 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Bilberry (Vaccinium myrtillus L.) is a medicinal fruit known for its high content of anthocyanins, flavonoids, vitamins, ascorbic acid, carotenoids, tocopherols and phenolic acid. Bilberry has long been used in oriental medicine for the prevention and treatment of various diseases, such as visual acuity, hyperglycemia, dyslipidemia, diabetes, cancer, obesity, inflammatory and cardiovascular diseases. In this study, we investigated using the antioxidant and anti-inflammatory of 70% ethanol extracts of bilberry in vitro. Antioxidant activities were measured with total phenolic, flavonoid and ascorbic acid contents. The bilberry extract (BE) showed significant free radical scavenging activities and inhibited oxidation of linoleic acid in a dose-dependent manner. BE also suppressed nitric oxide (NO) generation and reversed pro-inflammatory cytokines such as iNOS, COX-2, TNF-α and IL-6 in LPS-induced RAW 264.7 cells. From these results, we suggest that BE is a natural powerful antioxidant, and the anti-inflammatory agent being a rich source of anthocyanins.
Introduction
Bilberry (Vaccinium myrtillus L.) is a kind of berry that is a low-growing shrubby native to northern Europe and also grows in Asia and North America (Valentová et al., Citation2007). Bilberry is rich in anthocyanins with various health benefits. Anthocyanin is a kind of polyphenol and has strong biofunctional activities (Chu, Cheung, Lau, & Benzie, Citation2011). Anthocyanins can inhibit ROS generation at the cellular level (Tarozzi et al., Citation2007) and the inhibitory effect on NO production in PLS/IFN-γ-activated RAW 264.7 macrophages (Wang & Mazza, Citation2002). Therefore, bilberry could be used as potential therapeutics against various diseases and disorders, such as inflammation, cancer, diabetes, cardiovascular disease, dementia and other age-related diseases (Chu et al., Citation2011).
Excessive reactive oxygen species (ROS) are caused by oxidative and inflammatory reactions in mammalian cells. As we age, our bodies reduce antioxidant and anti-inflammatory capacities. To replenish it, we consume foods that have antioxidants and anti-inflammatory. In our body and metabolic process, oxygen is used during the process of producing energy by oxidizing the nutrients loses electrons resulting in the simultaneous production of unstable free radicals such as ROS and reactive nitrogen species (RNS). ROS also plays a positive role in the body such as regulation of cell growth, and intracellular signalling (Moskovitz et al., Citation2002). Excessive ROS generation produces harmful unstable free radicals which damage a variety of range of essential cellular biomolecules (Seitz & Stickel, Citation2006; Stocker et al., Citation1987). Antioxidants are necessary to prevent and protect the actions of ROS and RNS, which are generated in vivo and cause damage to biomolecules (such as DNA, lipids, proteins and so on). Many studies have been conducted on preventing the production of active ROS by studies on natural bioactive components derived from berries and plants (Rechner et al., Citation2002). Inflammation and inflammatory diseases are closely related to oxidative stress. Several studies have demonstrated that chronic inflammatory diseases are characterized by the excessive production of ROS (Harijith et al., Citation2014). Excessive ROS production is an indication and elicits many potent pathological events by damaging essential cellular proteins. In addition, ROS can induce pro-inflammatory cytokine production by acting as signalling factors (Reuter et al., Citation2010). Macrophages recognize as heterodimerization of Toll-like receptor 4(TLR4) in inflammatory responses. TLR4 activated by LPS initiates the activation of nuclear factor-κB (NF-κB). Activated NF-κB is considered the hallmark of inflammation. The translocated NF-κB into the nucleus from the cytoplasm induces gene expression of the pro-inflammatory cytokine such as (interleukins)ILs, iNOS, COX-2. Berries, like blueberries, bilberries, mulberry, and black raspberry contain large amounts of anthocyanins, which are well-known potent natural antioxidants (Mijan et al., Citation2019). Therefore, we designed our study to investigate the antioxidant and anti-inflammatory effects of BE on oxidation and inflammation caused by LPS on RAW 264.7 macrophage cells.
Oxidative stress and inflammatory cytokines trigger several chronic diseases. Antioxidant and anti-inflammatory properties of BE could be a very potent agent against several chronic diseases. Furthermore, bilberry is enriched in anthocyanins which are well known for their bio-functionalities.
Materials and methods
Chemicals and reagents
All chemicals, reagents and antibodies were of analytical grade and were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA), Fermentas Inc. (Glen Burnie, MD) and Wako Pure Chemical Industries Ltd. (Tokyo, Japan). All other chemicals and reagents were of analytical grade and used without any further purification.
Preparation of extracts
Bilberry (Vaccinium myrtillus L.) fruit lyophilized powder was obtained from AhnGook Health., Ltd. (Seoul, Korea) Then, the extract was filtered through Whatman No. 1 filter paper. Afterward, the solution was evaporated at 40°C under reduced pressure in a vacuum rotary evaporator (Eyela, Tokyo, Japan). Then, the concentrated extract was freeze-dried and stored at −20°C until use. The yield was calculated from the following equation:
Determination of total phenolic, flavonoid and ascorbic acid content
The phenolic content of bilberry extract (BE) was determined by the Folin–Ciocalteu method (Bayazid et al., Citation2020) where gallic acid (GA) is used for the standard curve. The result is expressed as mg gallic acid equivalents (GAE) per 100 g of dry mass. The flavonoid in BE was investigated by the aluminium colorimetric method. Ascorbic acid was quantitatively determined according to a slightly modified method of 2, 6-dichlorophenolindophenol (DCPIP) (Jeong et al., Citation2009). The data is expressed as mg ascorbic acid equivalent per 100 g dry weight (mg/100 g DW). The experiment was repeated with three independent assays.
Antioxidant activity
Antioxidant activities of BE were investigated against free radical scavenging activities like DPPH, ABTS, nitrite radical scavenging and ferric reducing activities) as earlier mentioned (Debnath et al., Citation2011) using the concentrations 50 to 400 μg/mL. The free radical scavenging activities were examined by the colorimetric method. The SOD-like scavenging activities of BE had been determined using the method described by Bayazid et al. (Citation2020) with slight modification.
Inhibition of lipid peroxidation
The inhibiting effect of BE in lipid peroxidation was assessed by the ferric thiocyanate (FTC) method with slight modification (Debnath et al., Citation2011) against linoleic acid oxidation. 2.5 mL of sample (1 mg/mL) or AA(1 mg/mL) used as a positive control was mixed with 1 mL of 0.1 M sodium phosphate buffer (pH 7.0), 1 mL of 50 mM linoleic acid solution, and 0.5 mL of DW. The mixture was incubated at 40°C in the dark. Afterward, an aliquot of 50 μL of the mixture was added with 2.5 mL of 75%(V/V) ethanol and 50 μL of 30%(V/V) ammonium thiocyanate. After 3 min, 50 μL of 20 mM ferrous chloride was added into the mixture followed by incubation for 3 min at room temperature. The absorbance of the reactant was measured at 500 nm wavelength. The experiment was carried out over time until the amount of peroxide production of the control group is reduced.
DNA protective activity
The DNA prevention against oxidative damage by H2O2 was performed as described by Bayazid et al. (Citation2020) with some minor modifications. Briefly, 1 μL of Plasmid pBR 322 DNA (Thermo Fisher Scientific) was mixed with FeSO4 (0.08 mM), 30% H2O2 (v/v), and 3 μL distilled water and 2 μL BE at different concentrations (50, 100, 150 μg/mL) and incubated at 37°C for 1 h. Afterward, 2 μL of 6X DNA loading dye was mixed and subjected to the 0.8% agarose gel. The electrophoresis was done at 70 V for 30 min and stained with EtBr.
Cell cultures
RAW 264.7 cell line, a murine macrophage, was purchased from the Korean Cell Line Bank (Seoul, Korea) and maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin and streptomycin (P.S) in a humidified atmosphere of 5% CO2 at 37°C.
Determination of cell viability
Cell viability test was investigated by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay Citation2021. Murine macrophage (RAW 264.7) cells were seeded at as 5 × 10⁴ cells/well in 96 well plates. Then, various concentrations (10–1000 μg/mL) of the BE were treated to the cell cultures with or without 1 μg/mL of LPS for 24 h. Then, the MTT (final conc. 0.5 mg/mL) solution was added to each well. After 2 h of incubation, the supernatant had been discarded and the formazan blue was formed in the cells, was dissolved in dimethyl sulfoxide (DMSO). The optical density was measured at 570 nm wavelength.
Measurement of nitric oxide
The nitric oxide (NO), released by RAW 264.7 cells against LPS-stimulation, was measured by collecting supernatant from each well and added the Griess reagent (Bayazid et al., Citation2021). The RAW cells (5 × 10⁴ cells) were seeded onto 96 well plates. The following day LPS (final concentration 1 μg/mL) with or without BE of different concentrations were treated. After 24 h of incubation, 80 μL of the supernatant and an equal amount of Griess reagent were mixed and the absorbance was measured at 540 nm. The nitrite concentration was determined by extrapolation from the sodium nitrite standard curve.
Western blot analysis
RAW 264.7 cells were seeded as 1 × 106 cells/well in a 6-well culture plate and incubated for 24 h. Then, the cells were treated with different concentrations of BE and LPS except for control groups well as previously described (Bayazid et al., Citation2021). The cells were washed twice with ice-cold PBS after 24 h of incubation and harvested proteins from the cell using radioimmunoprecipitation assay (RIPA) lysis buffer with 1% protease inhibitor cocktail using a PRO 200 homogenizer followed by sonication with a dismembrator 20 µg of protein were separated using SDS-polyacrylamide gel electrophoresis and transferred from the gel to a nitrocellulose membrane. The membranes were blocked in 5% BSA in TBS-T and incubated overnight with the corresponding primary antibodies each at 4°C. The membranes were then incubating with secondary antibody (anti-rabbit) at room temperature for 1 h, and the targeted proteins in the membrane were visualized by using enhanced chemiluminescence (ECL) reagents.
High-performance liquid chromatography (HPLC) analysis
Anthocyanin was analysed using Thermo Scientific Dionex Ultimate 3000 Series, equipped with syncronis C18, 5 μm, 250 mm × 4.6 mm column (Thermo Fisher Scientific, Waltham, MA, USA). The HPLC condition for anthocyanins analysis has been followed as described in .
Table 1. Conditions of HPLC analysis for anthocyanins profiling.
Statistical analysis
All data are presented as the mean ± standard deviation from triplicate experiments. All statistical analyses were computed by using GraphPad Prism5.0 software (GraphPad Software, Inc., San Diego, CA, USA). The significance of each group was determined by using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests or Bonferroni post-tests. A value of p < 0.05 was considered to represent a statistically significantly difference.
Results
Total phenolic, flavonoid and ascorbic acid content of bilberry
The yield was 70.32% for the bilberry extract (BE). Polyphenols are the most ubiquitous group of plant’s secondary metabolites and are well known for their biofunctional activities. The total phenolic content in BE was examined by using the regression equation of the calibration curve. The obtained values were 644.25 mg GAE per 100 g of dry mass for the BE (). Previous studies on the antioxidant potential of plants have shown that fruits are rich in phenolic compounds are also potent antioxidants. The flavonoid contents, calculated from a calibration curve and expressed in catechin equivalents (CE), were 542.39 mg CE per g of 100 g of dry mass ().
Table 2. Total phenolic, total flavonoid, and ascorbic acid contents and the yield of bilberry extract.
Antioxidant activities of bilberry extract (BE)
The DPPH scavenging activities were 17.7–95.2% for the BE in the concentration ranges were 50–400 μg/mL respectively (a). The median inhibitory concentration (IC50) value of DPPH scavenging activity was 151.98 μg/mL for the BE (). ABTS radical is widely used to assess antioxidant activity and BE showed a notable radical scavenging effect (b). The ABTS scavenging activities of BE were 99.17% at 200μg/mL concentration. The IC50 value of BE was 57.15 μg/mL (). Nitrite radical scavenging activity of BE was also investigated and showed in (c). BE also showed ferric reducing activities in a dose-dependent manner. (d) shows superoxide dismutase-like (SOD-like) activities of BE. The BE showed SOD-like activities in a dose-dependent manner as shown in (d). ROS occurs excessively due to an imbalance of metabolism, lack of natural antioxidants such as anthocyanins, flavonoids and polyphenols, which obstruct the formation of ROS, play a crucial role in the progression of many diseases (Mojica et al., Citation2015). (g) shows that the antioxidant activity of BE by inhibiting of oxidation of linoleic acid. Antioxidant assays in vitro can be used as the method that evaluates lipid peroxidation and that measures free radical scavenging ability. The inhibition rate of BE (2 mg/ml) in lipid peroxidation was 89.0% and that of ascorbic acid (2 mg/ml) was 97.7% at 88 h.
Figure 1. The antioxidant of bilberry extract. (a) DPPH radical scavenging, (b) ABTS radical scavenging activity of bilberry extract, (c) nitrite scavenging activity, (d) SOD-like activity, (e) oxidative DNA damage prevention activity of bilberry extract agarose gel electrophoretic patterns of plasmid DNA breaks by hydroxyl radical reaction in the presence of the extract of bilberry. Line 1, plasmid DNA control; Line2, negative plasmid DNA control with FeSO4 and H2O2 (DNA damage control); Line 3–5, plasmid DNA, FeSO4 and H2O2 in the presence of the extract with concentrations of 50, 100 and 200 µg/mL, respectively, (f) reducing power activity of bilberry extract, (g) inhibition of linoleic acid oxidation of bilberry extract. Sample and sodium phosphate buffer (pH 7.0), DW, and 50 mM linoleic acid was mixed and incubated at 40°C for 7 days. Then the above mixture was mixed with 2.5 mL of 75% ethanol, 50 μL of 30% ammonium thiocyanate and 50 μL of 20 mM ferrous chloride and incubation for 3 min at RT. The absorbance was measured at 500 nm wavelength every day. Ascorbic acid was used as a positive control. All data are expressed as mean ± standard deviation (n = 3).
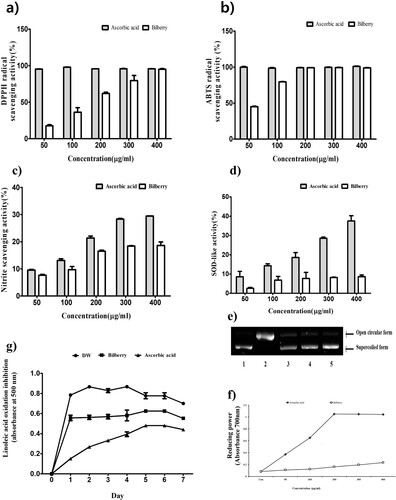
Table 3. DPPH, ABTS and nitrite radical scavenging activity of bilberry extract.
Effect of BE on oxidative DNA damage
Antioxidant compounds such as polyphenols and flavonoids scavenge hydroxyl radicals and thereby prevent the Fenton reaction. Oxidative DNA damage may cause aging, carcinogenesis, mutagenesis, cytotoxicity and several diseases. Line 1 is a control group, Line 2 is the negative control group where DNA was damaged by FeSO4 and H2O2. Lines 3–5 were treated with BE (50, 100 and 200 μg/mL) to protect the damaged DNA, where BE showed dose-dependently DNA protection. Treatment with 200 μg/mL BE had the greatest DNA protection effect. The protective capacity of BE on DNA damage caused by FeSO4 and H2O2 is demonstrated in (e). Previous studies reported that anthocyanins of bilberry stabilize and repair DNA.
Effect of BE on cell viability
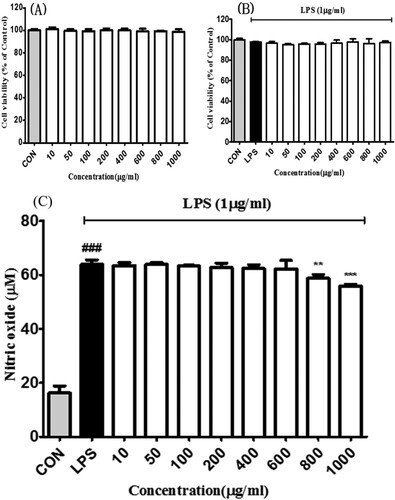
The cell viability of BE in RAW 264.7 cells has been elucidated in (a). The BE did not show any cytotoxic effect up to the concentration of 1000 μg/mL. On the other hand, (b) shows the effect of BE in LPS (1 μg/mL) induced RAW 264.7.
Figure 2 . Effects of bilberry extract on the (a) cell viability in RAW 264.7 and (b) LPS-treated RAW 264.7 cells. After 24 h incubation with the bilberry extracts, cell viability was examined by the MTT assay. (c) Effects of bilberry extract on NO production in LPS-induced Raw 264.7 cells. LPS-induced NO production was measured by Griess assays. All data are expressed as mean ± standard deviation (n = 3). ### P < 0.001 compared with the control group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the LPS-treated group.
Effect of BE on NO production LPS-induced RAW 264.7 cells
The anti-inflammatory activity of BE was screened by measuring the inhibition of the production of excessive nitric oxide (NO) in lipopolysaccharide (LPS) -energized RAW 264.7 cells (Mojica et al., Citation2015). NO production was significantly reversed by the treatment of BE of 800 and 1000 μg/mL respectively (c). Inflammation is a crucial biological response and a defense mechanism against harmful stimuli such as pathogens to protect from infection and injury in the body.
Effect of BE on inflammatory cytokines productions in LPS-induced RAW 264.7 cells
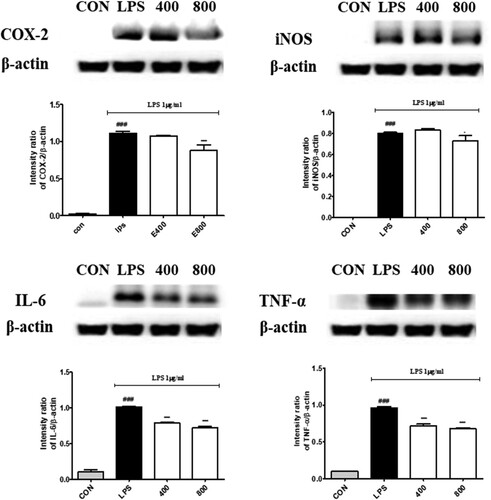
The effect of BE on the expression level of iNOS, COX-2, IL-6 and TNF-α was investigated from the LPS-induced RAW264.7 macrophages (). The protein expression of iNOS and COX-2 was significantly lowered by BE. Similarly, IL-6 and TNF-α protein expression were also influenced and substantially down-regulated by BE. iNOS, COX-2, IL-6 and TNF-α were inhibited by BE in LPS-induced RAW 264.7 cells remarkably. In the inflammatory response pathway, macrophages recognize as heterodimerization of Toll-like receptor 4 (TLR4) and initiate the activation of nuclear factor-κB (NF-κB). Activated NF-κB is considered the hallmark of inflammation. The translocated NF-κB into the nucleus from the cytoplasm induces gene expression of the inflammatory cytokine such as (interleukins) ILs, iNOS, COX-2. Inducible nitric oxide synthase (iNOS), endothelial NOS (eNOS) and neuronal NOS (nNOS) are three major NOS isoforms. NO is synthesized by iNOS and the chronic phase of inflammation is closely associated with an increase in iNOS activity.
Figure 3. Effects of bilberry extract on COX-2, iNOS, IL-6 and TNF-α protein expressions in LPS-induced RAW 264.7 cells treated with bilberry extract (400 and 800 μg/mL) and 1 µg/ml LPS for 24 h. The COX-2, iNOS, IL-6 and TNF-α proteins were analysed by Western blotting. ###P < 0.001 compared with the control group; *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the LPS-treated group.
Anthocyanins profile of BE
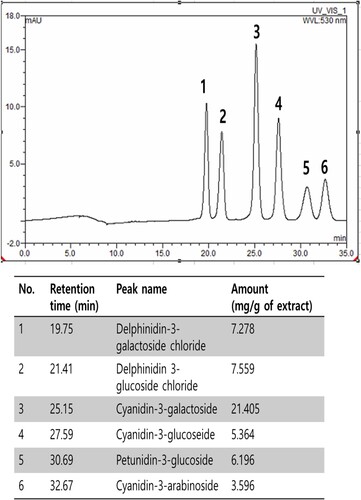
The anthocyanin compositions of BE were summarized in . Cyanidin, delphinidin, pelargonidin, peonidin, malvidin and petunidin are the most common anthocyanidins distributed in plants (Khoo et al., Citation2017). From the bilberry extract, 6 anthocyanins were identified by our HPLC analysis. Delphinidin-3-galactoside chloride, delphinidin 3-glucoside chloride, cyanidin-3-galactoside, cyanidin-3-glucoside, petunidin-3-glucoside and cyanidin-3-arabinoside. The contents of each compound in BE are shown in . Bilberry contains a lot of anthocyanins such as cyanidin, delphinidin, petunidin, peonidin, pelargonidin and malvidin. Anthocyanins, which have antioxidant properties, are highly correlated with antioxidant and anti-inflammatory activities (Chu et al., Citation2011). In the efficiency research of anthocyanin-rich extract on lipid peroxidation and DNA damage in vitamin E-depleted rats, they showed anthocyanin-rich extract including the 3-glucopyranoside forms of delphinidin, cyanidin, petunidin and malvidin strongly decreased vitamin E deficiency-induced elevations in DNA damage and increased plasma antioxidant capacity (Ramirez-Tortosa et al., Citation2001). In our current study, the results demonstrate that BE strongly inhibits linoleic acid oxidation due to high levels of anthocyanins and can be used as a natural antioxidant.
Figure 4. The identification and determination of anthocyanins contents in BE by High-Performance Liquid Chromatography: 1) delphinidin-3-galactoside chloride, (2) delphinidin 3-glucoside chloride, (3) cyanidin-3-galactoside, (4) cyanidin-3-glucoseide, (5) petunidin-3-glucoside and (6) cyanidin-3-arabinoside.
Discussion
Oxidative stress and inflammation cause cell death which leads to many chronic diseases and oxidative stress and inflammation are highly associated (Arnold, Citation2012; Bayazid et al., Citation2020; Reuter et al., Citation2010). ROS is the result of cellular functions but when it overwhelmed the cellular antioxidant systems, it leads to apoptosis. The BE showed remarkable abiotic antioxidant activities in this study and a previous study has shown the antioxidant activities of the bilberry in vivo (Popović et al., Citation2016). The BE could play a pivotal role in homeostasis by modulating antioxidant activities. On the other hand, oxidative stress also could trigger inflammations.
The immune cells are the body’s defense mechanism to protect cells and tissue. The immune cells, e.g. macrophages activate against pathogens, antigens, foreign invasion, cell debris and so on due to protection (Bayazid et al., Citation2020). Hence, the overactivation of immune cells causes inflammation and perpetuates to inflammatory diseases. In the inflammatory response pathway, macrophages recognize LPS (isolated gram bacterial membrane) as heterodimerization of TLR4 and initiate the activation of nuclear factor-κB (NF-κB). Activated NF-κB is considered the hallmark of inflammation. The translocated NF-κB into the nucleus from the cytoplasm induces gene expression of the inflammatory cytokine (Bayazid et al., Citation2020; Bellet et al., Citation2020; Reuter et al., Citation2010). Excessive NO synthesis from iNOS intensifies the inflammatory response, causing tissue and cell damage as genetic mutation. Many studies suggest that anthocyanins in bilberry have anti-inflammatory effects (Khoo et al., Citation2017) including inhibiting proteasome activity, which regulates cellular proteins degradation. Moreover, iNOS and COX-2 expression were significantly lowered by BE in LPS-induced RAW cells. TNF-α and IL-6 have been reported as pleiotropic cytokines (Bayazid et al., Citation2021). Pro-inflammatory cytokines IL-6 and TNF-α were suppressed by BE in LPS-induced RAW 264.7 cells, which envisaged the BE could be a potent natural agent against inflammation and inflammatory diseases.
Fruits and vegetables have been reported for their extensive health beneficial activities along supplying the nutrients. Bilberry is rich in anthocyanins and showed anti-cancer, anti-inflammatory, anti-oxidant, anti-diabetic activities and so on (Chu et al., Citation2011; Valentová et al., Citation2007). Anthocyanins have been reported as the most biologically active compounds in the bilberry (Popović et al., Citation2016). In this study, we identified and determined six anthocyanins in the hydroethanolic extract of bilberry. These anthocyanins are previously shown several biofunctional activities. The anthocyanin-rich BE showed strong antioxidant and anti-inflammatory activities.
Conclusion
In conclusion, this study has a significant elucidation of the antioxidant and anti-inflammatory effects of BE that showed a satisfactory outcome of radical scavenging activities like DPPH, ABTS, nitrite radicals and so on. The BE also had inhibitory activity against lipid peroxidation and preventive activity of oxidative DNA damage. Our results show that BE is a natural pivotal antioxidant and anti-inflammatory activities with 6 anthocyanins identified by HPLC analysis. BE also notably suppressed the excessive NO production in LPS-stimulated RAW 264.7 cells. In view of the positive outcomes from the consequences for hindrance of the statement of favourable to inflammatory mediators and cytokines, for example iNOS, COX-2, TNF-α and IL-6, the bilberry could be conceivably utilized as a calming specialist also. In any case, further exploration is needed on in vivo trials to reconfirm these outcomes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Arnold, S. (2012). Cytochrome c oxidase and its role in neurodegeneration and neuroprotection. In B. Kadenbach (Ed.), Mitochondrial oxidative phosphorylation: nuclear-encoded genes, enzyme regulation, and pathophysiology (Vol. 748, pp. 305–339). New York: Springer.
- Bayazid, A. B., Jang, Y. A., Kim, Y. M., Kim, J. G., & Lim, B. O. (2021). Neuroprotective effects of sodium butyrate through suppressing neuroinflammation and modulating antioxidant enzymes. Neurochemical Research, 46(9), 2348–2358. https://doi.org/10.1007/s11064-021-03369-z
- Bayazid, A. B., & Jang, Young Ah. (2021). The Role of Andrographolide on Skin Inflammations and Modulation of Skin Barrier Functions in Human Keratinocyte. Biotechnology and Bioprocess Engineering.
- Bayazid, A. B., Kim, J. G., Park, S. H., & Lim, B. O. (2020a). Antioxidant, anti-inflammatory, and antiproliferative activity of mori cortex radicis extracts. Natural Product Communications, 15(1), 1934578X–19899765. https://doi.org/10.1177/1934578X19899765
- Bayazid, A. B., Park, S. H., Kim, J. G., & Lim, B. O. (2020b). Green chicory leaf extract exerts anti-inflammatory effects through suppressing LPS-induced MAPK/NF-κB activation and hepatoprotective activity in vitro. Food Agricultural Immunology, 31(1), 513–532. https://doi.org/10.1080/09540105.2020.1742667
- Bellet, M. M., Pieroni, S., Castelli, M., Piobbico, D., Fallarino, F., Romani, L., … Servillo, G. (2020). HOPS/tmub1 involvement in the NF-kB-mediated inflammatory response through the modulation of TRAF6. Cell Death&Disease, 11(10), 1–14. https://doi.org/10.1038/s41419-020-03086-5
- Chu, W.-k., Cheung, S. C., Lau, R. A., & Benzie, I. F. (2011). Bilberry (Vaccinium myrtillus L.). Herbal Medicine, 20115386, 55–71. https://doi.org/10.1201/b10787-5
- Debnath, T., Park, P.-J., Deb Nath, N. C., Samad, N. B., Park, H. W., & Lim, B. O. (2011). Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chemistry, 128(3), 697–703. https://doi.org/10.1016/j.foodchem.2011.03.090
- Harijith, A., Ebenezer, D. L., & Natarajan, V. (2014). Reactive oxygen species at the crossroads of inflammasome and inflammation. Frontiers in Physiology, 5, 352. https://doi.org/10.3389/fphys.2014.00352
- Jeong, C.-H., Choi, G.-N., Kim, J.-H., Kwak, J.-H., Heo, H.-J., Shim, K.-H., … Choi, J.-S. (2009). In vitro antioxidative activities and phenolic composition of hot water extract from different parts of Cudrania tricuspidata. Preventive Nutrition Food Science, 14(4), 283–289. https://doi.org/10.3746/jfn.2009.14.4.283
- Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M. (2017). Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutrition Research, 61(1), 1361779. https://doi.org/10.1080/16546628.2017.1361779
- Mijan, M. A., Kim, J. Y., Moon, S.-Y., Choi, S.-H., Nah, S.-Y., & Yang, H.-J. (2019). Gintonin enhances proliferation, late stage differentiation, and cell survival from endoplasmic reticulum stress of oligodendrocyte lineage cells. Frontiers in Pharmacology, 10, 1211. https://doi.org/10.3389/fphar.2019.01211
- Mojica, L., Meyer, A., Berhow, M. A., & de Mejía, E. G. (2015). Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Research International, 69, 38–48. https://doi.org/10.1016/j.foodres.2014.12.007
- Moskovitz, J., Yim, M. B., Chock, P. B., & Biophysics. (2002). Free radicals and disease. Archives of Biochemistry and Biophysics, 397(2), 354–359. https://doi.org/10.1006/abbi.2001.2692
- Popović, D., Đukić, D., Katić, V., Jović, Z., Jović, M., Lalić, J., … Sokolović, D. (2016). Antioxidant and proapoptotic effects of anthocyanins from bilberry extract in rats exposed to hepatotoxic effects of carbon tetrachloride. Life Sciences, 157, 168–177. https://doi.org/10.1016/j.lfs.2016.06.007
- Ramirez-Tortosa, C., Andersen, ØM, Gardner, P. T., Morrice, P. C., Wood, S. G., Duthie, S. J., … Medicine. (2001). Anthocyanin-rich extract decreases indices of lipid peroxidation and DNA damage in vitamin E-depleted rats. Free Radical Biology, 31(9), 1033–1037. https://doi.org/10.1016/S0891-5849(01)00618-9
- Rechner, A. R., Kuhnle, G., Bremner, P., Hubbard, G. P., Moore, K. P., & Rice-Evans, C. A. (2002). The metabolic fate of dietary polyphenols in humans. Free Radical Biology Medicine, 33(2), 220–235. https://doi.org/10.1016/S0891-5849(02)00877-8
- Reuter, S., Gupta, S. C., Chaturvedi, M. M., Aggarwal, B. B., & Medicine (2010). Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biology, 49(11), 1603–1616. https://doi.org/10.1016/j.freeradbiomed.2010.09.006
- Seitz, H. K., & Stickel, F. (2006). Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biological Chemistry, 387(4), 349–360. https://doi.org/10.1515/BC.2006.047
- Stocker, R., Yamamoto, Y., McDonagh, A. F., Glazer, A. N., & Ames, B. N. (1987). Bilirubin is an antioxidant of possible physiological importance. Science, 235(4792), 1043–1046. https://doi.org/10.1126/science.3029864
- Tarozzi, N., Bizzaro, D., Flamigni, C., & Borini, A. (2007). Clinical relevance of sperm DNA damage in assisted reproduction. Reproductive BioMedicine Online, 14(6), 746–757. https://doi.org/10.1016/S1472-6483(10)60678-5
- Valentová, K., Ulrichová, J., Cvak, L., & Šimánek, V. (2007). Cytoprotective effect of a bilberry extract against oxidative damage of rat hepatocytes. Food Chemistry, 101(3), 912–917. https://doi.org/10.1016/j.foodchem.2006.02.038
- Wang, J., & Mazza, G. (2002). Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-γ-activated RAW 264.7 macrophages. Journal of Agricultural & Food Chemistry, 50(4), 850–857. https://doi.org/10.1021/jf010976a
