ABSTRACT
This research aims to explore the effect of L-arginine (Arg) on ovine intestinal epithelial cells (IOECs) from lipopolysaccharide (LPS)-induced intestinal barrier injury. IOECs were incubated for 16 h and classified as 4 groups with the replacement of various mediums, including (1) control (Con), Arg-free Dulbecco's modified Eagle's F12 Ham medium (DMEM); (2) Arg treatment, Arg-free DMEM supplemented with 100 μM Arg; (3) LPS treatment, Arg-free DMEM supplemented with 10 μg/mL LPS; (4) LPS with Arg treatment, Arg-free DMEM supplemented with both 10 μg/mL LPS and 100 μM Arg. Arg supplementation to LPS-treated IOECs resulted in lower (P < 0.05) relative Toll-like receptor 4 (TLR4) protein level. Arg supplementation to LPS-treated IOECs resulted in up-regulated (P < 0.05) zonula occludens-1 (ZO-1), peptide transporter 1 (PEPT1) protein levels, and the ratio of phosphorylated mechanistic target of rapamycin (phospho-mTOR)-to-mTOR. The results show that Arg protects IOECs from LPS induced intestinal barrier injury.
Introduction
Intestinal epithelium has been comprised of the proliferating and differentiating intestinal epithelial cells (IECs) that are formed in a continuous monolayer, and IECs can isolate intestinal mucosa away from the enteric cavity (Tang et al., Citation2016). IECs can form a semipermeable barrier, which can effectively absorb nutrients and water, in the meantime of limiting toxins, allergens and pathogens to penetrate from the enteric cavity to the mucosal tissue and the blood circulation (Suzuki, Citation2013; Zhao et al., Citation2015). Various stress conditions, like oxidative stress, can damage the intestinal barrier (Min et al., Citation2014; Sun et al., Citation2015), giving rise to the elevated permeation of toxins and allergens. Ovine intestinal is vulnerable to oxidative stress at several specific cases. Lipopolysaccharide (LPS) is the principal outer membrane component in Gram-negative bacteria, which can induce elevated oxidative injury to IECs through generating various reactive oxygen species (ROS), and the latter can lead to lipid peroxidation and apoptosis (Han et al., Citation2019; Talavera et al., Citation2017). In this study, LPS was employed to construct a cell injury model to study the protective mechanism of Arg in the intestinal epithelial barrier function of ovine intestinal epithelial cells (IOECs).
The role of L-arginine (Arg) on intestinal mucosal physiology has attracted much interest (Rhoads & Wu, Citation2009). Arg is investigated as one of the oral rehydration solution components, which can boost intestinal absorption and post-injury villous recovery; besides, it is suggested to show effectiveness in numerous intestinal injury models (Wang, Qiao, & Li, Citation2009). Arg can mitigate the LPS-induced inflammatory response and up-regulate the expression of casein in mammary epithelial cells in bovine (Wu et al., Citation2016). Tan et al. (Citation2010) demonstrated that Arg could protect the porcine enterocytes from the LPS-induced damage, which was achieved through the mechanistic target of rapamycin (mTOR) and Toll-like receptor 4 (TLR4) signalling pathways, along with the intracellular protein turnover. Zhu, Liu, Xie, Huang, and Hou (Citation2013) claimed that arginine supplementation protected and enhanced weaned pigs’ immune barrier function of the intestinal mucosa, in the meantime of maintaining the intestinal integrity following E. coli LPS challenged. Previous study has demonstrated that Arg supplementation was able to promote the growth of Hu suckling lambs with IUGR and maintain their intestinal integrity, immune function, and oxidative status (Zhang et al., Citation2018). A recent study revealed that Arg can prevent apoptosis induced by LPS in IOECs (Zhang et al., Citation2019). However, it remains largely unknown about the mechanism of Arg on intestinal epithelial barrier function in IOECs at damage conditions.
Therefore, it was hypothesized that Arg protects IOECs from LPS-induced intestinal barrier injury by attenuating the inflammatory response, enhancing tight junction integrity, and activating the mTOR signalling pathway and amino acid and peptide transporters. This study aimed to verify such hypothesis based on the IOECs model of LPS-induced intestinal barrier injury.
Material and methods
Reagents
Dulbecco's modified Eagle's F12 Ham medium (DMEM/F12), foetal bovine serum (FBS), and antibiotics had been provided by Invitrogen (Grand Island, NY, USA). In addition, the Arg-free custom-made DMEM (modified DMEM no.08–5009EF, GIBCO), and the 2.5% trypsin solution, was purchased from Gibco (Carlsbad, CA, USA). Meanwhile, epidermal growth factor was manufactured by BD Biosciences (Bedford, MA, USA), while the plastic culture plates had been bought from Corning Inc. (Corning, NY, USA). The remaining chemicals had been provided by Sigma-Aldrich (St. Louis, MO, USA) unless indicated elsewhere.
Cell culture
The intestinal ovine epithelial cells (IOECs), a cell line originated from the non-suckling neonatal lamb jejunum, was cultivated according to the method in previous study (Zhan, Lin, Liu, Sui, & Zhao, Citation2017). IOECs (at 20–25 passages) had been grown in plastic 75 cm2 flasks covered with the vented caps (BD Falcon) in the DMEM/F12 supplemented with FBS (5%), penicillin (100 U/mL), streptomycin (100 μg/mL), glutamine (4 mm/L), 1% NEAA, 1 × ITS, and EGF (15 ng/mL); meanwhile, cell passage was performed at an interval of 3 d, as depicted in previous study (Zhan, Jiang, Gong, & Zhao, Citation2018). All mediums were replaced at an interval of 2 days. After reaching confluence, cells were trypsinized and seeded in the 24-well cell culture plates at the density of about 2 × 104/well within the humidified incubator under 5% CO2 and 37°C. After reaching 85%–95% confluence, all cells were subject to routine passage (at the split ratio of 1:3). After an overnight incubation (16 h), the cells were subject to 6 h of starvation in the custom-made DMEM with no Arg (modified DMEM no.08–5009EF, GIBCO), so as to minimize Arg amount in the cells. 5% FBS could supply 10 μM Arg in the modified DMEM.
Assessment of Arg and LPS toxicities to IOECs
Cells were counted by the CCK-8 assay. IOECs were inoculated into the 96-well plates (1 × 104/well) covered with complete DMEM/F12. Cells were then incubated for 16 h, followed by 6 h of starvation in the modified DMEM to minimize Arg amount in the cells. Afterwards, cells were cultivated in the modified DMEM containing 0, 50, 100, 150, 200, 250 μM Arg, or treated with 0, 5, 10, 15 μg/mL LPS (Escherichia coli serotype 055: B5; Sigma Chemical Inc., St. Louis, MO, USA) for 24 h; later, CCK-8 reagent (10% concentration of CCK-8 in the medium) was added to culture for another 3 h, and the optical density (OD) value was detected at 450 nm using the enzyme-linked immune detector (Bio-Rad, Hercules, CA, USA). Cell viability was expressed as the proportion of control cells. All experiments had been repeated for 6 times (Zhao, Guo, Liu, Gao, & Nie, Citation2014).
Assessment of the time dependency of Arg and LPS in IOECs
Cells were subject to 6, 12, 24, 36, 48 h of 100 μM Arg treatment or 10 μg/mL LPS (assessed through toxicity experiment) treatment; later, CCK-8 reagent (10% concentration of CCK-8 in the medium) was added, respectively, to culture for another 3 h, and the OD value was measured at 450 nm using the enzyme-linked immune detector (Bio-Rad, Hercules, CA, USA). Cell viability was expressed as the proportion of control cells. All experiments had been repeated for 6 times.
Role of Arg in cell viability under LPS challenge
IOECs were followed by 24 h (evaluated through the time-dependent experiment) treatments shown below: (1) modified DMEM for Con group; (2) modified DMEM containing 100 μM Arg for Arg treatment group; (3) modified DMEM containing 10 µg/mL LPS (Escherichia coli serotype 055: B5; Sigma Chemical Inc., St. Louis, MO, USA) for LPS treatment group; (4), modified DMEM containing 10 µg/mL LPS and 100 μM Arg for LPS plus Arg treatment group. Afterwards, CCK-8 reagent (10% concentration of CCK-8 in the medium) was added into each treatment, respectively, to culture for another 3 h, and the OD value was measured at 450 nm using the enzyme-linked immune detector (Bio-Rad, Hercules, CA, USA). Cell viability was expressed as the proportion of control cells. All experiments were carried out for 6 times.
Transepithelial electrical resistance assay
Transepithelial electrical resistance (TEER) is an approach to measure the tight-junction (TJ) integrity using the epithelium volt-ohmmeter (Millicell ERS-2; Millipore Corporation) (Xia, Ye, Hou, & Yu, Citation2016). In brief, cells were inoculated in the cell culture transwells at the density of 5 × 104/well (with the membrane area of 0.33 cm2 and the pore size of 0.4 µm) and grown in the 24-well plates. Subsequently, TEER was measured based on the potential difference measured between the apical and the basolateral layers of cells using the epithelium volt-ohmmeter. Cells were then cultured in corresponding mediums for 24 h and measured. Resistance is expressed as KΩ cm2. We performed each experiment six times.
Determination of paracellular permeability
Cells were subject to the similar treatment to that for TEER measurement. Briefly, 1 g/L of the 20 kDa fluorescein isothiocyanate (FITC)–conjugated dextran was added into the apical cell monolayer. Afterwards, aliquot medium was eliminated out of the basolateral chamber; then, FITC-dextran concentration was detected using the SpectraMax M3 Multi-Mode Microplate Reader (Molecular Devices) at the excitation wavelength of 490 nm and the emission wavelength of 520 nm. Later, FITC-dextran amount transported from apical to basolateral chamber was deemed as the permeability of the monolayer cells. In addition, the FITC-dextran content was computed through subtracting the fluorescence value in FITC-free medium (Li et al., Citation2016).
Cytokine assay
Concentrations of IL-1β, IL-6, and TNF-αfrom cellular culture supernatants were determined by Enzyme Immunometric Assay (ELISA) kits (from R&D Systems, Oxford, UK). The examination steps in detail were carried out as the manufacturer's protocol described. The optical density of each well was read at 450 nm. The detection limits for IL-1β, IL-6, and TNF-α were 30.0, 9.0, and 8.0 pg/mL, respectively. Meanwhile, the inter-assay and intra-assay variation coefficient were ≤ 10%.
Analysis of the levels nitric oxide (NO) and nitric oxide synthase (NOS)
The culture supernatants were collected to be available for measurement of NO and NOS concentrations. The NO and NOS concentrations in IOECs were analyzed using the assay kits (Nanjing Jiancheng Biological Product, Nanjing, China) following the manufacturer's instructions (Guo et al., Citation2017). Optical densities were measured at 450 nm. The inter-assay CV was <15% at all instances.
mRNA level detected by real-time PCR
Cells were cultured in corresponding mediums for 24 h, then, the TRIzol (Invitrogen) reagent was added to extract the total RNA; meanwhile, the purity was evaluated with a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The reverse transcription conditions were shown below: at 37°C for 15 min and at 95°C for 5 s. The primers used in the current study had been designed using Primer 5.0 (). Target gene expression levels were normalized based on the house-keeping gene β-actin. Moreover, the PCR conditions were as follows, at 94°C for 40 s, 60°C for 30 s, and 72°C for 35 s for 36 cycles. Relative gene expression was displayed in the form of the target-control gene ratio according to the formula 2−(ΔΔCt), in which ΔΔCt = (CtTarget−Ctβ-actin)treatment−(CtTarget−Ctβ-actin)control (Stämmler et al., Citation2016). Afterwards, the relative expression was normalized and displayed in the form of target-control group ratio. At last, the mRNA expression of all target genes was set as 1.0 in control (Arg-free DMEM) (Zhang et al., Citation2018).
Table 1. The primer sequences used in the real-time PCR.
Protein abundance analysis by western blot
After 24 h incubation with challenge medium, cells were collected to analyze the protein abundance by the previously introduced western blotting (Wang et al., Citation2016). Briefly, equal amounts of proteins in the cellular culture supernatants were isolated on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer onto the blotting membrane for immunoblotting (Wang et al., Citation2015). Specific primary antibodies included β-actin (60008-1, diluted at 1:5000, Proteintech); phosphorylated α serine–threonine kinase (phospho-Akt; Ser473, sc 6732, diluted at 1:1000, Abcam); total Akt (sc1083, diluted at 1:1000, Abcam); phospho-mTOR (Ser2448, ab137133,diluted at 1:2000, Abcam); total mTOR (20657-1-AP, diluted at 1:500, Proteintech); phospho-S6 kinase 1 (phospho-S6K1; Thr389, sc16542, diluted at 1:1000, Abcam); total S6K1 (sc15098, diluted at 1:1000, Abcam);cationic amino acid transporter, y+ system, member 1 (CAT1; sc-66824, diluted at1:500, Santa); alanine–serine–cysteine amino acid transporter 2 (ASCT2; sc-87896, diluted at 1:500, Santa); neuronal/epithelial high-affinity glutamate transporter, system XAG, member 1 (EAAT3; ab41776, diluted at 1:1000, Abcam); peptide transporter 1 (PEPT1; sc19965, diluted at 1:500, Santa);amino acid transporter light chain, y+L system, member 7 (y+LAT2; sc35673, diluted at 1:500, Santa); Claudin-1 (sc-35672, diluted at 1:1000, Santa); zonula occludens-1 (ZO-1; sc-17892, diluted at 1:1000, Santa); TLR4 (Abcam, diluted at 1:1000);nuclear factor-κB p65 (NF-κB p65; cell signalling, diluted at 1:1000), and phosphorylated NF-κBp65 (Ser536,cell signalling, diluted at 1:1000). Primary antibody was then added into the membranes to incubate overnight at 4°C, and the membranes were rinsed with the tris-buffer saline solution containing 0.1% Tween-20 for 3*15 min. The ratio of phosphorylated form to total protein was presented next to the blots. Subsequently, the HRP-conjugated secondary antibodies, including goat anti-rabbit IgG HRP (1:5000, Antgene Biotech) and goat anti-mouse IgG HRP (1:5000, Antgene Biotech), were added into the membranes to incubate for 2 h under ambient temperature, followed by chemiluminescence using the ECL Plus Western Blotting Detection System (Amersham, Arlington Heights, IL, USA). After ECL plus system (Amersham Biosciences) incubation, the immunoreactive bands were visualized using the Image Quant LAS 4000 (GE Healthcare Bio-sciences). Chemifluorescence was quantified using the Image software (GE Healthcare Life Sciences) (Wang et al., Citation2016). Relative protein expression of target genes was normalized based on the β-actin internal gene for comparing target protein expression in different groups.
Statistical analyses
All data were presented in the form of means ± pooled standard error of the means (SEMs) and were analyzed through one-way ANOVA. Differences between various treatments were measured through Duncan's multiple range test. The SAS software (version 9.2) was employed for all statistical analyses, and a P-value ≤ 0.05 was deemed as statistically significant.
Results
Role of Arg in the cell viability of IOECs treated by LPS
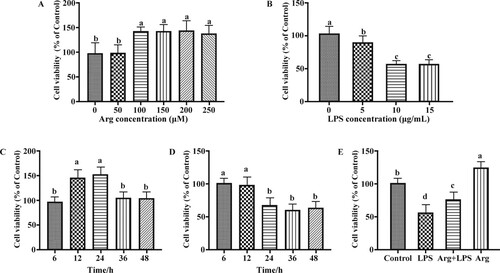
After 24 h, Arg at 100 μM could more remarkably elevate (P < 0.05) cell viability than those in 0 and 50 μM Arg groups ((A)); 10 μg/mL LPS markedly reduced (P < 0.05) the cell viability of IOECs ((B)). 24 h of 100 μ M Arg treatment led to a higher (P < 0.05) cell viability of IOECs ((C)); and the cell viability of IOECs were evidently decreased after 10 μg/mL LPS treatment for 24, 36, and 48 h (P < 0.05) ((D)). Therefore, 100 μM Arg, 10 μg/mL LPS, and 24 h of cell culture were selected for later experiments. Subsequently, IOECs were subject to 24 h of 100 μM Arg and/or 10 μg/mL LPS treatment, to examine the protection of Arg on cell viability. As was observed from (E), Arg remarkably (P < 0.05) improved the cell viability of LPS-challenged cells.
Figure 1. Effects of Arg on cell viability. (A) Toxicity of Arg on cell viability; (B) toxicity of LPS on cell viability; (C) time-dependent effects of Arg on cell viability; (D) time-dependent effects of LPS on cell viability; (E) effects of Arg on cell viability of IOECs challenged by LPS. Con, Arg-free DMEM; LPS, Arg-free DMEM supplemented with 10 μg/mL LPS; Arg + LPS, Arg-free DMEM supplemented with both 10 μg/mL LPS and 100 μM L-arginine; Arg, Arg-free DMEM supplemented with 100 μM L-arginine. Values are expressed as means ± SEM, n = 6/group. Mean values in columns without a common letter differ (P < 0.05).
Role of Arg in the intestinal barrier function of IOECs treated by LPS
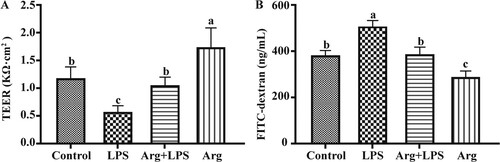
As shown, incubation of cells with 100 μM Arg resulted in the higher (P < 0.05) TEER at 24 h ((A)) relative to that of Con. Compared with the Con, TEER in LPS group was reduced (P < 0.05), while that in LPS plus Arg treatment group (P < 0.05) was higher than that in LPS group. Similar to the elevated TEER, cells under 100μM Arg treatment were associated with decreased (P<0.05) paracellular permeability, which could be verified by FITC dextran flux at 24 h ((B)) relative to that in the Con. Compared with the Con, paracellular permeability in LPS group (P < 0.05) was increased, while that in LPS plus Arg treatment group (P < 0.05) was lower than that in LPS group.
Figure 2. Effects of Arg on intestinal barrier function in LPS-treated IOECs for 24h. TEER (A) and paracellular permeability (B) were determined. TEER, transepithelial electrical resistance; Con, Arg-free DMEM; LPS, Arg-free DMEM supplemented with 10 μg/mL LPS; Arg + LPS, Arg-free DMEM supplemented with both 10 μg/mL LPS and 100 μM L-arginine; Arg, Arg-free DMEM supplemented with 100 μM L-arginine. Values are expressed as means ± SEM, n = 6/group. Mean values in columns without a common letter differ (P < 0.05).
Role of Arg in cytokine concentrations of IOECs treated by LPS
Relative to the Con, adding LPS markedly increased IL-1β, IL-6, and TNF-α concentrations (P < 0.05) (). And addition of arginine inhibited the IL-1β, IL-6, and TNF-α levels in LPS-treated cells, relative to those in LPS group (P < 0.05).
Table 2. Effects of Arg on concentrations of cytokines in LPS-treated IOECs for 24h.
Role of Arg in NO content and NOS activity of IOECs treated by LPS
Compared with Con group, the NO content and NOS activity were down-regulated (P < 0.05) in LPS group, but those were higher (P < 0.05) in LPS plus Arg treatment group than in LPS group (). Compared with Con group, the NO content and NOS activity (P < 0.05) in Arg group were greater.
Table 3. Effect of Arg on the content of NO and activity of NOS in LPS-treated IOECs for 24 ha.
Role of Arg in mRNA levels of IOECs treated by LPS
Compared with Con group, the MyD88, TLR-9, TLR-4, IL-6, NF-κB, IL-1β, and TNF-α mRNA levels were up-regulated (P < 0.05) in the LPS group (), while those were down-regulated (P < 0.05) in LPS plus Arg treatment group relative to those in LPS group.
Table 4. Effects of Arg on the mRNA abundance of genes in LPS-treated IOECs for 24 h.
Compared with Con group, the ZO-1, occluding and claudin-1mRNA levels were down-regulated (P < 0.05) in LPS group, but those were higher (P < 0.05) in LPS plus Arg treatment group than in LPS group. Compared with Con group, the ZO-1, occludin, and claudin-1 mRNA levels (P < 0.05) in Arg group were greater.
Compared with Con group, the SLC7A1, SLC1A1, SLC1A5, and SLC15A1 mRNA levels were reduced (P < 0.05) in LPS group, but enhanced (P < 0.05) in LPS plus Arg treatment group relative to that in LPS group. Compared with Con group, the SLC7A1, SLC1A1, and SLC15A1 mRNA levels were increased (P < 0.05) in Arg group.
Compared with Con group, the iNOS and eNOS mRNA levels were reduced (P < 0.05) in LPS group, but enhanced (P < 0.05) in LPS plus Arg treatment group relative to that in LPS group. Compared with Con group, the iNOS and eNOS mRNA levels were increased (P < 0.05) in Arg group.
The protein expressions for immune responses, barrier function, nutrients transporters and mTOR signalling pathways
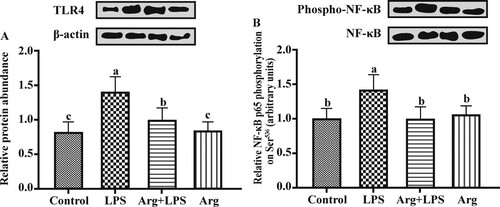
Compared to the Con groups, the relative TLR4 protein expression was up-regulated (P < 0.05) in LPS group ((A)). Arg supplementation to LPS-treated IOECs led to lower (P < 0.05) relative protein expression of TLR4 than that in LPS group. The Arg treatment (100 μg/mL) made no difference to (P > 0.05) total NF-κB protein expression, but it could evidently reduce (P < 0.05) the phosphorylated NF-κB level in IOECs treated by LPS ((B)). Representative Western blots of all detected proteins have been shown in .
Figure 3. The effect of Arg on TLR4 and NF-κB protein expression in LPS-treated IOECs for 24 h. Values are means, with standard errors represented by vertical bars. The protein expression value = densitometry units of selected protein/densitometry units of β-actin detected by western blotting. TLR4, Toll-like receptor 4; NF-κB Nuclear factor-κB; Con, Arg-free DMEM; LPS, Arg-free DMEM supplemented with 10 μg/mL LPS; Arg + LPS, Arg-free DMEM supplemented with both 10 μg/mL LPS and 100 μM L-arginine; Arg, Arg-free DMEM supplemented with 100 μM L-arginine. Values are expressed as means ± SEM, n = 6/group. Mean values in columns without a common letter differ (P < 0.05).
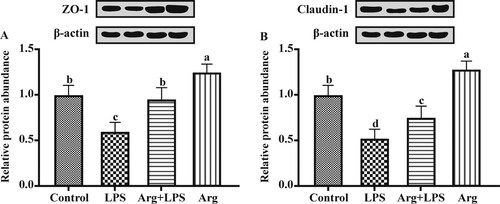
Compared to the Con groups, the relative protein expression of ZO-1 ((A)) and claudin-1 ((B)) was down-regulated (P < 0.05) in the LPS group. Arg supplementation to LPS-treated IOECs resulted in up-regulated (P < 0.05) levels of ZO-1 and claudin-1 proteins compared to those in LPS group. Compared with Con group, the relative protein expression of ZO-1 and claudin-1were up-regulated (P < 0.05) in Arg group.
Figure 4. The effect of Arg on the relative protein (A) ZO-1 and (B) claudin-1 expression levels in LPS-treated IOECs for 24 h. Values are means, with standard errors represented by vertical bars. The protein expression value = densitometry units of selected protein/densitometry units of β-actin detected by western blotting. ZO-1, zonula occludens-1; Con, Arg-free DMEM; LPS, Arg-free DMEM supplemented with 10 μg/mL LPS; Arg + LPS, Arg-free DMEM supplemented with both 10 μg/mL LPS and 100 μM L-arginine; Arg, Arg-free DMEM supplemented with 100 μM L-arginine. Values are expressed as means ± SEM, n = 6/group. Mean values in columns without a common letter differ (P < 0.05).
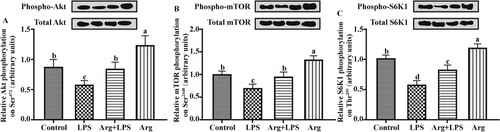
Compared with Con group, LPS-treated IOECs were associated with the deceased (P < 0.05) ratios of phosphorylated Akt to Akt ((A)), phosphorylated mTOR to mTOR ((B)) and of phosphorylated p70S6K to p70S6K ((C)) in the IOECs. Arg supplementation to LPS-treated IOECs led to greater (P < 0.05) ratios of pAkt to Akt, pmTOR-to-mTOR and pp70S6K-to-p70S6K compared with those in LPS group. Compared with Con group, the ratios of pAkt-to-Akt, pmTOR-to-mTOR and pp70S6K-to- p70S6K were higher (P < 0.05) in Arg group.
Figure 5. Effects of Arg on the mTOR signalling pathways in LPS-treated IOECs for 24 h. Phosphorylation state of (A) Akt, (B) mammalian target of rapamycin (mTOR) and (C) p70 S6 kinase (S6K1) in LPS-treated IOECs. Akt phosphorylation on Ser473, mTOR phosphorylation on Ser2448 and S6K1 phosphorylation on Thr389 were measured by western blot analysis using antibodies that recognize these proteins only when that residue was phosphorylated. Western blots are shown above each graph. Values for phosphorylation of each protein were normalized for total protein. Values are means, with their standard errors represented by vertical bars. Con, Arg-free DMEM; LPS, Arg-free DMEM supplemented with 10 μg/mL LPS; Arg + LPS, Arg-free DMEM supplemented with both 10 μg/mL LPS and 100 μM L-arginine; Arg, Arg-free DMEM supplemented with 100 μM L-arginine. Values are expressed as means ± SEM, n = 6/group. Mean values in columns without a common letter differ (P < 0.05).
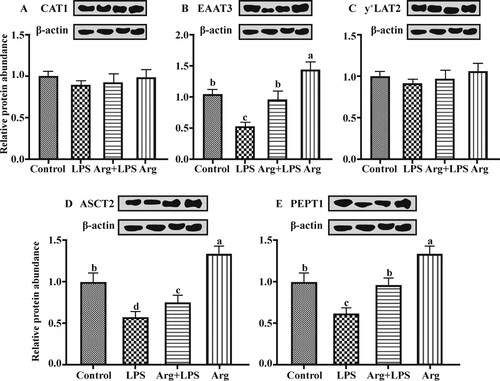
Compared to the Con groups, the relative protein expressions of EAAT3, ASCT2 and PEPT1 were down-regulated (P < 0.05) in the LPS group (). Arg supplementation to LPS-treated IOECs up-regulated (P < 0.05) the expression of EAAT3, ASCT2 and PEPT1 proteins compared with that in LPS group. Compared with Con group, the relative protein expressions of EAAT3, ASCT2 and PEPT1 was greater (P < 0.05) in the Arg group. No significant differences (P > 0.05) were observed in the CAT1 and y+LAT2 protein expression among various treatment groups.
Figure 6. Effects of Arg on the protein abundance of amino acid and peptide transporters in LPS-treated IOECs for 24 h.Values are means, with standard errors represented by vertical bars. The protein expression value = densitometry units of selected protein/densitometry units of β-actin detected by western blotting. (A) CAT1, (B) EAAT3, (C) y+LAT2, (D) ASCT2, and (E) PEPT1 were encoded by SLC7A1, SLC1A1, SLC7A7, SLC1A5, and SLC15A1, respectively. CAT1, cationic amino acid transporter, y+ system, member 1; EAAT3, neuronal/epithelial high-affinity glutamate transporter, system XAG, member 1; y+ LAT2, amino acid transporter light chain, y+ L system, member 7; ASCT2, alanine–serine–cysteine amino acid transporter 2; PEPT1, peptide transporter 1; Con, Arg-free DMEM; LPS, Arg-free DMEM supplemented with 10 μg/mL LPS; Arg + LPS, Arg-free DMEM supplemented with both 10 μg/mL LPS and 100 μM L-arginine; Arg, Arg-free DMEM supplemented with 100 μM L-arginine. Values are expressed as means ± SEM, n = 6/group. Mean values in columns without a common letter differ (P < 0.05).
Discussion
Intestinal mucosal epithelium is comprised of the proliferating and differentiating IECs formed in a continuous monolayer, which can serve as the critical barrier in the first-line defense of the body against the external world (Zhou, Zhang, et al., Citation2017). This study had first assessed Arg and LPS toxicities to IOECs; the results indicated that Arg at 100 µM can significantly increase cell viability, and 10 μg/mL LPS could dramatically reduce cell viability. Besides, our results indicated that 24 h of Arg treatment could increase the cell viability of IOECs, while 24 h of LPS treatment reduced the cell viability of IOECs. Therefore, 100 µM Arg, 10 μg/mL LPS, and 24 h of cell treatment were used for later experiments. To further elucidate the underlying mechanism of Arg in benefiting intestinal integrity, the inflammatory response of IOECs treated by LPS was measured. It is well known that LPS-induced inflammation events are characterized by eliciting and releasing a large number of pro-inflammatory cytokines (like TNF-α, IL-1β, and IL-6) in macrophages and epithelial cells (Xiao, Wang, Liu, & Wang, Citation2015). LPS-treated animals and cells are associated with higher inflammatory responses; as a result, they are the suitable model to study the anti-inflammation effects of dietary supplements (Liu et al., Citation2012; Zhou, Yuan, et al., Citation2017). More inflammatory cytokines will be synthesized and released, and more free radicals will be generated following LPS treatment, which are recognized to be the major markers to reflect the dysfunction in cells and organs (Khalifeh, Awaisheh, Alameri, & Hananeh, Citation2015; Wang et al., Citation2017). Our findings suggested that Arg could decrease the inflammatory cytokine concentrations (including IL-1β, TNF-α, and IL-6) and their mRNA levels in IOECs treated with LPS; in addition, it could also markedly reduce the relative TLR4 protein expression and the pNF-κB levels in IOECs treated with LPS. The above findings indicated that Arg could prevent the intestine from inflammatory response. Likewise, Arg supplement from diet could attenuate the oxidative stress in liver of obese rats (Jobgen et al., Citation2008) and intestinal tissue of weaned piglets (Zheng et al., Citation2017). Taken together, the above-mentioned results indicated that Arg could alleviate inflammatory bowel disease (IBD) and intestinal dysfunction.
Transepithelial electrical resistance (TEER) is an approach to measure the integrity and permeability of intestinal epithelium (Sun, Lei, Wang, Wu, & Wu, Citation2017). Enterocytes under Arg treatment have displayed elevated TEER whereas reduced FITC-dextran flux of LPS-treated IOECs, indicating that Arg could benefit the barrier function. Typically, the barrier function of epithelium, together with the paracellular permeability, can be mainly dependent on the epithelial tight junctions (TJ) proteins (Clayburgh, Shen, & Turner, Citation2004; Schneeberger & Lynch, Citation2004). TJs, which are comprised of the cytosolic (such as ZO-1) and transmembrane (like claudins and occludins) proteins, have been recognized as the crucial apical intercellular structures in IECs (Zhou, Zhang, Wu, Wan, & Yin, Citation2018). TJ proteins play crucial roles in keeping the intestinal integrity. Meanwhile, the gastrointestinal tract can serve as a physiologic barrier, which exerts a critical part in the interaction of the body with external environment (Lalles, Citation2012). TJ proteins have been recognized to modulate the paracellular barrier function in intestinal epithelium (Blasig et al., Citation2011). Moreover, the application of nutrients and prebiotics in restoring TJ proteins is able to enhance the integrity as well as function of mucosal barrier in both human and animal (Turner, Citation2009). It was recently discovered that Arg supplemented in diet could avoid the breakdown of the intestine-mucosa barrier induced by IUGR, which was achieved through reducing the levels of TJ proteins (Zhang et al., Citation2018). These findings suggested that Arg played certain functional roles in modulating the barrier function of mucosa. Our findings indicated that Arg could modulate the ZO-1, occludin, and claudin-1 mRNA levels in LPS-treated IOECs. To be specific, 100 µM L-arginine could increase the ZO-1 and claudin-1 protein levels, which showed potent correlation with the increased TEER values while reduced FITC-dextran flux in LPS-treated IOECs (). These results showed that Arg addition could serve as an efficient nutrition strategy for alleviating the dysfunction in the intestinal mucosal barrier.
The mTOR signalling pathways have been recognized to be critically involved in cell metabolism, growth, survival, and vesicular transport for a long time (Wu et al., Citation2016). This pathway was also proved to participate in the innate and adaptive immunity modulation (Xie et al., Citation2014). Toll-like receptors are able to activate the PI3K/AKT/mTOR pathway through enhancing the PI3K phosphorylation and the subsequent AKT and mTOR (Li et al., Citation2015). Our results showed that LPS-treated IOECs had lower ratios of phosphorylated Akt to Akt, phosphorylated mTOR to mTOR and of phosphorylated p70S6K to p70S6K compared with the Con group in the IOECs. These findings indicated that the mTOR pathway correlates closely to LPS-induced epithelial cells inflammation events. Our results also showed that arginine supplement increased the phosphorylation expression of AKT, mTOR and S6K1 (). The mTOR signalling pathways could negatively regulate the effects of inflammatory response induced by LPS through blocking NF-κB transactivation (Mendes et al., Citation2009; Wu et al., Citation2016). However, some previous reports claimed that inhibitors of PI3K and mTOR could remarkably suppress the secretion of cytokines by monocytes (Xie et al., Citation2014) and macrophages (Lorne et al., Citation2009) and human neutrophils (Fortin et al., Citation2011). For those paradoxical results, different cell types and treatments may answer. Little existing literature has examined the TJ barrier function regulated by Arg from the point of view of relevant signalling pathways in the LPS-treated IOECs. Glutamine supplementation increased TJ protein expression and intercellular junction integrity of Caco-2 cells by suppressing the PI3K/AKT signalling pathway (Li & Neu, Citation2009). By contrast, studies in vitro and in vivo suggest that TLR2 can preserve the TJ-related intestinal epithelial barrier function to resist the damage induced by stress by promoting cell survival that is regulated through activating the PI3K/AKT signalling (Cario, Gerken, & Podolsky, Citation2007). This study had first elucidated the role of Arg addition in regulating TJ by regulating the mTOR signalling pathway in LPS-treated IOECs. In this study, Arg supplementation promoted activation of mTOR signalling, which suggested the potential association of the mTOR signalling with the TJ barrier function enhanced by Arg. Herein, we found that phosphorylation of AKT, mTOR, and S6K1 was induced by Arg supplementation, indicating that Arg could activate the mTOR pathways in LPS-treated IOECs, which could account for the effect of Arg on enhancing ZO-1, occludin, and claudin-1 synthesis and intestinal epithelial integrity.
Apart from physically and functionally offering the barriers to avoid the bacteria, endotoxins, and other harmful materials from entering into the blood circulation, TJ proteins at suitable amount can keep the intestinal epithelial integrity; therefore, these proteins are necessary for nutrient absorption (Jacobi & Odle, Citation2012). Amino acids can be released into the small intestinal cavity through dietary protein and peptide hydrolysis, which can then be transported across the cell membrane through a complicated multi-amino acid transporter system (Broer, Citation2008). Numerous transporters are recognized on the small intestinal apical surface in mammals, which are involved in amino acid absorption by the intestine (Broer, Citation2008). Defects in the amino acid absorption by the intestine will result in the changed amino acids in plasma, growth retardation, and the Hartnup disorder (Broer, Citation2008). In our study, Arg could increase the protein levels of amino acids and peptide transporters, such as EAAT3, ASCT2 and PEPT1, in the LPS-induced IOECs, which might thereby enhance amino acid transport and absorption, while the latter stimulated protein synthesis and facilitate the growth of Hu lambs, as seen in previous study (Zhang et al., Citation2018). On the other hand, Arg is the eNOS substrate, which can generate NO, the critical vaso-protective molecule for regulating the vital metabolic pathways (Jobgen, Fried, Fu, Meininger, & Wu, Citation2006).NO exerts a vital part in regulating Arg in the growth and proliferation of intestinal epithelial cells. Arg can stimulate cell migration through the mechanism that requires NO and p70 S6 kinase phosphorylation (Rhoads, Liu, Niu, Surendran, & Wu, Citation2008). According to our results, Arg protects IOECs from LPS induced intestinal barrier injury, which might be related to the role of Arg as the NO precursor. To explore the role of the NO pathway in the Arg-enhanced intestinal barrier function, mRNA expression levels of eNOS and iNOS, NOS activity and NO content in IOECs were determined. Our results indicated that Arg could evidently increase the intestinal mRNA expression of eNOS and iNOS, NOS activity and NO content in IOECs. Specifically, these beneficial effects of Arg on protecting IOECs might be related to the enhanced intestinal barrier function depending on the NO pathway.
Conclusions
In summary, studies with IOECs induced by LPS suggest that Arg can improve the barrier function of the intestinal epithelium, which is verified by the elevated TEER while reduced paracellular permeability. Typically, such benefit of Arg is associated with the attenuated the inflammatory response and enhanced tight junction integrity in IOECs induced by LPS. Importantly, the mTOR signalling pathway and amino acid and peptide transporters were activated by Arg. Arg supplementation may serve as a possible nutrition strategy for boosting the barrier function and nutrient absorption of the intestine, so as to clinically manage the infants and neonatal lambs infected with endotoxin. In addition, the mechanisms are still not deciphered completely in our study, it would add a lot to prove that NO or mTOR signalling pathways are involved in the beneficial effects of Arg by using appropriate pharmacological inhibitors in the future study.
Acknowledgement
The authors thank all of the members of Hongrong Wang's laboratory who contributed to sample determination.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Blasig, I. E., Bellmann, C., Cording, J., Vecchio, G. D., Zwanziger, D., Huber, O., & Haseloff, R. F. (2011). Occludin protein family: Oxidative stress and reducing conditions. Antioxidants & Redox Signaling, 15(5), 1195–1219.
- Broer, S. (2008). Amino acid transport across mammalian intestinal and renal epithelia. Physiological Reviews, 88(1), 249–286.
- Cario, E., Gerken, G., & Podolsky, D. (2007). Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology, 132(4), 1359–1374.
- Clayburgh, D. R., Shen, L., & Turner, J. R. (2004). A porous defense: The leaky epithelial barrier in intestinal disease. Laboratory Investigation, 84(3), 282.
- Fortin, C. F., Cloutier, A., Ear, T., Sylvain-Prévost, S., Mayer, T. Z., Bouchelaghem, R., & McDonald, P. P. (2011). A class IA PI3 K controls inflammatory cytokine production in human neutrophils. European Journal of Immunology, 41(6), 1709–1719.
- Guo, Y. X., Nie, H. T., Sun, L. W., Zhang, G. M., Deng, K. P., Fan, Y. X., & Wang, F. (2017). Effects of diet and arginine treatment during the luteal phase on ovarian NO/PGC-1α signaling in ewes. Theriogenology, 96, 76–84.
- Han, C., Wei, Y., Wang, X., Cui, Y., Bao, Y., & Shi, W. (2019). Salvia Miltiorrhiza polysaccharides protect against lipopolysaccharide-induced liver injury by regulating NF-κb and Nrf2 pathway in mice. Food and Agricultural Immunology, 30(1), 979–994.
- Jacobi, S. K., & Odle, J. (2012). Nutritional factors influencing intestinal health of the neonate. Advances in Nutrition, 3(5), 687–696.
- Jobgen, W. S., Fried, S. K., Fu, W. J., Meininger, C. J., & Wu, G. (2006). Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. The Journal of Nutritional Biochemistry, 17(9), 571–588.
- Jobgen, W., Meininger, C. J., Jobgen, S. C., Li, P., Lee, M.-J., Smith, S. B., & Wu, G. (2008). Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. The Journal of Nutrition, 139(2), 230–237.
- Khalifeh, M. S., Awaisheh, S. S., Alameri, O. H., & Hananeh, W. M. (2015). Small intestine mucosal immune system response to high-fat-high-cholesterol dietary supplementation in male rats. Food and Agricultural Immunology, 26(2), 293–304.
- Lalles, J. (2012). Long term effects of pre-and early postnatal nutrition and environment on the gut. Journal of Animal Science, 90(suppl_4), 421–429.
- Li, T., Li, Z., Nan, F., Dong, J., Deng, Y., Yu, Q., & Zhang, T. (2015). Construction of a novel inducing system with multi-layered alginate microcapsules to regulate differentiation of neural precursor cells from bone mesenchymal stem cells. Medical Hypotheses, 85(6), 910–913.
- Li, N., & Neu, J. (2009). Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. The Journal of Nutrition, 139(4), 710–714.
- Li, W., Sun, K., Ji, Y., Wu, Z., Wang, W., Dai, Z., & Wu, G. (2016). Glycine regulates expression and distribution of claudin-7 and ZO-3 proteins in intestinal porcine epithelial cells. Journal of Nutrition, 146(5), 964–969.
- Liu, Y., Chen, F., Odle, J., Lin, X., Jacobi, S. K., Zhu, H., & Hou, Y. (2012). Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. The Journal of Nutrition, 142(11), 2017–2024.
- Lorne, E., Zhao, X., Zmijewski, J. W., Liu, G., Park, Y.-J., Tsuruta, Y., & Abraham, E. (2009). Participation of mammalian target of rapamycin complex 1 in Toll-like receptor 2–and 4–induced neutrophil activation and acute lung injury. American Journal of Respiratory Cell and Molecular Biology, 41(2), 237–245.
- Mendes, S. D. S., Candi, A., Vansteenbrugge, M., Pignon, M.-R., Bult, H., Boudjeltia, K. Z., & Raes, M. (2009). Microarray analyses of the effects of NF-κB or PI3 K pathway inhibitors on the LPS-induced gene expression profile in RAW264. 7 cells: Synergistic effects of rapamycin on LPS-induced MMP9-overexpression. Cellular Signalling, 21(7), 1109–1122.
- Min, L., Rui, G., Qingwei, M., Yuanyuan, Z., Chongpeng, B., Anshan, S., & Kartik, S. (2014). Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. Plos One, 9(9), e106412.
- Rhoads, J. M., Liu, Y., Niu, X., Surendran, S., & Wu, G. (2008). Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70 S6 kinase. The Journal of Nutrition, 138(9), 1652–1657.
- Rhoads, J. M., & Wu, G. (2009). Glutamine, arginine, and leucine signaling in the intestine. Amino Acids, 37(1), 111–122.
- Schneeberger, E. E., & Lynch, R. D. (2004). The tight junction: A multifunctional complex. American Journal of Physiology-Cell Physiology, 286(6), C1213–C1228.
- Stämmler, F., Gläsner, J., Hiergeist, A., Holler, E., Weber, D., Oefner, P. J., & Spang, R. (2016). Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome, 4(1), 28.
- Sun, T., Gao, G. Z., Li, R. F., Li, X., Li, D. W., Wu, S. S., & Jin, B. (2015). Bone marrow-derived mesenchymal stem cell transplantation ameliorates oxidative stress and restores intestinal mucosal permeability in chemically induced colitis in mice. American Journal of Translational Research, 7(5), 891.
- Sun, K., Lei, Y., Wang, R., Wu, Z., & Wu, G. (2017). Cinnamicaldehyde regulates the expression of tight junction proteins and amino acid transporters in intestinal porcine epithelial cells. Journal of Animal Science and Biotechnology, 8(1), 66.
- Suzuki, T. (2013). Regulation of intestinal epithelial permeability by tight junctions. Cellular and Molecular Life Sciences, 70(4), 631–659.
- Talavera, M. M., Sushma, N., Hongmei, C., Yi, J., Yusen, L., & Nelin, L. D. (2017). Immunostimulated arginase II expression in intestinal epithelial cells reduces nitric oxide production and apoptosis. Frontiers in Cell & Developmental Biology, 5, 15.
- Tan, B., Yin, Y., Kong, X., Li, P., Li, X., Gao, H., & Wu, G. (2010). L-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids, 38(4), 1227–1235.
- Tang, X., Hu, L., Shufen, Y., Zuohua, L., Jinfeng, Z., & Rejun, F. (2016). Epidermal growth factor and intestinal barrier function. Mediators of Inflammation, 2016(3), 1–9.
- Turner, J. R. (2009). Intestinal mucosal barrier function in health and disease. Nature Reviews Immunology, 9(11), 799.
- Wang, X., Liu, Y., Li, S., Pi, D., Zhu, H., Hou, Y., & Leng, W. (2015). Asparagine attenuates intestinal injury, improves energy status and inhibits AMP-activated protein kinase signalling pathways in weaned piglets challenged with Escherichia coli lipopolysaccharide. British Journal of Nutrition, 114(4), 553–565.
- Wang, W., Qiao, S., & Li, D. (2009). Amino acids and gut function. Amino Acids, 37(1), 105–110.
- Wang, B., Wu, Z., Ji, Y., Sun, K., Dai, Z., & Wu, G. (2016). L-Glutamine enhances tight junction integrity by activating CaMK kinase 2–AMP-activated protein kinase signaling in intestinal porcine epithelial cells. The Journal of Nutrition, 146(3), 501–508.
- Wang, Z., Xie, J., Yang, Y., Zhang, F., Wang, S., Wu, T., & Xie, M. (2017). Sulfated Cyclocarya paliurus polysaccharides markedly attenuates inflammation and oxidative damage in lipopolysaccharide-treated macrophage cells and mice. Scientific Reports, 7, 40402.
- Wu, T., Wang, C., Ding, L., Shen, Y., Cui, H., Wang, M., & Wang, H. (2016). Arginine relieves the inflammatory response and enhances the casein expression in bovine mammary epithelial cells induced by lipopolysaccharide. Mediators of Inflammation, 2016, 9618795.
- Xia, M., Ye, L., Hou, Q., & Yu, Q. (2016). Effects of arginine on intestinal epithelial cell integrity and nutrient uptake. British Journal of Nutrition, 116(10), 1675–1681.
- Xiao, H. B., Wang, C. R., Liu, Z. K., & Wang, J. Y. (2015). LPS induces pro-inflammatory response in mastitis mice and mammary epithelial cells: Possible involvement of NF-κB signaling and OPN. Pathologie Biologie, 63(1), 11–16.
- Xie, S., Chen, M., Yan, B., He, X., Chen, X., & Li, D. (2014). Identification of a role for the PI3 K/AKT/mTOR signaling pathway in innate immune cells. Plos One, 9(4), e94496.
- Zhan, K., Jiang, M., Gong, X., & Zhao, G. (2018). Effect of short-chain fatty acids on the expression of genes involved in short-chain fatty acid transporters and inflammatory response in goat jejunum epithelial cells. In Vitro Cellular & Developmental Biology-Animal, 54(4), 311–320.
- Zhan, K., Lin, M., Liu, M. M., Sui, Y. N., & Zhao, G. Q. (2017). Establishment of primary bovine intestinal epithelial cell culture and clone method. In Vitro Cellular & Developmental Biology-Animal, 53(1), 54–57.
- Zhang, H., Peng, A., Yu, Y., Guo, S., Wang, M., & Wang, H. (2019). L-arginine protects ovine intestinal epithelial cells from lipopolysaccharide-induced apoptosis through alleviating oxidative stress. Journal of Agricultural and Food Chemistry, 67(6), 1683–1690.
- Zhang, H., Zhao, F., Peng, A., Dong, L., Wang, M., Yu, L., & Wang, H. (2018). Effects of dietary l-arginine and N-carbamylglutamate supplementation on intestinal integrity, immune function, and oxidative status in intrauterine-growth-retarded suckling lambs. Journal of Agricultural and Food Chemistry, 66(16), 4145–4154.
- Zhao, X., Guo, Y., Liu, H., Gao, J., & Nie, W. (2014). Clostridium butyricumreduce lipogenesis through bacterial wall components and butyrate. Applied Microbiology & Biotechnology, 98(17), 7549–7557.
- Zhao, Y., Liu, D., Han, R., Zhang, X., Zhang, S., & Qin, G. (2015). Soybean allergen glycinin induced the destruction of the mechanical barrier function in IPEC-J2. Food and Agricultural Immunology, 26(4), 601–609.
- Zheng, P., Yu, B., He, J., Yu, J., Mao, X., Luo, Y., & Zeng, Q. (2017). Arginine metabolism and its protective effects on intestinal health and functions in weaned piglets under oxidative stress induced by diquat. British Journal of Nutrition, 117(11), 1495–1502.
- Zhou, Y., Yuan, H., Cui, L., Ansari, A. R., Xiao, K., Luo, Y., & Yang, Z. (2017). Effects of visfatin on the apoptosis of intestinal mucosal cells in immunological stressed rats. Acta Histochemica, 119(1), 26–31.
- Zhou, X., Zhang, Y., He, L., Wan, D., Liu, G., Wu, X., & Yin, Y. (2017). Serine prevents LPS-induced intestinal inflammation and barrier damage via p53-dependent glutathione synthesis and AMPK activation. Journal of Functional Foods, 39, 225–232.
- Zhou, X., Zhang, Y., Wu, X., Wan, D., & Yin, Y. (2018). Effects of dietary serine supplementation on intestinal integrity, inflammation and oxidative status in early-weaned piglets. Cellular Physiology and Biochemistry, 48(3), 993–1002.
- Zhu, H., Liu, Y., Xie, X., Huang, J., & Hou, Y. (2013). Effect of L-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immunity, 19(3), 242–252.
