ABSTRACT
Clonidine hydrochloride (CLO) is a new substitute for a traditionally used adrenergic agonist. The illegal use of CLO in the livestock industry possess potential harm to human health. Hence, it is an urgent need for the rapid detection of CLO residues. Here, we prepared a highly sensitive and specific monoclonal antibody (mAb) and it used to develop an indirect competitive ELISA (ic-ELISA) for the rapid screening of CLO residues. The limit of detection and limit of quantification values of ic-ELISA were as follows: 0.033 and 0.054 ng/mL for pig urine and 0.061 and 0.096 ng/mL for pork, respectively. Recovery experiment indicated that the ic-ELISA posed outstanding accuracy and precision. Furthermore, the results of ic-ELISA were strongly correlated to the results of HPLC. Thus, the ic-ELISA provided a sensitive and rapid on-site detection of CLO residues in pig urine and pork samples.
Introduction
Traditional β-agonists, such as clenbuterol hydrochloride (CLT), salbutamol (SAL), and ractopamine (RAC) have been used in animal feed or drinking water to promote the growth of livestock and improve the lean meat rate (Liu et al., Citation2016; Zhang et al., Citation2020). The excessive intake of these compounds by illegally treated animals that provide edible tissues can be harmful to humans (Peng et al., Citation2019). Most countries have strictly banned the addition of such compounds to animal feed or drinking water. In recent years, to evade regulation, several new substitutes for traditional adrenergic agonists have emerged, which possess similar receptor agonist effects (Feng et al., Citation2018; Wang et al., Citation2016). Clonidine hydrochloride (CLO) is one of the new substitutes for traditional adrenergic agonists, it’s an α2-adrenergic agonist of the central nervous system, is mainly used to treat clinical conditions, including hypertension, migraine, and attention-deficit hyperactivity disorders (Krieger et al., Citation2018; Naguy, Citation2016). However, the addition of CLO to animal feed or drinking water promotes the secretion of growth hormone, which then promotes animal growth (Klein et al., Citation2013). Consumption of CLO-containing edible tissues from CLO-treated animals can be harmful to humans. Side effects include dry mouth, drowsiness, dizziness, constipation, and sedation (Pelzer et al., Citation2002). The former Ministry of Agriculture of China, made a No.1519 announcement prohibiting the addition of CLO to animal feed or drinking water. But illegal addition still occurs from time to time. Therefore, there is an urgent need to develop detection methods for CLO monitoring, especially the practical and rapid method of on-site detection.
Several chromatographic methods have been developed to detect the presence of CLO residues, such as HPLC (AlRabiah et al., Citation2020; Yan et al., Citation2016), HPLC-MS/MS (Zhuang et al., Citation2015), and UHPLC-MS/MS (De Nicolò et al., Citation2016; Veigure et al., Citation2017). Although these methods possess several advantages, such as high sensitivity, accuracy, and reliability, there are still some limitations, such as expensive instrumentation, complex preprocessing procedures, and the need for sophisticated technical operators (Xiao et al., Citation2021; Yang et al., Citation2019). Therefore, it is necessary to develop an efficient, rapid, sensitive detection method for strengthening feed safety monitoring and ensuring animal-derived foods safety. Immunoassays are simple, rapid, low cost, and highly sensitivity and thus, are suitable for high-throughput and rapid on-site detection (Yin et al., Citation2020; Zhu et al., Citation2021). Enzyme-linked immunosorbent assay (ELISA), as a classical immunoassay, can meet the needs of the current situation (Li et al., Citation2020). However, ELISA for the detection of CLO is still lacking, and the reported indirect competitive ELISA (ic-ELISA) for CLO detection used rabbit polyclonal antibody (pAb) (Bai et al., Citation2015). In the study, we prepared a highly sensitive and specific monoclonal antibody (mAb) against CLO, which was then used to develop an ic-ELISA that was validated by HPLC.
Materials and methods
Chemicals, materials, and apparatus
Apraclonidine hydrochloride (ACLO) was purchased from National Institutes for Food and Drug Control (Beijing, China). CLO, Phenylethanolamine A (PA), Baclofen (BA), Cyproheptadine hydrochloride (CYP), SAL, RAC, CLT, Freund’s complete adjuvant (FCA), Freund’s incomplete adjuvant (FIA), Bovine serum albumin (BSA), ovalbumin (OVA), PEG1500, and murine mAb isotyping kits (MMIK) were purchased from Sigma (St. Louis, USA). Hypoxanthine aminopterin thymidine (HAT) and Hypoxanthine thymidine (HT) were purchased from Thermofisher (Grand Island, USA). Fetal bovine serum (FBS) was purchased from TransGen Biotech (Beijing, China). RPMI-1640 medium was procured from Solarbio (Beijing, China). Serum-free cell cryopreservation solution was purchased from NCM Biotech (Suzhou, China). Peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG (H + L) was obtained from Jackson (West Grove, USA). Ultrapure water was produced by Milli-Q (Millipore, Bedford). All other reagents and solvents were of analytical grade.
Female BALB/c mice were raised and treated at Henan Key Laboratory of Animal Immunology according to the principles of Institutional Animal Care and Use Committees of the Henan Academy of Agricultural Sciences. The murine myeloma SP2/0 cells were preserved in Henan Key Laboratory of Animal Immunology.
The microplate reader and Gel imager were purchased from Bio-Rad (Richmond, USA). The inverted microscope was purchased from Leica (Weztlar, Germany). The CO2 incubator and high-speed tabletop refrigerated centrifuge were purchased from Thermo Scientific (Osterode, Germany).
Synthesis of immunogen and coating antigen
CLO was conjugated with the carrier protein (BSA and OVA) using the diazotization method to prepare the artificial antigen using its structural analogues ACLO. a shows the coupling procedure of the immune antigen (CLO-BSA). First, ACLO (4 mg; 14.2 mmol) was dissolved in HCl (0.5 mL) (0.05 mol/L), followed by the addition of 20 μL of sodium nitrite solution (NaNO2, 0.2 mol/L), and stored at 0–4°C for 30 min in the dark. Second, BSA (10 mg; 0.15 mmol) was dissolved in 1 mL of carbonate–bicarbonate buffer (CBS, 0.05 mol/L, pH 9.6). Next, the above solution was added dropwise into the protein solution and stirred at room temperature for 5 h in the dark. Finally, the conjugates were dialyzed against phosphate-buffered saline (PBS, 0.01 mol/L, pH 7.4) for 3 days. The coating antigen (CLO-OVA) was similarly synthesized. The conjugated proteins were preliminary identified using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Figure 1. (a) The synthesis procedure of CLO-BSA; (b) Identification of CLO-BSA by SDS-PAGE; (c) The titer curve of the four mice pAb; (d) Sensitivity identification of the No.2 mouse pAb.
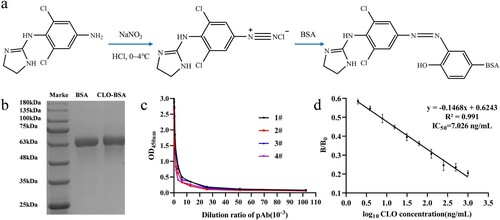
The procedure of immunization was as follows: Four female BALB/c mice (6–8 weeks) were subcutaneously immunized with immunogen CLO-BSA four times at an interval of 3 weeks to obtain pAb against CLO. For the first immunization, CLO-BSA (30 μg) was diluted with sterilized PBS (100 µL) and emulsified with FCA (100 µL). For the remaining immunizations, the same method was performed except that FCA was replaced by FIA. Ten days after the fourth immunization, the mouse polyclonal serum (pAb) was collected from the four immunized mice and analyzed by indirect ELISA (i-ELISA) and ic-ELISA to assess the titer and sensitivity. The serological analysis is the most accurate method for the identification of artificial antigens (Bai et al., Citation2012).
Preparation of mAb
The mouse that produced the highest titer and sensitivity of pAb was intraperitoneally injected with 100 µL sterilized PBS containing 30 μg of CLO-BSA. Three days later, the spleen cells of that mouse were collected and fused with SP2/0 cells. After seven days, the fused hybridoma cells were observed under the microscope. Ten days after the fusion, the cell supernatant was collected and detected by i-ELISA and ic-ELISA to screen for the positive hybridoma cells. Then, the positive hybridoma cells were subcloned by limited dilution to screen the monoclonal cell line that could steadily secrete high performance mAb. We intraperitoneally injected the expanded positive hybridoma cells into the paraffin-treated mouse to prepare the ascites, which were collected and purified by the caprylic acid-ammonium sulphate precipitation method (Wang et al., Citation2017). Finally, the immunological properties of the mAb were studied.
Establishment of ic-ELISA
For the coating step, the ELISA plates were coated with 100 µL of CBS buffer, containing CLO-OVA and incubated at 37°C for 2 h. Next, the ELISA plates were washed four times with PBST (PBS buffer containing 0.05% Tween-20). Finally, the plates were blocked with 300 µL of blocking fluid (PBST containing 0.05% of skim milk) and incubated at 37°C for 1 h. Next, the plates were washed and stored at 4°C for subsequent experiments.
First, the coated ELISA plates were added 100 µL of different concentrations of the standard solution and 100 µL of mAb solutions per well, and the blank wells were added the same dose of PBS. Next, the plates were incubated at 37°C for 15 min and washed with PBST. Second, we added Goat Anti-Mouse IgG (100 μL; 1:1000 dilution) into each well, and incubated at 37°C for 30 min, followed by washing with PBST. Third, we added the TMB substrate solution (100 μL) into each well, and kept at room temperature for 10 min. Subsequently, the reaction was stopped by the addition of 100 µL of 2 M H2SO4 solution. Finally, the optical density (OD450nm) was measured using a microplate reader at 450 nm.
Optimization of the ic-ELISA
The checkerboard titration experiments were performed to determine the optimal working conditions (optimal coated concentration of CLO-OVA and the optimal dilution of mAb) of ic-ELISA (Wang et al., Citation2015). First, the ELISA plates were coated with eight different concentrations of CLO-OVA (0.0156 to 2 µg/mL), followed by the determination of mAb titer and mAb sensitivity (IC50) at different coating concentrations using i-ELISA and ic-ELISA, respectively. Finally, the optimal working conditions were defined as the group with the highest sensitivity (the least IC50).
Under optimal working conditions, mAb sensitivity was determined by ic-ELISA as previously established, and the standard curve of the mAb against CLO was drawn by plotting the values of B/B0 (B: the OD450nm value of CLO at different concentrations, B0: the OD450nm value of CLO at 0 ng/mL) against the logarithm values of the concentrations of CLO. The sensitivity of the mAb was assessed based on the IC50 value, which indicated the CLO concentration that could reduce the specific binding reaction between mAb and CLO by half. The detection ranges (IC15∼IC85) were computed from the linear equation. The specificity of the mAb against other agonist drugs (PA, BA, CYP, SAL, RAC, and CLT) was determined by the ic-ELISA, and evaluated based on the values of cross-reactivity (CR), which were calculated according to the following equation: CR (%) = IC50 of CLO/IC50 of other compounds × 100%.
Preparation of the pig origin sample
The addition of CLO in feedstuff is known to cause the partial retention of CLO and its metabolite residue in the livestock, and the remaining are excreted via the urinary system. Therefore, the pig urine and pork samples were selected to detect CLO residues for real-time monitoring before slaughtering or post-slaughtering monitoring. The pig urine samples were obtained from pig farm affiliated to Henan Agricultural University; the pork samples were purchased from a local supermarket. All samples were confirmed as negative for CLO by HPLC. The pork samples were pretreated refer to the method of Wang et al. (Citation2021) as follows: first, the tissue samples (2 g) were chopped and homogenized, and moved into a polypropylene tube, and ultrasonicated for 10 min. Second, 2 mL of 0.1 mol/L HCl was added to the tube, and the mixture was vortexed for 5 min. Third, PBS (8 mL) was added to the mixture and centrifuged at 4500 × g for 20 min. Finally, the supernatant was collected, and the pH was adjusted to 7.4.
Matrix effects
The matrix components present in pig urine and pork extract samples are known to severely affect the specific recognition between antigen and antibody. Generally, the matrix effect could be eliminated by diluting with PBS or normal saline (NS). The standard curves obtained for the urine and pork extract samples at different dilution levels of PBS were compared with the standard curve of PBS to assess the matrix effect, followed by the identification of the optimal dilution limit to minimize or eliminate the matrix effects.
Validation of the ic-ELISA
The ic-ELISA was validated via the following parameters: the limit of detection (LOD), the limit of quantification (LOQ), accuracy, precision, and reliability. For determining the LOD and LOQ, 20 negative samples of pig urine and pork, in which the matrix effect had been eliminated, were analyzed by ic-ELISA, and the OD450nm values of the blank samples were recorded by the microplate reader. The concentrations of CLO were calculated based on the standard curve (0.0195, 0.039, 0.078, 0.156, 0.312, 0.625, and 1.25 ng/mL) obtained from the ic-ELISA, as well as the mean value (X) and the standard deviation (SD). The LOD value corresponded to the concentration of CLO calculated by the sum of X and 3 times of SD; the LOQ value was calculated by the sum of X and 6 times that of SD. The accuracy and precision of the ic-ELISA were assessed via the recovery experiment. The pig urine and pork extract samples with matrix effect eliminated containing 0.1, 0.2, 0.4, and 0.6 ng/mL of CLO were prepared and tested by ic-ELISA. For the intra-assay test, the samples were tested by the same batch of coated ELISA plates. For the inter-assay test, the samples were tested by three different batches of coated ELISA plates. Each test was performed in triplicates. The reliability was evaluated by comparing the correlation between ic-ELISA and HPLC (Jin et al., Citation2020). Four negative pig urine samples containing different concentrations (0.1, 0.2, 0.4, and 0.6 ng/mL) of CLO were prepared in duplicates and detected by ic-ELISA and HPLC, respectively.
Results and discussion
Identification of immunoreagents for CLO
The synthesis of high immunogenicity antigen is the key to the preparation of high-affinity mAb. CLO is reactive but lacks immunogenicity, due to its small molecular weight. The immunogenicity can be obtained by conjugate with carrier proteins to prepare artificial antigen. But CLO molecular structure has no active group (amino or carboxyl groups) for conjugation. Here, we conjugated ACLO, the analog of CLO, with the carrier proteins (BSA and OVA) using the amino group on the benzene ring. We used diazotization method for conjugation, and the prepared artificial antigen was preliminary identified by SDS-PAGE (b). The migration rate of CLO-BSA was slower than BSA, since the molecular weight of the artificial antigen was larger than carrier protein (BSA). These observations confirmed the successful coupling of CLO with BSA. c,d shows the results of the serological experiments. The titer of four immunized mice was all high than 1:2.56 × 104 and could be specifically recognized by CLO. Furthermore, the titer of mouse No. 2 had the highest sensitivity with IC50 of 7.02 ng/mL. The serological experiment further verified the successful synthesis of the artificial antigen. The artificial antigen and serological experiments laid a good foundation for the preparation of mAb and the establishment of ELISA.
Identification of mAb
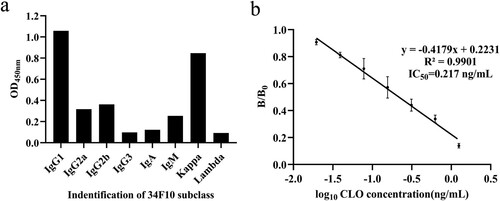
We selected 34F10 cell line by cell fusion and the positive hybridoma cells screening to produce mAb by the inducing ascites in vivo method. The mAb produced by the 34F10 cell line was verified to be IgG1sub-class by MMIK (a) and the titer was higher than 1:5.12 × 105. The standard inhibition curves of mAb against CLO was y = −0.4179x + 0.2231 (R2 = 0.9901) (b). The IC50 of mAb was 0.217 ng/mL, with a detection range (IC15–IC85) of 0.056–1.496 ng/mL. Antibody is the key reagent to establish immunoassay, and the sensitivity of the antibody is a decisive factor. In this study, we obtained mAb with high titer and sensitivity. Therefore, a sensitive ic-ELISA can be established for the determination of CLO.
Optimization and specificity of the ic-ELISA
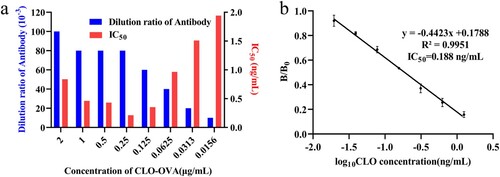
The sensitivity of the mAb is the key factor for the optimization of the ic-ELISA under different working conditions. Different concentrations of CLO-OVA (0.0156 to 2 μg/mL) with different dilutions of mAb (1:1 × 103 to 1:1.28 × 105, v/v) regulate the specific binding reaction between antigen and antibody, resulting in different mAb sensitivity. The optimal working conditions were determined as 0.25 μg/mL of CLO-OVA and 1:8 × 104 of mAb dilution (a). Under the optimal working conditions, the standard inhibition equation of mAb was y = −0.4423x + 0.1788 (R2 = 0.9951) with an IC50 of 0.188 ng/mL (b). Compared with previous studies, the prepared mAb against CLO showed higher sensitivity (Feng et al., Citation2018). The specificity of mAb was evaluated by the cross-reactivity experiment (). We found that mAb had no cross-reactivity with other agonist drugs, such as PA, BA, CYP, SAL, RAC, and CLT. The results indicated that the developed ic-ELISA exhibited high sensitivity and specificity and could be used to detect CLO residues.
Table 1. The cross-reaction of mAb with other agonist drugs.
Matrix effects
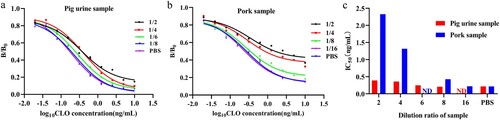
Pig urine and pork are commonly used as samples for agonist drugs residue detection, but the matrix effect in pig urine and pork extract samples was found to significantly affect the sensitivity and accuracy in testing of CLO residues using the developed ic-ELISA, resulting in an erroneous result. Usually, the matrix could be eliminated by PBS dilution; this method is simple and suitable for immunoassay methods. In this study, the standard curves obtained in pig urine and pork extract samples were compared with standard curve obtained in PBS to evaluate the matrix effects. The B/B0 and IC50 values in pig urine and pork extract samples were higher than that in PBS (). These results indicated that the matrix components significantly affected the specific recognition between mAb and antigen. However, the matrix effect could be eliminated by the above-mentioned sample pretreatment method. The matrix effect in pig urine and pork extract samples was eliminated after diluting 8-fold and 16-fold with PBS, respectively (c). This dilution procedure of samples is a simplification of pretreatment, and suitable for rapid on-site detection. Thus, the ic-ELISA met the detection requirements for CLO in the process of feeding or the slaughtered pork products.
Validation of the ic-ELISA
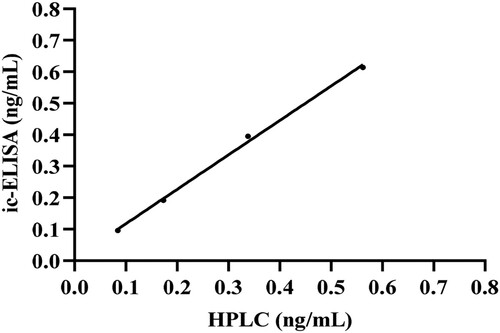
The values of LOD and LOQ reflected the sensitivity of the ic-ELISA, which were defined as the minimum concentrations of CLO that could be detected and the quantified, respectively. Two types of negative samples (pig urine and pork) were used to evaluate the values of LOD and LOQ by the ic-ELISA. The LOD values of the pig urine and pork samples were 0.033 and 0.061 ng/mL, respectively; the LOQ values were 0.054 and 0.096 ng/mL, respectively. The accuracy and precision were evaluated by the recovery experiment. Two types of negative samples (pig urine and pork), spiked with 0.1, 0.2, 0.4, and 0.6 ng/mL of CLO, were tested by the ic-ELISA for the intra-assay and the inter-assay, respectively. In the intra-assay, the recoveries for CLO were from 84.04% to 99.55%, with the highest CV at 11.27% (). In the inter-assay, the recoveries ranged from 87.18% to 99.81%, with the highest CV at 9.72% (). Usually, the ic-ELISA was considered precise and acceptable when the recovery was within 25% and the highest CV was within 15%. These results indicated that the developed ic-ELISA possessed satisfactory accuracy and precision for the detection of CLO residues in the pig urine and pork samples. Additionally, four negative pig urine samples containing different concentrations of CLO were detected by ic-ELISA and HPLC, respectively, the results of HPLC and ic-ELISA showed a good correlation (R2 = 0.9974) (), indicating that the developed ic-ELISA had good practicability. Compared with the chromatography method, the ic-ELISA was simple, and the results could be obtained within 2 h without the use of large instruments. The developed ic-ELISA could adequately meet the detection requirements for CLO residues in pig urine and pork samples.
Table 2. The recovery experiment of the ic-ELISA for CLO in pig urine and pork samples.
Conclusions
In the study, we developed an ic-ELISA for the rapid detection of CLO residues in the pig urine and pork samples. This ic-ELISA exhibited high sensitivity and excellent specificity for CLO and lacked cross-reactivity with other agonist drugs. The results of recovery experiment indicated that the developed ic-ELISA posed outstanding accuracy and precision. Meanwhile, the developed ic-ELISA showed high correlation with the result of HPLC for the detection of CLO in the pig urine samples. Thus, the proposed ic-ELISA provided a serviceable and rapid tool for the detection of CLO residues in real samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AlRabiah, H., Attia, S. M., Al-Shakliah, N. S., & Mostafa, G. A. (2020). Development and validation of an HPLC-UV detection assay for the determination of clonidine in mouse plasma and its application to a pharmacokinetic study. Molecules, 25(18), 4109. https://doi.org/10.3390/molecules25184109
- Bai, Y., Liu, Z., Bi, Y., Wang, X., Jin, Y., Sun, L., Wang, H., Zhang, C., & Xu, S. (2012). Preparation of polyclonal antibodies and development of a direct competitive enzyme-linked immunosorbent assay to detect residues of phenylethanolamine a in urine samples. Journal of Agricultural and Food Chemistry, 60(46), 11618–11624. https://doi.org/10.1021/jf3036066
- Bai, Y., Liu, Z., Wang, H., Bi, Y., Huang, Y., Sun, L., Su, F., Jin, Y., Zhang, C., & Xu, S. (2015). Development of a rapid and sensitive enzyme-linked immunosorbent assay to detect clonidine residues in swine urine samples. Food and Agricultural Immunology, 26(1), 1–12. https://doi.org/10.1080/09540105.2013.858663
- De Nicolò, A., Avataneo, V., Rabbia, F., Bonifacio, G., Cusato, J., Tomasello, C., Perlo, E., Mulatero, P., Veglio, F., Di Perri, G., & D’Avolio, A. (2016). UHPLC–MS/MS method with protein precipitation extraction for the simultaneous quantification of ten antihypertensive drugs in human plasma from resistant hypertensive patients. Journal of Pharmaceutical and Biomedical Analysis, 129, 535–541. https://doi.org/10.1016/j.jpba.2016.07.049
- Feng, M., Suryoprabowo, S., Tao, H., Liu, L., Zheng, Q., & Kuang, H. (2018). Rapid detection of clonidine and its cross-reactivity with apraclonidine in pig urine using an immunochromatographic test strip. Food and Agricultural Immunology, 29(1), 821–832. https://doi.org/10.1080/09540105.2018.1460325
- Jin, G., Wu, X., Cui, G., Liu, L., Kuang, H., & Xu, C. (2020). Development of an ic-ELISA and immunochromatographic strip assay for the detection of diacetoxyscirpenol in rice. ACS Omega, 5(29), 17876–17882. https://doi.org/10.1021/acsomega.9b02496
- Klein, R. H., Alvarez-Jimenez, R., Sukhai, R. N., Oostdijk, W., Bakker, B., Reeser, H. M., Ballieux, B. E. P. B., Hu, P., Klaassen, E. S., Freijer, J., Burggraaf, J., Cohen, A. F., & Wit, J. M. (2013). Pharmacokinetics and pharmacodynamics of orally administered clonidine: A model-based approach. Hormone Research in Paediatrics, 79(5), 300–309. https://doi.org/10.1159/000350819
- Krieger, E. M., Drager, L. F., Giorgi, D. M., Pereira, A. C., Barreto-Filho, J. A. S., Nogueira, A. R., Mill, J. G., Lotufo, P. A., Amodeo, C., Batista, M. C., Bodanese, L. C., Carvalho, A. C. C., Castro, I., Chaves, H., Costa, E. A. S., Feitosa, G. S., Franco, R. J. S., Fuchs, F. D., Guimarães, A. C., … ReHOT Investigators. (2018). Spironolactone versus clonidine as a fourth-drug therapy for resistant hypertension: The ReHOT randomized study (Resistant Hypertension Optimal Treatment). Hypertension, 71(4), 681–690. https://doi.org/10.1161/HYPERTENSIONAHA.117.10662
- Li, S., Wu, X., Kuang, H., & Liu, L. (2020). Development of an ic-ELISA and an immunochromatographic strip assay for the detection of aconitine. Food and Agricultural Immunology, 31(1), 243–254. https://doi.org/10.1080/09540105.2020.1714555
- Liu, A., Lin, J., Dai, M., Ma, B., Wu, Y., Fang, J., & Zhang, M. (2016). Development of a monoclonal antibody-based immunochromatographic assay detecting ractopamine residues in swine urine. Food Analytical Methods, 9(7), 2016–2025. https://doi.org/10.1007/s12161-015-0382-5
- Naguy, A. (2016). Clonidine use in psychiatry: Panacea or panache Pharmacology, 98(1-2), 87–92. https://doi.org/10.1159/000446441
- Pelzer, M., Addison, T., Li, W., Jiang, X., & Weng, N. (2002). Development and validation of a liquid chromatography-tandem mass spectrometry method, using silica column and aqueous-organic mobile phase, for the analysis of clonidine as low as 10 pg/ml in human serum. Journal of Liquid Chromatography & Related Technologies, 25(7), 1019–1032. https://doi.org/10.1081/JLC-120003421
- Peng, D., Zhao, L., Zhang, L., Pan, Y., Tao, Y., Wang, Y., Sheng, F., & Yuan, Z. (2019). A novel indirect competitive enzyme-linked immunosorbent assay format for the simultaneous determination of ractopamine and phenylethanolamine a residues in swine urine. Food Analytical Methods, 12(5), 1077–1085. https://doi.org/10.1007/s12161-019-01445-3
- Veigure, R., Aro, R., Metsvaht, T., Standing, J. F., Lutsar, I., Herodes, K., & Kipper, K. (2017). A highly sensitive method for the simultaneous UHPLC-MS/MS analysis of clonidine, morphine, midazolam and their metabolites in blood plasma using HFIP as the eluent additive. Journal of Chromatography B, 1052, 150–157. https://doi.org/10.1016/j.jchromb.2017.03.007
- Wang, A., Li, J., Liu, H., Chen, Y., Zhou, J., Liu, Y., Qi, Y., Jiang, W., & Zhang, G. (2021). Quantum dot-labelled antibody based on fluorescence immunoassays for the determination of arsanilic acid in edible pork and liver. Food Additives & Contaminants: Part A, 38(5), 820–829. https://doi.org/10.1080/19440049.2021.1885751
- Wang, X., Liufu, T., Beloglazova, N. V., Luo, P., Qu, J., & Jiang, W. (2016). Development of a competitive indirect enzyme-linked immunosorbent assay for screening phenylethanolamine A residues in pork samples. Food Analytical Methods, 9(11), 3099–3106. https://doi.org/10.1007/s12161-016-0500-z
- Wang, Y., Deng, R., Zhang, G., Li, Q., Yang, J., Sun, Y., Li, Z., & Hu, X. (2015). Rapid and sensitive detection of the food allergen glycinin in powdered milk using a lateral flow colloidal gold immunoassay strip test. Journal of Agricultural and Food Chemistry, 63(8), 2172–2178. https://doi.org/10.1021/jf5052128
- Wang, Y., Li, Z., Pei, Y., Li, Q., Sun, Y., Yang, J., Yang, Y., Zhi, Y., Deng, R., Hou, Y., & Hu, X. (2017). Establishment of a lateral flow colloidal gold immunoassay strip for the rapid detection of soybean allergen β-conglycinin. Food Analytical Methods, 10(7), 2429–2435. https://doi.org/10.1007/s12161-017-0800-y
- Xiao, X., Hu, S., Lai, X., Peng, J., & Lai, W. (2021). Developmental trend of immunoassays for monitoring hazards in food samples: A review. Trends in Food Science & Technology, 111, 68–88. https://doi.org/10.1016/j.tifs.2021.02.045
- Yan, K., Zhang, H., Hui, W., Zhu, H., Li, X., Zhong, F., Tong, X., & Chen, C. (2016). Rapid screening of toxic salbutamol, ractopamine, and clenbuterol in pork sample by high-performance liquid chromatography-UV method. Journal of Food and Drug Analysis, 24(2), 277–283. https://doi.org/10.1016/j.jfda.2015.12.002
- Yang, X., Wang, Y., Yang, J., Sun, Z., Yue, Z., Li, L., He, L., & Hu, X. (2019). An immunochromatographic lateral flow strip test for the rapid detection of danofloxacin in milk. Food Analytical Methods, 12(11), 2430–2437. https://doi.org/10.1007/s12161-019-01601-9
- Yin, M., Hu, X., Sun, Y., Xing, Y., Chai, S., Xing, G., Yang, Y., Teng, M., Li, Q., Wang, Y., Deng, R., & Zhang, G. (2020). The broad-spectrum and ultra-sensitive detection of zeranol and its analogues by an enzyme-linked immunosorbent assay in cattle origin samples. RSC Advances, 10(35), 20809–20816. https://doi.org/10.1039/D0RA02936J
- Zhang, H., Wang, L., Yao, X., Wang, Z., Dou, L., Su, L., Zhao, M., Sun, J., Zhang, D., & Wang, J. (2020). Developing a simple immunochromatography assay for clenbuterol with sensitivity by one-step staining. Journal of Agricultural and Food Chemistry, 68(52), 15509–15515. https://doi.org/10.1021/acs.jafc.0c05972
- Zhu, J., Li, Q., Yu, X., Zhang, X., Li, H., Wen, K., Ke, Y., Zhang, S., & Wang, Z. (2021). Synthesis of hapten, production of monoclonal antibody, and development of immunoassay for ribavirin detection in chicken. Journal of Food Science, 86(7), 2851–2860. https://doi.org/10.1111/1750-3841.15789
- Zhuang, J., Chen, J., Wang, X., Pang, Y., Bi, H., Huang, L., Zeng, G., Liao, X., Ma, Z., Chen, X., Zhong, G., Huang, M., & Zhao, X. (2015). Validation of a HPLC-ESI MS/MS method for the determination of clonidine in human plasma and its application in a bioequivalence study in Chinese healthy volunteers. Biomedical Chromatography, 29(10), 1506–1513. https://doi.org/10.1002/bmc.3450