ABSTRACT
Aim
To evaluate the occurrence of food allergy to walnuts and hazelnuts in atopic dermatitis patients.
Methods
The examination of specific IgE to molecular components with Multiplex ISAC testing was performed. The diagnosis of food allergy was confirmed in patients suffering from food reactions to walnuts/hazelnuts together with positive results of specific IgE to molecular components.
Results and conclusion
: Altogether 100 atopic dermatitis patients were examined – 48 men, 52 women, the average age 40.9 years. Hazelnuts allergy was confirmed in 25% of patients; these patients are majorly allergic to PR 10 protein, minorly to storage proteins. Walnut allergy was confirmed in 14% of patients; these patients are majorly allergic to storage proteins and to non-specific lipid transfer proteins. Sensitization to Bet v 1 homologues and profilins was associated with mild symptoms; nsLTPs and seed storage proteins were related to severe reactions.
Introduction
Tree nuts are a potent and frequent allergen source, inducing IgE-mediated food allergic reactions, which can cause serious and sometimes even fatal reactions. Tree nut allergy has been reported from all over the world with a prevalence of up to 4.9% of the general population (McWilliam et al. (Citation2015)). Food allergy (FA) and atopic dermatitis (AD) are often co-expressed, and a causal link of foods, triggering skin lesions, has been shown (McWilliam et al. (Citation2015), Weidinger and Novak (Citation2016)). Many studies reveal the prevalence of allergic diseases; however, most studies analysed a limited period from infancy to later childhood and/or to early adolescence. The incidence of new-onset food allergy in adult atopic dermatitis patients is currently unknown as are the main routes of sensitization (Weidinger and Novak (Citation2016)). It is evident that the direct contact of skin with allergens could trigger signals to initiate Th2 allergic response (Weidinger and Novak (Citation2016)). Emerging data now suggest that epithelial cell-derived cytokines, such as thymic stromal lymphopoietin (TSLP), IL-33, and IL-25, may drive the progression from atopic dermatitis to asthma and food allergy (Weidinger and Novak (Citation2016)). AD affects up to 20% of children and up to 3% of adults; recent data show that its prevalence is still increasing (Weidinger and Novak (Citation2016)). AD patients, who are sensitized to a food protein, can show three distinct reaction patterns: non-eczematous reactions, isolated atopic dermatitis flares or a combination of both reactions (McWilliam et al. (Citation2015), Weidinger and Novak (Citation2016), Manam et al. (Citation2014)).
Hazelnuts (Corylus avellana) are classified as tree nuts belonging to the family of Betulaceae. They are consumed worldwide due to their nutritional value and beneficial health effect. Although there are significant geographic and age-related variations regarding the severity of symptoms, hazelnut allergy has been reported to be the most common tree nut allergy in Europe (McWilliam et al. (Citation2015)). So far, 8 hazelnut allergens have been recognized: Cor a 1 (Bet v 1 like, PR -10 protein), Cor a 2 (Profilin), Cor a 8 (Non-specific lipid transfer protein), Cor a 9 (Legumin), Cor a 11 (Vicilin), Cor a 12, Cor a 13 (Oleosin), Cor a 14 (2S albumin), (Masthoff et al. (Citation2013), Pastorello et al. (Citation2004), Burney et al. (Citation2014), Matricardi et al. (Citation2016)).
The common walnut (Juglans regia) belongs to the family of Juglandaceae and is cultivated around the world, predominantly in temperate climates. The edible kernels are part of healthy, balanced food diets because of their high content of polyunsaturated fatty acids, phytochemicals, and antioxidants. Generally, there is little information concerning the prevalence of walnut allergy. In contrast to Europe, where the prevalence of walnut allergy is rather low with an overall incidence of 2.2% (Sato et al. (Citation2017)), it is the most frequently reported tree nut allergy in the US (Tuano and Davis (Citation1998)). So far, eight walnut allergens have been recognized: Jug r 1 (2S albumin), Jug r 2 and Jug r 6 (Vicilin), Jug r 3 and Jug r 8 (Non-specific lipid transfer proteins - nsLTP), Jug r 4 (11S globulin), Jug r 5 (PR-10), and Jug r 7 (Profilin), (Masthoff et al. (Citation2013), Pastorello et al. (Citation2004), Burney et al. (Citation2014), Matricardi et al. (Citation2016), Sato et al. (Citation2017), Tuano and Davis (Citation1998)). Hazelnut and walnut allergy diagnosis is based on a clinical history supported by positive results from IgE sensitization tests. Extract-based hazelnut tests are sensitive, but moderate predictors of allergy (Masthoff et al. (Citation2012), Clark and Ewan (Citation2003)). The current gold standard for diagnosing food allergy is the double-blind placebo-controlled food challenge (DBPCFC), a time-consuming and costly procedure (McWilliam et al. (Citation2015)).
Molecular diagnosis provides a major step in improving the accuracy of diagnosing IgE-mediated sensitizations in food allergy (Uotila et al. (Citation2015), van Hage et al. (Citation2016)). This approach has been developed when highly purified or recombinant allergen molecules have become available. These molecules are the allergenic proteins towards which the specific and clinically relevant IgE immune response is directed (Uotila et al. (Citation2015), van Hage et al. (Citation2016)). The ability to identify and characterize single allergens at a molecular level has added a significant body of understanding as to the mechanism of sensitization to foods (Uotila et al. (Citation2015), van Hage et al. (Citation2016)). Research into the structural similarity between allergens and the amino acid sequence homology between food allergens also helps to explain cross-reactivity between allergens, which may be clinically relevant or irrelevant. Certain pan-allergen molecules can indicate broad cross-sensitization and unfold particular pollen-food or plant-food syndromes (Uotila et al. (Citation2015), van Hage et al. (Citation2016), Costa et al. (Citation2016), Ebo et al. (Citation2012), Weidinger and Novak (Citation2012)). The examination of molecular components is used in both singleplex ImmunoCAP and multiplex ImmunoCAP ISAC assays. The major advantage of ISAC is the comprehensive IgE pattern obtained with a minute amount of serum (Verweij et al. (Citation2017)). ImmunoCAP ISAC (Thermo Fisher), based on 112 different molecular components (both extracted and recombinant), is the most studied and most frequently used molecular diagnostic tool based on a microarray (Melioli et al. (Citation2011)). Overview of the different proteins per protein families for the hazelnut/walnut allergens is recorded in , (Matricardi et al. (Citation2016)); molecular components examined in ISAC Multiplex testing are shown extra bold.
Table 1. Tree nuts allergens. Overview of the different proteins per protein families for the tree nut allergens (Matricardi et al. (Citation2016)). Molecular components in ISAC Multiplex testing are shown extra bold*.
In our previous studies, we evaluated the occurrence of sensitization to food and inhalant allergens in patients suffering from AD; these patients were subjected to skin prick test, atopy patch tests and extract specific IgE to different food and inhalant allergens (Čelakovská and Bukač (Citation2011), Čelakovská and Bukač (Citation2017), Čelakovská et al. (Citation2018 Jul–Aug)).
Aim
The aim of our study is to evaluate the occurrence of hazelnut/walnut allergy in atopic dermatitis patients. Our aim is also to analyze the results of specific IgE to molecular components to show the role of species – specific and cross-reactive molecular components in the walnut/hazelnut allergy.
Method
Patients and methods
During 2018–2019, 100 patients suffering from atopic dermatitis at the age of 14 years and older were examined. All these patients were examined in the Department of Dermatology, Faculty Hospital Hradec Králové, Charles University, Czech Republic. The diagnosis of atopic dermatitis was made with the Hanifin-Rajka criteria (Hanifin and Rajka (Citation1980)). Exclusion criteria were systemic therapy (cyclosporin, systemic corticoids, biological therapy), pregnancy, breastfeeding. Patients with atopic dermatitis, having other systemic diseases, were excluded from the study as well. Complete dermatological and allergological examination (evaluating the occurrence of asthma bronchiale and allergic rhinitis) was performed in all patients included in the study. This study was approved by Ethics committee of Faculty Hospital Hradec Králové, Charles University of Prague, Czech Republic.
Examination of specific IgE to molecular components
The serum level of the specific IgE was measured by the component-resolved diagnosis microarray-based sIgE detection assay ImmunoCAP ISAC (Phadia, Thermo Fisher Scientific, Uppsala, Sweden). ImmunoCAP ISAC is a solid-phase multiple immunoassay, which enables to determine 112 different components from 51 allergen sources (Verweij et al. (Citation2017), Melioli et al. (Citation2011)). The allergens are applied in triplicates to ensure the test reproducibility. The specific IgE values are presented in arbitrary units called ISAC Standardized Units (measuring range of 0.3–100 ISU-E). The level of specific IgE higher than 0.3 ISU–E was assessed as positive. The level of molecular components in ISU – E was evaluated: < 0.3 – negative, 0.3–0.9 low positivity, 0.9–15 moderate positivity, above 15 ISU–E very high positivity (Verweij et al. (Citation2017), Melioli et al. (Citation2011)).
Scoring of AD
Severity of atopic dermatitis was scored in agreement with SCORAD (Scoring of atopic dermatitis) with the assessment of topography items (affected skin area), intensity criteria and subjective parameters. The severity of atopic dermatitis was evaluated with SCORAD as a mild form to 25 points, as moderate over 25–50 points, as a severe form over 50 points (European Task Force on Atopic Dermatitis (Citation1993)). This examination was performed during one year every two months and the average SCORAD index was calculated.
Anamnestic data
A detailed personal history of possible food allergy was taken in all included patients. The patients indicate whether they suffer from immediate or late food reactions affecting the skin, gastrointestinal tract, or respiratory tract, not only after the ingestion of hazelnuts and walnuts, but also after the ingestion of other foods. We recorded previous reactions to hazelnuts and/or walnuts; symptoms; affected organ system, onset and course, cofactors (exercise, NSAID, alcohol). The open exposure test was not performed in patients with the early reaction after the ingestion of hazelnuts and/or walnuts in their history because of anaphylactic reaction danger. All of these patients have been carefully examined and they are always monitored.
Confirmation of food allergy
The diagnosis of hazelnut/walnut allergy was confirmed according to the convincing history with the food reactions after hazelnut/walnut ingestion (gastrointestinal symptoms – oral allergy syndrome, vomiting, cramps, abdominal pain, skin symptoms – pruritus and worsening of AD), together with the positive results of specific IgE to molecular components of hazelnuts/walnuts in ISAC Multiplex testing.
Sensitization to hazelnuts and/or walnuts was confirmed in patients with positive results of specific IgE to molecular components in ISAC Multiplex testing and with no clinical reaction after the ingestion of hazelnuts and/or walnuts.
Patients with clinical reactions after hazelnut and/or walnut ingestion with no positive results to molecular components in ISAC Multiplex testing are always monitored and the clinical reactions are observed.
Statistical analysis
The analysis of the results in the examination of ISAC Multiplex testing in patients with walnut/hazelnut allergy was performed. Venn diagram was used to show the positivity to molecular components of walnuts/ hazelnuts in patients suffering from food allergy.
Results
Patients
One hundred patients suffering from atopic dermatitis were included in the study – 48 men and 52 women with the average age 40.9 years and with the average SCORAD 39, s.d.13.1 (points). The mild form of AD was recorded in 14 patients (14%), moderate form in 58 patients (58%), severe form in 28 patients (28%).
From one hundred patients included in the study, the diagnosis of asthma bronchiale was confirmed in 55 patients (55%), the diagnosis of allergic rhinitis in 78 patients (78%). The food reactions after the ingestion of walnuts were recorded in 49 patients (49%), after the ingestion of hazelnuts in 46 patients (46%). The food reactions after the ingestion of hazelnuts and walnuts at the same time were recorded in 35 patients (35%). The positive results to molecular components of walnuts were recorded in 16 patients (16%), of hazelnuts in 43 patients (43%), of hazelnuts and walnuts at the same time in 11 patients (11%). The characteristics of patients are shown in .
Table 2. The characteristic of patients with atopic dermatitis.
In patients suffering from food reactions to walnuts (49 patients = 100%) and to hazelnuts (46 patients = 100%), we recorded in rare cases only late reactions with the worsening of AD (to walnuts in 2.1%, to hazelnuts in 4.3%). Only early reactions were observed in 24.5% of patients to walnuts and in 21.8% of patients to hazelnuts. In majority of patients, we recorded combined reactions (early + late reactions) – in 73.5% of patients to walnuts and in 73.9% of patients to hazelnuts. Patients observed oral allergy syndrome, abdominal pain, pruritus of the skin and in rare cases the worsening of atopic dermatitis, . In patients suffering from clinical reactions after the ingestion of walnuts and/or hazelnuts, we recorded the mild form of AD only in minority of them; majority of these patients suffer from moderate form of AD and more than one-third of patients suffer from severe form of AD ().
Table 3. Characteristic of patients with clinical reaction to walnuts and hazelnuts. Severity of atopic dermatitis (AD), clinical reactions (early, late, combined) after the ingestion of walnuts (49 patients = 100%) and hazelnuts (46 patients = 100%) OAS – oral allergy syndrome, GIT – gastrointestinal symptoms (nausea, abdominal pain).
The food allergy to walnuts/hazelnuts was confirmed in the case of convincing clinical symptoms after the ingestion of walnuts and/or hazelnuts together with the positive results of specific IgE to molecular components of walnuts/hazelnuts. We analyzed these data in the whole group of 100 patients ( and ). The food allergy to hazelnuts was confirmed in 25 patients (25%); in all of these patients, we recorded the positive results of specific IgE to Cora1.0401 (PR – 10 protein); the positive result of specific IgE to Cor a 9 (Storage protein, 11S globulin) was recorded only in 1 patient (1%). The food allergy to walnuts was confirmed in 14 patients (14%) – the positive result of specific IgE to storage proteins, Jug r 2 and Jug r 1, was confirmed in 8% of patients, the positivity of specific IgE to nsLTP Jug r 3 was confirmed in another 6% of patients. We show the Venn diagram of positive results of specific IgE to molecular components in patients suffering from walnut allergy () and hazelnut allergy ().
Diagram 1. Venn diagram (areas proportional to frequencies) of positive results to molecular components in patients suffering from food allergy to walnuts A – altogether 7 patients with positivity to Jug r 2, B – altogether 3 patients with positivity to Jug r 1, C – altogether 6 patients with positivity to Jug r 3.
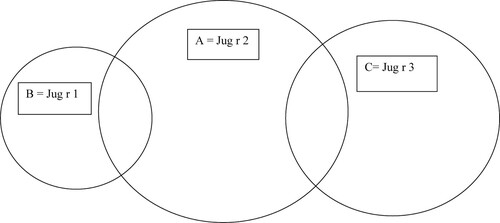
Diagram 2. Venn diagram (areas proportional to frequencies) of positive results to molecular components in patients suffering from food allergy to hazelnuts A – altogether 25 patients with the positivity to Cor a 1.0401, B – 2 patients with the positivity to Cor a 8, C – 1 patient with positivity to Cor a 9.
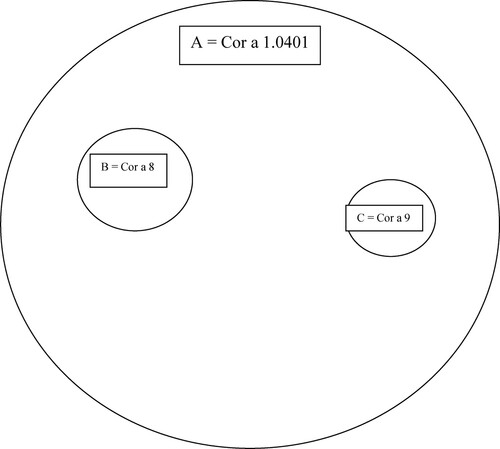
Table 4. Analysis of sensitization to molecular components of walnuts in 100 atopic dermatitis patients (100 patients = 100%). The positive results of specific IgE to molecular components of walnuts.
Table 5. Analysis of sensitization to molecular components of hazelnuts in 100 atopic dermatitis patients (100 patients = 100%). The positive results of specific IgE to molecular components of hazelnuts.
The positive results of specific IgE to molecular components of hazelnuts with no clinical reaction in these patients (= sensitization) were confirmed in another 18% of patients; majority of these patients have the positive results of specific IgE to Cor a 1.0401 (PR – 10 protein), the positive results of specific IgE to Cor a 8 (nsLTP) are rare (in 2%). The positive results of specific IgE to molecular components of walnuts with no clinical reaction (= sensitization) were confirmed only in 2% of patients (to Jug r 2 and to Jug r 3), ( and ).
The negative results of specific IgE to molecular components of walnuts were recorded in 35 patients (71.4%) from 49 patients suffering from clinical symptoms after walnut ingestion. The negative results of specific IgE to molecular components of hazelnuts were recorded in 21 patients (45.6%) from 46 patients suffering from clinical symptoms after hazelnut ingestion (). These ratios could be formally called sensitivity, but we don't use this terminology because it would remind us of its connection with making a decision. We are more interested in the role of species – specific and cross-reactive molecular components in the walnut/hazelnut allergy.
Table 6. The results of specific IgE to molecular components of walnuts/hazelnuts in ISAC Multiplex testing in patients suffering from food reactions to walnuts (49 patients = 100%) and hazelnuts (46 patients = 100%).
Regarding the kind of clinical reaction to hazelnuts, in patients with positive results to Cor a 1.0401 (PR – 10 protein) we observed mild oral allergy syndrome and pruritus with the worsening of atopic dermatitis in predilection sites such as in flexural involvement of the elbows and knees and on the face. In patients with positive results Cor a 8* (Non-specific lipid transfer protein), we observed oral allergy syndrome and abdominal pain, pruritus and worsening of atopic dermatitis. In patients with positive results to Cor a 9 (Storage protein, 11S globulin), we recorded severe symptoms. Regarding the kind of clinical reaction to walnuts, in patients with positive results to Jug r 2 (Storage protein, 7S globulin) and Jug r 1 (Storage protein, 2S albumin), we observed abdominal pain, pruritus and worsening of atopic dermatitis. In patients with positive results to Jug r 3 (Lipid transfer protein), we observed oral allergy syndrome, pruritus and the worsening of atopic dermatitis.
In 8 patients (8%), we confirmed food allergy to walnuts and to hazelnuts; in another 35 patients (35%) we recorded clinical reactions to walnuts and to hazelnuts, but without and/or with the positive results to molecular components of walnuts and/or hazelnuts (). The level of specific IgE in ISU-E to molecular components of walnuts and hazelnuts is recorded in .
Table 7. The overview of patients suffering from clinical reactions to walnuts and hazelnuts at the same time.
Discussion
Despite their clinical importance, tree nut allergy epidemiology remains understudied and the prevalence of tree nut allergy in different regions of the world has not yet been well characterized (McWilliam et al. (Citation2015)). Data are limited to largely European, US and UK studies using self-reported prevalence in children and adolescents. There is a need for further studies to determine tree nut allergy and differentiate between the primary and secondary tree nut allergy (McWilliam et al. (Citation2015)).
In our study with atopic dermatitis patients, we confirmed the positive results of specific IgE to cross-reactive molecular component Cor a 1.0401 (PR – 10 protein) in majority of patients suffering from hazelnuts allergy, only minority of patients are allergic to specific molecular components of hazelnuts. On the other hand, in patients suffering from allergy to walnuts, the positivity to specific molecular components Jug r 1 and Jug r 2 predominates. In the case of positivity to cross-reacting components, the observed symptoms were mild (mild form of oral syndrome, itching of the skin and worsening of atopic dermatitis). Eczema lesions appeared mainly in flexural involvement of the elbows and knees and on the face. In the case of positivity to species-specific components, we observed more pronounced symptoms – abdominal pain and severe oral allergy syndrome. Our results confirm that sensitization to Bet v 1 homologues and profilins are associated with mild symptoms (pollen food syndrome), on the other hand, nsLTPs and seed storage proteins are related to severe reactions. Interesting finding is the confirmation of the late allergic reaction; all these patients observe the late skin reaction, such as new eczematic lesions and worsening of AD during 24–48 h after a casual ingestion of walnuts/hazelnuts. Generally, late eczematous reaction on the skin, in patients with a persistent moderate or severe form of AD, can be observed with difficulty. The worsening of AD occurs several hours after the ingestion of food allergen and other factors can contribute to the deterioration of AD at the same time also. The psychological stress and inhalant allergens, such as animal dander, trees and grass pollen, along with environmental stressors, such as reduced humidity and lower outdoor temperatures, are the other factors for the worsening of AD. The dysregulation of the skin barrier also predisposes individuals to colonization of microbial pathogens.
On the other hand, in some patients suffering from clinical symptoms after walnuts and/or hazelnuts ingestion, we did not confirm the positivity of specific IgE to molecular components of hazelnuts/walnuts. The explanation can be in the fact that these patients suffer from pollen food syndrome and/or other molecular components of walnuts and hazelnuts may play the role in these patients. In our last study (Celakovska et al. (Citation2021 in press)), we evaluated the relation between the sensitization to molecular components of inhalant allergens and the occurrence of food reactions to peanuts, walnuts, hazelnuts, wheat, soy, celery, apple, peach and kiwi in atopic dermatitis patients (Celakovska et al. (Citation2021 in press)). In patients suffering from food reaction to hazelnuts, the significant relation (p-value <0.05) was confirmed to sensitization to molecular components Aln g1 (Alder, PR – 10 Protein), Bet v 1 (Birch, PR – 10 Proteins), Ole e 1 (Olive pollen, common olive group 1), Phl p 1, 2 (grass group 1, 2), Phl p 4 (Berberine bridge enzyme). The significantly higher occurrence of sensitization (p-value <0.05) to molecular components Bet v 1 (Birch, PR 10 protein), Alt a 1 (Alternaria, Acidic glycoprotein), Ole e 9 (Olive pollen, Beta-1,3-glucanase), Phl p 1, 2, 5, 6 (Grass group 1, 2, 5, 6), Phl p 4 (Timothy, Berberine bridge enzyme) and Mer a 1 (Annual Mercury, profilin) was confirmed in patients suffering from food reaction to walnuts (Celakovska et al. (Citation2021 in press)). Pathogenesis-related protein group 10 (PR-10) molecules (i.e. Bet v 1 and homologous allergens) are the major allergens in Fagales pollen and are recognized by virtually all allergic patients, thus representing the major cause of clinical allergy (Matricardi et al. (Citation2016)). Sensitization to Phl p 1 usually precedes sensitization to other grass pollen allergen and it is the most prevalent component sensitization in grass pollen-allergic patients (Matricardi et al. (Citation2016)). We recommend to examine other molecular components, such as Cor a 11 (7S globulin), Cor a 14 (2S albumin), Cor a 12 (Oleosin), Cor a 2 (Profilin), Jug r 4 (11S globulin), Jug r 5 (Profilin) and Jug r 6 (7S globulin), Jug r 7 (Profilin, Jug r 8 (Non-specific lipid transfer proteins)) in patients suffering from clinical reaction to walnuts and/or hazelnuts without positive results in ISAC Multiplex testing. Another possibility for the examination of specific IgE to molecular components is the examination with ALEX Multiplex testing (ALEX 2–Allergy Explorer). The macro-array nanotechnology-based immunoassay used as a molecular allergy explorer (ALEX®; MacroArray Diagnostics, Wien, Austria) is the latest launched in vitro multiplex tool for precision medicine in allergy diagnosis. It is based on a state-of-the-art proprietary nano-bead technology. This new array contains 295 allergen reagents (117 allergenic extracts and 178 molecular components), with a large majority of aeroallergen families and cross-reactive food allergens being represented. The molecular component, such as Cor a 11 (7 S globulin), Cor a 14 (2 S albumin), Jug r 4 (11 S globulin) and Jug r 6 (7 S globulin), are included in this ALEX 2 – Allergy explorer (Verweij et al. (Citation2017)). Other components, such as Cor a 12 (Oleosin) and Cor a 2 (Profilin) and Jug r 5 (Profilin), are not included in multiplex testing examination. The sensitization to these molecular components can be examined in singleplex examination.
According to the literature, there are is knowledge of sensitization to tree nuts and seeds in the general population and in patients suffering from atopic dermatitis. Most reports on tree nut and seed allergy are based on clinical cases (McWilliam et al. (Citation2015 )). McWilliam V. et al. aimed to systematically review the population prevalence of tree nut allergy in children and adults. The authors searched three electronic databases (OVID MEDLINE, EMBASE and PubMed) from January 1996 to December 2014 (McWilliam et al. (Citation2015)). Eligible studies were categorized by age, region and method of assessment of tree nut allergy. Of the 36 studies identified most were in children (n = 24) and from Europe (n = 18), UK (n = 8) or USA (n = 5). Challenge-confirmed IgE-mediated tree nut allergy prevalence was less than 2% (although only seven studies used this gold standard), while probable tree nut allergy prevalence ranged from 0.05% to 4.9%. Prevalence estimates that included oral allergy syndrome (OAS) reactions to tree nut were significantly higher (8%–11.4%) and were predominantly from Europe. Prevalence of individual tree nut allergies varied significantly by region with hazelnut the most common tree nut allergy in Europe, walnut and cashew in the USA and Brazil nut, almond and walnut most commonly reported in the UK (McWilliam et al. (Citation2015), Skypala et al. (Citation2010), Datema et al. (Citation2018), Masthoff et al. (Citation2015), Maloney et al. (Citation2008), Nwaru et al. (Citation2014)). Patients, reporting hazelnut allergy (n = 423) from 12 European cities, were tested for IgE against individual hazelnut allergens; a model, combining CRD with clinical background and extract-based serology, was superior to CRD alone in assessing the risk of severe reactions to hazelnut, particular in ruling out severe reactions (Datema et al. (Citation2018 Mar)). Some studies show that sIgE to hazelnut storage proteins, Cor a 14 and Cor a 9, confers higher specificity in the diagnosis of hazelnut allergy in children compared with Cor a 1 or hazelnut extract, and could be used clinically to improve the identification of allergic children and reduce the number of confirmatory food challenges, (Nilsson et al. (Citation2020 Feb)); according to the authors, future analyses of the contribution of additional hazelnut allergen components are needed, as well as evaluations in adult populations (Nilsson et al. (Citation2020 Feb)). The sensitivity and specificity of both Cor a 9 and Cor a 14-sIgE were consistent across paediatric studies, and the diagnostic parameters calculated showed low data variation. This likely reflects the role of storage proteins as early sensitizers and markers of hazelnut allergy (Faber et al. (Citation2014), Verweij et al. (Citation2011), van Erp et al. (Citation2019)). Storage protein 2S albumins have high accuracy in identifying children with allergies and objective symptoms (Flores Kim et al. (Citation2018), Klemans et al. (Citation2015), Sicherer et al. (Citation2015), Lange et al. (Citation2017), Sato et al. (Citation2017)). Another study reflects the cross-reactive nature of Cor a 1-sIgE, where sensitization is driven by birch or birch-related tree pollen allergies (Valcour et al. (Citation2016)) rather than primary hazelnut allergy. The diverse geographic locations of the studies as well as age differences may contribute to the data variation, as pollen cross-reactive sensitizations become increasingly common when children reach school age (Ballmer-Weber et al. (Citation2015)). Cor a 8 is a common sensitizer in the Mediterranean area although LTP sensitization is also increasingly demonstrated in non-Mediterranean areas, but its clinical relevance is unclear (Teuber et al. (Citation2019)). The quality of an applied protein extract is important in both serological and in vivo diagnosis of allergy, and for allergen detection methods (Dooper et al. (Citation2008)).
In the research project MeDALL, IgE reactivities towards a large number of micro-arrayed allergen molecules have been determined in several European birth cohorts using the MeDALL allergen chip (Melioli et al. (Citation2011)). Data obtained in the MeDALL project seem to confirm that patients with atopic dermatitis are often polysensitized towards a large number of different allergen molecules and thus exhibit extremely complex IgE sensitization profiles (Anto et al. (Citation2017), Bousquet et al. (Citation2015)).
Conclusion
The food allergy to hazelnuts was confirmed in 25% of atopic dermatitis patients; these patients are majorly allergic to PR 10 proteins (Cora1.0401), minorly to Cor a 9 (Storage protein, 11S globulin). The food allergy to walnuts was confirmed in 14% of atopic dermatitis patients; these patients are in 8% allergic to storage proteins (Jug r 2 and Jug r 1) and in 6% to nsLTP (Jug r 3). The positive results of specific IgE to molecular components of hazelnuts (walnuts) with no clinical reaction were confirmed in another 18%, resp. 2% of patients. In the case of positivity to cross-reacting components, the symptoms are milder (mild form of oral syndrome, itching of the skin and worsening of atopic dermatitis). In the case of positivity to species-specific components, more pronounced symptoms were observed. We recommend to examine other molecular components, such as Cor a 11 (7S globulin), Cor a 14 (2S albumin), Cor a 12 (Oleosin), Cor a 2 (Profilin), Jug r 4 (11S globulin), Jug r 5, Jug r 7 (Profilin) and Jug r 6 (7S globulin), Jug r 8 (nsLTP), in patients suffering from clinical reaction to walnuts and/or hazelnuts without positive results in ISAC Multiplex testing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anto, J. M., Bousquet, J., Akdis, M., Auffray, C., Keil, T., Momas, I., Postma, D. S., Valenta, R., Wickman, M., Cambon-Thomsen, A., Haahtela, T., Lambrecht, B. N., Lodrup Carlsen, K. C., Koppelman, G. H., Sunyer, J., Zuberbier, T., Annesi-Maesano, I., Arno, A., Bindslev-Jensen, C., … Guerra, S. (2017). Mechanisms of the development of allergy (MeDALL): introducing novel concepts in allergy phenotypes. Journal of Allergy and Clinical Immunology, 139(2), 388–399. https://doi.org/10.1016/j.jaci.2016.12.940
- Ballmer-Weber, B. K., Lidholm, J., Fernández-Rivas, M., Seneviratne, S., Hanschmann, K.-M., Vogel, L., Bures, P., Fritsche, P., Summers, C., Knulst, A. C., Le, T.-M., Reig, I., Papadopoulos, N. G., Sinaniotis, A., Belohlavkova, S., Popov, T., Kralimarkova, T., de Blay, F., Purohit, A., … Dubakiene, R. (2015). Ige recognition patterns in peanut allergy are age dependent: Perspectives of the EuroPrevall study. Allergy, 70(4), 391–407. https://doi.org/10.1111/all.12574
- Bousquet, J., Anto, J. M., Wickman, M., Keil, T., Valenta, R., Haahtela, T., Lodrup Carlsen, K., van Hage, M., Akdis, C., Bachert, C., Akdis, M., Auffray, C., Annesi-Maesano, I., Bindslev-Jensen, C., Cambon-Thomsen, A., Carlsen, K. H., Chatzi, L., Forastiere, F., Garcia-Aymerich, J., … Guerra, S. (2015). Are allergic multimorbidities and IgE polysensitization associated with the persistence or re-occurrence of foetal type 2 signalling? The MeDALL hypothesis. Allergy, 70(9), 1062–1078. https://doi.org/10.1111/all.12637
- Burney, P. G. J., Potts, J., Kummeling, I., Mills, E. N. C., Clausen, M., Dubakiene, R., Barreales, L., Fernandez-Perez, C., Fernandez-Rivas, M., Le, T. M., Knulst, A. C., Kowalski, M. L., Lidholm, J., Ballmer-Weber, B. K., Braun-Fahlander, C., Mustakov, T., Kralimarkova, T., Popov, T., Sakellariou, A., … van Ree, R. (2014). The prevalence and distribution of food sensitization in European adults. Allergy, 69 (3), 365–371. https://doi.org/10.1111/all.12341
- Čelakovská, J., & Bukač, J. (2011). Food adverse reactions in patients over 14 years of age suffering from atopic eczema. Food and Agricultural Immunology, 22(2), 105–114. https://doi.org/10.1080/09540105.2010.533751
- Čelakovská, J., & Bukač, J. (2017). Severity of atopic dermatitis in relation to food and inhalant allergy in adults and adolescents. Food and Agricultural Immunology, 28(1), 121–133. https://doi.org/10.1080/09540105.2016.1228838
- Čelakovská, J., Bukač, J., Ettler, K., Vaneckova, J., & Ettlerova, K. (2018). Allergy to peanuts in atopic dermatitis patients 14 year or older and the Association with food Hypersensitivity, inhalant allergens, asthma bronchiale and rhinitis. Indian Journal of Dermatology, 63(4), 317–322. PMID: 30078876; PMCID: PMC6052752. https://doi.org/10.4103/ijd.IJD_576_17
- Celakovska, J., Vankova, R., Bukac, J., Krejsek, J., & Andrys, C. (2021). The relation between the sensitisation to molecular components of inhalant allergens and food reactions in patients suffering from atopic dermatitis. Food and Agricultural Immunology, 32(1), 33–53. https://doi.org/10.1080/09540105.2020.1865281
- Clark, A. T., & Ewan, P. W. (2003). Interpretation of tests for nut allergy in one thousand patients, in relation to allergy or tolerance. Clinical and Experimental Allergy, 33(8), 1041–1045. https://doi.org/10.1046/j.1365-2745.2003.01624.x
- Costa, J., Mafra, I., Carrapatoso, I., & Oliveira, M. B. (2016). Hazelnut allergens: Molecular characterization, detection, and clinical relevance. Critical reviews in food science and nutrition, 56(15), 2579–2605. https://doi.org/10.1080/10408398.2013.826173
- Datema, M. R., van Ree, R., Asero, R., Barreales, L., Belohlavkova, S., de Blay, F., Clausen, M., Dubakiene, R., Fernández-Perez, C., Fritsche, P., Gislason, D., Hoffmann-Sommergruber, K., Jedrzejczak-Czechowicz M, M., & Ballmer-Weber, B. (2018 Mar). Component-resolved diagnosis and beyond: Multivariable regression models to predict severity of hazelnut allergy. Allergy, 73(3), 549–559. Epub 2017 Nov 24. Erratum in: Allergy. 2020 Aug;75(8):2145-2145. PMID: 28986984. https://doi.org/10.1111/all.13328
- Dooper, M. M. B. W., Plassen, C., Holden, L., Moen, L. H., Namork, E., & Egaas, E. (2008). Antibody binding to hazelnut (Corylus avellana) proteins: The effects of extraction procedure and hazelnut source. Food and Agricultural Immunology, 19(3), 229–240. https://doi.org/10.1080/09540100802243325
- Ebo, D. G., Verweij, M. M., Sabato, V., Hagendorens, M. M., Bridts, C. H., & De Clerck, L. S. (2012). Hazelnut allergy: A multi-faced condition with demographic and geographic characteristics. Acta Clinica Belgica, 67, 317–321. https://doi.org/10.2143/ACB.67.5.2062683. PMID: 23189537.
- European Task Force on Atopic Dermatitis. (1993). Severity scoring of atopic dermatitis: The SCORAD index (consensus report of the European Task Force on atopic dermatitis). Dermatology (Basel, Switzerland), 186(1), 23–31. https://doi.org/10.1159/000247298
- Faber, M. A., De Graag, M., Van Der Heijden, C., Sabato, V., Hagendorens, M. M., Bridts, C. H., De Clerck, L. S., & Ebo, D. G. (2014). Cor a 14: Missing link in the molecular diagnosis of hazelnut allergy? International Archives of Allergy and Immunology, 164(3), 200–206. https://doi.org/10.1159/000365050
- Flores Kim, J., McCleary, N., Nwaru, B. I., Stoddart, A., & Sheikh, A. (2018). Diagnostic accuracy, risk assessment, and cost-effectiveness of component-resolved diagnostics for food allergy: A systematic review. Allergy, 73(8), 1609–1621. https://doi.org/10.1111/all.13399
- Hanifin, J., & Rajka, G. (1980). Diagnostic features of atopic dermatitis. Acta Dermato-Venereologica, 92, 44–47.
- Klemans, R. J., van Os-Medendorp, H., Blankestijn, M., Bruijnzeel-Koomen, C. A., Knol, E. F., & Knulst, A. C. (2015). Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: A systematic review. Clinical Experimental Allergy, 45(4), 720–730. https://doi.org/10.1111/cea.12412
- Lange, L., Lasota, L., Finger, A., Vlajnic, D., Büsing, S., Meister, J., Broekaert, I., Pfannenstiel, C., Friedrichs, F., Price, M., Trendelenburg, V., Niggemann, B., & Beyer, K. (2017). Ana o 3-specific IgE is a good predictor for clinically relevant cashew allergy in children. Allergy, 72(4), 598–603. https://doi.org/10.1111/all.13050
- Maloney, J. M., Rudengren, M., Ahlstedt, S., Bock, S. A., & Sampson, H. A. (2008). The use of serum specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. Journal of Allergy and Clinical Immunology, 122(1), 145–151. https://doi.org/10.1016/j.jaci.2008.04.014
- Manam, S., Tsakok, T., Till, S., & Flohr, C. (2014). The association between atopic dermatitis and food allergy in adults. Current Opinion in Allergy and Clinical Immunology, 14(5), 423–429. https://doi.org/10.1097/ACI.0000000000000095
- Masthoff, L. J. N., Mattsson, L., Zuidmeer-Jongejan, L., Lidholm, J., Andersson, K., Akkerdaas, J. H., Versteeg, S. A., Garino, C., Meijer, Y., Kentie, P., Versluis, A., den Hartog Jager, C. F., Bruijnzeel-Koomen, C. A. F. M., Knulst, A. C., van Ree, R., van Hoffen, E., & Pasmans, S. G. M. A. (2013). Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. Journal of Allergy and Clinical Immunology, 132(2), 393–399. https://doi.org/10.1016/j.jaci.2013.02.024
- Masthoff, L. J., Pasmans, S. G., Hoffen, E., Knol, M. J., Bruijnzeel-Koomen, C. A., Flinterman, A. E., Kentie, P., Knulst, A. C., & Meijer, Y. (2012). Diagnostic value of hazelnut allergy tests including rCor a 1 spiking in double-blind challenged children. Allergy, 67(4), 521–527. https://doi.org/10.1111/j.1398-9995.2011.02766.x
- Masthoff, L. J., van Hoffen, E., Mattsson, L., Lidholm, J., Andersson, K., Zuidmeer-Jongejan, L., Versteeg, S. A., Bruijnzeel-Koomen, C. A., Knulst, A. C., Pasmans, S. G., & van Ree, R. (2015). Peanut allergy is common among hazelnut-sensitized subjects but is not primarily the result of IgE cross-reactivity. Allergy, 70(3), 265–274. https://doi.org/10.1111/all.12554
- Matricardi, P. M., Kleine-Tebbe, J., Hoffmann, H. J., Valenta, R., Hilger, C., Hofmaier, S., Aalberse, R. C., Agache, I., Asero, R., Ballmer-Weber, B., Barber, D., Beyer, K., Biedermann, T., Bilò, M. B., Blank, S., Bohle, B., Bosshard, P. P., Breiteneder, H., Brough, H. A., … Lopata, A. L. (2016). EAACI molecular Allergology User‘s guide. Pediatric Allergy and Immunology, 27(Suppl 23), 1–250. https://doi.org/10.1111/pai.12563
- McWilliam, V., Koplin, J., Lodge, C., Tang, M., Dharmage, S., & Allen, K. (2015). The prevalence of tree Nut allergy: A Systematic review. Current Allergy and Asthma Reports, 15(9), 54. https://doi.org/10.1007/s11882-015-0555-8. PMID: 26233427.
- Melioli, G., Bonifazi, F., Bonini, S., Maggi, E., Mussap, M., Passalacqua, G., Rossi, E. R., Vacca, A., & Canonica, G. W. (2011). The ImmunoCAP ISAC molecular allergology approach in adult multi-sensitized Italian patients with respiratory symptoms. Clinical Biochemistry, 44(12), 1005–1011. https://doi.org/10.1016/j.clinbiochem.2011.05.007
- Nilsson, C., Berthold, M., Mascialino, B., Orme, M., Sjölander, S., & Hamilton, R. (2020 Feb). Allergen components in diagnosing childhood hazelnut allergy: Systematic literature review and meta-analysis. xx, 31(2), 186–196. Epub 2020 Jan 1. PMID: 31301691. https://doi.org/10.1111/pai.13110
- Nwaru, B. I., Hickstein, L., Panesar, S. S., Roberts, G., Muraro, A., & Sheikh, A. (2014). Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy, 69(8), 992–1007. https://doi.org/10.1111/all.12423
- Pastorello, E. A., Farioli, L., Pravettoni, V., Robino, A. M., Scibilia, J., Fortunato, D., Conti, A., Borgonovo, L., Bengtsson, A., & Ortolani, C. (2004). Lipid transfer protein and vicilin are important walnut allergens in patients not allergic to pollen. Journal of Allergy and Clinical Immunology, 114(4), 908–914. https://doi.org/10.1016/j.jaci.2004.06.020
- Sato, S., Yamamoto, M., Yanagida, N., Ito, K., Ohya, Y., Imai, T., Nagao, M., Borres, M. P., Movérare, R., Ebisawa, M. (2017). Jug r 1 sensitization is important in walnut-allergic children and youth. The Journal of Allergy and Clinical Immunology: In Practice, 5(6), 1784–1786.e1. https://doi.org/10.1016/j.jaip.2017.04.025
- Sicherer, S. H., Mu∼noz-Furlong, A., Godbold, J. H., & Sampson, H. A. (2010). US prevalence of selfreported peanut, tree nut, and sesame allergy: 11-year follow-up. Journal of Allergy and Clinical Immunology, 125(6), 1322–1326. https://doi.org/10.1016/j.jaci.2010.03.029
- Skypala, I. J., Cecchi, L., Shamji, M. H., Scala, E., & Till, S. (2019 Jul). Lipid transfer protein allergy in the United Kingdom: Characterization and comparison with a matched Italian cohort., 74(7), 1340–1351. https://doi.org/10.1111/all.13747. Epub 2019 Mar 14. PMID: 30762886; PMCID: PMC6767535
- Teuber, S. S., Dandekar, A. M., Peterson, W. R., & Sellers, C. L. (1998). Cloning and sequencing of a gene encoding a 2S albumin seed storage protein precursor from English walnut (Juglans regia), a major food allergen. Journal of Allergy and Clinical Immunology, 101(6), 807–814. https://doi.org/10.1016/S0091-6749(98)70308-2
- Tuano, K. S., & Davis, C. M. (2015). Utility of component-resolved diagnostics in food allergy. Current Allergy and Asthma Reports, 15(6), 32. https://doi.org/10.1007/s11882-015-0534-0
- Uotila, R., Kukkonen, A. K., Pelkonen, A. S., & Makela, M. J. (2016). Cross-sensitization profiles of edible nuts in a birch-endemic area. Allergy, 71(4), 514–521. https://doi.org/10.1111/all.12826
- Valcour, A., Lidholm, J., Borres, M. P., & Hamilton, R. G. (2019). Sensitization profiles to hazelnut allergens across the United States. Annals of Allergy, Asthma Immunology, 122(1), 111–116.e1. https://doi.org/10.1016/j.anai.2018.09.466
- van Erp, F. C., Klemans, R. J., Meijer, Y., van der Ent, C. K., & Knulst, A. C. (2016). Using component-resolved diagnostics in the management of peanut-allergic patients. Current Treatment Options in Allergy, 3(2), 169–180. https://doi.org/10.1007/s40521-016-0080-6
- van Hage, M., Hamsten, C., & Valenta, R. (2017). ImmunoCAP assays: Pros and cons in allergology. Journal of Allergy and Clinical Immunology, 140(4), 974–977. https://doi.org/10.1016/j.jaci.2017.05.008
- Verweij, M. M., Hagendorens, M. M., De Knop, K. J., Bridts, C. H., De Clerck, L. S., Stevens, W. J., & Ebo, D. G. (2011). Young infants with atopic dermatitis can display sensitization to Cor a 9, an 11S legumin-like seed-storage protein from hazelnut (Corylus avellana). Pediatric Allergy and Immunology, 22(2), 196–201. https://doi.org/10.1111/j.1399-3038.2010.01088.x
- Verweij, M. M., Hagendorens, M. M., Trashin, S., et al. (2012). Age-dependent sensitization to the 7S-vicilin-like protein Cor a 11 from hazelnut (Corylus avellana) in a birch-endemic region. Journal of Investigational Allergology & Clinical Immunology, 22, 245–251.
- Weidinger, S., & Novak, N. (2016). Atopic dermatitis. The Lancet, 387(10023), 1109–1122. doi:10.1016/S0140-6736(15)00149-X
