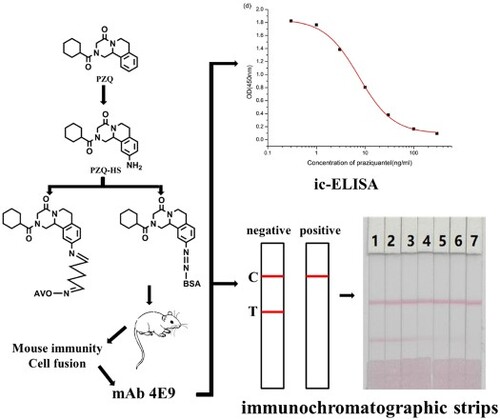
ABSTRACT
Praziquantel (PZQ) is a broad-spectrum antiparasitic drug used in mammals and fish. In this study, we developed an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) for the detection of PZQ residues. The hapten PZQ-HS produced by introducing an amino group at a benzene ring synthesized the immunogen and the coating antigen with carrier proteins. Highly sensitive monoclonal antibodies (mAbs) were prepared following mouse immunization and cell fusion. Under optimum conditions, the half maximal inhibitory concentration of the cell line 4E9 was 7.4 ng/mL, and the working range was 2.4–22.8 ng/mL. Immunochromatographic strips (ICS) were developed for the rapid detection of PZQ residues in mackerel. The reliability of the ICS assay was verified with PZQ-negative mackerel and confirmed by HPLC/MS. The cutoff value for ICS was 50 ng/mL PZQ. Our findings revealed that ic-ELISA and ICS can be used for the rapid detection of PZQ in mackerel.
Introduction
Praziquantel (PZQ) is a broad-spectrum antiparasitic drug used in mammals and fish, especially for the treatment of aphids and trematodes (Bader, Chelladurai, Thompson, Starling, & Brewer, Citation2017; Chuah, Gobert, Latif, Heo, & Leow, Citation2019; Qian, Utzinger, Keiser, & Zhou, Citation2016). Due to its rapid absorption, efficacy, and ease of use in aquatic organisms, its application has widely expanded (Xu, Dong, Yang, & Ai, Citation2016). However, the misuse and abuse of PZQ in aquaculture may result in drug residues in aquatic products (Bader, Starling, Jones, & Brewer, Citation2019) and in bio-resistance in fish and other organisms (Gold et al., Citation2017; Maeder et al., Citation2016; Vale et al., Citation2017). In humans, PZQ residues have detrimental effects on liver function (Hong, Citation2018). Japan and South Korea have set the maximum residue limit of PZQ at 20 µg/kg in poultry, livestock, and aquatic products. In China, the national standard for PZQ in the edible portion of aquatic products is 10 µg/kg. Therefore, it is imperative to establish a PZQ detection method that is rapid, sensitive, and simple.
PZQ detection methods are based on instrumental analysis such as spectroscopic methods (Piantavini, Pontes, Weiss, Sena, & Pontarolo, Citation2015), mass spectrometry, and chromatography (He et al., Citation2017; Yan, Citation2016; Zheng, Shao, & Jiang, Citation2016; Zrncic et al., Citation2014). Due to cost, cumbersome sample preparation steps, and large amount of manpower and material resources, these detection methods are not suitable for the detection of PZQ in aquatic products. The enzyme-linked immunosorbent assay (ELISA) and immunochromatographic strip assay are suitable options.
Few studies have focused on the production of specific antibodies against PZQ. In this study, we developed a highly sensitive monoclonal antibody (mAb) against PZQ by designing a hapten containing a PZQ fragment. This mAb was used in the development of immunochromatographic strip (ICS) and indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) for the detection of PZQ residues in mackerel.
Materials and methods
Chemicals and materials
PZQ was purchased from J&K Scientific Ltd. (Beijing, China). Bovine serum albumin (BSA), ovalbumin (OVA), glutaraldehyde (GA), sodium nitrite, Freund’s complete adjuvant, Freund’s incomplete adjuvant, 3,3′,5,5′-tetramethylbenzidine (TMB), Tween-20, horseradish peroxidase, and gelatin were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Enzyme immunoassay-grade horseradish peroxidase-labeled goat anti-mouse immunoglobulin was acquired from Kang-cheng Bioengineering Co. (Shanghai, China). Cell culture reagents, including polyethylene glycol 1500, HT supplement, HAT supplement, RPMI-1640 cell culture medium, and fetal calf serum were supplied by Life Technologies Corporation (Shanghai, China). Other reagents were of analytical grade.
Jieyi Biotech. Co., Ltd. (Shanghai, China) supplied the sample pad (glass fiber membrane), nitrocellulose (NC) membrane, absorbent pad, and polyvinyl chloride pad. The hapten and antigen were characterised by ultraviolet/visible (UV/vis) spectrophotometry (Agilent, Santa Clara, CA, USA). The results from ic-ELISA were analyzed using a Multiskan MKS microplate reader (Thermo Labsystems Company, Beijing, China).
Solutions
Coating buffer and blocking buffer consisted of 50 mM hydrogen carbonate (CB, pH 9.6) and 0.2% gelatin in CB, respectively. Phosphate-buffered saline (PBS, 0.01 M, pH 7.4) was prepared, and washing buffer was obtained by adding 0.05% Tween-20 (v/v) to 0.01 M PBS. Substrate solution was prepared from solution A (36.8 g Na2HPO4, 9.33 g citric acid, and 180 μL 30% H2O2) and solution B (0.06% v/v TMB in ethylene glycol) at a ratio of 5:1 (v/v). Stop solution was prepared by diluting 12 M sulfuric acid to 2 M.
Synthesis of hapten
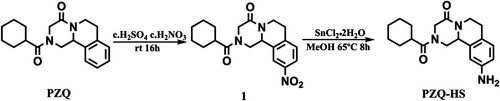
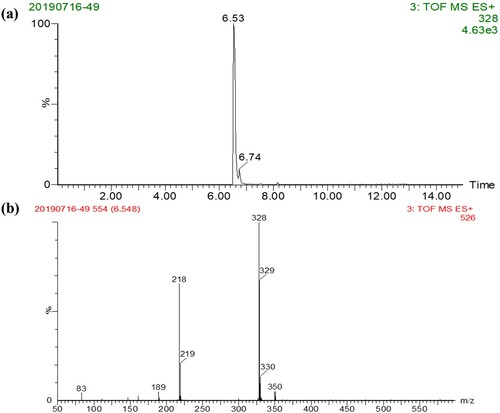
PZQ-HS hapten () was synthesised as previously reported with a slight modification (Wu, Wu, Zheng, Xu, & Kuang, Citation2018). Concentrated sulfuric acid (4.7 g, 48 mmol) and concentrated nitric acid (1.5 g, 24 mmol) were slowly added to PZQ (1.5 g, 4.8 mmol) and stirred at room temperature for 16 h. We added water (60 mL) and solid sodium bicarbonate until the pH of the aqueous layer reached 8. The mixture was extracted with ethyl acetate (60 mL) and eluted with three volumes of 50 mL ethyl acetate. The organic layer was washed with sodium chloride solution, dried over Na2SO4, and concentrated under vacuum to give a yellow solid. The solid was purified by flash column chromatography resulting in compound 1. We added SnCl2•H2O (1.0 g, 4.4 mmol) to compound 1 (0.54 g, 1.6 mmol) previously dissolved in 20 mL of methanol. The mixture was refluxed at 65°C for 8 h, and methanol was removed under vacuum. The pH of the residue was adjusted to 8 with 10% NaHCO3, and extracted with three volumes of 30 mL ethyl acetate. The organic layer was washed with sodium chloride solution, dried with Na2SO4, filtered, and concentrated under vacuum yielding a pale yellow solid, which was purified by flash column chromatography to obtain hapten PZQ-HS. We used LC-MS to confirm that the derivative () met the requirements.
Preparation of immunogen and coating antigen
Immunogen PZQ-HS-Dia-BSA was synthesised by conjugating the hapten to BSA using the diazonium method (Min et al., Citation2018; Wei, Lu, Liu, Song, & Hua, Citation2017). Briefly, PZQ-HS (5.5 mg), previously dissolved in 600 μL of N,N-dimethylformamide (DMF), was mixed with 50 μL of 1M HCl, incubated at 4°C for 30 min in the dark, and mixed with 5.56 μL of 30% NaNO2 at 4°C (solution A). BSA (10 mg) was dissolved in 2 mL of CB and kept at 4°C (solution B). Solution A was slowly added dropwise to solution B to maintain a pH of ∼8 and incubated a 4°C for 6 h. Finally, the solution was dialyzed against PBS for 3 d and stored at −20°C.
To synthesise the coating antigen PZQ-HS-GA-OVA, the hapten first was bound to OVA by the glutaraldehyde method (He et al., Citation2018; Peng, Liu, Hua, Gang, & Xu, Citation2016; Yan, Liu, Xu, Hua, & Xu, Citation2015). PZQ-HS (5.5 mg), previously dissolved in 600 μL of DMF, was mixed with 50 μL of 5% glutaraldehyde and incubated at 4°C for 10 min in the dark (solution A). OVA (10 mg) was dissolved in 2 mL of CB and kept at 4°C (solution B). Solution A was added dropwise to solution B and incubated at 4°C for 1 h. The conjugate was purified as previously reported to obtain PZQ-HS-OVA. The immunogen and coating antigen were characterised by UV/Vis spectroscopy.
Production of mAb against PZQ
Female BALB/c mice (∼6 weeks old) were used in this experiment (Guo et al., Citation2015; Lei, Xu, Song, Liu, & Hua, Citation2017; Xu, Xu, Wei, Hua, & Xu, Citation2015). The immunogen PZQ-HS-Dia-BSA (100 μg) was emulsified with Freund's complete adjuvant for the first immunisation and subcutaneously injected into the neck of each mouse. Immunogen (50 μg) was emulsified with Freund's incomplete adjuvant, and booster immunizations were performed every three weeks. Serum was collected from the tail vein one week after the third immunisation and analyzed by ic-ELISA. The most effective positive mice were selected for intraperitoneal immunisation with 25 μg of immunogen dissolved in 100 μL of physiological saline. Three days later, spleen cells of positive mice were fused with SP2/0 cells by a hybridoma technique (Sun, Liu, Song, Cui, & Kuang, Citation2018; Ye, Wu, Xu, Zheng, & Kuang, Citation2018). Three subclones were performed, and the selected cell lines were expanded and injected into mice for ascites production. Ascites were purified by octanoic acid-ammonium sulfate precipitation (Khaemba et al., Citation2016), dialyzed against PBS for 3 d, and stored at −20°C. All animal studies in this work were performed according to institutional ethical guidelines and were approved by the Committee on Animal Welfare of Jiangnan University.
Development of ic-ELISA
We determined the optimum coating antigen and mAb concentrations in ic-ELISA by a checkerboard assay (M. Guo et al., Citation2018). Coating antigen was diluted to 0.03, 0.1, 0.3, and 1 μg/mL, and mAb was diluted to 0.03, 0.1, 0.3, and 1 μg/mL. Additionally, we evaluated the effects of different variables on the test results to improve sensitivity and stability: NaCl concentration (0, 0.4, 0.8, 1.6, and 3.2%), pH (5.5, 6.5, 7.5, and 8.5), and methanol concentration (0, 10, 20, and 30%, v/v). A standard mAb curve was generated by plotting OD450 values (y-axis) against PZQ concentration (x-axis; log 10). Antibody performance was evaluated based on half maximal inhibitory concentration (IC50) and detection limits (Yao, Liu, Song, Hua, & Xu, Citation2017).
PZQ recovery by ic-ELISA
The study by He et al. was referenced during the pretreatment of the sample. PZQ-negative mackerel obtained from the local market (confirmed by HPLC/MS) was used for this experiment. Mackerel (0.5 g) was placed in 15-mL centrifuge tubes. PZQ was added at a final concentration of 5, 10, 25, and 50 ng/g, i.e. 2.5, 5, 12.5, and 25 ng of PZQ standard were added per 0.5 g of sample. The PZQ-spiked mackerel was immersed in 1 mL of ethyl acetate and thoroughly mixed (i.e. diluted three times). The corresponding concentrations at the time of detection were 1.67, 3.33, 8.33, and 16.67 ng/mL. After 15 min of vigorous shaking, the mixture was centrifuged at 10,000 rpm for 10 min at 4°C. The resulting supernatant was transferred to a clean centrifuge tube. The residue was extracted by adding 1 mL of ethyl acetate, and the two supernatants were pooled and dried at 40°C under warm nitrogen. The residual material was dissolved in 1 mL of 0.5% PBST for analysis (L. He et al., Citation2017).
Development of ICS assay
Preparation of gold nanoparticles
Gold nanoparticles were synthesised by the sodium citrate reduction method (Wang et al., Citation2017). First, 100 mL of chloroauric acid (HAuCl4, 0.01%, w/v) was heated to boiling in an Erlenmeyer flask. Second, freshly prepared trisodium citrate solution (1%, w/v) was immediately added under continuous stirring. Third, the solution was boiled until the color was burgundy. Finally, the solution was allowed to cool to room temperature and stored at 4°C until use. Transmission electron microscopy was used to characterise gold nanoparticles.
Preparation of gold-labelled anti-PZQmAb
The principle of this reaction is based on the fact that positively charged mAbs and negatively charged colloidal gold nanoparticles can combine by electrostatic interactions. In this study, anti-PZQ mAb was added to the colloidal gold solution by adjusting the pH of the colloidal gold solution to 8 with 0.1 M K2CO3. BSA (10%) dissolved in ultrapure water was added to seal the unoccupied sites on the colloidal gold and to ensure the stability of the colloidal gold, and the mixture was incubated at room temperature for 2 h. Free blocking agent and excess mAb were removed by centrifugation at 8,000 rpm for 25 min at 4°C. The precipitate was dissolved in 1 mL of ultrapure water and centrifuged. Finally, gold-labeled anti-PZQ mAb was re-suspended in borate buffer (0.002 M BB, 1% sucrose, and 0.01% Tween-20, pH 8, w/v) and stored at 4°C (Suryoprabowo, Liu, Peng, Hua, & Xu, Citation2015).
Preparation of ICS
ICS consists of four sections: a polyvinyl chloride (PVC) backing liner, a sample pad, a nitrocellulose (NC) membrane, and an absorbent pad. Goat anti-mouse IgG antibody and an optimum concentration of coating antigen were sprayed onto the NC membrane resulting in a test line (T-line) and control line (C-line). The NC membrane was dried at 37°C for 1 h and attached to the centre of the PVC backing liner. The sample pad and the absorbent pad were attached to the NC film near the ends of the T-line and the C-line, respectively. Finally, the pad was cut longitudinally into 2.8-mm strips (Lu, Wei, Liu, Song, & Hua, Citation2017).
ICS assay
The sample solution and gold-labeled anti-PZQ mAb were mixed and allowed to react for 5 min. The assembled test strip was inserted into the mixture, and the ends of the sample pad were quickly wetted. During the assay, the solution moves quickly due to capillary action. In negative samples, gold-labeled mAb binds to the coating antigen on the T-line and to the goat anti-mouse IgG antibody on the C-line, and both lines turn red. In positive samples, gold-labeled mAb binds to PZQ and does not completely bind to the coating antigen on the T-line, resulting in a weak color intensity or complete disappearance of the T-line. The color intensity of the T-line is inversely proportional to the concentration of PZQ in the sample (Xu, Li, et al., Citation2015).
Sample pretreatment and spiked sample analyses
Sample pretreatment was similar to the recovery test, except that PZQ final concentrations were 0, 1, 2.5, 5, 10, 25, and 50 ng/g.
Results and discussion
Synthesis of hapten and artificial antigen
PZQ has no groups that may directly react with protein. Therefore, by retaining the basic structure of the original drug, we introduced a side chain amino group on the benzene ring and converted it into a derivative containing a reactive group (). The derivative was analyzed by LC-MS to give a main product having a retention time of 8.96 min ((a)), indicating that the yield of the derivative was high. Meanwhile, as shown in (b), the MS data showed that a fragment having a molecular weight of 328.2 Da was detected, which was consistent with the molecular weight of the target derivative. These results indicated that the derivatization of the hapten is very successful. The immunogen was synthesised by combining BSA with PZQ-HS by the diazonium method. The coating antigen was synthesised by coupling PZQ-HS with OVA by the glutaraldehyde method. The results of the UV-Vis spectroscopy confirmed the successful synthesis of the immunogen and the coated antigen.
Antibody sensitivity in ic-ELISA
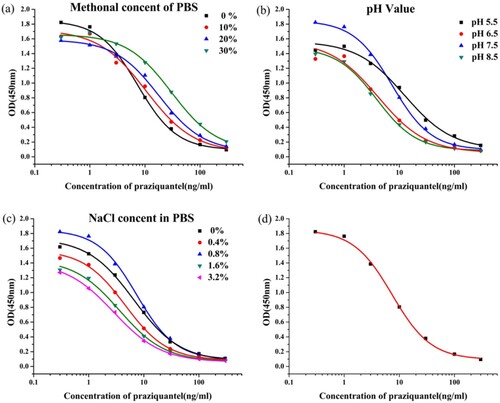
Three cell lines (4E9, 2E4, and 4G10) were obtained in our experiment. We selected 4E9 mAb, because it had the lowest IC50 value (7.4 ng/mL). The mAb concentration was set to 0.3 μg/mL, and the coating antigen concentration was set to 0.03 μg/mL. Factors affecting ic-ELISA performance include buffer pH, methanol concentration, and ionic strength. Our findings revealed that IC50 increased with increasing methanol concentration (a), and the IC50 value was the lowest at 0% methanol. Additionally, IC50 was the lowest at pH 7.5 (b). b shows that IC50 values and Absmax were affected by pH. Finally, the lowest IC50 value and highest Absmax were obtained at 0.8% NaCl (c). Therefore, the optimum ic-ELISA conditions consisted of pH 7.5, 0.8% NaCl, and 0% methanol in PBS buffer. A standard curve was generated under optimum conditions (d) resulting in an IC50 value of 7.4 ng/mL and a detection limit of 2.4–22.8 ng/mL.
PZQ recovery by ic-ELISA
During sample pretreatment, the ethyl acetate extract was finally dissolved in PBS. To investigate the interference of the sample matrix, we diluted PZQ-spiked mackerel by a factor of three to obtain different concentrations of PZQ and analyzed the amount of PZQ recovered from mackerel by ic-ELISA. shows that PZQ recovery ranged between 87 and 96% with a standard deviation of 0.046–0.251 and a coefficient of variation of 1.506–2.875%. Therefore, the pretreatment method was suitable for PZQ detection in mackerel.
Table 1. Recovery of PZQ in mackerel by ic-ELISA and strip assay.
PZQ detection by ICS
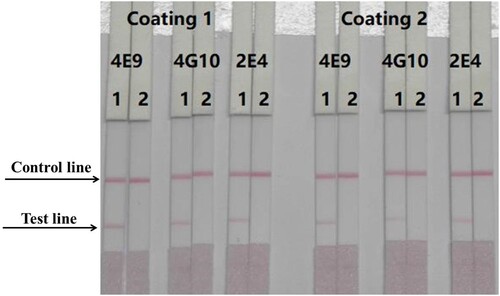
We evaluated three different antibodies (4E9, 2E4, and 4G10) and two coating antigens (PZQ-HS-GA-OVA 30 and PZQ-HS-GA-OVA 60). Optimised, 1 mL gold nanoparticles were coupled with 4 μL K2CO3 and 8 μg/mL antibody. The results are shown in . In this experiment, 50 μL of 4E9-gold nanoparticles were mixed with 150 μL of PBS (0 ppb). The coating antigen concentration was 0.3 mg/mL. The standard concentrations of PZQ were 0 and 50 ng/mL. According to our findings, the optimum mAb and coating antigen were 4E9 mAb and PZQ-HS-GA-OVA 30, respectively.
Figure 4. Optimisation of different antibodies and coating antigens. Antibody 4E9 and coating 1 were better. 1 = 0 ng/mL, 2 = 50 ng/mL.
The PZQ standard was diluted from stock solution (1 mg/mL) with PBS to give a titer range of 0–25 ng/mL. Under optimum conditions, the cutoff value of the T-line was 25 ng/mL (a).
Figure 5. Immunochromatography strips. (a) Images of the detection of PZQ in PBS. 1 = 0 ng/mL, 2 = 0.5 ng/mL, 3 = 1 ng/mL, 4 = 2.5 ng/mL, 5 = 5 ng/mL, 6 = 10 ng/mL, 7 = 25 ng/mL. (b) Images of the detection of PZQ in mackerel. 1 = 0 ng/mL, 2 = 1 ng/mL, 3 = 2.5 ng/mL, 4 = 5 ng/mL, 5 = 10 ng/mL, 6 = 25 ng/mL, 7 = 50 ng/mL.
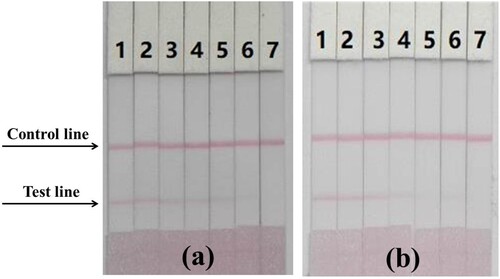
Gradient PZQ-spiked mackerel was prepared according to ic-ELISA preparation protocol, and the extraction solution was analyzed by ICS assay within 5 min. The sample extract was diluted three times during the pretreatment step to reduce matrix interference. (b) and show that the ICS results were consistent with the ic-ELISA results. At 0 ng/mL PZQ, the T-line was detected by the naked eye. As PZQ concentration increased, the T-line color decreased in intensity until disappearing at 50 ng/mL PZQ. The PZQ cutoff value in mackerel was < 50 ng/mL, which meets the requirements for on-site rapid detection.
Conclusion
We developed 4E9 mAb using a newly synthesised PZQ hapten with an IC50 value of 7.4 ng/mL under optimum conditions. Additionally, we developed ic-ELISA and ICS for the rapid detection of PZQ in mackerel. These methods are stable and sensitive for PZQ analysis in mackerel. The working range of ic-ELISA was 2.4–22.8 ng/mL. In ICS, the PZQ cutoff value was 25 ng/mL in PBS and 50 ng/mL in mackerel. We obtained similar results from ic-ELISA and ICS; therefore, these simple methods can be used to rapidly detect PZQ residues in aquatic products. In future studies, we will generate mAbs with higher sensitivity by derivatizing the amino group on the benzene ring on the hapten to form a carboxyl group.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bader, C., Chelladurai, J. J., Thompson, K., Starling, D., & Brewer, M. T. (2017). Outbreak of Cleidodiscus in juvenile black crappies (Pomoxis nigromaculatus) and bath treatment with praziquantel. Journal of Fish Diseases, 40(11), 1737–1739.
- Bader, C., Starling, D. E., Jones, D. E., & Brewer, M. T. (2019). Use of praziquantel to control platyhelminth parasites of fish. Journal of Veterinary Pharmacology and Therapeutics, 42(2), 139–153.
- Chuah, C., Gobert, G. N., Latif, B., Heo, C. C., & Leow, C. Y. (2019). Schistosomiasis in Malaysia: A review. Acta Tropica, 190, 137–143.
- Gold, D., Alian, M., Domb, A., Karawani, Y., Jbarien, M., Chollet, J., … Golenser, J. (2017). Elimination of Schistosoma mansoni in infected mice by slow release of artemisone. International Journal for Parasitology-Drugs and Drug Resistance, 7(2), 241–247.
- Guo, J., Liu, L., Feng, X., Xing, C., Song, S., Hua, K., & Xu, C. (2015). Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food & Agricultural Immunology, 26(2), 282–292.
- Guo, M., Sun, L., Liu, L., Song, S., Kuang, H., & Cui, G. (2018). Ultrasensitive immunochromatographic strip for detection of cyproheptadine. Food and Agricultural Immunology, 29(1), 941–952.
- He, L., Gao, F., Li, E., Lee, J. T., Bian, L., & Armstrong, D. W. (2017). Chromatographic separation of racemic praziquantel and its residual determination in perch by LC-MS/MS. Talanta, 174, 380–386.
- He, F., Tian, Y., Xu, Z., Luo, L., Yang, J., Wang, H., … Shen, Y. (2018). Development of an immunochromatographic assay as a screen for detection of total phthalate acid esters in cooking oil. Journal of Toxicology and Environmental Health-Part a-Current Issues, 81(4), 80–88.
- Hong, S.-T. (2018). Albendazole and praziquantel: Review and safety monitoring in Korea. Infection and Chemotherapy, 50(1), 1–10.
- Khaemba, G. W., Tochi, B. N., Mukunzi, D., Joel, I., Guo, L., Suryobrobowo, S., … Xu, C. (2016). Development of monoclonal antibody and lateral test strip for sensitive detection of clenbuterol and related β2-agonists in urine samples. Food & Agricultural Immunology, 27(1), 111–127.
- Lei, X., Xu, L., Song, S., Liu, L., & Hua, K. (2017). Development of an ultrasensitive ic-ELISA and immunochromatographic strip assay for the simultaneous detection of florfenicol and thiamphenicol in eggs. Food & Agricultural Immunology, 29(1), 254–266.
- Lu, Z., Wei, J., Liu, L., Song, S., & Hua, K. (2017). Development of ic-ELISA and lateral-flow immunochromatographic strip for detection of vitamin B2 in an energy drink and vitamin tablets. Food & Agricultural Immunology, 29(1), 121–132.
- Maeder, P., Blohm, A. S., Quack, T., Lange-Gruenweller, K., Gruenweller, A., Hartmann, R. K., … Schlitzer, M. (2016). Biarylalkyl Carboxylic acid derivatives as novel antischistosomal agents. ChemMedChem, 11(13), 1459–1468.
- Min, F., Suryoprabowo, S., Hong, T., Liu, L., Zheng, Q., & Hua, K. (2018). Rapid detection of clonidine and its cross-reactivity with apraclonidine in pig urine using an immunochromatographic test strip. Food & Agricultural Immunology, 29(1), 821–832.
- Peng, J., Liu, L., Hua, K., Gang, C., & Xu, C. (2016). Development of an icELISA and immunochromatographic strip for detection of norfloxacin and its analogs in milk. Food & Agricultural Immunology, 28(2), 288–298.
- Piantavini, M. S., Pontes, F. L. D., Weiss, L. X., Sena, M. M., & Pontarolo, R. (2015). Comparison between ultraviolet and infrared spectroscopies for the simultaneous multivariate determination of pyrantel and praziquantel. Journal of the Brazilian Chemical Society, 26(7), 1387–1395.
- Qian, M.-B., Utzinger, J., Keiser, J., & Zhou, X.-N. (2016). Clonorchiasis. Lancet, 387(10020), 800–810.
- Sun, J., Liu, L., Song, S., Cui, G., & Kuang, H. (2018). Development of an immunochromatographic strip assay for three major capsaicinoids based on an ultrasensitive monoclonal antibody. Food and Agricultural Immunology, 29(1), 930–940.
- Suryoprabowo, S., Liu, L., Peng, J., Hua, K., & Xu, C. (2015). Antibody for the development of specific immunoassays to detect nadifloxacin in chicken muscles. Food & Agricultural Immunology, 26(3), 317–324.
- Vale, N., Gouveia, M. J., Rinaldi, G., Brindley, P. J., Gartner, F., & da Costa, J. M. C. (2017). Praziquantel for Schistosomiasis: Single-drug metabolism revisited, mode of action, and resistance. Antimicrobial Agents and Chemotherapy, 61(5), e02582.
- Wang, Z., Xie, Z., Cui, G., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of an indirect competitive enzyme-linked immunosorbent assay and immunochromatographic assay forhydrocortisone residues in milk. Food and Agricultural Immunology, 28(3), 476–488.
- Wei, J., Lu, Z., Liu, L., Song, S., & Hua, K. (2017). Immunochromatographic strip for rapid detection of phenylethanolamine A. Food & Agricultural Immunology, 29(1), 182–192.
- Wu, A., Wu, X., Zheng, Q., Xu, L., & Kuang, H. (2018). Preparation of an anti-4,4′-dinitrocarbanilide monoclonal antibody and its application in an immunochromatographic assay for anticoccidial drugs. Food and Agricultural Immunology, 29(1), 1162–1172.
- Xu, N., Dong, J., Yang, Y., & Ai, X. (2016). Pharmacokinetics and residue depletion of praziquantel in rice field eels Monopterus albus. Diseases of Aquatic Organisms, 119(1), 67–74.
- Xu, N., Li, L., Song, S., Xu, L., Hua, K., & Xu, C. (2015). Development of a lateral flow immunoassay for the detection of total malachite green residues in fish tissues. Food & Agricultural Immunology, 26(6), 870–879.
- Xu, N., Xu, L., Wei, M., Hua, K., & Xu, C. (2015). Development and characterisation of an ultrasensitive monoclonal antibody for chloramphenicol. Food & Agricultural Immunology, 26(3), 440–450.
- Yan, D. (2016). Determination of Fenbendazole and praziquantel in compound Fenbendazole tablets by HPLC. China Pharmacist, 19(5), 1011–1013.
- Yan, H., Liu, L., Xu, N., Hua, K., & Xu, C. (2015). Development of an immunoassay for carbendazim based on a class-selective monoclonal antibody. Food & Agricultural Immunology, 26(5), 659–670.
- Yao, L., Liu, L., Song, S., Hua, K., & Xu, C. (2017). Development of indirect competitive enzyme-linked immunosorbent and immunochromatographic strip assays for carbofuran detection in fruits and vegetables. Food & Agricultural Immunology, 28(4), 639–651.
- Ye, L., Wu, X., Xu, L., Zheng, Q., & Kuang, H. (2018). Preparation of an anti-thiamethoxam monoclonal antibody for development of an indirect competitive enzyme-linked immunosorbent assay and a colloidal gold immunoassay. Food and Agricultural Immunology, 29(1), 1173–1183.
- Zheng, X., Shao, Q., & Jiang, H. (2016). Enantiomers detection of praziquantel intermediates by RP-HPLC after GITC Pre-column derivatization. World Sci-Tech R&D, 38(3), 626–629. 641.
- Zrncic, M., Gros, M., Babic, S., Kastelan-Macan, M., Barcelo, D., & Petrovic, M. (2014). Analysis of anthelmintics in surface water by ultra high performance liquid chromatography coupled to quadrupole linear ion trap tandem mass spectrometry. Chemosphere, 99, 224–232.