?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
To improve the detection sensitivity of neomycin (NEO) in milk, we produced a sensitive monoclonal antibody (mAb) against NEO and developed a lateral flow immunoassay based on amorphous carbon nanoparticles (ACNPs-LFA). First, we conjugated NEO to carrier protein to prepare mAbs. We obtained six mAbs: mAb 1C6, mAb 1D3, mAb 2D3, mAb 4D5, mAb 5D1, and mAb 5H1. We characterised the mAbs by indirect competitive enzyme-linked immunosorbent assay and selected the most sensitive mAb based on the half-maximal inhibitory concentration (IC50). We selected mAb 4D5 (IC50 = 0.15 ng/mL) for the development of LFA. MAb 4D5 was labelled with ACNPs (ACNPs-mAb 4D5) by electrostatic absorption. Under optimised conditions, 5.4 μg mAb 4D5 coupling with 1 mL ACNPs, NEO-OVA at concentration of 80 μg/mL, 3 μL ACNPs-mAb 4D5 were used to develop LFA. The cut-off value was 8 ng/mL. Therefore, our developed ACNPs-LFA is suitable for on-site detection of NEO residues.
Introduction
Neomycin sulfate (NEO), an aminoglycoside antibiotic produced by Streptomyces fradiae, is widely used in veterinary medicine and animal husbandry. NEO inhibits the growth of both Gram-positive and Gram-negative bacteria (Jana & Deb, Citation2006; Waksman & Lechevalier, Citation1949). However, the abuse or misuse of NEO during the lactation stage of dairy cattle, especially intramammary or intrauterine use, contributes to the presence of NEO residues in milk, which have ototoxic and nephrotoxic effects in humans and animals (Manners & Stewart, Citation1982; Tunc et al., Citation2018; Wang et al., Citation2009). NEO residues in milk can be transferred to humans through the food chain and increase the risk of bacterial resistance in humans (Liu et al., Citation2021). To minimise these harmful effects, the maximum residue limit (MRL) of NEO in milk set by China and the European Union is 500 μg/L. (Commission Regulation, EC No. 1183/Citation2002). Therefore, it is important to have a rapid and reliable NEO detection method.
Several analytical methods have been developed to detect NEO residues, such as chromatographic assays, microbiological methods and immunoassays. Due to the absence of chromophores or fluorophores in NEO, liquid chromatography generally requires pre- or post-column derivatization for detection using UV detector (Zhang et al., Citation2021a). To avoid sample derivatization, a universal mode of detection could be used as evaporative light (Clarot et al., Citation2005), pulse amperometry (Adams et al., Citation1996), and mass spectrometry detectors (Zu et al., Citation2018). Microbiological methods are time-consuming and have poor sensitivity (Wang et al., Citation2009). In contrast, immunoassays, i.e. enzyme-linked immunosorbent assays (ELISAs) and lateral flow immunoassays (LFAs) based on antibodies, are the most common method for screening veterinary drug residues due to their sensitivity, low cost, simplicity, and high sample throughput (He et al., Citation2016; Zhang et al., Citation2020). Researchers have developed ELISA for the detection of NEO. Burkin and Galvidis (Citation2011) developed an indirect competitive ELISA (icELISA), which requires a 100-fold sample dilution in the least sensitive assay, for the detection of NEO at MRL levels (Burkin & Galvidis, Citation2011). Chen et al. (Citation2007) established a sensitive icELISA with a limit of detection (LOD) of 0.69 ng/mL in milk (Chen et al., Citation2007). Wang et al. (Citation2009) developed an icELISA for detecting NEO residues in pork, chicken muscle, egg, fish, milk and pig kidney, with LOD values of 5, 10, 10, 10, 10, and 20 μg/kg, respectively (Wang et al., Citation2009). Luo et al. (Citation2016) established a chemiluminescent ELISA for the detection of NEO residues in milk with an LOD of 9.4 μg/kg (Luo et al., Citation2016). In addition, Liu et al. (Citation2021) reported an ultratrace analysis of NEO using flow–through immunoaffinity chromatography with an LOD of 162.5 pg/mL in milk (Liu et al., Citation2021). However, these methods are time consuming, and milk requires a 10-fold dilution to prevent column blocking and reduce matrix interference. In comparison to ELISA and flow-through immunoaffinity chromatography, LFA is more suitable for on-site screening because it does not require tedious washing steps. In conventional LFA, colloidal gold nanoparticles (CGNPs) have been mainly used as labels (Li et al., Citation2016). However, this type of LFA fails to meet detection requirements.
To improve the sensitivity of LFA, the indirect-labelling format which secondary antibodies specific to the primary antibodies are labelled with nanomaterials, is an effective way. Recently, Hendrickson et al. (Citation2021) reported a sensitive LFA for NEO in milk with a cut-off value of 10 ng/mL by indirect labelling method (Hendrickson et al., Citation2021). However, this marking procedure is complicated. An alternative method to improve the sensitivity of LFA requires novel nanomaterial labels, such as quantum dots (Beloglazova et al., Citation2014), fluorescent microsphere (Zhang et al., Citation2016), and time-resolved fluorescence(Wang et al., Citation2016). However, these fluorescent LFA requires additional light sources to provide excitation wavelengths. Similar to CGNPs, novel materials that allow results under natural light will be effective for on-site screening. Amorphous carbon nanoparticles (ACNPs) as labels in LFA may improve the sensitivity of LFA due to its strong dark colour and high contrast against light backgrounds under natural light (Linares et al., Citation2012). However, no studies have evaluated the feasibility of ACNPs-LFA in NEO detection.
In this study, we developed an ACNPs-LFA to detect NEO residues in milk under natural light. First, we conjugated NEO with a carrier protein to prepare monoclonal antibodies (mAbs). We obtained the most sensitive mAb based on the half-maximal inhibitory concentration (IC50) by icELISA. Second, we used anti-NEO mAb coupled with ACNPs to develop a sensitive LFA for the detection of NEO residues in milk. To the best of our knowledge, this is the first study that reports LFA based on ACNPs for NEO detection in milk.
Materials and methods
Chemicals and apparatus
NEO, 1-(3-Dimethylaminopropyl)−3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxythiosuccinimide (NHS) were purchased from Aladdin Reagents Co., Ltd (Beijing, China). Amorphous Carbon Nanoparticles (ACNPs) were supplied by Beijing Najing Biotechnology Co., Ltd (Beijing, China). Bovine serum albumin (BSA), ovalbumin(OVA), polyethylene glycol 2000 (PEG 2000) and hypoxanthine-aminopterin-thymidine (HAT) were obtained from Sigma-Aldrich (St. Louis. MO, U.SA). Nitrocellulose (NC) membranes were obtained from Shantou Yineng Membrane Industry Co., Ltd (Gangdong, China). Absorbent pads were obtained from Huaiyuan Tongcheng Paper Products Co., Ltd (Beijing, China); Polyvinylchloride backing cards were obtained from Shanghai Jinbiao Biotechnology Co., Ltd (Shanghai, China). Sample pads were obtained from Shanghai Jieyi Biotechnology Co., Ltd (Shanghai, China). The commercial icELISA kit for the detection of NEO in milk was supplied from Beijing Wedewekang Biotechnology Co., Ltd, (Beijing, China).
Strip cutter ZQ2002 was purchased from Shanghai Jinbiao Biotechnology Co., Ltd, (Shanghai, China). The dispensing platform XYZ-HGS510 was purchased from Hangzhou Autokun (Hangzhou, China). A portable test strip reader FD-600 was supplied from Shanghai Femdetection Bio-tech Co., Ltd. (Shanghai, China). SpectraMax M2e microplate reader MK3 was purchased from Thermo Fisher Instruments Co., Ltd, (Shanghai, China).
Preparation of antigens
We synthesised the immunogen (NEO-BSA) and coating antigen (NEO-OVA) using the active ester method (Wang et al., Citation2009). Briefly, to 72 mg NEO in 2 mL PBS (pH 7.4) we added 260 mg BSA (or 180 mg OVA) in 20 mL PBS (pH 7.4). Subsequently, we added 20 mg EDC and 20 mg NHS in 2 mL PBS (pH 7.4) dropwise. The reaction mixture reacted at 4°C for 12 h. NEO-BSA and NEO-OVA were dialysed in PBS for 48 h and stored at −20°C.
Production of mAbs
We immunised three BALB/c mice (8 weeks old) by subcutaneous injection (Yang et al., Citation2016). For the first immunisation, we emulsified 0.6 mg NEO-BSA (1 mg/mL) in 0.6 mL Freund’s complete adjuvant and administered 0.2 mg NEO-BSA/mouse. During three weeks, we administered two booster shots at the same dose emulsified in Freund’s incomplete adjuvant. Seven days after the third immunisation, we collected the serum from the caudal vein and subjected it to icELISA. The mouse with the highest antibody titre and inhibition ratio was used for cell fusion. The inhibition ratio was calculated using the following equation,
where B0 and B represent the absorbance values without NEO and with NEO standard, respectively.
We conducted icELISA as previously described (Zhang et al., Citation2021b). First, 96-well ELISA plates were coated with 0.1 μg/mL NEO-OVA at 4°C for 10 h. We discarded the coating antigen and sealed the wells with 200 μL of 3% skimmed milk powder in PBS at 37°C for 2 h. Second, we diluted 50 μL PBS (or NEO standard solution) and 50 μL anti-serum 1,000-fold with PBS and incubated the plates at 37°C for 30 min. Third, we discarded the solution and washed the plate three times with PBS containing 0.05% Tween-20. We added 100 μL goat anti-mouse IgG (diluted 1:5,000) and incubated the plate for 30 min at 37°C. Fourth, the second antibody solution was discarded, and the plate was washed three times. We added 100 μL/well substrate solution and incubated the plate for 10 min. Finally, we added 50 μL of 2 M H2SO4 to stop the reaction and measured absorbance at 450 nm on a Multiskan MK3 microplate reader.
Hybridoma cells secreting anti-NEO antibodies were prepared using cell fusion technology (Clarot et al., Citation2005). Briefly, we fused the spleen of the mouse with the highest antibody titre and inhibition ratios with SP2/0 murine myeloma cells using PEG 2000 and cultured it in HAT selective medium. The cell supernatant was analyzed by icELISA. Cells that secreted anti-NEO antibodies were sub-cloned three times by the limiting dilution method for the production of ascites.
Production of ACNPs-mAb conjugates
The anti-NEO mAb labelled with ACNPs (ACNPs-mAb) was synthesised as previously described (Zhang et al., Citation2017). Berifly, 1 mL ACNPs and 5.4 μg mAb 14D5 in a 1.5-mL EP-tube were incubated at 37°C for 30 min. Subsequently, 20 μL 1% BSA was added into the mixture reaction soultion for blocking unreaction sites at 37°C for another 30 min. Following centrifugation at 8,000 g for 10 min, we dispersed the precipitate (ACNPs-mAb) in 1 mL of 1% BSA and stored it at 4°C.
Preparation of LFA
LFA consists of four sections: sample pad, conjugation pad, nitrocellulose (NC) membrane, and absorbent pad (Zhang et al., Citation2016). First, NC membranes were coated with 0.08 μL/mm NEO-OVA (80 μg/mL) and goat anti-mouse antibodies (0.5 mg/mL) to generate the T and C lines, respectively. The assembly procedure was performed as previously reported (Zhang et al., Citation2020). The assembled plate was cut into 3.2-mm wide strips and stored under dry conditions at 25°C.
We obtained milk samples from a local supermarket. The samples were NEO-negative based on a commercial icELISA kit. The milk samples were detected without any pre-sample processing. The detection procedure was the following, 3 μL ACNPs-mAb and 200 μL NEO-negative milk (or NEO-spiked milk) were added into a 96 well-plate and incubated for 3 min. We inserted the strips into the wells for 8 min. Qualitative results were obtained with the naked eye, and quantitative results were obtained using a hand-held strip scan reader.
Results and disscussion
Preparation of antigen
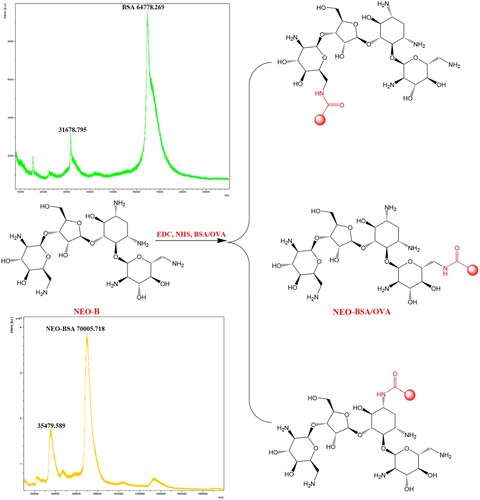
The quality of artificial antigens affects antibody titres, specificity (cross-reactivity), and sensitivity (IC50 value). NEO (MW = 614.65 Da) does not cause immune responses in animals due to its low molecular weight. To attain immunogenicity, we must conjugate NEO to a carrier protein. NEO-B, a marker residue, was selected to prepare antibodies against NEO. NEO-B has several active groups, such as primary and secondary amines and a hydroxy group. In principle, the active ester method and glutaraldehyde method could be used to synthesise antigens between the amine groups of NEO-B and carrier proteins (Jin et al., Citation2006; Liu et al., Citation2021; Wang et al., Citation2009). In addition, the hydroxy group could be converted an aldehyde group by sodium periodate prior to carrier protein coupling (Chen et al., Citation2007).
In this study, we used the ester method to prepare antigens without introducing a spacer arm between NEO-B and the carrier protein. All primary and secondary amines could react with the carboxyl groups of the carrier proteins (). To avoid simultaneous linking of multiple amino groups of NEO-B to a carrier protein, we set the reaction ratio of NEO-B to BSA (or OVA) at 25:1, which was lower than that reported by Liu et al. (Citation2021) (Liu et al., Citation2021). It was challenging to characterise the conjugates by UV-visible absorption due to the absence of chromophores or fluorophores in NEO-B. Therefore, we characterised the antigen and hapten-to-carrier protein molar ratios by matrix-assisted laser desorption time-of-flight mass spectrometry. The mass of NEO-BSA was 70,005.8, which was higher than that of BSA, indicating that the antigen was successfully synthesised. The hapten-to-carrier protein molar ratios was 8.5: NEO-BSA (MW 70,005.8) − BSA (MW 64,778.3)/NEO-B (MW 614.65 Da). This ratio was beneficial to produce antibodies (the appropriate coupling ratios range between 3 and 15) (Mari et al., Citation2021; Li et al., Citation2017).
Preparation of mAbs
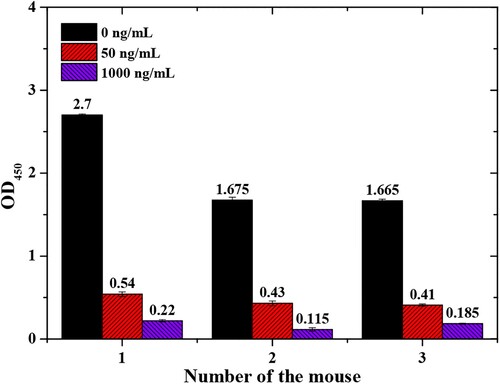
Following the third immunisation, we characterised the serum using icELISA. The concentration of NEO-OVA was 0.5 μg/mL, and the serum was diluted 3,000-fold. When the concentration of NEO was 50 μg/L, the antiserum inhibition ratios of mouse No. 1, mouse No. 2, and mouse No. 3 were 20.66%, 24.12%, and 24.24%, respectively. All three mice produced antibodies against free NEO (). Mouse No. 1 was sacrificed for cell fusion due to higher antibody and inhibition ratio.
After cell fusion, we obtained six cell lines (1C6, 1D3, 2D3, 4D5, 5C1, and 5H1), which were used for ascites production. We characterised these mAbs by ELISA and icELISA. We used antibody titres, absorbance of NEO at 0 ng/mL, IC50, IC20, and IC80 as the evaluation criteria to obtain the best mAbs for subsequent experiments. As shown in , the six mAbs recognised free NEO, and mAb 4D5 was the best antibody against NEO with lower IC50 value (0.15 μg/L) and higher antibody titre (1×105). Therefore, mAb 4D5 and NEO-OVA were selected for preparing the LFA.
Table 1. mAbs properties characterised by ELISA and icELISA.
Optimisation of the LFA
ACNPs-LFA is based on indirect competition. In NEO-negative samples, ACNPs-mAb 4D5 binds to NEO-OVA and goat anti-mouse IgG, and the T and C lines appear as black bands. In NEO-positive samples, ACNPs-mAb 4D5 binds to NEO, and the colour of the T line gradually decreases with increasing NEO concentration in the sample.
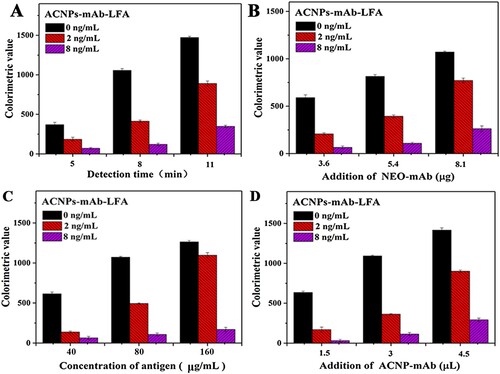
Reaction time, amount of mAb 4D5 for ACNP conjugation, concentration NEO-OVA, and amount of ACNPs-mAb affect the sensitivity of ACNPs-LFAs. We used 2 and 8 ng/mL NEO for the inhibition test. We could visualise the T-line with the naked eye (the value of the T line was higher than 800 at 0 ng/mL NEO as the evaluation standard). After 8 min (A), the values of the T line (concentration of NEO at 0 ng/mL) was 1,060.5 ± 6.4. The inhibition ratios were 39.4% and 11.6% at 2 and 8 ng/mL NEO, respectively. Therefore, the detection time was set at 8 min. By this criterion, 5.4 μg mAb 4D5 coupled with 1 mL ACNPs (B), 80 μg/mL NEO-OVA (C), and 3 μL ACNPs-mAb 4D5 (D) were selected as the best reaction conditions for developing the LFA.
Detection of NEO using ACNPs-LFA
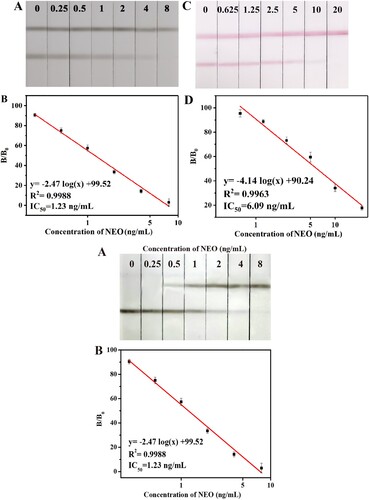
Based on the optimised conditions, we spiked milk samples with a series of NEO concentrations (0, 0.25, 0.5, 1, 2, 4 and 8 ng/mL) to assess the sensitivity of the LFA. The cut-off value was defined as the lowest concentration of NEO that resulted in the disappearance of the T line. A shows that the cut-off value was 8 ng/mL for the detection of NEO in milk samples using the developed ACNPs-LFA and anti-NEO mAb 4D5. Subsequently, we measured the reflectance values of the T lines using a hand-held strip scan reader, thereby calculating B/B0 (ratio between the reflectance value of the T line of a positive sample to that of a negative sample; B). The IC50 value was 1.23 ng/mL, and the working range (IC20−IC80) was 0.26–3.06 ng/mL. As shown in C and D (the preparation of the CGNPs-LFA was not shown in this study), the cut-off value of the CGNPs-LFA assay to the naked eye was 20 ng/mL, IC50 value was 6.09 ng/mL. These results indicated that the sensitivity of the ACNPs-LFA was five times higher than that of the CGNPs-LFA. In addition, the sensitivity for detecting NEO in milk samples was higher in ACNPs-LFA than in icELISA (LOD was 5 ng/mL) and LAF (cut-off value was 10 ng/mL) (Hendrickson et al., Citation2021; Wang et al., Citation2009).
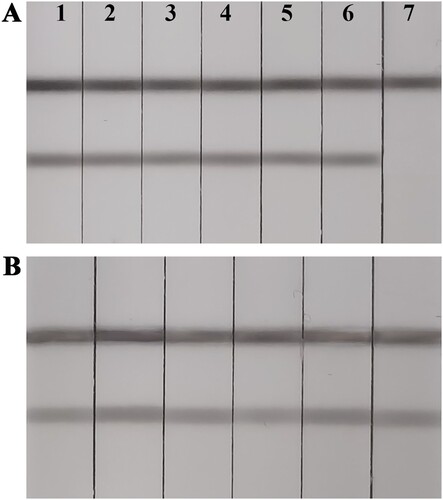
We assessed specificity by evaluating cross reactivity with other antibiotics, e.g. gentamycin, streptomycin, kanamycin, ampicillin, chloramphenicol, and ciprofloxacin at 50 ng/mL. ACNPs-LFA was specific for NEO (A). To further evaluate the parctivability of the ACNPs-LFA, we evaluated six milk samples using a commercial icELISA and our developed ACNPs-LFA. All milk samples were NEO-negative based on the icELISA results (data not shown). Subsequently, milk samples were analyzed by ACNPs-LFA. All milk samples were negative based on the naked eye (B). Therefore, the detection results obtained with ACNPs-LFA were consistent with those obtained with the commercial icELISA. ACNPs-LFA is reliable for on-site detection of NEO residues in milk samples.
Conclusion
In this study, we prepared a sensitive anti-NEO mAb 4D5 and developed an LFA based on ACNPs for the rapid detection of NEO residues. ACNPs-LFA had high sensitivity (cut-off value = 8 ng/mL) in milk samples without any pre-sample processing. In addition, we compared the sensitivity of the ACNPs-LFA and CGNPs-LFA, and the results showed that the sensitivity of the ACNPs-LFA was five times higher than that of the CGNPs-LFA. Our developed ACNPs-LFA can be used as a practical tool for the on-site detection of NEO in milk samples.
Ethical approval
BALB/c mice were purchased from the Experimental Animal Centre of Zhengzhou University and raised in the Animal Experimental Centre of the University of Traditional Chinese Medicine. Animal experiments were approved by the animal ethics committee and carried out in accordance with the guidelines of the animal experiment centre.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adams, E., Schepers, R., Roets, E., & Hoogmartens, J. (1996). Determination of neomycin sulfate by liquid chromatography with pulsed electrochemical detection. Journal of Chromatography A, 741(2), 233–240. https://doi.org/10.1016/0021-9673(96)00207-5
- Beloglazova, N. V., Speranskaya, E. S., Wu, A., Wang, Z., Sanders, M., Goftman, V. V., Zhang, D., Goryacheva, I. Y., & Saeger, S. D. (2014). Novel multiplex fluorescent immunoassays based on quantum dot nanolabels for mycotoxins determination. Biosensors and Bioelectronics, 62, 59–65. https://doi.org/10.1016/j.bios.2014.06.021
- Burkin, M. A., & Galvidis, I. A. (2011). Development and application of indirect competitive enzyme immunoassay for detection of neomycin in milk. Applied Biochemistry and Microbiology, 47(3), 321–326. Available online: https://www.webofscience.com/wos/alldb/full-record/MEDLINE:21790038. https://doi.org/10.1134/S0003683811030045
- Chen, Y. Q., Shang, Y. H., Wu, X. P., Qi, Y. T., & Xiao, X. L. (2007). Enzyme-linked immunosorbent assay for the detection of neomycin in milk: Effect of hapten heterology on assay sensitivity. Food and Agricultural Immunology, 18(2), 117–128. https://doi.org/10.1080/09540100701579829
- Clarot, I., Regazzeti, A., Auzeil, N., Laadani, F., Cittonet, M., Netter, P., & Nicolas, A. (2005). Analysis of neomycin sulfate and framycetin sulfate by high-performance liquid chromatography using evaporative light scattering detection. Journal of Chromatography A, 1087(1-2), 236–244. https://doi.org/10.1016/j.chroma.2005.05.054
- Commission Regulation, EC. (2002). No. 1183/2002 of 1 July 2002. amending Annex I of Council Regulation (EEC), No. 2377/90.
- He, J. J., Wang, Y., & Zhang, X. Y. (2016). Preparation of artificial antigen and development of IgY-based indirect competitive ELISA for the detection of kanamycin residues. Food Analytical Methods, 9(3), 744–751. https://doi.org/10.1007/s12161-015-0248-x
- Hendrickson, O. D., Byzova, N. A., Zvereva, E. A., Zherdev, A. V., & Dzantiev, B. B. (2021). Sensitive lateral flow immunoassay of an antibiotic neomycin in foodstuffs. Journal of Food Science and Technology, 58(7), 292–301. https://doi.org/10.1007/s13197-020-04541-z
- Jana, S., & Deb, J. K. (2006). Molecular understanding of aminoglycoside action and resistance. Applied Microbiology and Biotechnology, 70(2), 140–150. https://doi.org/10.1007/s00253-005-0279-0
- Jin, Y., Jang, J. W., Lee, M. H., & Han, C. H. (2006). Development of ELISA and immunochromatographic assay for the detection of neomycin. Clinica Chimica Acta, 364(1-2), 260–266. https://doi.org/10.1016/j.cca.2005.07.024
- Li, J., Duan, H., Xu, P., Huang, X. L., & Xiong, Y. H. (2016). Effect of different-sized spherical gold nanoparticles grown layer by layer on the sensitivity of an immunochromatographic assay. RSC Advances, 6(31), 26178–26185. https://doi.org/10.1039/C6RA03695C
- Li, Z. Z., Wang, Y., Li, D. M., Chen, X. J., Li, Z. L., Gao, H. L., Cao, L., Li, S. B., & Hou, Y. Z. (2017). Development of an indirect competitive enzyme-linked immunosorbent assay for screening ethopabate residue in chicken muscle and liver. RSC Advances, 7(57), 36072–36080. https://doi.org/10.1039/C6RA20736G
- Linares, E. M., Kubota, L. T., Michaelis, J., & Thalhammer, S. (2012). Enhancement of the detection limit for lateral flow immunoassays: Evaluation and comparison of bioconjugates. Journal of Immunological Methods, 375(1-2), 264–270. https://doi.org/10.1016/j.jim.2011.11.003
- Liu, C., Jiang, Y. L., Xiu, L. Y., Qian, R. J., Zhao, M. X., Lou, P. J., Ke, Y. B., Li, G. M., & Jiang, W. X. (2021). Ultratrace analysis of neomycin residues in milk at femtogram levels by flow-through immunoaffinity chromatography test. Food Analytical Methods, 14(11), 2298–2307. https://doi.org/10.1007/s12161-021-02058-5
- Luo, P. J., Zhang, J. B., Wang, H. L., Chen, X., Wu, N., Zhao, Y. F., & Jiang, W. X. (2016). Rapid and sensitive chemiluminescent enzyme immunoassay for the determination of neomycin residues in milk. Biomedical and Environmental Sciences, 29(5), 374–378. Available online: https://doi.org/10.3967/bes2016.048.
- Manners, J. G., & Stewart, R. (1982). Presence of dihydrostreptomycin and penicillin in cows, milk following intrauterine administration. Australian Veterinary Journal, 58(5), 203–204. https://doi.org/10.1111/j.1751-0813.1982.tb00655.x
- Mari, G. M., Li, H. F., Dong, B. L., Yang, H. J., Talpur, A., Mi, J. F., Guo, L. C., Yu, X. Z., Ke, Y. B., Han, D. G., & Wang, Z. H. (2021). Hapten synthesis, monoclonal antibody production and immunoassay development for direct detection of 4-hydroxybenzehydrazide in chicken, the metabo-lite of nifuroxazide. Food Chemistry, 355, 129598. https://doi.org/10.1016/j.foodchem.2021.129598
- Tunc, C., Sehnaz, Y., Vildan, E. A., Hakan, B., & Selma, O. (2018). Monitoring of neomycin sulfate antibiotic in microbial fuel cells. Bioresource Technology, 268, 116–120. https://doi.org/10.1016/j.biortech.2018.07.122
- Waksman, S. A., & Lechevalier, H. A. (1949). Neomycin, a new antibiotic active against streptomycin-resistant bacteria, including tuberculosis organisms. American Journal of Obstetrics and Gynecology, 109(2830), 305–307. Available online: https://doi.org/10.1126/science.109.2830.305
- Wang, D., Zhang, Z. W., Li, P. W., Zhang, Q., & Zhang, W. (2016). Time-resolved fluorescent immunochromatography of aflatoxin b1 in soybean sauce: A rapid and sensitive quantitative analysis. Sensors, 16(7), 1094. https://doi.org/10.3390/s16071094
- Wang, S., Xu, B., Zhang, Y., & He, J. X. (2009). Development of enzyme-linked immunosorbent assay (ELISA) for the detection of neomycin residues in pig muscle, chicken muscle, egg, fish, milk and kidney. Meat Science, 82(1), 53–58. https://doi.org/10.1016/j.meatsci.2008.12.003
- Yang, H. J., Dai, R., Zhang, H. Y., Li, C. L., Zhang, X. Y., Shen, J. Z., Wen, K., & Wang, Z. H. (2016). Production of monoclonal antibodies with broad specificity and development of an immunoassay for microcystins and nodularin in water. Analytical and Bioanalytical Chemistry, 408(22), 6037–6044. https://doi.org/10.1007/s00216-016-9692-8
- Zhang, Q., He, L. Y., Rani, K. K., Wu, D. Y., Han, J. J., Chen, Y. H., & Su, W. J. (2021a). Colorimetric detection of neomycin sulfate in tilapia based on plasmonic core–shell Au@PVP nanoparticles. Food Chemistry, 356, 129612. https://doi.org/10.1016/j.foodchem.2021.129612
- Zhang, X. Y., Ding, M. Y., Zhang, C. S., Mao, Y. X., Wang, Y. Y., Li, P. P., Jiang, H. Y., Wang, Z. H., & Yu, X. Z. (2021b). Development of a New monoclonal antibody against brevetoxins in oyster samples based on the indirect competitive enzyme-linked immunosorbent assay. Foods (basel, Switzerland), 10(10), 2398. https://doi.org/10.3390/foods10102398
- Zhang, X. Y., Wen, K., Wang, Z. H., Jiang, H. Y., Beier, R. C., & Shen, J. Z. (2016a). An ultra-sensitive monoclonal antibody-based fluorescent microsphere immunochromatographic test strip assay for detecting aflatoxin M 1 in milk. Food Control, 60, 588–595. https://doi.org/10.1016/j.foodcont.2015.08.040
- Zhang, X. Y., Wu, C., Wen, K., Jiang, H. Y., Shen, J. Z., Zhang, S. X., & Wang, Z. H. (2016b). Comparison of fluorescent microspheres and colloidal gold as labels in lateral flow immunochromatographic assays for the detection of T-2 toxin. Molecules, 21(1), 27. https://doi.org/10.3390/molecules21010027
- Zhang, X. Y., Yu, X. Z., Wen, K., Li, C. L., Mari, G. M., Jiang, H. Y., Shi, W. M., Shen, J. Z., & Wang, Z. H. (2017). Multiplex lateral flow immunoassays based on amorphous carbon nanoparticles for detecting threefusariummycotoxins in maize. Journal of Agricultural and Food Chemistry, 65(36), 8063–8071. https://doi.org/10.1021/acs.jafc.7b02827
- Zhang, X. Y., Zhao, F. F., Sun, Y. W., Mi, T. J., Wang, L. Y., Li, Q., Li, J. Y., Ma, W. T., Liu, W. J., Zuo, J. N., Chu, X. Y., Chen, B., Han, W. M., & Mao, Y. X. (2020). Development of a highly sensitive lateral flow immunoassay based on receptor-antibody-amorphous carbon nanoparticles to detect 22 β-lactams in milk. Sensors and Actuators B: Chemical, 321, 128458. https://doi.org/10.1016/j.snb.2020.128458
- Zu, M., Jiang, J., Zhao, H., Zhang, S. L., Yan, Y., Qiu, S. W., Yuan, S. L., Han, J. W., Zhang, Y., Guo, W. W., & Yang, S. M. (2018). Rapid analysis of neomycin in cochlear perilymph of Guinea pigs using disposable SPE cartridges and high performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography B, 1093-1094, 52–59. https://doi.org/10.1016/j.jchromb.2018.06.055