?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A rapid, ultra-sensitive and simple fluorescence immunoassay (FLISA) on CdSe/ZnS Quantum dots (QDs) for recognition pesticide triazophos have been established. In this assay, the immunoassay uses (CdSe/ZnS) Quantum dots (QDs) as a probe that carries monoclonal antibody (mAb). The free triazophos and OVA-haptens were competed for binding the mAb on the surface of the QDs. Therefore, the concentration of TRIAZ can get by calculation of the detection of fluorescence value. The assay has a linear response in the 10 ng L−1–25 μg L−1 TRIAZ concentration range, and limit of detection (IC10) was 0.508 ng L−1. The recovery rate in the case of spiked samples ranges from 82.6% to 96.6%, and the RSD is <20%. FLISA can accurately and sensitively screen out triazophos in representative actual samples that contain trace levels of pesticides, it can play a prominent part in agricultural product and environmental detection.
Introduction
Organophosphorus Pesticides (OPs) are extremely important compounds in pesticides due to their diverse varieties, high efficacy, wide application and easy decomposition. However, some varieties are highly toxic, and their widespread use can effectively reduce pests and diseases and increase crop yields, but at the same time, their trace residues in the environment and agricultural products endanger the health of animals and humans (Hua et al., Citation2006; Lai, Citation2017; Vermeire, MacPhail, & Waters, Citation2003). Triazophos (O,O-diethyl O-(1-phenyl-1H-1,2,4-triazol-3-yl) phosphorothioate) is a broad-spectrum, moderately toxic insecticide that has been mainly used in China on fruit trees, cotton, and grain crops. This pesticide is moderately toxic to mammals and exhibits inhibition of acetylcholinesterase activity, which reduces its activity and affects central nervous function (Chen et al., Citation2015; Du, Cai, Song, & Zhang, Citation2007). In recent years as a good alternative to highly toxic organophosphates such as parathion, parathion methyl, and methamidophos (Liu et al., Citation2013; Rainina, Efremenco, Varfolomeyev, Simonian, & Wild, Citation1996). In nowadays, the traditional analytical methods for detection of triazophos include gas chromatography (GC) (Andrade et al., Citation2015; Matyus et al., Citation2012) and liquid or gas chromatography coupled with mass spectrometry (MS), and the methods have good stability and high sensitivity. However, the detection takes a long time, requires professional operation and complicated sample pre-treatment, it is not suitable for real-time detection and on-side field detection, and more importantly, the reagents required during sample processing will be polluted environment again (Du et al., Citation2015; Gong et al., Citation2017). In a large number of instances, immunoassays have been identified as a special tool for pesticide detection due to its simple, inexpensive, and rapid characteristics. Among these immunoassays, enzyme linked immunosorbent assay (ELISA) is widely popular; however, sometimes its sensitive undesirable (Tochi et al., Citation2015; Wang et al., Citation2017; Zhang et al., Citation2018). Therefore, establish a highly sensitive detection may be increasingly important.
Quantum dots (QDs) are semiconductor fluorescent nanomaterials developed in the past 20 years with excellent spectral characteristics and photochemical stability (Ji et al., Citation2005;Rainina, et al.,Citation1996; Zhang et al., Citation2015). Compared with other bioluminescent dyes, it has a wide range of excitation lines, narrow emission lines, high luminous efficiency, adjustable luminescent colour and good light stability, and is very suitable as a fluorescent marker (Frigerio et al., Citation2012; Liu et al., Citation2013; Rainina et al., Citation1996; Tochi et al., Citation2015). Using it as a marker can significantly reduce the detection limit of the detection method, thereby improving the sensitivity and resolution of the analytical method. When a quantum dot is used as a fluorescent probe, the surface thereof needs to be modified with a biomolecule such as an antibody, a polypeptide, a saccharide or the like. Currently, there are some types of coupling techniques used quantum dots conjugates with the antibody. Among them, the most commonly used covalent bonding method is to directly couple an antibody and a quantum dot together using a crosslinking agent 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) (Anastassiades, Lehotay, Stajnbaher, & Schenck, Citation2003; Piloto et al., Citation2018; Ji et al., Citation2005; Zhang et al., Citation2017). In this reaction, the carboxyl group on the surface of the quantum dot is first activated by N-Hydroxysuccinimide (NHS), and the activated carboxyl group forms an amide bond with the original amino group on the surface of the antibody under the action of EDC (Ji et al., Citation2005; Wang et al., Citation2017; Wu et al., Citation2019). In addition, there are non-covalent bond binding methods, such as biotin and avidin non-covalent bond coupling, covalent bond bonding and amino group and sulfhydryl covalent bond bonding (Chen et al., Citation2010; Taton, Citation2000; Zajac, Song, Qian, & Zhukov, Citation2007).
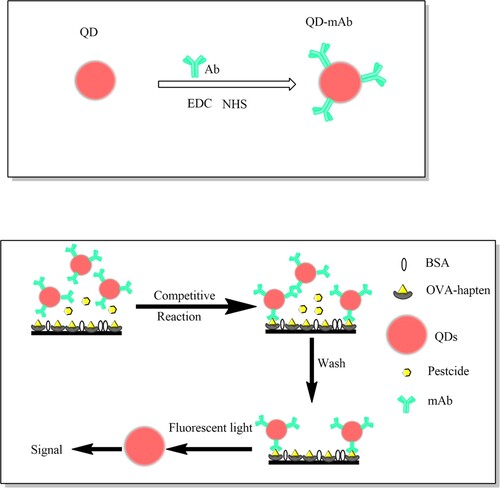
In this study, we developed a CdSe/ZnS QDs-based FLISA for the simple, sensitive and quantitative detection of triazophos in representative actual samples based on an anti-Triazophos monoclonal antibody. The QDs were modified with mAb, and after the competitive reaction between ovalbumin-linked haptens (OVA-haptens) and triazophos in the sample, the haptens-mAb-QDs were formed at the bottom of the microplate, as shown in Scheme 1. Used reliable and sensitive instrument analysis method UPLC-MS as the confirmed method of QD-based FLISA method. Our developed CdSe/ZnS QD-based FLISA displayed a highly sensitive, stable method for detection of triazophos residues in agro-products and environmental samples.
Materials and methods
Chemicals and materials
The standard for triazophos (98%) and structural analogues (>95%), bovine serum albumin (BSA), 1-ethy-3[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) and N-Hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The quantum dots (QDs) were purchased from Shanghai Kundao Biotech Co., Ltd (Shanghai, China). The triazophos haptens and monoclonal antibodies (mAbs) from mouse ascites were generously gifted by the Institute of Pesticide and Environmental Toxicology (IPET), Zhejiang University, China. Polyethylene glycol sorbitan monolaurate (Tween-20) were purchased from Beijing Solarbio Life Sciences. Octadecyl silyl (C18, 40–60 mm), and primary secondary amine (PSA, 40–60 mm) were obtained from Agela (Tianjin, China). LC-grade acetonitrile and methanol were supplied by Fisher Scientific (Pittsburgh, PA, USA). All other chemicals, such as anhydrous magnesium sulphate (MgSO4) and sodium acetate (NaCl) and organic solvents of analytical grade or higher were purchased from Beijing Chemical Industry Group Co., Ltd (Beijing, China). The Size exclusion chromatography (SEC)'s pillar was purchased from Biocomma Co., Ltd (Shenzhen, China) and SEC's filler (Superdex 200 prep grade) were obtained from GE Healthcare, Inc. (2018 General electric company, USA). Ultrafiltration centrifuge tube (4ML 100KD) were purchased form Millipore (Millipore, USA). Ninety-six well plates (transparent and black, flat bottom) were obtained from Corning, Inc. (Corning, NY, USA). Ultrapure water (18.2 MU cm) produced by a Milli-Q water purification system (Millipore, Bedford, MA, USA) was used in all e experimental works.
Buffers solution
The buffers used in this study were as follows: salting buffer (100 mM phosphate buffered saline (PBS)); blocking and storage buffer (consisting of 10 mM PBS (pH 7.4), 1% BSA); coating buffer (consisting of 50 mM carbonate buffer solution (CBS, pH 9.6)); washing buffer (PBST) (consisting of 10 mM PBS (pH 7.4) and 0.05% Tween 20); blocking buffer (consisting of 10 mM PBS (pH 7.4) and 2% BSA); assay buffer (consisting of 10 mM PBS (pH 7.4) and 0.05% BSA).
Antibody conjugation
Used a previously reported method conjugates of hydrophilic CdSe/ZnS QDs surfaced by carboxyl, and an-triazophos antibody. Briefly, added the 25 μL QDs (8 μM) dissolved in phosphate buffer saline (PBS 10 mM, pH 7.4), 20 μL sulfo-NHS (500 nmol), 140 μL EDC (500 nmol), 40 μL PBST buffer (PBS with 0.01% Tween-20) in to a tube, incubated for 30 min. After that, mAb (4.53 μg) were added into the mixture was incubated at 37°C for 2 h. Then, BSA (1 mg) was added into the solution, and shaken for another 30 min. The solution was purified by centrifugation at 3700 rpm for 10 min in an ultrafiltration centrifuge tube (100 KD) concentrate solution to 100 μL. After that, used SEC to remove free antibodies and small molecules and under UV lighter (365 nm) to make sure all the QDs-mAb move off. The purified QDs-mAb were dispersed in PBS buffer and stored at 4°C.
Competitive QDs-based fluorescence immunoassay
Add 100 μL per well of OVA-triazophos hapten in CBS to a black polystyrene microplate and incubated overnight at 4°C. The coated plate was washed three times with PBST followed by blocking with 300 μL blocking buffer for 1 h at 37°C. Then 50 μL of sample extract or triazophos standard solution, in 10% (v/v) methanol-PBS (0.01 M, pH 7.4), and 50 μL QDs-mAb probes, diluted in assay buffer, were sequentially added together into the microplate. During incubation at 37°C for 1 h, the OVA-triazophos hapten coating the bottom of the microplate and the triazophos in the sample compete for binding with the mAb adsorbed on the surface of the QDs. During incubation at 37°C for 1 h, the hapten coating was compete with the triazophos in the sample to binding the mAb adsorbed on the surface of the QDs in the bottom of the microplate. After that, PBST wash three times removed the unbound pesticide and QDs-mAb probes. The inhibition of fluorescence intensity (Ex341 nm/Em635 nm, Infinite M200 PRO microplate reader, TECAN, Switzerland) has a linear relationship with the logarithm of the pesticide concentration.
Sample treatment
The pesticide-free triazophos samples of apples, pear, cucumbers and rice purchased from local markets in Beijing. All samples were confirmed to be free from triazophos by using LC Scheme MS/MS. The pre-treatments samples were carried out according to the QuEChERS (quick, easy, cheap, effective, rugged, and safe) method (Anastassiades et al., Citation2003). In the standard curve and recovery studies, an appropriate volume of triazophos standard solutions in methanol was added to the samples and the fortification level of recovery assay was 10, 50, 100 μg kg−1. First, 10 g (5 g for rice) samples were weighed into 50 mL centrifuge tubes and added triazophos into the tube left them alone overnight. Mixed thoroughly by vortexing for 1 min with 10 mL acetonitrile (5 mL of water was added to the rice samples before acetonitrile). Then adding 4 g MgSO4 and 1 g NaCl to the mixture was vigorously shaken for 1 min and then centrifuged for 5 min at 5000 rpm. Afterward, 2 mL of supernatant was transferred to a 10 mL centrifuge tube containing 150 mg PSA, 150 mg C18 and vortexing for 1 min. The tubes were centrifuged for 5 min at 10,000 rpm. Half of the supernatant was transferred to injection vials for LC-MS/MS detection and the other half of the supernatant was concentrated under a stream of nitrogen. The residue was dissolved in 10% methanol-PBS and examined for competitive QDs-based fluorescent immunoassay.
Results and discussion
QDs probe preparation and optimisation
Quantum dots are often used in laboratories as labelling probe detection because of its simple conjugate methods, stability, wide range of excitation lines and narrow emission lines. The QDs coupled with mAb was based on the carboxyl group in the surface of the quantum dots to the amino group on the antibody. And used size exclusion chromatography (SEC)'s to purify the solution. After the QDs has conjugation with mAb, the QDs-mAb can visualise with the unaided eye under UV light. A previous study indicated that mAb can improve remarkably fluorescence intensity of CdSe QDs. The mechanism is attributed to sulphurs in mAb, which possess a strong affinity towards Cd2+, can improve greatly recombination fluorescence of CdSe QDs by creating more radiative centres at CdSe/Cd-SR complex.(Gong et al., Citation2016; Khantaw, Boonmee, Tuntulani, & Ngeontae, Citation2013) This paper we use QDs was 10 nm. The raw QDs exhibited a maximum absorption at 322 nm.
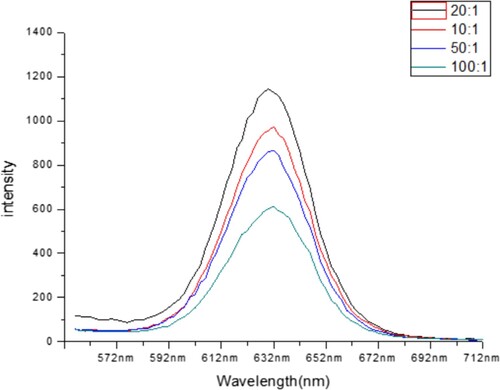
Otherwise, we have a concentration of mAb and QDs were optimised by adding a series amount of mAb to the final molar ratio of mAb and QDs of 10:1, 20:1, 50:1 and 100:1. As the results shown in , the fluorescence increased as the ratio increased from the 100:1 to 10:1 and in 20:1 has better fluorescence. Therefore, 4.53 mg L−1 mAbs and 1 μM QDs was used in subsequent experiments.
Characterisation of QDs-mAb probe
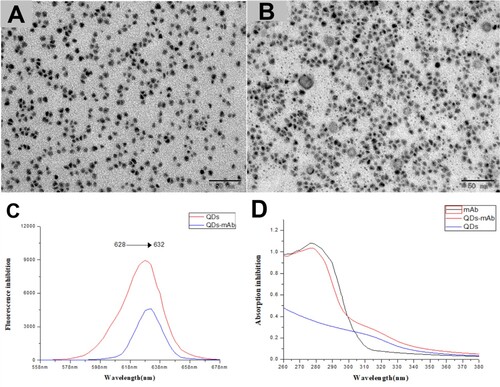
The morphologies of synthesised microspheres were characterised by transmission electron microscopy (TEM) (JEOL-JEM- 2100F, Japan), UV/Vis and fluorescent spectroscopy to qualitatively characterise the successful conjugation of QDs-mAb. The TEM () results indicate that the QDs (A), QDs-mAb(B) were well dispersed with uniform size. As shown in (A), the raw QDs ((A)) was coated by a grey diaphragm, which indicate the formation of QDs and mAb composites. As shown in (A), the maximum absorption wavelength of QDs and QDs-mAb displayed a redshift from 628 to 632 nm.
Figure 2. Characterisation of QDs-mAb probes. (A) TEM micrographs of QDs, (B) TEM micrographs of QDs-mAb. Normalised fluorescent spectra (C) of QDs (upper line) and QDs-mAb (lower line). The UV–Vis spectrum (D) of bare mAb (upper line), QDs modified with antibodies (middle line), and bare QDs (lower line).
Method establishment
Optimisation of the immune reagents
In this experiment, the concentrations of the reagents participating were performed use chessboard assay to select the best working concentrations of the OVA-hapten and QDs-mAb probes in an immunoassay. It can have a strong influence on the detection sensitivity and the linear range of competition reactions (Yan, Shi, & Wang, Citation2012). The concentrations of OVA-hapten were 1.25, 0.63, 0.31 and 0.16 mg L−1 and the QDs-mAb probes were prepared before. The results are shown in . In this work, used the inhibition rate (Formula 1), IC50 (the concentration of analyte that produced 50% inhibition of the maximum fluorescence intensity), IC10 (the concentration of analyte that produced 10% inhibition of the maximal fluorescence, defined as the limit of detection (LOD)), and ratio of maximal fluorescence to IC50 (Fmax/IC50) to evaluate the performance of the immunoassays. Lower IC50 and IC10 values indicate higher sensitivity, as do higher Fmax/IC50 values. The optimal concentrations of OVA-hapten and QDs-mAb were 1.25 and 4.53 mg L−1, respectively.
Table 1. Optimized working concentrations of QDs-mAb and OVA-hapten.
Standard curve construction
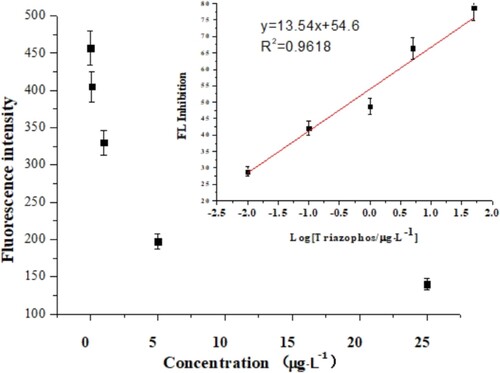
After the optimised conditions, a standard curve of the fluorescence immunoassay was generated using inhibition with the logarithm of the concentration of triazophos. As shown in , the competition curve (y = 13.54×+54.6, R2 = 0.9618, n = 3) showed a linear range of detection from 0.01 to –25 μg L−1. The average IC50 value was 0.457 μg L−1 and the LOD was 0.508 ng L−1:(1)
(1) where “I%” represents the inhibition rate, “Fx” is the signal related with the concentration of triazophos standard, “Fmax” represents the maximum signal (the concentration of triazophos was zero), and “Fmin” represents the signal of blank wells (no antibody).
Accuracy and precision
Recovery tests for spiked samples by QDs-based fluorescence immunoassay. The blank samples (apple, pear, cucumber and rice) were spiked with the triazophos standard solutions. The three levels (10, 50, and 100 μg kg−1) of triazophos were spiked in test samples. After sample pre-treatment, All the samples were screened the extracts by QDs-based fluorescence immunoassay (). The mean recovery rates with the competitive QDs-based FLISA were determined to range from 82.6% to 96.6% for triazophos, and the RSD (n = 5) ranged from 5.0% to 15.2%. In addition, the average of QDs-based FLISA recovery and RSD were 89.4% and 8.8%. The recoveries of the LC-MS/MS range from 82.1% and 107.1%, and the RSD (n = 5) range from 0.2% to 1.9%, respectively. The result indicates that the QDs-based fluorescence immunoassay is a reliable method for triazophos detection.
Table 2. Recoveries and relative standard deviations (RSDs) of the fluorescence immunoassay and LC-MS/MS.
Comparison between various fluorescence immunoassays
Comparison of the characteristics of this method and other fluorescence immunoassays is provided in . Those studies have a different method based on fluorescence immunoassays, such as fluorescence lateral flow test strip, fluorescence polarisation immunoassay, dual-label time-resolved fluorescence immunoassay, plate-based FLISA and nitrocellulose membrane-based flow-through FLISA. In this study, an approach using CdSe/ZnS quantum dots, which contained carboxyl, was used to labelled antibody. The developed QDs-based FLISA can avoid to use an enzyme-labelled secondary antibody, and reduce one washing step which saves time. Moreover, it compared with other method have lower LOD. Moreover, the currently developed method accurate and sensitive, and could be applied as convenient tools for the rapid detection of pesticide residue.
Table 3. Comparison of various fluorescence immunoassays.
Conclusions
In conclusion, we here report plate-based and QDs-based fluorescent immunoassays, which are sensitive and effective in detection of triazophos. The detection limit and IC50 were 0.508 and 457.4 ng L−1 and the working range is from 0.01 to –25 μg L−1. The QDs-based fluorescence immunoassay can apply as detection of pesticides residue in the environment, agricultural products and food safety.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anastassiades, M., Lehotay, S. J., Stajnbaher, D., & Schenck, F. J. (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. Journal of Aoac International, 86(2), 412–431.
- Andrade, G. C., Monteiro, S. H., Francisco, J. G., Figueiredo, L. A., Botelho, R. G., & Tornisielo, V. L. (2015). Liquid chromatography-electrospray ionization tandem mass spectrometry and dynamic multiple reaction monitoring method for determining multiple pesticide residues in tomato. Food Chemistry, 175, 57–65. doi: 10.1016/j.foodchem.2014.11.105
- Chen, Y. P., Ning, B., Liu, N., Feng, Y., Liu, Z., Liu, X., & Gao, Z. X. (2010). A rapid and sensitive fluoroimmunoassay based on quantum dot for the detection of chlorpyrifos residue in drinking water. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes, 45(6), 508–515. doi: 10.1080/03601234.2010.493476
- Chen, G., Yang, L., Jin, M., Du, P., Zhang, C., Wang, J., … Wang, J. (2015). The rapid screening of triazophos residues in agricultural products by chemiluminescent enzyme immunoassay. PLoS One, 10(7), e0133839. doi: 10.1371/journal.pone.0133839
- Du, D., Cai, J., Song, D., & Zhang, A. (2007). Rapid determination of triazophos using acetylcholinesterase biosensor based on sol–gel interface assembling multiwall carbon nanotubes. Journal of Applied Electrochemistry, 37(8), 893–898. doi: 10.1007/s10800-007-9328-y
- Du, P. F., Jin, M. J., Yang, L. H., Du, X. W., Chen, G., Zhang, C., … Wang, J. (2015). A rapid immunomagnetic-bead-based immunoassay for triazophos analysis. Rsc Advances, 5(99), 81046–81051. doi: 10.1039/c5ra15106f
- Frigerio, C., Ribeiro, D. S. M., Rodrigues, S. S. M., Abreu, V. L. R. G., Barbosa, J. A. C., Prior, J. A. V., … Santos, J. L. M. (2012). Application of quantum dots as analytical tools in automated chemical analysis: A review. Analytica Chimica Acta, 735, 9–22. doi: 10.1016/j.aca.2012.04.042
- Gong, T. T., Liu, J. F., Liu, X. X., Liu, J., Xiang, J. K., & Wu, Y. W. (2016). A sensitive and selective sensing platform based on CdTe QDs in the presence of L-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chemistry, 213, 306–312. doi: 10.1016/j.foodchem.2016.06.091
- Gong, T., Liu, J., Wu, Y., Xiao, Y., Wang, X., & Yuan, S. (2017). Fluorescence enhancement of CdTe quantum dots by HBcAb-HRP for sensitive detection of H2O2 in human serum. Biosensors & Bioelectronics, 92, 16–20. doi: 10.1016/j.bios.2017.01.048
- Hua, X. F., Liu, T. C., Cao, Y. C., Liu, B., Wang, H. Q., Wang, J. H., … Zhao, Y. D. (2006). Characterization of the coupling of quantum dots and immunoglobulin antibodies. Analytical and Bioanalytical Chemistry, 386(6), 1665–1671. doi: 10.1007/s00216-006-0807-5
- Ji, X. J., Zheng, J. Y., Xu, J. M., Rastogi, V. K., Cheng, T. C., DeFrank, J. J., & Leblanc, R. M. (2005). (Cdse)ZnS quantum dots and organophosphorus hydrolase bioconjugate as biosensors for detection of paraoxon. Journal of Physical Chemistry B, 109(9), 3793–3799. doi: 10.1021/jp044928f
- Khantaw, T., Boonmee, C., Tuntulani, T., & Ngeontae, W. (2013). Selective turn-on fluorescence sensor for Ag+ using cysteamine capped CdS quantum dots: Determination of free Ag+ in silver nanoparticles solution. Talanta, 115, 849–856. doi: 10.1016/j.talanta.2013.06.053
- Lai, W. (2017). Pesticide use and health outcomes: Evidence from agricultural water pollution in China. Journal of Environmental Economics and Management, 86, 93–120. doi: 10.1016/j.jeem.2017.05.006
- Li, S., Dong, M., Li, R., Zhang, L., Qiao, Y., Jiang, Y., & Qi, W. (2015). A fluorometric microarray with ZnO substrate-enhanced fluorescence and suppressed “coffee-ring” effects for fluorescence immunoassays. Nanoscale, 7(44), 18453–18458. https://doi.org/10.1039/C5NR06070B
- Liu, Y., Liu, R., Boroduleva, A., Eremin, S., Guo, Y., & Zhu, G. (2016). A highly specific and sensitive fluorescence polarization immunoassay for the rapid detection of triazophos residue in agricultural products. Analytical Methods, 8(36), 6636–6644. https://doi.org/10.1039/C6AY00908E
- Liu, J., Zhang, Q., Zhang, W., Ding, X., Hu, X., Zhao, F., & Li, P. (2013). Development of a fluorescence-linked immunoassay based on quantum dots for fenvalerate. Food and Agricultural Immunology, 25(1), 82–93. doi: 10.1080/09540105.2012.749220
- Mao, Q., Liu, X., Chen, C., Ye, J., Liang, H., Li, B., & Sun, X. (2018). A dual‐label time‐resolved fluorescence immunoassay for the simultaneous determination of carcinoembryonic antigen and squamous cell carcinoma antigen. Biotechnology and Applied Biochemistry, 65(6), 816–821. https://doi.org/10.1002/bab.2018.65.issue-6 doi: 10.1002/bab.1663
- Matyus, M., Kocsis, G., Boldis, O., Karvaly, G., Magyar, E., Furesz, J., & Gachalyi, A. (2012). Determination of morphine and codeine in serum after poppy seed consumption using Gas chromatography-mass spectrometry. Acta Chromatographica, 24(3), 351–365. doi: 10.1556/AChrom.24.2012.3.2
- Piloto, A. M., Ribeiro, D. S. M., Rodrigues, S. S. M., Santos, C., Santos, J. L. M., & Sales, M. G. F. (2018). Plastic antibodies tailored on quantum dots for an optical detection of myoglobin down to the femtomolar range. Scientific Reports, 8), doi:ARTN 4944 10.1038/s41598-018-23271-z
- Rainina, E. I., Efremenco, E. N., Varfolomeyev, S. D., Simonian, A. L., & Wild, J. R. (1996). The development of a new biosensor based on recombinant E-coli for the direct detection of organophosphorus neurotoxins. Biosensors & Bioelectronics, 11(10), 991–1000. doi: 10.1016/0956-5663(96)87658-5
- Taton, T. A. (2000). Scanometric DNA array detection with nanoparticle probes. Science, 289(5485), 1757–1760. doi: 10.1126/science.289.5485.1757
- Tochi, B. N., Khaemba, G., Isanga, J., Mukunzi, D., Liu, L., Peng, J., … Xu, C. (2015). Monoclonal antibody for the development of specific immunoassays to detect Enrofloxacin in foods of animal origin. Food and Agricultural Immunology, 27(4), 435–448. doi: 10.1080/09540105.2015.1089844
- Vermeire, T., MacPhail, R., & Waters, M. (2003). Integrated human and ecological risk assessment: A case study of organophosphorous pesticides in the environment. Human and Ecological Risk Assessment, 9(1), 343–357. doi:10.1080/718990537.
- Wang, J., Wang, Q., Zheng, Y., Peng, T., Yao, K., Xie, S., … Jiang, H. (2017). Development of a quantitative fluorescence-based lateral flow immunoassay for determination of chloramphenicol, thiamphenicol and florfenicol in milk. Food and Agricultural Immunology, 29(1), 56–66. doi: 10.1080/09540105.2017.1359498
- Wu, J., Ma, J., Wang, H., Qin, D., An, L., Ma, Y., … Wu, X. (2019). Rapid and visual detection of benzothiostrobin residue in strawberry using quantum dot-based lateral flow test strip. Sensors and Actuators B: Chemical, 283, 222–229. doi: 10.1016/j.snb.2018.11.137
- Yan, X., Shi, H., & Wang, M. (2012). Development of an enzyme-linked immunosorbent assay for the simultaneous determination of parathion and imidacloprid. Analytical Methods, 4(12), 4053. doi: 10.1039/c2ay25760b
- Zajac, A., Song, D., Qian, W., & Zhukov, T. (2007). Protein microarrays and quantum dot probes for early cancer detection. Colloids and Surfaces. B, Biointerfaces, 58(2), 309–314. doi: 10.1016/j.colsurfb.2007.02.019
- Zhang, C., Du, P., Jiang, Z., Jin, M., Chen, G., Cao, X., … Wang, J. (2018). A simple and sensitive competitive bio-barcode immunoassay for triazophos based on multi-modified gold nanoparticles and fluorescent signal amplification. Analytica Chimica Acta, 999, 123–131. doi: 10.1016/j.aca.2017.10.032
- Zhang, C., Han, Y., Lin, L., Deng, N., Chen, B., & Liu, Y.. (2017). Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. Journal of Agricultural and Food Chemistry, 65(6), 1290–1295. https://doi.org/10.1021/acs.jafc.6b05305
- Zhang, C., Hu, R. F., Shi, G. M., Jin, Y. H., Robson, M. G., & Huang, X. S. (2015). Overuse or underuse? An observation of pesticide use in China. Science of the Total Environment, 538, 1–6. doi: 10.1016/j.scitotenv.2015.08.031