays an important role in both innate and adaptive immunity.
The probiotic Lactobacilli, which predominantly colonizes the chicken small intestine, interacts with the epithelial cells and immune cells associated with the lamina propria of the small intestine and elicits local mucosal and systemic immune responses. The immunomodulatory effects of the probiotic Lactobacilli on the intestinal and systemic immune system have been extensively studied (Han et al., Citation2018; Jeong, Lee, Jang, Han, & Kim, Citation2018; Ng, Hart, Kamm, Stagg, & Knight, Citation2009; Śliżewska & Markowiak, Citation2018). Nevertheless, the underlying molecular mechanisms by which different probiotic strains influence the immune system are not completely understood. Our previous study demonstrated that specific probiotic Lactobacillus strains dose-dependently enhanced AvBD9 gene expression in primary intestinal epithelial cells of chicken embryos. Moreover, Lactobacillus rhamnosus MLGA from the healthy chicken small intestine induced the strongest expression of AvBD9, among all of the tested Lactobacillus strains (Li et al., Citation2012). Additionally, heat-inactivated L. ramous MLGA also upregulated the expression of AvBD9, indicating that specific components from this probiotic bacterium confer beneficial effects to host defense. However, the underlying molecular mechanism regarding the L. rhamnosus MLGA-dependent expression of AvBD9 in chicken intestinal epithelial cells, as well as the specific component from L. rhamnosus MLGA that is responsible for this effect, is unclear. To the best of our knowledge, there is no information regarding the effect of cell wall components from probiotic Lactobacillus strains isolated from chicken intestines on the induction of the chicken avian β-defensins. Thus, the present study aimed to investigate how cellular components from L. ramous MLGA, which was isolated from the gut of healthy chickens, influenced the expression of AvBD9 in primary chicken intestinal epithelial cells, and to explore the signalling pathway involved in the induction of AvBD9 by L. ramous MLGA.
Materials and methods
Probiotic bacterial strain
The probiotic Lactobacillus rhamnosus MLGA strain was isolated from the small intestine of a healthy chicken (Li et al., Citation2012). L. rhamnosus MLGA was cultured statically in Mann-Rogosa-Sharpe (MRS) broth (Solarbio, Beijing, China) overnight at 37°C under anaerobic conditions. The bacteria were grown until log phase and were then harvested by centrifugation at 8000 × g for 10 min and washed three times with sterile phosphate buffer saline (PBS) (pH 7.4). The optical density at 600 nm (OD600) was measured using NanoVue-100 UV-vis spectrophometer (GE Healthcare, Pittsburgh, PA, USA), and the bacterial concentration was calculated from a standard curve using the following equation in order to determine the number of colony-forming units per mL (CFU/mL) = (2.5 × 108) × OD600. Heat-inactivation of bacteria was performed in a water bath at 65°C for 30 min. Bacteria were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) without fetal bovine serum (FBS) and antibiotics. The bacterial suspension was then adjusted to the required concentration for the following stimulation experiments.
Preparation of the cellular components from L. ramous MLGA
Whole peptidoglycan (WPG) was prepared according to the previously described method (Sekine et al., Citation1985). Briefly, heat-inactivated bacteria were treated with 0.5% Triton X-100 for 1 h with continuous stirring at 80–85°C, washed thoroughly with double distilled water, and then successively washed with methanol-water (2:1, vol/vol), methanol, and acetone to remove the detergent from the insoluble residue. The residual sample was incubated with trypsin (1 mg/mL; Solarbio, Beijing, China), DNase (100 μg/mL; New England Biolabs, Ipswich, MA, USA), and RNase (150 μg/mL, New England Biolabs) at 37°C for 14 h, and then centrifuged at 20,000 × g for 40 min to collect the insoluble residue. The residue was digested with trypsin (0.5 mg/mL) and α-chymotrypsin (0.5 mg/mL, Solarbio) at 37°C for 14 h. The sample was then treated with pepsin (1 mg/mL in 0.01 mol/L HCl, Amresco, Suwanee, GA, USA) and then with Pronase E (1 mg/mL in Tris-HCl buffer, pH 7.4; Roche, Shanghai, China) at 37°C for 14 h. The Pronase-digested residue was delipidified by successive refluxing with methanol, methanol:chloroform (1:1, vol/vol), and chloroform. The delipidified material was digested three times with Pronase E (1 mg/mL in Tris-HCl buffer, pH 7.4) at 37°C for 14 h and then dialyzed against double distilled water for 3 days. Finally, the insoluble material was treated with 0.005 mol/L H2SO4 at 85–95°C for 5 min. The mixture was immediately cooled and centrifuged at 15,000 × g for 30 min. The sediment was dialyzed against double distilled water for 7 days and then lyophilized.
Extraction of lipoteichoic acid (LTA) from L. rhamnosus MLGA was carried out as previously described (Miörner, Johansson, & Kronvall, Citation1983). In brief, L. rhamnosus MLGA was suspended in distilled water with an equal volume of 90% (wt/vol) phenol at 65°C and mixed for 5 min. The suspension was then centrifuged at 12,000 × g for 30 min. The aqueous phase was removed and dialyzed against six changes of distilled water for 3 days.
The whole cell wall components (WCW) and cell wall proteins (CWP) from L. rhamnosus MLGA were prepared as previously described (Wang et al., Citation2003). Lyophilized L. rhamnosus MLGA were dissolved in lysis buffer containing 20 mM PBS (pH 7.4) and 0.025% phenylmethanesulfonyl fluoride (PMSF), and then lysed by intermittent ultrasonication (20 kHz) for a total of 15 times for 2 min each time. The suspension thus obtained was centrifuged at 2000 × g for 5 min. The supernatants were then centrifuged at 55,000 × g for 30 min, and the precipitate formed was designated as the WCW and dissolved in 20 mmol/L PBS for stimulation experiments. The WCW extract was dissolved in PBS containing 2% sodium dodecyl sulfate (SDS) and incubated for 30 min at room temperature (approximately 25°C), and then centrifuged at 55,000 × g for 30 min. The soluble proteins present in the supernatants were designated as the CWP. The supernatants were dialyzed for three days. The SDS in the sample was removed using SDS-OutTM Sodium Dodecyl Sulfate Precipitation Kit (Pierce, Rockford, IL, USA), according to the manufacture’s instruction and then lyophilized. The protein content of the lyophilized samples was determined using a BCA protein assay kit (Viagene Biotech Inc., Changzhou, China).
L. rhamnosus MLGA culture supernatants (S) were obtained from growing bacterial cultures in MRS broth for 16 h at 37°C under anaerobic conditions. Bacteria were removed by centrifugation at 12,000 g for 5 min. Supernatants were collected and passed through a 0.22-μm pore size filter unit (Dalian Meilun Biotechnology Co., LTD., Dalian, China) and lyophilized. Prior to the experiments, the lyophilized cell-free supernatant was resuspended in DMEM medium.
All of the cellular components prepared from L. rhamnosus MLGA were diluted in FBS- and antibiotic-free DMEM to the desired concentration for stimulating the intestinal epithelial cells.
Intestinal epithelial cell preparation and culture
Primary chicken embryo intestinal epithelial cells were prepared from 18-day-old specific pathogen free (SPF) chicken embryos (Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin, China) according to the process described previously (Hong, Jia, Qiu, Li, & Liu, Citation2011). Briefly, SPF chicken embryos were killed by cervical rupture. Intestines were separated and cut into small pieces in D-Hank’s buffered salt solution (Solarbio, Beijing, China) supplemented with 200 U/mL penicillin (Sigma-Aldrich, St. Louis, MO, USA) and 200 μg/mL streptomycin (Sigma-Aldrich). The small pieces of intestine were washed twice with D-Hank’s buffered salt solution and then digested with 1 mg/mL collagenase type I in D-Hank’s buffered salt solution (pH 7.4) twice at 37°C with gentle shaking, 50 min for each. The pellets were then collected by centrifugation at 500 × g for 10 min at room temperature (approximately 25°C) and resuspended in DMEM supplemented with 5% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 10 ng/mL epidermal growth factor, 100 μg/mL heparin sodium salt, and 5 μg/mL insulin. Undigested intestinal tissues were removed by filtering the suspension with a sterile 200-mesh sieve. Monocytes and fibroblasts were discarded by incubating the solution in a tissue culture flask for 1.5 h in order to allow these cells to adhere to the flask. Non-adherent cells were seeded into 6-well culture plates in complete DMEM and incubated in a humidified environment at 37°C with 5% CO2. Cell culture media were changed with fresh medium every 2 days.
Stimulation experiments
Intestinal epithelial cells were seeded into 6-well culture plates. Cells grown until approximately 80%–90% confluence and then were washed three times with pre-warmed sterile PBS (pH 7.4). The cells were then stimulated with live L. rhamnosus MLGA, heat-inactivated L. rhamnosus MLGA, or cellular components from L. rhamnosus MLGA at indicated concentrations (2 mL per well) for 4 h. The FBS- and antibiotic-free DMEM medium alone served as the control. For signalling pathway studies, cells were pre-treated with a rabbit anti-human Toll-like receptor 2 (TLR2) antibody (Bioss, Beijing, China) for 40 min, or a specific p38 inhibitor SB203580 (Sigma, USA), specific c-Jun N-terminal kinase (JNK) inhibitor SP600125 (Sigma, USA), specific extracellular signal regulated kinase 1/2 (ERK1/2) inhibitor PD98059 (Sigma, USA), or nuclear factor (NF)-κB inhibitor pyrrolidine dithiocarbamate (PDTC) (Beyotime, Nantong, China) for 1 h prior to stimulation with bacteria or cellular components from bacteria, respectively. The inhibitors were added directly to the culture medium at a final concentration of 20 μmol/L for SB203580, SP600125, and PD98059, and 50 μmol/L for PDTC. After stimulation, cells were collected for RNA extraction. Each treatment was performed in triplicates, and all of the experiments were repeated at least twice.
Electrophoretic mobility shift assay (EMSA) for NF-κB and activator protein-1 (AP-1)
Nuclear extracts for the EMSA were prepared from cells stimulated with live L. rhamnosus MLGA, heat-inactivated L. rhamnosus MLGA, or WPG from L. rhamnosus MLGA for 4 h using the Viagene extraction kit (Viagene Biotech Inc), following the manufacturer’s instructions. Protein concentration in nuclear extracts was measured with a BCA protein assay kit (Viagene Biotech Inc.). The nuclear translocation of NF-κB and AP-1 was determined by EMSA using a non-radioactive EMSA kit (Viagene Biotech Inc.) with NF-κB and AP-1 oligonucleotide probes labelled with biotin, respectively. The competition reactions were performed by the addition of 100-fold excess of unlabelled NF-κB p65 and AP-1 to the reaction mixture. Extracts were electrophoresed on 6.5% polyacrylamide gel on ice at 180 V for one h. The DNA-protein complexes resolved in the gel were transferred to Hybond N+ nylon membrane by electroblotting with 0.5x TBE at 300 mA for 20 min. DNA-protein complexes were fixed to the membrane by incubation in an UV cross-linker (UV Stratalinker 1800, Stratagene, USA) for 10 min. The complexes were detected by incubation of the membrane with the substrate solution and then visualized with a CoolImager imaging system (IMGR002) (exposure to radiographic film). Densitometric analysis of the optical density (OD) of the bands and background was performed using Scion Image software.
Quantitative real-time RT-PCR
Total RNA was extracted from cells using AxyPrep Total RNA Isolation Reagent Kit (Axygen Scientific Inc., Union City, CA, USA), according to the manufacturer’s instructions. Reverse transcription (RT) was performed using PrimeScriptTM RT Reagent Kit (TaKaRa Biotechnology, Dalian, China), following the manufacturer’s instructions. Amplification and detection were carried out using the LightCycler® 480 II Real-Time PCR System (Roche, USA). Quantification of reference and target gene expressions was estimated using the LightCycler 1.5 software (Roche, USA). The primers and probes used in quantitative real-time PCR (qPCR) reactions were designed as follows: β-actin (GenBank accession number L08165.1), 5′-TTGTGATGGACTCTGGTGATGG-3′ (forward), 5′-TGTCAGGATCTTCATGAGGTAGTC-3′ (reverse), FAM-TCACGGCCAGCCAGATCCAGACGG-BHQ (probe); AvBD9 (GenBank accession number NM_001001611), 5′-CCACGGCTCCTGCTCTTTT-3′ (forward), 5′-ACCACGGCAGGTCCCAAT-3′ (reverse), FAM-TTGCATGCCGTGCTCCTTCAGTTG-BHQ (probe); TLR2 (GenBank accession number AB046533), 5′-CGATCAGGAAGATAAATCAC-3′ (forward), 5′-AGAGGAAGACATAATTCTCA-3′ (reverse), FAM-AGGATTGACGTATTCTCAGCAGTCATC-BHQ (probe). The levels of individual transcripts were normalized to the reference β-actin gene. Exact amplification efficiencies of target and reference genes were verified separately before normalizing the expression of the target gene to that of the housekeeping gene, and efficiency (E) was calculated as: E = 10−1/slope of standard curve. Each analysis was performed in triplicate. With the gene of interest as the target and β-actin as the reference, the relative expression ratio (R) was determined as: , where ETarget and ERef are the efficiencies of the target gene and β-actin, respectively, and Ct is the logarithmic-scaled threshold cycle value.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) from triplicate experiments. Data were analyzed using one-way analysis of variance or paired sample t tests. Differences were considered to be statistically significant when the P-value was <0.05.
Results
Induction of AvBD9 in intestinal epithelial cells by cellular components of L. rhamnosus MLGA
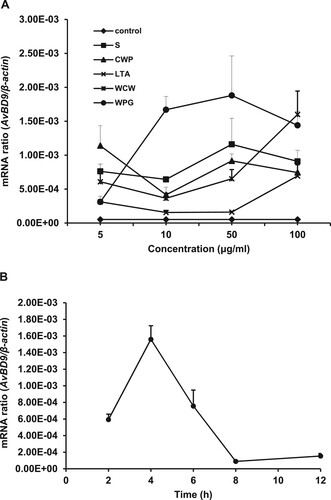
We previously demonstrated that both live and heat-killed L. rhamnosus MLGA induced AvBD9 mRNA expression in primary chicken intestinal epithelial cells (Li et al., Citation2012), indicating that specific cellular components from this probiotic bacterium induced AvBD9 expression. To determine the specific cellular components responsible for the induction of AvBD9 expression, we treated chicken intestinal epithelial cells with various L. rhamnosus MLGA preparations, including S, CWP, LTA, WCW, and WPG. All of the cellular components tested promoted AvBD9 mRNA expression in chicken intestinal epithelial cells in a dose-dependent manner ((A)). The greatest induction of AvBD9 expression was observed with WPG, followed by the culture supernatant of L. rhamnosus MLGA at the concentration of 50 μg/mL. LTA showed only a weak effect on AvBD9 expression at low concentrations of 10 and 50 μg/mL. Next, we determined the time-course expression of AvBD9 mRNA upon exposure to 50 μg/mL of WPG. The gene expression of AvBD9 was induced in a time-dependent manner, peaking at 4 h post-stimulation with 50 μg/mL of WPG and returning to basal levels after 8 h of incubation ((B)).
Figure 1. Cellular components from L. rhamnosus MLGA-induced AvBD9 mRNA expression in primary chicken intestinal epithelial cells. (A) Intestinal epithelial cells were incubated with five different components at the indicated concentrations for 4 h each. (B) Intestinal epithelial cells were stimulated with WPG at a concentration of 50 µg/mL for 2, 4, 6, 8, and 12 h. AvBD9 mRNA expression was determined by qPCR. Values are given as the mean ± SD. n = 3 for each treatment. S: cultural supernatant of L. rhamnousus; CWP: cell wall protein; LTA: lipoteichoic acid; WCW: whole cell wall; WPG: whole peptidoglycan.
TLR2-mediated induction of AvBD9 in intestinal epithelial cells by L. rhamnosus MLGA and WPG
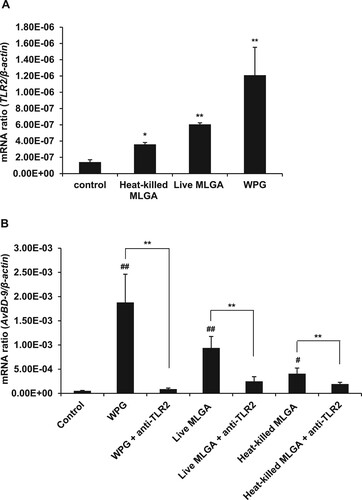
Accumulating evidence indicates that the inducible expression of defensins and other antimicrobial factors depend on TLR-dependent signalling in epithelial cells (Vaishnava, Behrendt, Ismail, Eckmann, & Hooper, Citation2008; Vora et al., Citation2004). TLR2 is a well-known pattern recognition receptor that recognizes a wide variety of microbe-associated molecular patterns (MAMPs), including bacterial peptidoglycan, lipoproteins, lipopeptides, and LTA from gram-positive bacterial cell walls (St Paul, Paolucci, & Sharif, Citation2013). In order to determine the role of TLR2 in the induction of AvBD9 expression by L. rhamnosus MLGA preparations, we first investigated the effect of live L. rhamnosus MLGA, heat-killed L. rhamnosus MLGA, or WPG from L. rhamnosus MLGA on TLR2 expression in chicken intestinal epithelial cells. The expression level of TLR2 mRNA was significantly enhanced in all live and heat-killed L. rhamnosus MLGA- and WPG-treated cells, and the greatest increase in expression level was observed in WPG-treated cells ((A)). Next, we used a TLR2 neutralizing antibody to block TLR2 activation. Pretreatment with a TLR2 neutralizing antibody significantly inhibited AvBD9 mRNA expression induced by live and heat-killed L. rhamnosus MLGA, as well as WPG ((B)).
Figure 2. Role of TLR2 in the induction of L. rhamnosus MLGA-dependent AvBD9 mRNA expression in primary chicken intestinal epithelial cells. (A) Upregulation of TLR2 mRNA expression by L. rhamnosus MLGA preparations. Intestinal epithelial cells were treated with live or heat-killed L. rhamnosus MLGA at a concentration of 2 × 106 CFU/mL or WPG at a concentration of 50 µg/mL for 4 h. TLR2 mRNA expression was determined by qPCR. Values are given as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with the control group. (B) Blocking TLR2 inhibited AvBD9 mRNA expression induced by L. rhamnosus MLGA preparations. Intestinal epithelial cells were pretreated with 10 µg/mL anti-TLR2 antibody for 40 min and then stimulated with live or heat-killed L. rhamnosus MLGA or WPG. **P < 0.01 between the indicated treatments; #P < 0.05, ##P < 0.01 compared with control group. Values are given as the mean ± SD of three independent experiments.
Involvement of transcription factors NF-κB and AP-1 in the induction of AvBD9 in intestinal epithelial cells by L. rhamnosus MLGA and WPG
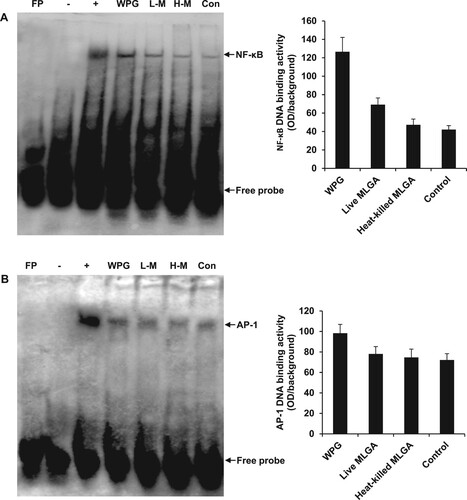
In order to determine whether the transcription factors NF-κB and AP-1 were involved in the induction of AvBD9 in chicken intestinal epithelial cells, the DNA-binding activities of NF-κB and AP-1 were characterized using an EMSA. The DNA-binding activities of NF-κB ((A)) and AP-1 ((B)) were increased in intestinal epithelial cells following treatment with L. rhamnosus MLGA and WPG, suggesting that induction of AvBD9 by L. rhamnosus MLGA and WPG was both NF-κB- and AP-1-dependent. As shown in (A), the strongest binding activity was observed in WPG-stimulated cells, followed by live L. rhamnosus MLGA. Furthermore, the NF-κB signalling in the induction of AvBD9 was more important than the AP-1 signalling.
Figure 3. EMSA analysis of DNA-binding activities of NF-κB and AP-1 in chicken intestinal epithelial cells. Cells were stimulated with L. rhamnosus MLGA or WPG for 4 h. NF-κB and AP-1 DNA-binding activities in the nuclear extracts were assessed by EMSA. EMSA results demonstrate that NF-κB (A) and AP-1 (B) DNA-binding activities were increased by stimulation with WPG, live L. rhamnosus MLGA, or heat-killed L. rhamnosus MLGA, with WPG-treated cells being strongly positive. Lane FP: the free probe only control; lane -: negative control without nuclear protein; lane +: positive control with nuclear proteins from Mo7e cells; lane WPG: nuclear extracts from WPG-treated cells; lane L-M: nuclear extracts from live L. rhamnosus MLGA-treated cells; lane H-M: nuclear extracts from heat-killed L. rhamnosus MLGA-treated cells; lane Con: nuclear extracts from untreated control cells. Data are representative of three independent experiments.
Involvement of JNK and NF-κB pathways in the induction of AvBD9 in intestinal epithelial cells by L. rhamnosus MLGA and WPG
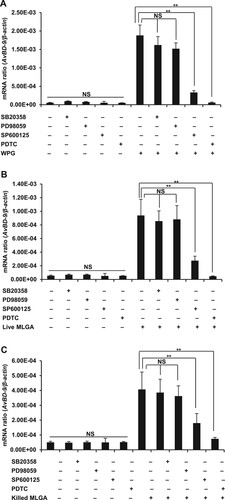
It has been reported that stimulation of TLR2 results in activation of multiple downstream molecules, including NF-κB and mitogen-activated protein kinases (MAPKs) (Vasselon, Hanlon, Wright, & Detmers, Citation2002). Therefore, we investigated the effect of specific inhibitors of NF-κB and the three main MAPKs on L. rhamnosus MLGA preparation-induced AvBD9 gene expression. Exposure to SP600125, a specific inhibitor of JNK, significantly blocked AvBD9 induction by WPG ((A)), live ((B)), or heat-killed L. rhamnosus MLGA ((C)), while p38 MAPK inhibitor SB203580 and ERK1/2 inhibitor PD98059 showed a minimal effect ((A, B, C)). Furthermore AvBD9 induction by WPG, live, and heat-killed L. rhamnosus MLGA was almost completely blocked by pre-treatment of epithelial cells with the specific NF-κB inhibitor PDTC ((A, B, C)).
Figure 4. Induction of AvBD9 in intestinal epithelial cells by L. rhamnosus MLGA and WPG is dependent on NF-κB or JNK, but not ERK1/2 and p38 MAPK pathways. PDTC, an inhibitor of NF-κB, completely inhibited AvBD9 expression induced by WPG (A), live L. rhamnosus MLGA (B), and heat-killed L. rhamnosus MLGA (C) in intestinal epithelial cells. SP600125, an inhibitor of JNK, potentially inhibited WPG- (A), live L. rhamnosus MLGA- (B), and heat-killed L. rhamnosus MLGA (C)-induced AvBD9 expression in intestinal epithelial cells, whereas p38 MAPK inhibitor SB203580 and ERK 1/2 inhibitor PD98059 showed little or no effect. Intestinal epithelial cells were pretreated with PDTC (50 μM), SP600125 (20 μM), SB203580 (20 μM), and PD98059 (20 μM) for 1 h and then treated with L. rhamnosus MLGA preparations for 4 h. AvBD9 mRNA expression was determined by qPCR. NS, not significant between the indicated treatments, **P < 0.01 between the indicated treatments. Values are given as the mean ± SD of three independent experiments.
Discussion
It is generally accepted that probiotic bacteria must adhere to host cells in order to colonize and multiply in the intestine and confer beneficial effects to the host (Galdeano, de Moreno de LeBlanc, Vinderola, Bonet, & Perdigón, Citation2007). Therefore, the interaction or crosstalk between live probiotic bacteria and the intestinal epithelium is a key determinant for the initiating event in probiotic immunomodulatory activity (Delcenserie et al., Citation2008). However, it has been well documented that heat-killed probiotic bacteria and probiotic-derived products, including chromosomal DNA, cell wall components, and soluble metabolites, have the capacity to regulate local mucosal and systemic immune responses (Corthésy, Gaskins, & Mercenier, Citation2007; Giahi, Elmadfa, Hoseini, & Klein, Citation2013; Jensen, Cash, Farmer, & Keller, Citation2017; Lee et al., Citation2016; O’Flaherty, Saulnier, Pot, & Versalovic, Citation2010; Sanchez, Urdaci, & Margolles, Citation2010). We previously reported that both viable and heat-inactivated L. rhamnosus MLGA significantly promoted AvBD9 expression in chicken intestinal epithelial cells (Li et al., Citation2012). Based on this observation, in this study, we further investigated the specific bacterial components of the probiotic bacteria, L. rhamnosus MLGA, that was responsible for the induction of AvBD9 expression and delineated the mechanism by which L. rhamnosus MLGA and its components regulated the expression of the antimicrobial peptide AvBD9. Our results showed that all five cell components (WPG, WCW, WCP, LTA, and S) derived from L. rhamnosus MLGA have the capability to induce AvBD9 mRNA transcription in a dose-dependent manner. Peptidoglycan, a major component of the gram-positive bacterial cell wall (accounting for approximately 90% of its dry weight), plays crucial roles in bacterial growth and survival, as well as in the modulation of host immune responses (Sukhithasri, Nisha, Biswas, Anil Kumar, & Biswas, Citation2013). Peptidoglycan can be recognized by TLR2, leading to activation of a variety of host signalling cascades and ultimately production of immune effector molecules, including cytokines, chemokines, and antimicrobial peptides (Brownlie & Allan, Citation2011; Maynard, Elson, Hatton, & Weaver, Citation2012). Therefore, we presumed that the AvBD9-inducing activity of the cell wall from L. rhamnosus MLGA might be mainly attributed to peptidoglycan fractions. As expected, the WPG exhibited the highest induction of AvBD9 expression among the five components from L. rhamnosus MLGA. Moreover, WPG showed stronger activity in promoting TLR2 expression than live and heat-inactivated L. rhamnosus MLGA. Interestingly, we observed that the bacterial culture supernatant of L. rhamnosus MLGA also potently promoted AvBD9 expression, implying that soluble factor(s) released by L. rhamnosus MLGA into the supernatant may be responsible for the induction of AvBD9 in chicken intestinal epithelial cells. Further investigations are required to characterize the bioactive factor(s) produced by L. rhamnosus MLGA, which potently induces the expression of AvBD9 in chicken intestinal epithelial cells, and to explore the mechanism of action on AvBD9 induction.
Several studies have shown that heat-killed probiotic lactic acid bacteria and probiotic Escherichia coli strongly enhanced β-defensin gene expression and protein production in intestinal epithelial cells (Habil, Abate, Beal, & Foey, Citation2014; Möndel et al., Citation2009; Rizzo, Losacco, Romano, & Carratelli, Citation2013; Schlee et al., Citation2008; Wehkamp et al., Citation2004). Specifically some heat-killed strains exhibited β-defensin induction comparable to viable bacteria (Rizzo et al., Citation2013; Wehkamp et al., Citation2004). Induction of β-defensin expression in epithelial cells by bacterial components from probiotics or commensal microorganisms has also been previously reported (Bhattacharyya et al., Citation2016; Ghadimi et al., Citation2011; Gupta et al., Citation2010; Krisanaprakornkit, Kimball, & Dale, Citation2002; Li et al., Citation2013; Schlee et al., Citation2007; Schlee et al., Citation2008; Schmitt et al., Citation2015; Zhang, Jin, & Yang, Citation2019). Probiotics are generally viable beneficial microbes that confer a health benefit on the host. However, our results, along with other observations, suggest that probiotic bacteria-dependent antimicrobial peptide induction in the gastrointestinal tract does not require live probiotic bacteria. When taking AvBD9 induction property and stability, as well as the colonization ability of probiotics in the gastrointestinal tract, into consideration, our results provide promising evidence that cell wall preparations from probiotic bacteria could behave as effective inducers of antimicrobial peptides, thereby reinforcing intestinal mucosal barrier defenses.
Epithelial cells are a major interaction site between the host and the environment and they are involved in the regulation of innate mucosal immunity through different pathways, including specific receptors, such as Toll-like receptors (TLRs) (Ahn et al., Citation2016; Maldonada-Contreras & McCormick, Citation2011). TLRs have been shown to play an essential role in host immune defense. TLR2, as one of the TLR family members, recognizes a broad range of ligands, especially ligands of cell wall components from gram-positive bacteria, such as LTA, lipoproteins, and peptidoglycan. Thus, the interactions between TLR2 and its ligands lead to immediate innate immune responses, such as production of antimicrobial peptides, pro-inflammatory cytokines, and chemokines, preventing the spread of infection (Brownlie & Allan, Citation2011; St Paul et al., Citation2013). The role of TLR2 in the regulation of β-defensin induction in response to probiotic bacteria, commensal microorganisms, and bacterial cell wall components, as well as pathogen infection, has been demonstrated in various epithelial cells, including human intestinal epithelial cells (Paolillo, Carratelli, Sorrentino, Mazzola, & Rizzo, Citation2009; Vora et al., Citation2004), human vaginal epithelial cells (Rizzo et al., Citation2013), human oral epithelial cells (Bhattacharyya et al., Citation2016; Gupta et al., Citation2010; Ji et al., Citation2009), human ear epithelial cells (Lee et al., Citation2008), human corneal epithelial cells (Kumar, Zhang, & Yu, Citation2006), human epidermal keratinocytes (Li et al., Citation2013), and ovine ruminal epithelial cells (Jin et al., Citation2018). Herein, we show for the first time that TLR2 mediates induction of AvBD9 by probiotic L. rhamnosus and its cell wall component WPG in chicken intestinal epithelial cells. Our investigation demonstrated an increase in the mRNA expression of TLR2 and AvBD9 in epithelial cells following treatment with viable or heat-killed L. rhamnosus MLGA, or derived WPG, among which the WPG was the most potent inducer of both TLR2 and AvBD9. Pre-treatment with a TLR2 neutralizing antibody almost completely inhibited WPG-induced AvBD9 expression and partially suppressed the expression of AvBD9 in cells stimulated with viable or heat-killed L. rhamnosus MLGA. These findings provided evidence for a critical role of TLR2 in L. rhamnosus MLGA- and WPG-dependent induction of the potent antimicrobial innate immune molecule, AvBD9, in primary chicken intestinal epithelial cells. However, the partial inhibition of AvBD9 expression by anti-TLR2 in L. rhamnosus MLGA-stimulated cells suggested that other mechanisms (i.e. L. rhamnosus binding other pattern recognition receptors) exist for the induction and regulation of AvBD9 in chicken intestinal epithelial cells treated with L. rhamnosus MLGA. Further investigation is warranted to verify this statement.
Stimulation of TLRs activates downstream signalling pathways of the MAPK superfamily cascade and the transcription factor NF-κB (Banerjee & Gerondakis, Citation2007). MAPK is an essential component of the signal transduction machinery that consists of at least three distinct subfamilies: p38, JNK, and ERK1/2 – all of which are upstream mediators for AP-1 activation (Kaminska, Citation2005). Previous studies have indicated that several MAPKs are involved in the signalling cascades leading to expression of defensins upon various stimuli (Gan et al., Citation2014; Jang et al., Citation2004; Ju et al., Citation2012; Kim et al., Citation2013; Li et al., Citation2013; Madi et al., Citation2013). Among these studies, all three main MAPKs, p38, JNK, and ERK1/2, or two of them, were reported to be involved in mediating induction of defensins in response to a variety of stimuli. Specifically, increasing evidence has demonstrated that the JNK pathway plays a critical role in the upregulation of β-defensins in epithelial cells by probiotics (Fan et al., Citation2016; Jin et al., Citation2018; Schlee et al., Citation2007, Citation2008; Wehkamp et al., Citation2004) or commensal microorganisms (Krisanaprakornkit et al., Citation2002). The present study showed that a JNK inhibitor, but not ERK or p38 MAPK inhibitors, inhibited L. rhamnosus MLGA- and WPG-induced AvBD9 mRNA expression. This suggests that JNK is the critical downstream molecule of TLR2, which induces AvBD9 expression in chicken intestinal epithelial cells in response to L. rhamnosus MLGA and WPG, and that ERK and p38 MAPKs are not necessary. A similar result was reported by Wehkamp et al. (Citation2004), which demonstrated that among the three main MAPKs, only JNK was involved in human β-defensin-2 (hBD-2) regulation in intestinal epithelial cells by probiotic Escherichia coli Nissle 1917. The involvement of JNK activity in L. rhamnosus MLGA- and WPG-induced AvBD9 mRNA expression suggests that the AP-1 pathway contributes to the induction of AvBD9 expression due to the fact that there are three binding sites for AP-1 in the promoter region of the AvBD9 gene (van Dijk et al., Citation2007). This was confirmed by EMSA analysis demonstrating that DNA-binding activity of AP-1 was increased in intestinal epithelial cells following treatment with L. rhamnosus MLGA and WPG.
NF-κB is another well-studied protein involved in signalling pathways. Following activation, the NF-κB heterodimer rapidly translocates to the nucleus, where it activates the transcription of target genes, including genes encoding cytokines, interferons, and defensins – all of which participate in the innate immune response (Gan et al., Citation2014; Lajczak et al., Citation2017; Pahl, Citation1999). The importance of NF-κB signalling for β-defensin expression in epithelial cells of mammals and humans has been well documented (Gan et al., Citation2014; Jin et al., Citation2018; Kaiser & Diamond, Citation2000; Ogushi et al., Citation2001; O’Neil et al., Citation1999; Schlee et al., Citation2007, Citation2008; Wehkamp et al., Citation2004). In addition to several binding sites for AP-1, there is one binding site for NF-κB in the promoter region of the AvBD9 gene. We observed that PDTC, a specific inhibitor of NF-κB, almost completely suppressed AvBD9 transcription induced by L. rhamnosus MLGA and its cell wall component WPG, indicating that NF-κB plays a critical role in the regulation of the L. rhamnosus MLGA- and WPG-mediated AvBD9 gene induction. The involvement of NF-κB in the signalling pathway leading to induction of AvBD9 gene expression by L. rhamnosus MLGA and WPG was further supported by our EMSA data, which showed that the DNA-binding activity of NF-κB was increased in L. rhamnosus MLGA- and WPG-treated cells. Moreover, our results showed that NF-κB inhibitor PDTC exhibited a more pronounced inhibition on the induction of AvBD9 by L. rhamnosus MLGA and WPG than the JNK inhibitor SP600125, and that activation of the NF-κB pathway upon L. rhamnosus MLGA or WPG stimulation is more potent than AP-1 pathway, as demonstrated by EMSA data. Collectively, these data suggest that the induction of AvBD9 expression in chicken intestinal epithelial cells by L. rhamnosus MLGA and its cell wall component is co-mediated by the NF-κB and JNK-AP-1 signalling pathways, with the NF-κB-dependent induction of AvBD9 being more important than AP-1 signalling. However, different bacterial stimuli, including probiotic and pathogenic bacteria, may have opposite effects on NF-κB signalling. Krisanaprakornkit et al. (Citation2002) reported that NF-κB is neither essential nor sufficient for the induction of hBD-2 in gingival epithelial cells by the cell wall of the commensal Fusobacterium nucleatum, and that hBD-2 induction by F. nucleatum was regulated via p38- and JNK-AP-1 dependent pathways. Similarly, Li et al. (Citation2013) found that the induction of hBD-2 and hBD-3 by a lipopeptide from the skin commensal Staphylococcus epidermidis in human epidermal keratinocytes required p38 MAPK activation, but not NF-κB activation. Several studies have shown that pathogen-dependent hBD-3 expression in various types of epithelial cells is independent of NF-κB and is mediated through a MAPK-AP-1-dependent pathway (Haarmann et al., Citation2015; Menzies & Kenoyer, Citation2006; Scharf et al., Citation2010; Scharf et al., Citation2012). Schlee et al. (Citation2008) observed a different contribution of NF-κB or AP-1 to hBD-2 induction for each probiotic Lactobacillus strain. Thus, the importance of the AP-1 or NF-κB signalling pathway in the regulation of defensin induction appears to be dependent on the cell type and stimulant source, most probably due to the differences in the MAMP molecules of specific species and expression patterns of pattern recognition receptors in different cells.
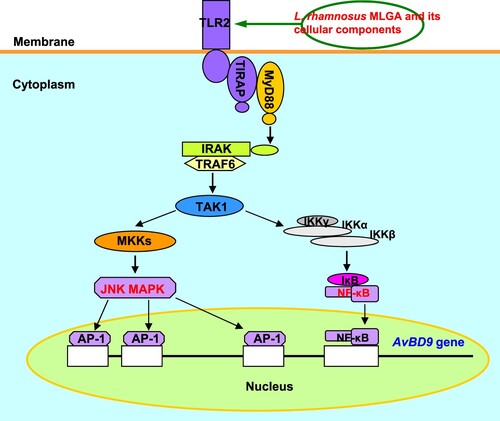
In conclusion, our data indicate that TLR2-mediated signalling pathways, including MAPK-AP-1 and NF-κB, are involved in the AvBD9 induction by L. rhamnosus MLGA and its cell wall component WPG, with NF-κB being more important than AP-1 (). Our study revealed that probiotic bacteria, including their cell wall components and secreted factors, may potentially exert beneficial effects on the host by inducing the expression of antimicrobial peptides, such as β-defensins, via TLR-mediated signalling pathways in the small intestinal epithelium. Furthermore, our results provide insight into understanding the underlying molecular mechanism by which probiotic bacteria modulate the gastrointestinal immune system.
Figure 5. Schematic diagram of the role of TLR2-mediated signalling in AvBD9 induction in chicken intestinal epithelial cells by L. rhamnosus MLGA and its whole cell wall peptidoglycan. The NF-κB and JNK MAPK signalling pathways are activated by TLR2 stimulation with L. rhamnosus MLGA or its whole cell wall peptidoglycan, leading to the induction of AvBD9 expression.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahn, S. I., Kim, J. S., Hong, C. Y., Gu, G. J., Shin, H. M., Paek, J. H., … Youn, H. S. (2016). Eupatorium makinoi suppresses toll-like receptor signaling pathways. Food & Agricultural Immunology, 27, 242–250. doi: 10.1080/09540105.2015.1086315
- Avila, E. E. (2017). Functions of antimicrobial peptides in vertebrates. Current Protein & Peptide Science, 18, 1098–1119. doi: 10.2174/1389203717666160813162629
- Banerjee, A., & Gerondakis, S. (2007). Coordinating TLR-activated signaling pathways in cells of the immune system. Immunology & Cell Biology, 85, 420–424. doi: 10.1038/sj.icb.7100098
- Bhattacharyya, S., Ghosh, S. K., Shokeen, B., Eapan, B., Lux, R., Kiselar, J., … Weinberg, A. (2016). FAD-I, a Fusobacterium nucleatum cell wall-asociated diacylated lipoprotein that mediates human beta defensin 2 induction through Toll-like receptor-1/2 (TLR-1/2) and TLR-2/6. Infection & Immuity, 84, 1446–1456. doi: 10.1128/IAI.01311-15
- Brownlie, R., & Allan, B. (2011). Avian Toll-like receptors. Cell and Tissue Research, 343, 121–130. doi: 10.1007/s00441-010-1026-0
- Corthésy, B., Gaskins, H. R., & Mercenier, A. (2007). Cross-talk between probiotic bacteria and the host immune system. The Journal of Nurtrition, 137, 781S–790S. doi: 10.1093/jn/137.3.781S
- Cuperus, T., Coorens, M., van Dijk, A., & Haagsman, H. P. (2013). Avian host defense peptides. Developmental & Comparative Immunology, 41, 352–369. doi: 10.1016/j.dci.2013.04.019
- Delcenserie, V., Martel, D., Lamoureux, M., Amiot, J., Boutin, Y., & Roy, D. (2008). Immunomodulatory effects of probiotics in the intestinal tract. Current Issues in Molecular Biology, 10, 37–54.
- Fan, Y. R., Jin, X., Tian, Q. Z., Zhang, M., Liu, J., & Yang, Y. F. (2016). Preliminary study on the pathway of SBD-1 expression in sheep rumen epithelial cells induced by Lactobacillus plantarum. Acta Veterinaria et Zootechnica Sinica, 47, 1026–1032. doi: 10.11843/j.issn.0366-6964.2016.05.021
- Galdeano, C. M., de Moreno de LeBlanc, A., Vinderola, G., Bonet, M. E., & Perdigón, G. (2007). Proposed model: Mechanisms of immunomodulation induced by probiotic bacteria. Clinical & Vaccine Immunology, 14, 485–492. doi: 10.1128/CVI.00406-06
- Gan, Y., Cui, X., Ma, T., Liu, Y., Li, A., & Huang, M. (2014). Paeoniflorin upregulates β-defensin-2 expression in human bronchial epithelial cell through the p38 MAPK, ERK, and NF-κB signaling pathways. Inflammation, 37, 1468–1475. doi: 10.1007/s10753-014-9872-7
- Ghadimi, D., Hassan, M., Njeru, P. N., de Vrese, M., Geis, A., Shalabi, S. I., … Schrezenmeir, J. (2011). Suppression subtractive hybridization identifies bacterial genomic regions that are possibly involved in hBD-2 regulation by enterocytes. Molecular Nutrition & Food Research, 55, 1533–1542. doi: 10.1002/mnfr.201100052
- Giahi, L., Elmadfa, I., Hoseini, M., & Klein, P. (2013). Heat-inactivated Lactobacillus rhamnosus and Lactobacillus delbrueckii induce efficient maturation and differential cytokine production in human monocyte derived dendritic cells. Food & Agricultural Immunology, 24, 95–109. doi: 10.1080/09540105.2011.651445
- Gupta, S., Ghosh, S. K., Scott, M. E., Bainbridge, B., Jiang, B., Lamont, R. J., … Weinberg, A. (2010). Fusobacterium nucleatum-associated beta-defensin inducer (FAD-I): identification, isolation, and functional evaluation. Journal of Biological Chemistry, 285, 36523–36531. doi: 10.1074/jbc.M110.133140
- Haarmann, H., Steiner, T., Schreiber, F., Heinrich, A., Zweigner, J., Die N’Guessan, P., & Slevogt, H. (2015). The role and regulation of Moraxella catarrhalis-induced human beta-defensin 3 expression in human pulmonary epithelial cells. Biochemical and Biophysical Research Communications, 467, 46–52. doi: 10.1016/j.bbrc.2015.09.126
- Habil, N., Abate, W., Beal, J., & Foey, A. D. (2014). Heat-killed probiotic bacteria differentially regulate colonic epithelial cell production of human β-defensin-2: Dependence on inflammatory cytokines. Beneficial Microbes, 5, 483–495. doi: 10.3920/BM2013.0061
- Han, J., Wang, Y., Song, D., Lu, Z., Dong, Z., Miao, H., … Li, A. (2018). Effects of Clostridium butyricum and Lactobacillus plantarum on growth performance, immune function and volatile fatty acid level of caecal digesta in broilers. Food & Agricultural Immunology, 29, 797–807. doi: 10.1080/09540105.2018.1457013
- Hong, Z. M., Jia, Y. J., Qiu, M. R., Li, G. H., & Liu, S. G. (2011). Isolation and primary culture of chicken embryo small intestinal epithelial cells. Acta Agriculturae Universitatis Jiangxiensis, 33, 1164–1170. doi: 10.13836/j.jjau.2011209
- Jang, B. C., Lim, K. J., Paik, J. H., Kwon, Y. K., Shin, S. W., Kim, S. C., … Suh, S. I. (2004). Up-regulation of human beta-defensin 2 by interleukin- 1β in A549 cells: Involvement of PI3K, PKC, p38 MAPK, JNK, and NF-kappaB. Biochemical & Biophysical Research Communications, 320, 1026–1033. doi: 10.1016/j.bbrc.2004.06.049
- Jensen, G. S., Cash, H. A., Farmer, S., & Keller, D. (2017). Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro. Joural of Inflammation Research, 10, 107–117. doi: 10.2147/JIR.S141660
- Jeong, J. J., Lee, H. J., Jang, S. E., Han, M. J., & Kim, D. H. (2018). Lactobacillus plantarum C29 alleviates NF-κB activation and Th17/Treg imbalance in mice with TNBS-induced colitis. Food & Agricultural Immunology, 29, 577–589. doi: 10.1080/09540105.2017.1418841
- Ji, S., Shin, J. E., Kim, Y. S., Oh, J. E., Min, B. M., & Choi, Y. (2009). Toll-like receptor 2 and NALP2 mediate induction of human beta-defensins by Fusobacterium nucleatum in gingical epithelial cells. Infection & Immunity, 77, 1044–1052. doi: 10.1128/IAI.00449-08
- Jin, X., Zhang, M., Zhu, X. M., Fan, Y. R., Du, C. G., Bao, H. E., … Yang, Y. F. (2018). Modulation of ovine SBD-1 expression by Saccharomyces cerevisiae in ovine ruminal epithelial cells. BMC Veterinary Research, 14, 134. doi: 10.1186/s12917-018-1445-9
- Ju, S. M., Goh, A. R., Kwon, D. J., Youn, G. S., Kwon, H. J., Bae, Y. S., … Park, J. (2012). Extracellular HIV-1 Tat induces human beta-defensin-2 production via NF-kappaB/AP-1 dependent pathways in human B cells. Molecules & Cells, 33, 335–341. doi: 10.1007/s10059-012-2287-0
- Kaiser, V., & Diamond, G. (2000). Expression of mammalian defensin genes. Journal of Leukocyte Biology, 68, 779–784. doi: 10.1189/jlb.68.6.779
- Kaminska, B. (2005). MAPK signaling pathways as molecular targets for anti-inflammatory therapy - from molecular mechanisms to therapeutic benefits. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1754, 253–262. doi: 10.1016/j.bbapap.2005.08.017
- Kim, Y. J., Shin, H. S., Lee, J. H., Jung, Y. W., Kim, H. B., & Ha, U. H. (2013). Pneumolysin-mediated expression of beta-defensin 2 is coordinated by p38 MAP kinase-MKP1 in human airway cells. Journal of Microbiology, 51, 194–199. doi: 10.1007/s12275-013-2579-x
- Krisanaprakornkit, S., Kimball, J. R., & Dale, B. A. (2002). Regulation of human β-Defensin-2 in gingival epithelial cells: The involvement of mitogen-activated protein kinase pathways, but not the NF-κB transcription factor family. The Journal of Immunology, 168, 316–324. doi: 10.4049/jimmunol.168.1.316
- Kumar, A., Zhang, J., & Yu, F. S. (2006). Toll-like receptor 2-mediated expression of β-defensin-2 in human corneal epithelial cells. Microbes & Infection, 8, 380–389. doi: 10.1016/j.micinf.2005.07.006
- Lai, Y., & Gallo, R. L. (2009). AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends in Immunology, 30, 131–141. doi: 10.1016/j.it.2008.12.003
- Lajczak, N. K., Saint-Criq, V., O’Dwyer, A. M., Perino, A., Adorini, L., Schoonjans, K., & Keely, S. J. (2017). Bile acids deoxycholic acid and ursodeoxycholic acid differentially regulate human β-defensin-1 and -2 secretion by colonic epithelial cells. The FASEB Journal, 31, 3848–3857. doi: 10.1096/fj.201601365R
- Lee, I. C., Caggianiello, G., van Swam, I. I., Taverne, N., Meijerink, M., Bron, P. A., … Schaffner, D. W. (2016). Strain-specific features of extracellular polysaccharides and their impact on Lactobacillus plantarum-host interactions. Applied & Environmental Microbiology, 82, 3959–3970. doi: 10.1128/AEM.00306-16
- Lee, H. Y., Takeshita, T., Shimada, J., Akopyan, A., Woo, J. I., Pan, H., … Lim, D. (2008). Induction of beta defensin 2 by NTHi requires TLR2 mediated MyD88 and IRAK-TRAF6-p38MAPK signaling pathway in human middle ear epithelial cells. BMC Infectious Diseases, 8, 87. doi: 10.1186/1471-2334-8-87
- Li, G. H., Hong, Z. M., Jia, Y. J., You, J. M., Zhang, J. H., & Liu, B. S. (2012). Probiotic Lactobacilli stimulate avian beta-defensin 9 expression in cultured chicken small intestinal epithelial cells. Proceedings of the Nutrition Society, 71, E239. doi: 10.1017/S0029665112003308
- Li, D., Lei, H., Li, Z., Li, H., Wang, Y., Lai, Y., & Ryffel, B. (2013). A novel lipopeptide from skin commensal activates TLR2/CD36-p38MAPK signaling to increase antibacterial defense against bacterial infection. PLoS One, 8, e58288. doi: 10.1371/journal.pone.0058288
- Lynn, D. J., Higgs, R., Gaines, S., Tierney, J., James, T., Lloyd, A. T., … O’Farrelly, C. (2004). Bioinformatic discovery and initial characterization of nine novel antimicrobial peptides genes in the chicken. Immunogenetics, 56, 170–177. doi: 10.1007/s00251-004-0675-0
- Madi, A., Alnabhani, Z., Leneveu, C., Mijouin, L., Feuilloley, M., & Connil, N. (2013). Pseudomonas fluorescens can induce and divert the human beta-defensin-2 secretion in intestinal epithelial cells to enhance its virulence. Archives of Microbiology, 195, 189–195. doi: 10.1007/s00203-012-0865-3
- Maldonada-Contreras, A. L., & McCormick, B. A. (2011). Intestinal epithelial cells and their role in innate mucosal immunity. Cell & Tissue Research, 343, 5–12. doi: 10.1007/s00441-010-1082-5
- Maynard, C. L., Elson, C. O., Hatton, R. D., & Weaver, C. T. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature, 489, 231–241. doi: 10.1038/nature11551
- Menzies, B. E., & Kenoyer, A. (2006). Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human beta-defensin 3 in skin keratinocytes. Infection & Immunity, 74, 6847–6854. doi: 10.1128/IAI.00389-06
- Miörner, H., Johansson, G., & Kronvall, G. (1983). Lipoteichoic acid is the major cell wall component responsible for surface hydrophobicity of group A streptococci. Infection & Immunity, 39, 336–343
- Möndel, M., Schroeder, B. O., Zimmermann, K., Huber, H., Nuding, S., Beisner, J., … Wehkamp, J. (2009). Probiotic E. coli treatment mediates antimicrobial human beta-defensin synthesis and fecal excretion in humans. Mucosal Immunology, 2, 166–172. doi: 10.1038/mi.2008.77
- Ng, S. C., Hart, A. L., Kamm, M. A., Stagg, A. J., & Knight, S. C. (2009). Mechanisms of action of probiotics: Recent advances. Inflammatory Bowel Diseases, 15, 300–310. doi: 10.1002/ibd.20602
- O’Flaherty, S., Saulnier, D. M., Pot, B., & Versalovic, J. (2010). How can probiotics and prebiotics impact mucosal immunity? Gut Microbes, 1, 293–300. doi: 10.4161/gmic.1.5.12924
- Ogushi, K., Wada, A., Niidome, T., Mori, N., Oishi, K., Nagatake, T., … Hirayama, T. (2001). Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells. Journal of Biological Chemistry, 276, 30521–30526. doi: 10.1074/jbc.M011618200
- O’Neil, D. A., Porter, E. M., Elewaut, D., Anderson, G. M., Eckmann, L., Ganz, T., & Kagnoff, M. F. (1999). Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. Journal of Immunology, 163, 6718–6724.
- Pahl, H. L. (1999). Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene, 18, 6853–6866. doi: 10.1038/sj.onc.1203239
- Paolillo, R., Carratelli, C. R., Sorrentino, S., Mazzola, N., & Rizzo, A. (2009). Immunomodulatory effects of Lactobacillus plantarum on human colon cancer cells. International Immunopharmacology, 9, 1265–1271. doi: 10.1016/j.intimp.2009.07.008
- Rizzo, A., Losacco, A., Romano, C., & Carratelli, C. R. (2013). Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human β-defensins 2 and 3. Immunology Letters, 156, 102–109. doi: 10.1016/j.imlet.2013.08.013
- Sanchez, B., Urdaci, M. C., & Margolles, A. (2010). Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa-bacteria interactions. Microbiology, 156, 3232–3242. doi: 10.1099/mic.0.044057-0
- Scharf, S., Vardarova, K., Lang, F., Schmeck, B., Opitz, Z., Flieger, A., … N’Guessan, P. D. (2010). Legionella pneumophila induces human beta defensin-3 in pulmonary cells. Respiratory Research, 11, 93. doi: 10.1186/1465-9921-11-93
- Scharf, S., Zahlten, J., Szymanski, K., Hippenstiel, S., Suttorp, N., & N’Guessan, P. D. (2012). Streptococcus pneumoniae induces human β-defensin-2 and -3 in human lung epithelium. Experimental Lung Research, 38, 100–110. doi: 10.3109/01902148.2011.652802
- Schlee, M., Harder, J., Köten, B., Stange, E. F., Wehkamp, J., & Fellermann, K. (2008). Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clinical & Experimental Immunology, 151, 528–535. doi: 10.1111/j.1365-2249.2007.03587.x
- Schlee, M., Wehkamp, J., Altenhoefer, A., Oelschlaeger, T. A., Stange, E. F., & Fellermann, K. (2007). Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infection & Immunity, 75, 2399–2407. doi: 10.1128/IAI.01563-06
- Schmitt, P., Wacyk, J., Morales-Lange, B., Rojas, V., Guzmán, F., Dixon, B., & Mercado, L. (2015). Immunomodulatory effect of cathelicidins in response to a β-glucan in intestinal epithelial cell from rainbow trout. Develpomental & Comparative Immunology, 51, 160–169. doi: 10.1016/j.dci.2015.03.007
- Sekine, K., Toida, T., Saito, M., Kubyama, M., Kawashima, T., & Hashimoto, Y. (1985). A new morphologically characterized cell wall preparation (whole peptidoglycan) from Bifidobacterium infantis with a higher efficacy on the regression of an established tumor in mice. Cancer Research, 45, 1300–1307.
- Śliżewska, K., & Markowiak, P. (2018). The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathogens, 10, 21. doi: 10.1186/s13099-018-0250-0
- St Paul, M., Paolucci, S., & Sharif, S. (2013). Characterization of responses initiated by different Toll-like 2 ligands in chicken spleen cells. Research in Veterinary Science, 95, 919–923. doi: 10.1016/j.rvsc.2013.06.025
- Sukhithasri, V., Nisha, N., Biswas, L., Anil Kumar, V., & Biswas, R. (2013). Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiological Research, 168, 396–406. doi: 10.1016/j.micres.2013.02.005
- Sunkara, L. T., Achanta, M., Schreiber, N. B., Bommineni, Y. R., Dai, G., Jiang, W., … Zhang, G. (2011). Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One, 6, e27225. doi: 10.1371/journal.pone.0027225
- Vaishnava, S., Behrendt, C. L., Ismail, A. S., Eckmann, L., & Hooper, L. V. (2008). Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host microbial interface. Proceedings of the National Academy of Sciences, 105, 20858–20863. doi: 10.1073/pnas.0808723105
- van Dijk, A., Veldhuizen, E. J. A., & Haagsman, H. P. (2008). Avian defensins. Veterinary Immunology and Immunopathology, 124, 1–18. doi: 10.1016/j.vetimm.2007.12.006
- van Dijk, A., Veldhuizen, E. J., Kalkhove, S. I., Tjeerdsmavan Bokhoven, J. L., Romijn, R. A., & Haagsman, H. P. (2007). The β-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrobial Agents & Chemotherapy, 51, 912–922. doi: 10.1128/AAC.00568-06
- Vasselon, T., Hanlon, W. A., Wright, S. D., & Detmers, P. A. (2002). Toll-like receptor 2 (TLR2) mediates activation of stress-activated MAP kinase p38. Journal of Leukocyte Biology, 71, 503–510. doi: 10.1189/jlb.71.3.503
- Vora, P., Youdim, A., Thomas, L. S., Fukata, M., Tesfay, S. Y., Lukasek, K., … Abreu, M. T. (2004). Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. The Journal of Immunology, 173, 5398–5405. doi: 10.4049/jimmunol.173.9.5398
- Wang, G., Feng, Y., Wang, Y., Huang, N., Wu, Q., & Wang, B. (2003). Bifidobacterium cell wall proteins induced beta-defensin 2 mRNA expression in human intestinal epithelial cells. Journal of Sichuan University. Medical Science Edition, 34, 622–624.
- Wehkamp, J., Harder, J., Wehkamp, K., Wehkamp-von Meissne, B., Schlee, M., Enders, C., … Stange, E. F. (2004). NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: A novel effect of a probiotic bacterium. Infection & Immunity, 72, 5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004
- Xiao, Y., Hughes, A. L., Ando, J., Matsuda, Y., Cheng, J. F., Skinner-Noble, D., & Zhang, G. (2004). A genome-wide screen identifies a single β-defensin gene cluster in the chicken: Implications for the origin and evolution of mammalian defensins. BMC Genomics, 5, 56–66. doi: 10.1186/1471-2164-5-56
- Yacoub, H. A., El-Hamidy, S. M., Mahmoud, M. M., Nabih Baeshen, M., Almehdar, H. A., Uversky, V. N., … Elazzazy, A. M. (2016). Biocidal activity of chicken defensin-9 against microbial pathogens. Biochemistry & Cell Biology, 94, 176–187. doi: 10.1139/bcb-2015-0121
- Yuan, W., Jin, H. T., Ren, Z. H., Deng, J. L., Zuo, Z. C., Wang, Y., … Deng, Y. T. (2015). Effects of antibacterial peptide on humoral immunity in weaned piglets. Food & Agricultural Immunology, 26, 682–689. doi: 10.1080/09540105.2015.1007448
- Zasloff, M. (2002). Antimicrobial peptides of multicellular organisms. Nature, 415, 389–395. doi: 10.1038/415389a
- Zhang, M., Jin, X., & Yang, Y. F. (2019). β-Glucan from Saccharomyces cerevisiae induces SBD-1 production in ovine ruminal epithelial cells via the Dectin-1-Syk-NF-κB signaling pathway. Cellular Signalling, 53, 304–315. doi: 10.1016/j.cellsig.2018.10.018
