Abstract
Mercury (Hg) and its compounds are much concerned for their high toxicity and wide presence in the environment. Since the toxicity of Hg is species dependent, various methods have been developed for the speciation analysis of Hg. This review focus on the determination and speciation analysis of Hg chemical species in water, sediment, and soil samples. Recent developments on sample pre-treatment and extraction/pre-concentration, separation, and quantification of Hg chemical species, and associated analytical challenges have been reviewed and briefly discussed based on recent reports.
Abbreviations:
- [C6MIM][PF6]: 1-hexyl-3-methylimidazolium hexafluorophosphate
- [C8MIM][PF6]: 3-methyl-1-octylimidazolium hexafluorophosphate
- AFS: atomic fluorescence spectrometry
- APDC: ammonium pyrrolidine dithiocarbamate
- CE: capillary electrophoresis
- CPE: cloud point extraction
- CV-AAS: cold vapor generation atomic absorption spectrometry
- CV-ILA-HS-SDME: cold vapor ionic liquid assisted head space single drop microextraction
- DAD: diode array detector
- DDTC: diethyldithiocarbamate
- DGT: diffusive gradient technique
- DLLME: dispersive liquid liquid microextraction
- ET-AAS: electrothermal atomic absorption spectrometry
- EtHg: ethylmercury
- FLPE: fluorinated polyethylene
- GC: gas chromatography
- GC-AFS: gas chromatography atomic fluorescence spectrometry
- GC–ICP-MS: gas chromatography inductively coupled plasma mass spectrometry
- GC-MS: gas chromatography mass spectrometry
- GC-Pyr-AFS: gas chromatography pyrolysis atomic fluorescence spectrometry
- HF-LLLME: hollow fiber liquid liquid liquid microextraction
- Hg: mercury
- HG/CV-AFS: hydride generation/cold vapor generation atomic fluorescence spectrometry
- Hg0: elemental mercury
- HPLC: high performance liquid chromatography
- HPTLC: high performance thin layer chromatography
- HS-GC-AFS: head space gas chromatography atomic fluorescence spectrometry
- IC: ion chromatography
- ICP-MS: inductively coupled plasma mass spectrometry
- ICP-OES: inductively coupled plasma optical emission spectrometry
- ID: isotope dilution
- IL-SDME: ionic liquid based single drop microextraction
- LC: liquid chromatography
- LOD: limit of detection
- LPME: liquid phase microextraction
- LVSS: large volume sample stacking
- MeHg: methylmercury
- MSC: multi syringe chromatography
- OrgHg: organic mercury
- PAN: 1-(2-pyridylazo)-2-naphthol
- P&T: purge and trap
- P&T-GC-Pyr-AFS: purge and trap gas chromatography pyrolysis atomic fluorescence spectrometry
- PE: polyethylene
- PET: polyethylene terephthalate
- PhHg: phenylmercury
- PJDA: plasma jet desorption atomization
- PJD-AFS: plasma jet desorption atomic fluorescence spectrometry
- POSS-SH: polyhedral oligomeric silsesquioxane
- PTFE: polytetrafluoroethylene
- PVG: photochemical vapor generation
- RSD: relative standard deviation
- SDME: single drop microextraction
- SERS: surface enhanced Raman spectroscopy
- SnCl2: stannous chloride
- SPE: solid phase extraction
- SPME: solid phase microextraction
- TLC: thin layer chromatography
- UV: ultraviolet
- UV–vis: ultraviolet visible
Introduction
Mercury, formerly named as hydrargyrum, is a chemical element which exists throughout the environment. It is one of the most toxic pollutants which can cause various health complications due to its characteristic properties, undergo long-range transport in the atmosphere,[Citation1] long last in the environment and accumulate in the food web.[Citation2–4] Despite its high toxicity, Hg has been used and still in use for different purpose.[Citation1]
Hg can exist as Hg0, inorganic Hg (Hg+ and Hg2+) and various OrgHg forms (e.g. MeHg, EtHg, and PhHg) in the environment. Hg2+ is the dominant form of Hg in water, soil, and sediment while MeHg and Hg0 are the major species in biota and atmosphere, respectively. All forms of Hg are highly toxic to organisms. Organic compounds of Hg are highly toxic, particularly, MeHg is the most toxic chemical species due to its bioaccumulating ability, high affinity to macromolecules and slow metabolism. It can easily diffuse from cell membrane and deposit in blood, therefore affect the central nervous system.[Citation5]
The biogeochemical transformation, toxicity, bioavailability, mobility, and fate of Hg rely not only on its total concentration but also on its chemical forms.[Citation6] For all toxic heavy metals, which undergo physical and/or chemical transformations, the total concentration of an element in general cannot reflect the hazard or benefit of their individual species. Korbas et al. [Citation7] studied the difference in toxicity between OrgHg and inorganic Hg species towards Zebrafish larvae and demonstrated that OrgHg resulted in overall higher Hg burdens and also targeted different cells and tissues than their inorganic counterparts. This indicates that the toxicity depends on the chemical forms of an element than its total quantity. Thus, only monitoring the concentration of total Hg in the environment is insufficient. Therefore, speciation analysis, which provides useful information on the toxicity and health risks of Hg, should be considered to further understand its emission, transport, deposition, and biogeochemical cycling. Consequently, tremendous efforts have been expended to develop reliable methodologies for Hg determination and speciation analysis.
Since the concentration of Hg in environmental samples might be at trace level, the Hg speciation analysis requires the sample pre-treatment and pre-concentration methods such as SPE,[Citation8] SPME,[Citation9] HF-LLLME,[Citation10,11] DLLME,[Citation12–16] SDME,[Citation17,18] CPE,[Citation19] and P&T.[Citation20–23] Separation and determination techniques including LC, GC, and CE coupled to ICP-MS,[Citation3,10,12,24–26] PJD-AFS,[Citation27] AFS (HG/CV-AFS),[Citation9,28] and CV-AAS [Citation9] have been used. As well, particular attention has to be paid to the sampling, transportation, and proper sample storage procedures.[Citation29]
A lot of recent advancements have been reported on the sample preparation, separation, and detection steps in speciation analysis of the element. However, only few review papers [Citation6,30,31] have been published. Therefore, this review focuses on selected research papers conducted on the speciation analysis of Hg chemical species in environmental samples, covering the major developments in the sample pre-concentration, separation, and detection techniques.
Speciation analysis of Hg
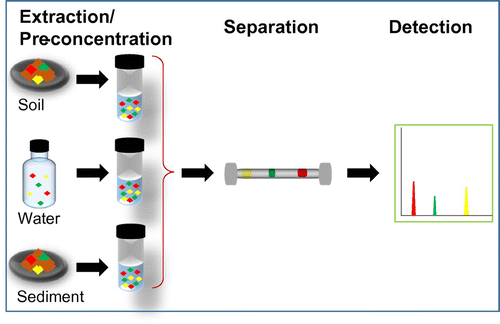
Speciation analysis of Hg chemical species in environmental samples is a sequential process involving extraction/pre-concentration, separation, and detection steps (Figure ). Each of the analysis stages require from simple to sophisticated analytical tools and should be carried out cautiously to generate the intended information. Under this section, the basic principles, detailed strategies and recent advancements on speciation analysis of Hg have been discussed.
Sample preservation and storage
Parameters like initial concentration of the analytes, sample composition, storage materials and other conditions (light, temperature, and pH) usually affect the stability of Hg chemical species and should be monitored as much as possible to investigate the species contained in the samples. The amount of Hg in environmental samples is usually low. Therefore, risk of contamination due to minor impurity/contamination of the sampling materials, additives, and the storage containers is probable. In addition, some forms of Hg are either volatile, have strong affinity for surface adsorption and/or leak in/out through the storage materials which can affect the original sample constituent.[Citation32] Furthermore, processes like transformation and degradation of the analytes, and inter-conversion between the chemical species should be avoided during sample collection, preservation, and storage. Therefore, appropriate sample collection, pre-treatment, and storage procedures are critical.[Citation29,30,32–34]
Apparatuses made of polymer,[Citation5,35] glass,[Citation17] stainless steel, and aluminum are popular in collection, pre-treatment, and storage of environmental samples. The use of PE containers in sample collection and storage of water samples has been reported.[Citation5] However, PE containers are not recommended in speciation analysis due to rapid degradation of OrgHg, species inter-conversion, and surface adsorption.[Citation34] PTFE,[Citation36] FLPE,[Citation21,35] PET, Pyrex glass, and quartz [Citation37] containers are advisable for the storage of Hg sample solutions.[Citation34] Quartz is not economical, and hence cannot be used for routine analysis. PET apparatuses are inexpensive, recyclable, light, and don’t breakdown easily compared to glass containers. Furthermore, analyte loss due to adsorption on surface of the containers can be minimized using PTFE vessels.[Citation34]
In addition, sample preservation techniques such as sample pre-treatment, addition of various preservatives, and container pre-treatment, acid bath and repeated cleaning, are strongly recommended to minimize analyte loss during the storage time.
Water sample is usually filtered with 0.22 μm [Citation12,26,37–40] or 0.45 μm [Citation5,9,14,17,36,41–44] pore size membrane filters to avoid particulate materials and treated with acidic solutions of HNO3 [Citation9,36,45] or HCl [Citation17,21,35,38,46] to preserve the analytes and also to prevent the formation of microbes. The appropriate sample storage place and temperature has been reported to be in dark and 4 °C, respectively.[Citation5,12,14,17,21,39,44–46] In recent, SPME [Citation30,47] and DGT [Citation48–50] methods have shown to be the promising approach for the storage of Hg species in the analysis of environmental samples. In these techniques, the analytes are adsorbed and stabilized on the solid materials in the field which is easy to transport and store rather than in a liquid phase, and it also minimize the analyte loss.
Sediment and soil samples could be collected and stored in different manner. The PE containers cleaned by acid have been commonly used for the storage of homogenized and frozen sediment samples until analysis.[Citation4,22,46,51] Stainless steel and glass containers can be also used.[Citation4] Pre-treatment of the samples with an acidic solutions can preserve the analytes and can avoid the species inter-conversion.[Citation52] In addition, sediment samples should be covered with thin layer of overlaying water from the site and sealed with no headspace.[Citation51] Soil samples are usually packed in PE bags in cooler containing ice, and transported to laboratory,[Citation20,53,54] dried, homogenized, sieved and stored in high density PE containers at 4 °C.[Citation54] The drying conditions also have great influence on the Hg contents of soil samples and freeze-drying is highly recommended than air and oven drying.[Citation55]
The apparatuses employed in the analysis process (sample collection and storage containers, glassware, and filtration devices) should be pre-treated before use, usually soaked in 10% (v/v) HNO3 bath for 24 h and rinsed with purified water several times before use.[Citation5,17,36,39,44]
In general, the use of appropriate sampling procedure, transportation, and storage materials, pretreatment of the apparatuses, and sample pretreatment strategies should be known in speciation analysis of Hg in environmental samples. In situ preconcentration techniques like SPME and DGT seem promising as much of the processes are simplified and hence we recommend researchers to emphasis on their development and applications.
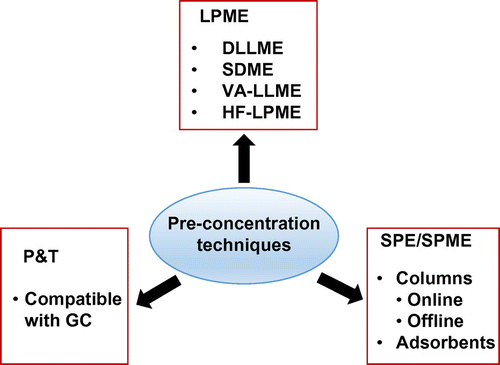
Pre-concentration techniques
Environmental samples usually contain trace (ng/L level) of Hg and its compounds.[Citation47] This indicates that its determination and speciation analysis in complex samples is a challenging job even using modern instruments with high sensitivity. Therefore, different sample preparation and pre-concentration strategies should be employed before the separation and detection stages. In fact, speciation analysis of Hg in water samples without pre-concentration has been reported.[Citation3] However, to report reliable analytical data, actually which depends on the instrumentation and sample type, either of the pre-concentration and pre-treatment approaches (Figure ) have been used. In this section, the strategies and their advancements are addressed.
Liquid phase microextraction
LPME is a miniaturized pre-concentration technique in which the target analytes are extracted to small volume of an extraction solvent (<100 μL). The features of this technique include high pre-concentration factor and minimum consumption of organic solvents. DLLME, Hollow fiber liquid phase microextraction (HF-LPME), SDME, and Vortex assisted liquid liquid microextraction (VA-LLME) are the most popular LPME techniques which have been employed for the enrichment of Hg chemical species in various environmental samples (Figure ).
Dispersive liquid liquid microextraction
Involves the extraction of analytes in to the extraction solvent dispersed in the sample using dispersive solvent and then separated for analysis. The technique has been widely applied as a pre-concentration purpose in speciation analysis of Hg in environmental samples.[Citation12,14,44,56] In DLLME, application of dispersive solvent, usually which dissolve in both sample and extraction solvent, have been practiced for complete transfer of the target analytes between the two phases which can be stated as one of its drawbacks since solvent consumption should be minimized in analysis from environmental point of view and it also decreases the distribution ratio from analytical perspective.[Citation57] To avoid this, other processes like ultrasonication [Citation58,59] and vortexing [Citation15,16,59] can be used for effective agitation of the mixtures. For high enrichment of the analytes, chelating agents like sodium diethyldithiocarbamate,[Citation12] APDC,[Citation16] dithizone,[Citation44,56] 1-(2-pyridylazo)-2-naphthol and L-cysteine [Citation14] have been used to complex the species from the matrix for DLLME. After DLLME, the samples could be injected to HPLC [Citation12,44,56] or CE [Citation14] for separation and then to detection and quantification techniques.
Jia et al. [Citation12] applied DLLME in speciation analysis of Hg2+ and MeHg in water samples using carbon tetrachloride and methanol as extraction and disperser solvents, respectively, and sodium DDTC as chelating agent followed by HPLC-ICP-MS. Accordingly, high enrichment factors, 350 and 138, and low LOD, 1.4 and 7.6 ng/L, were reported for Hg2+ and MeHg, respectively from 5 mL sample solution. Ethanol and benzyl bromide were used as a disperser and extraction solvents, respectively in speciation analysis of Hg2+, MeHg, EtHg, and PhHg in water samples.[Citation14] The method is based on the formation of Hg chemical species complex with 1-(2-pyridylazo)-2-naphthol to form hydrophobic chelates and L-cysteine to displace 1-(2-pyridylazo)-2-naphthol to form hydrophilic chelates with the analytes. Accordingly, enrichment factor, LOD, and RSD values were in the range of 46–547, 230–1790 ng/L, and 1.98–7.18%, respectively.
The use of ILs as an extraction solvent in DLLME is becoming common in recent reports. Namely, [HMIM][PF6] has been employed as an extraction solvent in speciation analysis of Hg2+, MeHg, and PhHg using dithizone (chelating agent) and methanol as dispersive solvent with HPLC-DAD and exhibited 320–1910 ng/L LOD values.[Citation56] Another IL, [C8MIM][PF6], as extraction solvent and acetone as dispersive solvent were used in the work of Song et al. and LOD values between 16 and 92 ng/L was reported for Hg2+, MeHg, EtHg, and PhHg speciation analysis using HPLC-HG-AFS.[Citation44]
In general, short extraction time (few minutes), high enrichment factor, inexpensive, consumption of low extraction solvents and its simplicity can be stated as the main advantages of DLLME. However, the use of dispersive solvent, impossible of automation and limited applicability (used for aqueous samples) could be stated as the main disadvantages. In recent, the use of relatively “green” solvents like ILs and DLLME without dispersive solvents have been reported which should be stated as recent advancements of the strategy. Therefore, future research works are expected to synthesize more “green” solvents and other approaches to avoid the use of dispersive solvents.
Vortex assisted liquid liquid microextraction and ultrasonic assisted liquid liquid microextraction
Are the two LPME techniques developed to avoid the consumption of dispersive solvent using vortex and ultrasonic mechanical emulsifications, respectively.
Leng et al. used VA-LLME for the speciation analysis of Hg2+, MeHg, and EtHg in sediment.[Citation15] Carbon tetrachloride and 1% (m/v) L-cysteine were consumed as collecting and extraction solvents, respectively. Low LOD values, 29, 57, and 28 ng/kg, respectively, for Hg2+, MeHg, and EtHg analytes, were obtained. In recent, we have selectively extracted Hg2+ in environmental waters using APDC (chelating agent) and zinc oxide nanofluid in ILs as extraction solvent.[Citation16] Even though Hg2+ was considered as the only target analyte, it is “green” technique (no dispersive solvent and nontoxic extraction solvent was used), fast, simple, sensitive (LOD, 0.019 ng/mL) and compatible with AFS detection. VA-LLME have been also used in the speciation analysis of Hg2+, MeHg, and EtHg in sediment samples using dithizone and [HMIM][PF6] as chelating and extraction solvents, respectively.[Citation60] Therefore, it can be said that the mechanical emulsification technique is a promising strategy to advance the application of VA-LLME in analysis of environmental solid samples.
The possibility of extraction of Hg2+ and CH3Hg+ by TSIL, methyltrioctylammonium thiosalcylate, as an extraction solvent without chelating agent has been reported using ultrasonic assisted liquid liquid microextraction (USA-LLME) adopted with CV-AAS [Citation58] which can be stated as further one step advancement in the field of LPME based sample preparation for speciation analysis. In addition, the speciation analysis was effected without dispersive solvent which is highly recommended as green analytical chemistry principles.
In summary, VA-LLME and USA-LLME are the two LPME techniques in which the use of dispersive solvent is completely avoided and the analyte is dispersed to the extraction solvent with mechanical emulsification techniques, vortexing, and sonication, respectively. Hence, these techniques are highly recommended in future works.
Hollow fiber liquid phase microextraction
A technique in which analytes are extracted to a liquid membrane sustained in the pores of hollow fiber and then to an acceptor solution,[Citation61] has been used as another pre-concentration option in speciation analysis of Hg.[Citation10,11,37,62] HF-LPME is simple and inexpensive technique which provides excellent analyte pre-concentration and sample cleanup. However, long extraction time and memory effect when reusing the hollow fiber could be stated as its shortcomings. The technique has been employed with HPLC-ICP-MS [Citation10,62] and CE-UV [Citation11,37] analysis techniques in Hg speciation analysis.
In the work of Chen et al. [Citation10] the application of HF-LLLME for enrichment of Hg2+, MeHg, EtHg, and PhHg in lake water and sediment samples was reported. In the method, using PAN as a chelating agent, the analytes were transferred to acceptor phase (Na2S2O3 solution) using toluene as an extraction solvent and injected to HPLC-ICP-MS for subsequent analysis. Accordingly, low LOD, below 5.6 ng/L, and precise (RSD, 5.6–10.6%) was reported. Moreno et al. [Citation62] used HF-LPME and reported <230 ng/L and <15% LOD and RSD values, respectively, prior to HPLC separation and ICP-MS detection for Hg2+, MeHg, and Se4+ speciation analysis. The method employed bromobenzene as an extraction solvent and the extraction time was reported to be 20 min and applied for the speciation analysis of the analytes in tap water, river water, estuarine water, and serum samples. Chen et al. [Citation37] also reported on speciation analysis of Hg2+, MeHg, EtHg, and PhHg analytes after pre-concentration with HF-LLLME using PAN, cyclohexane, and L-cysteine solution as a chelating agent, extraction solvent, and acceptor phase, respectively followed by CE analysis. Even though long extraction time (40 min) is required, the method exhibited LOD between 70–1000 ng/L, and repeatable data (RSD, 1.7–4.4%). In very recent, Li et al. [Citation11] proposed automatic dynamic HF-LLLME coupled to LVSS-CE-UV for simultaneous extraction of Hg2+, MeHg, and PhHg in lake water samples. In the method, 18-crown-6 was used to complex the analytes for their efficient extraction to chlorobenzene and then back extracted to 0.1% (m/v) 3-mercapto-1-propanesulfonic acid in aqueous solution followed by LVSS-CE-UV analysis. The method exhibited low LOD values (210–1290 ng/L and precise (RSD, 3.8–8.1) analytical results, and its applicability on environmental waters was ascertained using lake water samples and spiking recoveries of 87.9–111% was reported.
Single drop microextraction
Is another microextraction technique in which small volume of suspended extraction solvent (microdrop) is exposed to a sample solution for effective extraction process. SDME has been applied in speciation analysis of Hg due to its unique properties like reduced solvent consumption, permit automation, and provide high enrichment factor.[Citation63] Various forms of SDME, head space [Citation17] and direct immersion,[Citation18] have been employed in speciation analysis of Hg in environmental samples. Martinis and Wuilloud [Citation17] reported the application of CV-ILA-HS-SDME in speciation analysis of inorganic Hg and OrgHg, using suspended microdrop of tetradecyl(trihexyl)phosphonium chloride as extraction solvent and SnCl2 as reductant solution. Enhanced sensitivity (by a factor of 75), low LOD (10 ng/L) and good precision (RSD, 4.6%) was reported based on the technique. Complete separation and speciation analysis of Hg2+, MeHg, EtHg, and PhHg was reported with IL-SDME by using [C6MIM][PF6] and dithizone as extraction solvent and complexing agent, respectively before HPLC-DAD analysis.[Citation18] The authors reported LOD value of <22800 ng/L and precise result, RSD <11.6%, by using 4 μL drop volume from 12 mL sample solution.
In general, these LPME techniques enhance the enrichment factor and sensitivity, and reduce the amount of volatile organic solvents consumed for the pre-concentration purpose. Despite these qualities, most of the techniques still require the volatile organic solvents directly or indirectly. Therefore, invention of greener techniques for the same purpose is highly demanded.
Solid phase extraction and solid phase microextraction
These techniques involve the adsorption of target analytes onto the surface of solid materials, adsorbents, and then eluting with appropriate solvent for analysis. The approach is simple, appropriate and environmental friendly since it avoid the consumption of toxic organic reagents. For effective pre-concentration purpose, in the case of Hg analysis, the adsorbents could be pretreated with functional groups having high affinity towards Hg chemical species.[Citation64]
SPE with adsorbents like commercial C18 column,[Citation8] Amberlyst 36,[Citation9] polymer-supported ionic liquid,[Citation39] thiol modified magnetic silica sorbent,[Citation42] 1,5-bis(2-pyridyl) methylene thiocarbohydrazide functionalized mesoporous silica,[Citation65] and γ-mercaptopropyltrimethoxysilane modified Fe3O4@SiO2 magnetic nanoparticles [Citation66] have been investigated to retain various Hg species in environmental samples.
Our group developed a novel and simple SPE for speciation analysis of Hg2+, MeHg, and EtHg in environmental water samples,[Citation8] the analytes were complexed in C18 column with dithizone and eluted by 3 mL Na2S2O3 solution (100 mmol/L) before HPLC-ICP-MS determination. Based on the strategy, low LOD (3 ng/L) and precise data was obtained from 100 mL of water sample, and average recoveries of 93.7, 83.4, and 71.7% for MeHg, Hg2+, and EtHg, respectively, were obtained upon application on various environmental waters (tap, river, sea, and coal-washing wastewater). Türker et al. [Citation9] used Amberlyst 36 filled glass column (150 mm length and 10 mm inner diameter) to retain Hg2+ and MeHg in water samples and then sequentially eluted with 10 mL of 0.2 mol/L thiourea in 3 mol/L HCl and 10 mL of 0.1 mol/L HCl solutions, respectively before CV-AAS determination. The significance of this technique can be addressed as absence of complexing agents and satisfactory LOD values, 440 and 560 ng/L for Hg2+ and MeHg, respectively. The method was applied on tap and hot spring water samples and precise determination (relative error, <10%) of the analytes were reported. Even though the method seem simple and economical, its limited sensitivity compared to others [Citation8,39,40] could be stated. Escudero et al. [Citation39] made a column filled with IL impregnated Amberlite XAD-1180 polymer resin to quantitatively retain Hg2+ complexed with chloride ions (). The trapped species was reduced and eluted by 5% (w/v) SnCl2 solution in 2.4 mol/L HCl at a flow rate of 6 mL/min directly into the gas–liquid separator of CV-AAS for detection. By using the proposed method, the authors reported reasonable extraction efficiency (95%) and low LOD (2.4 ng/L) for Hg2+ from 40 mL sample. The authors tested the applicability of the method on mineral, tap, and river waters and obtained 96.2–103 and 97.3–101% recovery values which indicted the high capability of the method. In recent, Rodriguez-Reino et al. [Citation67] synthesized a novel ionic imprinted polymer adsorbent material and used in SPE for preconcentration of Hg2+, MeHg, and EtHg species in waters. The analytes were retained by loading 200 mL of sample solution on a column packed with 200 mg of the material and eluted with 4 mL solution containing 0.8% (v/v) 2-mercaptoethanol and 20% (v/v) methanol and investigated with HPLC-ICP-MS. Applicability of the method on real samples was assessed by analysis of sea waters. After chelating Hg2+ and MeHg analytes on mini-column filled with synthesized nanosorbent based on mesoporous silica functionalized with 1,5-bis(2-pyridyl)methylene thiocarbohydrazide (adsorption capacity, 173.1 μmol/g, pH 5), Trujillo et al. [Citation65] reported the preconcentration (time, 2 min) and speciation analysis of Hg in sea waters. The retained Hg2+ and MeHg analytes were sequentially eluted using 0.1% thiourea in 0.5% HCl and 0.2% HCl, respectively and investigated with ICP-MS. The analytical performance of the method was reported to be 11 and 4.7 enrichment factor, 4 and 2 ng/L LODs, and 2.6 and 2.8% precision (RSD, n = 10) for Hg2+ and MeHg, respectively. The method was successfully applied on sea water and 94.5–105% recovery values was reported. In addition to these qualities, additional separation stage was not required for the speciation analysis which drastically decreases the time of analysis and solvent consumption. Ma et al. [Citation66] reported the effective adsorption of Hg2+ and MeHg with γ-mercaptopropyltrimethoxysilane modified Fe3O4@SiO2 magnetic nanoparticles which can be eluted using 1.5 mol/L HCl containing 0.01% (m/v) thiourea for MeHg and 1.5 mol/L HCl containing 3% (m/v) thiourea for total Hg followed by ICP-MS determination and then the amount of Hg2+ was obtained from the difference of total Hg and MeHg.
SPME is another tiny SPE in which limited amount of the adsorbent and necessary solvents are consumed. Wang et al. [Citation40] prepared a POSS-SH modified with thiol group and used in SPME for pre-concentration of Hg2+ and MeHg in water samples. Using POSS-SH, the analytes were retained from 4 mL of sample solution and recovered with 400 μL thiourea (2% (m/v)) followed by AFS analysis. Accordingly, LOD of <4 ng/L and RSD <3.5% were reported along with satisfactory spiking recovery (98–101%) of the analytes from lake and sea waters. Polymeric ion-imprinted nanoparticles as adsorbent materials were prepared and used by Yordanova et al. [Citation68] in SPME technique for speciation of Hg2+ and MeHg in water samples. The method exhibited LOQ of 15 ng/L for Hg2+ and 20 ng/L for MeHg and spiking recovery in the range of 92–96% when applied on river, sea, and mineral water samples.
More or less, SPE and SPME are compatible with samples from various sources. Therefore, the techniques can be installed online with separation and detection techniques for Hg pre-concentration in the speciation analysis. Reports concerning this approach have been reviewed and discussed under online pre-concentration techniques.
Online pre-concentration techniques
Materials having high affinity towards Hg species can be installed online for pre-concentration purpose.[Citation24,26–28,38,69,70] Different types of column could be used to adsorb and concentrate the complexed analytes from the sample solutions [Citation24,26,38,70] and eluted with solvents like L-cysteine,[Citation24] a mixture of L-cysteine (0.02 mol/L) and 3% (v/v) nitric acid (4.0 mol/L),[Citation70] 2-merchaptoethanol,[Citation26,71] and 1.5 mM APDC in 75% methanol [Citation38] from the column. The technique is simple, fast, and exhibited high preconcentration factors.
Using IonPac CG5A guard column, Jia et al. [Citation24] developed simple, fast, and convenient online pre-concentration method for trace speciation analysis of Hg2+, MeHg, and EtHg in seawater by HPLC-ICP-MS. The strong electrostatic interaction between the analytes and CEC resin avoided the consumption of extraneous solvent, and increased the pre-concentration efficiency to an enrichment factor of 1250 from 30 mL sample solution. The LOD values (0.008–0.042 ng/L) and percent recovery values (89–102%) for all analytes from sea waters confirmed the high potential of the method. In recent, strong anion exchange guard column (Zorbax SAX) preconditioned with sodium 3-mercapto-1-propanesulfonate was used in speciation analysis of Hg+, Hg2+, MeHg, and EtHg.[Citation26] The adsorbed analytes were eluted with 5 mL of 3% (v/v) 2-mercaptoethanol before HPLC-ICP-MS analysis. The authors obtained satisfactory enrichment factor (>1000), LOD (<0.016 ng/L) and precision (RSD, <5%) and its applicability was tested using fresh water, drinking water, and sea water samples (recovery, 95–102%). Empty HPLC column packed with sulfur based sorption material (a mixture of thiosilica and thioureasilica) has been applied as pre-concentration column for speciation analysis of Hg2+ and MeHg.[Citation38] The determination of Hg in water samples at pg/L level was reported based on this technique when coupled to HPLC-ICP-MS and 1.5 mM APDC in 75% ethanol is used as a mobile phase and to desorb the retained analytes from the column. In addition to this, the sensitivity can be modified by loading large volume of sample solution (with 200 mL of sample solution, 0.04 ng/L LOD as Hg was reported) and spiking recovery values of 91.4–101.8% were reported for sea, river, and crude sewage water samples. A mini-column packed with Cys-fiber, cellulose fiber functionalized by L-cysteine, to significantly improves its Hg2+ and MeHg sorption capacity, was used for on-line separation and pre-concentration of Hg species in a sequential injection system.[Citation70] The retained species was reconstituted by a mixture of L-cysteine (0.02 mol/L) and nitric acid (1 and 4 mol/L for Hg2+ and MeHg, respectively), and analyzed with VG-AFS. With 1 mL of sample solution and 0.1 mL of eluent, LOD of 1 ng/L for Hg2+ and 3 ng/L for MeHg were reported. The method was used for the speciation analysis of the Hg2+ and MeHg in sea, river, and lake waters and recoveries of 99–104% were reported for both analytes.
Gao et al. [Citation69] proposed an online pre-concentration and in situ PVG in a coiled reactor for speciation analysis of ultratrace Hg2+ and MeHg. In the method, DDTC was used as a chelating reagent to form hydrophobic complex of Hg for on-line pre-concentration and as a reductant for in situ PVG and desorption of Hg from the coiled reactor. At optimal conditions, reliable (RSD, 4.5%) and <4 ng/L LOD were reported for Hg2+, and real samples (mineral and tap waters) and certified reference materials have been used to validate the developed method.
Different strategies were also developed for on-line treatment of the separated Hg species before feeding to the instrument for detection. Liu et al. [Citation27] developed PJDA source for AFS and coupled on-line with TLC. The authors tested the applicability on spiked lake water and 90–103% recovery were reported for the selected species. Using Fe3O4 magnetic nanoparticles, Ai et al. [Citation28] developed a novel, “green” and efficient post-column oxidation method to convert HG/CV inactive species to their active species on-line without microwave/UV irradiation and installed to HPLC-HG/CV-AFS to enable sensitive speciation analysis of Hg2+, MeHg, EtHg, and PhHg. The method is sensitive and reliable, and successfully applied on pond and river waters (recovery, 85–115%).
Purge and trap technique
P&T is a technique which involve purging a liquid/solid samples with inert gases such as argon,[Citation20] helium [Citation21,72] or nitrogen [Citation23,52] at ambient or slightly elevated temperature and trap the volatile analytes on Tenax,[Citation20,21,23,52,72] as a solid sorbent followed by desorption for GC analysis. It is the most effective and commonly used extraction/pre-concentration technique in speciation analysis of Hg by GC. It can be easily coupled to GC-ICP-MS [Citation20,21,23,52,72] and GC-AFS.[Citation23,73] Efficiency of the pre-concentration technique usually depends on the instrument configurations like column and detector types. Taylor et al. [Citation23] evaluated these effects and reported that capillary column and ICP-MS detector are superior than packed column and AFS detector, respectively. It was also reported that a reagent cleaning step and type of the derivatizing reagents play great role to enhance the pre-concentration purpose.[Citation73] Besides, the possibility of automation and capability of running numerous samples sequentially can be stated as the main advantages of the technique.
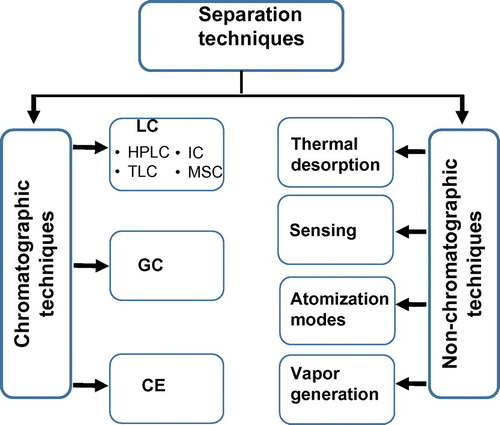
Separation techniques
Chromatographic techniques
In Hg speciation analysis, different chromatographic techniques can be used for the separation purpose. The three commonly used chromatographic techniques are LC, GC, and CE (Figure ).
Liquid chromatography
Various LC techniques, viz., HPLC,[Citation8,10,12,18,24,26,28,38,44,56,62,74] TLC,[Citation27] IC,[Citation25,39] and MSC [Citation43] have been used to separate the target analytes in speciation analysis of Hg. Recent reports carried out based on these separation techniques have been compiled and presented in Table .
Table 1. LC based separation of Hg chemical species in speciation analysis.
The main advantage of using HPLC separation in Hg speciation analysis is its compatibility with various detectors like ICP-MS,[Citation8,10,12,24,26,62,67,74] CV-AFS,[Citation15,28,38] HG-AFS,[Citation44] DAD,[Citation18,56] CV-AAS,[Citation9] and others like MS, AES, and UV. In addition, the most laborious, time and chemical consuming derivatization step is not necessary in HPLC separation unlike in GC. Reversed phase HPLC, with hydrophobic stationary phase C18 [Citation8,10,12,15,18,24,26,28,38,44,62,67] and C8 [Citation56,74] columns, are the most widely employed HPLC technique. For efficient separation, various mixtures of solvents have been used as a mobile phase (Table ).
Separation of Hg2+, MeHg, and PhHg chemical species with TLC in speciation analysis of Hg was reported.[Citation27] The analytes were allowed to react with a solution of complexing agents (0.02%, m/v, dithizone solution in chloroform in the report) to enhance the separation and to obtain clear and visible spots. The TLC separation was carried out on a glass backed silica gel HPTLC and developed in n-hexane/triethylamine (5:1, v/v) solution. A novel PJD atomization source was used in AFS detection of the differentiated analytes. As TLC is the simplest technique of its kind and the PJD atomization own simple setup, the coupling exhibit advantages like simple, cost effective, quick and ease of implementation in speciation analysis. It is also promising technique in development of portable instruments for field speciation analysis.
IC is another alternative technique suggested for analytes separation in speciation analysis. Chen et al. [Citation25], reported “green” and rapid separation method for Hg2+, MeHg, EtHg, and PhHg species in which the separation was carried out with two consecutive 12.5 mm strong cation exchange guard columns, with 2.0 mM L-cysteine or thiourea (pH 2.0) as the mobile phase, coupled to ICP-MS for detection. Accordingly, effective separation of the analytes was achieved within 2–2.5 min speciation time. After using lab made column as pre-concentration strategy, Türker et al. [Citation9] performed the separation of Hg2+ and MeHg using 10 mL of 0.2 mol/L thiourea in 3 mol/L HCl and 10 mL of 0.1 mol/L HCl eluents, respectively followed by addition of 1.5% (w/v) NaBH4 in 0.5% (w/v) NaOH as a reducing agent for CV-AAS investigation. A column packed with IL-impregnated resin was used to retain Hg2+ complex () by ion exchange mechanism and separate from OrgHg, which do not interact with the resin.[Citation39] The target analytes were eluted, underwent reduction by SnCl2 and CV-AAS detection. The amount of MeHg was obtained from the difference between total Hg and Hg2+.
A novel automatic approach using MSC and CV-AFS was investigated in speciation analysis of Hg2+, MeHg, and EtHg.[Citation43] The analytes were eluted by multi-isocratic elusion with 0.005% 2-mercapthoethanol in 240 mM ammonium acetate (pH 6) and acetonitrile as a mobile phase. The eluted targeting analytes were oxidized, reduced, separated from the reaction mixture and atomized for detection. Under optimized conditions, the reported LOD and RSD values were in the range of 30–110 ng/L and 2.4–4.0%, respectively.
Gas chromatography
Is a chromatographic technique which is effective in separating analytes and can be hyphenated to various detectors.[Citation75] Recent reports on speciation analysis of Hg based on GC separation from water, soil, and sediment samples have been tabulated and presented in Table .
Table 2. GC based separation of Hg chemical species in speciation analysis.
To employ GC for speciation analysis, derivatization is necessary for the safety of the analytical column and to convert the aqueous Hg analytes to volatile derivatives prior to GC. The commonly used derivatization processes are aqueous phase ethylation,[Citation21,73,75–77] propylation,[Citation73] and phenylation.[Citation20,45] Thermal desorption technique was also proposed by Kim et al. [Citation78] instead of such unfriendly processes.
The output of GC depends on the type of column and detector.[Citation23] Nevado et al. compared three hyphenated techniques, GC-MS, GC-IC-PMS, and GC-Pyro-AFS, for Hg speciation analysis in different matrices using aqueous ethylation in all cases with NaBEt4 ethylation reagent.[Citation75] All techniques found to be robust, precise (RSD, <5%) and sensitive (LODs, 2–6 pg for GC-pyro-AFS, 1–4 pg for GC-MS, and 0.05–0.21 pg for GC-ICP-MS). Besides, GC-MS and GC-ICP-MS offer the use of species-specific isotope dilution analysis and GC-Pyro-AFS is the most cost effective when compared to the other two systems. Better sensitivity (lower LOD) were also reported when GC-ICP-MS (0.06 pg) is compared with GC-AFS (0.25 pg) for the same analyte.[Citation23]
In recent, Pietilä et al. [Citation21] employed GC based separation for the determination of MeHg in humic rich natural water samples using capillary column with helium mobile phase followed by ICP-MS detection. Based on the technique, sensitive, and precise data, 0.05 ng/L and better than 10% values for LOD and RSD, respectively was obtained from 20 mL sample volume. Sharif et al. [Citation73] reported the possibility of Hg2+ and MeHg speciation analysis by P&T-GC-Pyr-AFS and GC-ICP-MS in water samples at trace level (LOD, <0.01 ng/L). NaEtB4 and NaBPr4 were consumed as derivatizing reagents. In general, standard addition and/or isotope dilution is recommended to avoid the matrix effect in GC, particularly for samples containing significant amounts of salts and/or organic matter.[Citation21,73] In addition, the application of DGT in HS-GC-AFS analysis, which works independent of ionic strength and in wide pH range (3–8), was reported in recent. Using DGT probe, consisting agarose (which has low affinity towards MeHg) as hydrogel and 3-merchaptopropyl functionalized silica resin as resin gel, Gao et al. [Citation77] investigated the trace level of MeHg in aquatic system. The extraction involves liberation of MeHg by simple acidic extraction followed by ethylation reaction with NaBEt4, before HS-GC-AFS analysis. Accordingly, as low as 0.08 ng/L and 5.5% LOD and RSD values were reported.
Kadlecova et al. [Citation76] developed an automated method for the determination of MeHg in sediments by using HS only or HS trap coupled to GC with AFS detection. The analytes were thermally decomposed and determined as Hg0. Based on the technique, 1.12 ng/L LOD value as Hg was reported. From similar matrix, Avramescu et al. [Citation52] proposed a simplified sample pre-treatment approach for the determination of MeHg by GC-ICP-MS. The method is based on the use of acid leaching-ion exchange-thiosulfate extraction to isolate and purify the target analyte from the samples. It bridges acid leaching and distillation derivatization procedures and hence is cost effective. LOD of 0.001 ng was reported for MeHg.
Capillary electrophoresis
Is another chromatographic technique which is extensively demonstrated for its application in various areas of analytical chemistry. Even though it has been indicated to be complementary tool to other separation strategies, the prospects of CE in Hg speciation analysis is most likely because of advantages like effective separation efficiency, speed of separation time, high resolution, lower consumption of sample and reagents, and no interaction between analytes and the capillary. Timerbaev [Citation79] reported a complete review on the development and applications of CE in speciation analysis over 20 years. There are recent reports employing CE in speciation analysis of Hg and has been compiled as presented in Table .
Table 3. CE based separation of Hg chemical species in speciation analysis.
Detectors like UV,[Citation11,37,80] DAD,[Citation14] and ICP-MS [Citation3,41] are widely used to detect the analytes separated on fused silica capillary. L-cysteine solution is widely used to form Hg chemical species complexes to absorb UV incase when UV detector is used.[Citation37] A gradient mixture of boric acid and methanol [Citation14,37,41] or boric acid and sodium tetra borate [Citation3] solutions can be used as a running separation buffer.
Non-chromatographic techniques
Different non-chromatographic approaches (Figure ) in which the target analytes are separated based on their chemical and physical behavior have been reported (Table ).
Table 4. Non-chromatographic techniques based separation of Hg chemical species in speciation analysis.
Atomization modes
Speciation analysis of Hg based on the advantage of atomization modes in AFS detection is one of the non-chromatographic techniques. The principle is based on the fact that the OrgHg species require more heat to form volatile species and Hg0 for its determination. Chen et al. [Citation70] carried out speciation analysis of Hg2+ and MeHg by using the advantage of atomization modes. Hg2+ was selectively reduced by a mixture of 0.01% (m/v) NaBH4 and 1 mol/L HNO3 and quantified by cold atomization AFS. After determining total Hg, reduced with a mixture of 2.5% (m/v) NaBH4 and 4 mol/L HNO3, and quantified by flame/heat atomization AFS, the concentration of MeHg was obtained from the difference. Using a mixture of KBH4 and HCl in the reduction process, our research group determined the amount of MeHg from Hg2+ and total Hg values obtained by flame less and flame AFS, respectively.[Citation81] Wang et al. also used the same approach to separately quantify the analytes in water samples.[Citation40] Hou’s group [Citation69] used in situ PVG in a coiled reactor for speciation analysis of Hg2+ and MeHg by controlling the desorption and PVG conditions before AFS detection. The speciation of the analytes is possible since MeHg can be more easily converted into Hg0 with the proposed method, PVG, than Hg2+ as decomposition of MeHg may yield CH3 radicals under UV radiation which assist the conversion. Martinis and Wuilloud [Citation17] invented a novel technique, CV-ILA-HS-SDME, for Hg species determination at trace levels. The concentration of Hg2+ was assessed by reducing it to volatile Hg0 with SnCl2 to separate from OrgHg followed by HS-SDME and ET-AAS detection. Total Hg concentration was determined after photoxidation of the pretreated sample (3 h irradiation using 15 W UV lamp) and amount of OrgHg was evaluated from the difference between total Hg and Hg2+concentrations. However, the OrgHg species was reported as single species in the report which can be stated as the main shortcoming of the technique.
Adsorption
Selective adsorption and separation of Hg2+ from other species, MeHg, was reported which might be considered as a promising strategy for the advancement of Hg speciation analysis.[Citation68] The report of Yordanova et al. [Citation68] indicated the use of polymeric ion-imprinted nanoparticles in SPME technique for selective separation of Hg2+. The adsorption and interaction of MeHg to the functional groups might be hindered due to its large size. A mixture of 2 mL 0.1 mol/L thiourea and 0.1 mol/L HCl can be used to elute the retained species before CV-AAS determination by using 0.04% (m/v) NaBH4 as reductant. Microwave digestion made the total Hg determination possible by CV-AAS after reducing with 0.4% (m/v) NaBH4, so that the difference is MeHg. As mentioned earlier, recent reports by Trujillo et al. [Citation65] and Ma et al. [Citation66] also shown the use of adsorbents for speciation analysis of Hg2+ and MeHg chemical species in water samples.
Sensing
The application of sensors in speciation analysis is the current research interest. Even though sensor application for Hg determination is not sensitive than other techniques which can be addressed as its main shortcoming, use of such techniques is clearly advantageous, don’t require physical separation of the target analytes, simple, environmental friendly, conserve the analytes, and shorten the analysis time and procedures. Xu et al. [Citation82] applied ZIF-7 and ZIF-60 as fluorescence sensing probe for speciation analysis of Hg2+ and MeHg in aqueous media and reported LOD values of 3000 and 6000 ng/L for Hg2+ and MeHg, respectively. SERS based sensor was also investigated for speciation analysis of Hg.[Citation83] Hg2+ and MeHg were differentiated with SERS by using 4-merchaptopyride (MPY) which coordinates with both analytes via its nitrogen atom as a chemoreceptor. The two complexes, MPY-Hg2+ and MPY-(Hg-CH)+ yield different spectra which make the speciation analysis practical. Based on the method, 100 and 1500 ng/L LOD values were reported for Hg2+ and MeHg, respectively. Chen et al. [Citation84] reported colorimetric nanosensor on the basis of the analyte-induced aggregation of Au NPs for Hg speciation analysis. By displacing Cu2+ from Cu-DDTC with Hg2+ due to the stronger interaction between Hg and DDTC, as well as the masking effect of EDTA, three OrgHg species (MeHg, EtHg, and PhHg) could be easily discriminated from Hg2+. The LODs, 2.9, 2.6, 8.5, and 30 nM for Hg2+, MeHg, EtHg, and PhHg is almost comparable to the conventional methods. However, difficulty to discriminate the OrgHg species and its less applicability in complex matrix, the method become invalid at high cations concentration (like sea water samples), and yield false results in the presence of protein molecules (like in urine samples) are the main weaknesses of this technique. Zhang et al. [Citation85] proposed cost effective and powerful molecular probe for selective detection of MeHg in the presence of Hg2+ and applied for their speciation analysis in aqueous media with a single kinetic trace exploiting their differential reactivity toward a single probe. Based on the strategy, low LODs, 0.0046 nM and 0.16 nM were reported for Hg2+ and MeHg, respectively.
Smartphones
The presentation of smartphones as analytical tool is emerging in recent. A recent report [Citation86] indicated the possibility of employing smartphone to digitally quantify Hg2+ using a plasmonic Au NPs and aptamer based colorimetric transmission assay. The authors quantified the amount of Hg2+ in water by using a two-color ratiometric method employing light-emitting diodes at 523 and 625 nm, where a custom-developed smart application was utilized to process each acquired transmission image on the same phone. Based on the method, LOD value of 3500 ng/L was reported. The selectivity of the method was assessed in the presence of various cations and confirmed to be specific to Hg2+. However, its applicability in the presence of other Hg cations was not articulated. For sure, we expect further innovations on this area as it is promising technique to replace those expensive, backbreaking, time consuming and sophisticated strategies.
Thermal desorption
Speciation analysis of three Hg chemical species, HgCl2, Hg bound to humic acid and HgS, in soil and sediment samples has been reported by Reis et al. [Citation87] using direct Hg analyser. Speciation was performed by the continuous thermal-desorption of the Hg chemical species (temperature range 76–770 °C). The thermo-desorption curves of each material showed a well-resolved peak at specific temperature intervals: 125–225, 100–250, and 225–325 °C for HgCl2, Hg bound to humic acid, and HgS, respectively. The method exhibit many qualities over conventional methods and is suitable fashion to carry out speciation analysis from such complex samples.
Isotope dilution analysis
ID is a method in which known amount of an enriched isotope of the analyte species is added to sample solution prior to pre-treatment procedures and the ratio of the added isotope to the naturally occurring isotopes is then measured.[Citation30] It improves the accuracy and precision of elemental speciation, and has been suggested as the primary method in speciation analysis of trace chemical species. Even though more isotopes of Hg exists, the stable isotopes reported for the preparation of isotope-enriched species are 198Hg,[Citation74] 199Hg,[Citation23,52] 200Hg,[Citation52,74] 201Hg,[Citation23,74] and 202Hg.[Citation52,74] ID has been used in speciation analysis of Hg chemical species with techniques like HPLC-ICP-MS,[Citation74,88] GC-MS,[Citation75] and GC-ICP-MS.[Citation52,73,75,89] The use of ID is highly suggested, specially incase when the matrix effect is high.[Citation73] Pietilä et al. used nitrogen distillation assisted ID for efficient separation of MeHg from non-filtered humic-rich natural water samples before GC-ICP-MS.[Citation21] The technique was also used with GC-ICP-MS in speciation analysis of Hg2+ and MeHg chemical species in sediment samples.[Citation89] The use of ID is obtained to be more effective with GC-ICP-MS than with GC-MS.[Citation75]
Detection techniques
Various detection techniques have been used in speciation analysis of Hg in environmental samples. The detectors can be selected based on their sensitivity, specificity, cost and time of analysis, and the nature of the samples we handle. Besides, the separation technique to be used also decide the type of the detector we use.
In LC separation techniques, CV-AAS,[Citation9,39] HG-AAS,[Citation74] CV-AFS,[Citation15,38,43] HG-AFS,[Citation44] CV/HG-AFS,[Citation28] PJDA-AFS,[Citation27] ICP-MS,[Citation8,10,12,24–26,62,74] and DAD [Citation18,56] detectors have been used. GC has been hyphenated to CV-AFS,[Citation76] pyro-AFS,[Citation73,75] MS,[Citation75] TOF-MS,[Citation78] ICP-MS,[Citation20,21,73,75] and FID [Citation45] detectors. ICP-MS,[Citation3,41,65] UV,[Citation11,37,80] and DAD [Citation14] are the commonly used detectors in CE. In non-chromatographic techniques, AFS,[Citation40,69,70,82] volatile species generation flame less/flame atomization AFS (VSG-FL/FA-AFS),[Citation81] ET-AAS,[Citation5,17] CV-AAS,[Citation68] ICP-MS,[Citation66] ICP-OES,[Citation82] and UV–vis [Citation83–86] detectors have been used.
The sensitivity of these detectors is dependent on sample introduction, which remains a frequent impediment to their optimum performance. Among these detectors, AFS and ICP-MS are the two detectors widely used in speciation analysis of Hg owing to high sensitivity and selectivity. In addition to these qualities, AFS is cost-effective and ICP-MS can be applied in ID analysis to enhance the precision and accuracy of the experimental results. The other quality of ICP-MS detector is its ability to provide isotopic information of the analyte. However, the derivatization process in AFS and long clean up procedure in ICP-MS detectors are in violation of the principle of green analytical chemistry.[Citation90] More or less, the detection techniques usually used for the speciation analysis of Hg in environmental analysis have been indicated under the pre-concentration and separation techniques.
Conclusions
Hg is one of the toxic elements among environmental contaminants. Particularly its organic forms are the most poisonous to living things. Hence, attention has been given to the speciation analysis of Hg chemical species rather than its total content in the environmental samples. There are advanced instruments which can be used for ease determination of Hg and its chemical species at very trace level, even at a single digit pg/L concentration. However, most of those instruments usually require different sample pre-treatment and pre-concentration approaches which involve time, labor and chemical consuming steps, especially in LC and GC techniques. Therefore, less reagent consuming and more reliable pre-treatment and pre-concentration techniques which are portable, robust, and simple to handle are encouraged.
Non-chromatographic techniques in which the target Hg chemical species is separated based on their chemical and physical behaviors are potentially greener than the chromatographic techniques. The strategies meet the aforementioned specifications; but most of them are Hg2+ species based and often have insufficiently low LOD. Therefore, the development of sensitive, simple and multi Hg species oriented methods are highly desirable.
Application of sensors like fluorescence, SERS, colorimetric nanosensor and smart phones in speciation analysis is clearly advantageous, don’t require physical separation of the target analytes, which shorten the analysis procedures. However, since these strategies are newly emerging and not well developed, the reported sensitivity and applicability are limited. Therefore, further innovation and advancements are expected to shine the utilities of sensor based discrimination of the target analytes in speciation analysis of Hg in environmental samples.
| Abbreviations | ||
| [C6MIM][PF6] | = | 1-hexyl-3-methylimidazolium hexafluorophosphate |
| [C8MIM][PF6] | = | 3-methyl-1-octylimidazolium hexafluorophosphate |
| AFS | = | atomic fluorescence spectrometry |
| PDC | = | ammonium pyrrolidine dithiocarbamate |
| CE | = | capillary electrophoresis |
| CPE | = | cloud point extraction |
| CV-AAS | = | cold vapor generation atomic absorption spectrometry |
| CV-ILA-HS-SDME | = | cold vapor ionic liquid assisted head space single drop microextraction |
| DAD | = | diode array detector |
| DDTC | = | diethyldithiocarbamate |
| DGT | = | diffusive gradient technique |
| DLLME | = | dispersive liquid liquid microextraction |
| ET-AAS | = | electrothermal atomic absorption spectrometry |
| EtHg | = | ethylmercury |
| FLPE | = | fluorinated polyethylene |
| FFF | = | flow field fractionation |
| GC | = | gas chromatography |
| GC-AFS | = | gas chromatography atomic fluorescence spectrometry |
| GC-ICP-MS | = | gas chromatography inductively coupled plasma mass spectrometry |
| GC-MS | = | gas chromatography mass spectrometry |
| GC-Pyr-AFS | = | gas chromatography pyrolysis atomic fluorescence spectrometry |
| HF-LLLME | = | hollow fiber liquid liquid liquid microextraction |
| Hg | = | mercury |
| HG/CV-AFS | = | hydride generation/cold vapor generation atomic fluorescence spectrometry |
| Hg0 | = | elemental mercury |
| HPLC | = | high performance liquid chromatography |
| HPTLC | = | high performance thin layer chromatography |
| HS-GC-AFS | = | head space gas chromatography atomic fluorescence spectrometry |
| IC | = | ion chromatography |
| ICP-MS | = | inductively coupled plasma mass spectrometry |
| ICP-OES | = | inductively coupled plasma optical emission spectrometry |
| ID | = | isotope dilution |
| IL-SDME | = | ionic liquid based single drop microextraction |
| IUPAC | = | International Union for Pure and Applied Chemistry |
| LC | = | liquid chromatography |
| LOD | = | limit of detection |
| LPME | = | liquid phase microextraction |
| LVSS | = | large volume sample stacking |
| MeHg | = | methylmercury |
| MSC | = | multi syringe chromatography |
| OrgHg | = | organic mercury |
| PAN | = | 1-(2-pyridylazo)-2-naphthol |
| P&T | = | purge and trap |
| P&T-GC-Pyr-AFS | = | purge and trap gas chromatography pyrolysis atomic fluorescence spectrometry |
| PE | = | polyethylene |
| PET | = | polyethylene terephthalate |
| PhHg | = | phenylmercury |
| PJDA | = | plasma jet desorption atomization |
| PJD-AFS | = | plasma jet desorption atomic fluorescence spectrometry |
| POSS-SH | = | polyhedral oligomeric silsesquioxane |
| PTFE | = | polytetrafluoroethylene |
| PVG | = | photochemical vapor generation |
| RSD | = | relative standard deviation |
| SDME | = | single drop microextraction |
| SERS | = | surface enhanced Raman spectroscopy |
| SnCl2 | = | stannous chloride |
| SPE | = | solid phase extraction |
| SPME | = | solid phase microextraction |
| TLC | = | thin layer chromatography |
| UV | = | ultraviolet |
| UV–vis | = | ultraviolet visible |
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the National Basic Research Program of China [2013CB43001]; the Strategic Priority Research Program of the Chinese Academy of Sciences [XDB14020101]; the Chinese Academy of Sciences [YSW2013A01], [YSW2013B01].
Acknowledgement
Meseret Amde acknowledges the support of CAS-TWAS President’s Fellowship for his PhD study.
References
- Rafaj P, Bertok I, Cofala J, et al. Scenarios of global mercury emissions from anthropogenic sources. Atmos. Environ. 2013;79:472–479.10.1016/j.atmosenv.2013.06.042
- Krystek P, Favaro P, Bode P, et al. Methyl mercury in nail clippings in relation to fish consumption analysis with gas chromatography coupled to inductively coupled plasma mass spectrometry: a first orientation. Talanta. 2012;97:83–86.10.1016/j.talanta.2012.03.065
- Zhao Y, Zheng J, Fang L, et al. Speciation analysis of mercury in natural water and fish samples by using capillary electrophoresis-inductively coupled plasma mass spectrometry. Talanta. 2012;89:280–285.10.1016/j.talanta.2011.12.029
- Sedlackova L, Kruzikova K, Svobodova Z. Mercury speciation in fish muscles from major Czech rivers and assessment of health risks. Food Chem. 2014;150:360–365.10.1016/j.foodchem.2013.10.041
- Sarıca DY, Türker AR. Speciation and determination of inorganic mercury and methylmercury by headspace single drop microextraction and electrothermal atomic absorption spectrometry in water and fish. Clean – Soil Air Water. 2012;40:523–530.10.1002/clen.201100535
- Gao Y, Shi Z, Long Z, et al. Determination and speciation of mercury in environmental and biological samples by analytical atomic spectrometry. Microchem. J. 2012;103:1–14.10.1016/j.microc.2012.02.001
- Korbas M, Macdonald TC, Pickering IJ, et al. Chemical form matters: differential accumulation of mercury following inorganic and organic mercury exposures in zebrafish larvae. ACS Chem. Biol. 2012;7:411–420.10.1021/cb200287c
- Yin YG, Chen M, Peng JF, et al. Dithizone-functionalized solid phase extraction-displacement elution-high performance liquid chromatography–inductively coupled plasma mass spectrometry for mercury speciation in water samples. Talanta. 2010;81:1788–1792.10.1016/j.talanta.2010.03.039
- Türker AR, Çabuk D, Yalçınkaya Ö. Preconcentration, speciation, and determination of mercury by solid phase extraction with cold vapor atomic absorption spectrometry. Anal. Lett. 2013;46:1155–1170.
- Chen B, Wu Y, Guo X, et al. Speciation of mercury in various samples from the micro-ecosystem of East Lake by hollow fiber-liquid–liquid–liquid microextraction-HPLC-ICP-MS. J. Anal. At. Spectrom. 2015;30:875–881.
- Li P, He M, Chen B, et al. Automated dynamic hollow fiber liquid–liquid–liquid microextraction combined with capillary electrophoresis for speciation of mercury in biological and environmental samples. J. Chromatogr. A. 2015;1415:48–56.10.1016/j.chroma.2015.08.062
- Jia X, Han Y, Liu X, et al. Speciation of mercury in water samples by dispersive liquid–liquid microextraction combined with high performance liquid chromatography–inductively coupled plasma mass spectrometry. Spectrochim. Acta, Part B. 2011;66:88–92.10.1016/j.sab.2010.12.003
- Liang P, Yu J, Yang E, et al. Determination of mercury in food and water samples by displacement-dispersive liquid–liquid microextraction coupled with graphite furnace atomic absorption spectrometry. Food Anal. Method. 2015;8:236–242.10.1007/s12161-014-9899-2
- Yang F, Li J, Lu W, et al. Speciation analysis of mercury in water samples by dispersive liquid–liquid microextraction coupled to capillary electrophoresis. Electrophoresis. 2014;35:474–481.10.1002/elps.201300409
- Leng G, Yin H, Li S, et al. Speciation analysis of mercury in sediments using vortex-assisted liquid–liquid microextraction coupled to high-performance liquid chromatography–cold vapor atomic fluorescence spectrometry. Talanta. 2012;99:631–636.10.1016/j.talanta.2012.06.051
- Amde M, Liu J-F, Tan Z-Q, et al. Ionic liquid-based zinc oxide nanofluid for vortex assisted liquid liquid microextraction of inorganic mercury in environmental waters prior to cold vapor atomic fluorescence spectroscopic detection. Talanta. 2016;149:341–346.10.1016/j.talanta.2015.12.004
- Martinis EM, Wuilloud RG. Cold vapor ionic liquid-assisted headspace single-drop microextraction: a novel preconcentration technique for mercury species determination in complex matrix samples. J. Anal. At. Spectrom. 2010;25:1432.10.1039/c004678g
- Pena-Pereira F, Lavilla I, Bendicho C, et al. Speciation of mercury by ionic liquid-based single-drop microextraction combined with high-performance liquid chromatography-photodiode array detection. Talanta. 2009;78:537–541.10.1016/j.talanta.2008.12.003
- Yuan C-G, Lin K, Chang A. Determination of trace mercury in environmental samples by cold vapor atomic fluorescence spectrometry after cloud point extraction. Microchim. Acta. 2010;171:313–319.10.1007/s00604-010-0429-7
- Mao Y, Yin Y, Li Y, et al. Occurrence of monoethylmercury in the Florida Everglades: identification and verification. Environ. Pollut. 2010;158:3378–3384.10.1016/j.envpol.2010.07.031
- Pietilä H, Perämäki P, Piispanen J, et al. Determination of methyl mercury in humic-rich natural water samples using N2-distillation with isotope dilution and on-line purge and trap GC-ICP-MS. Microchem. J. 2014;112:113–118.10.1016/j.microc.2013.10.002
- Li X, Wang Y, Li B, et al. Distribution and speciation of heavy metals in surface sediments from the Yangtze estuary and coastal areas. Environ. Earth Sci. 2012;69:1537–1547.
- Taylor VF, Carter A, Davies C, et al. Trace-level automated mercury speciation analysis. Anal. Methods. 2011;3:1143–1148.10.1039/c0ay00528b
- Jia XY, Gong DR, Han Y, et al. Fast speciation of mercury in seawater by short-column high-performance liquid chromatography hyphenated to inductively coupled plasma spectrometry after on-line cation exchange column preconcentration. Talanta. 2012;88:724–729.10.1016/j.talanta.2011.10.026
- Chen X, Han C, Cheng H, et al. Rapid speciation analysis of mercury in seawater and marine fish by cation exchange chromatography hyphenated with inductively coupled plasma mass spectrometry. J. Chromatogr. A. 2013;1314:86–93.
- Cheng H, Wu C, Shen L, et al. Online anion exchange column preconcentration and high performance liquid chromatographic separation with inductively coupled plasma mass spectrometry detection for mercury speciation analysis. Anal. Chim. Acta. 2014;828:9–16.10.1016/j.aca.2014.04.042
- Liu Z, Zhu Z, Zheng H, et al. Plasma jet desorption atomization-atomic fluorescence spectrometry and its application to mercury speciation by coupling with thin layer chromatography. Anal. Chem. 2012;84:10170–10174.10.1021/ac3028504
- Ai X, Wang Y, Hou X, et al. Advanced oxidation using Fe(3)O(4) magnetic nanoparticles and its application in mercury speciation analysis by high performance liquid chromatography–cold vapor generation atomic fluorescence spectrometry. Analyst. 2013;138:3494–3501.10.1039/c3an00010a
- Braaten HFV, de Wit HA, Harman C, et al. Effects of sample preservation and storage on mercury speciation in natural stream water. Int. J. Environ. Anal. Chem. 2014;94:381–384.10.1080/03067319.2013.823489
- Leopold K, Foulkes M, Worsfold P. Methods for the determination and speciation of mercury in natural waters – a review. Anal. Chim. Acta. 2010;663:127–138.10.1016/j.aca.2010.01.048
- Xu X, Li YF, Zhao J, et al. Nanomaterial-based approaches for the detection and speciation of mercury. Analyst. 2015;140:7841–7853.10.1039/C5AN01519G
- Parker JL, Bloom NS. Preservation and storage techniques for low-level aqueous mercury speciation. Sci. Total Environ. 2005;337:253–263.10.1016/j.scitotenv.2004.07.006
- Devai RD, Delaune WHP, Jr., Gambrell RP. Changes in methylmercury concentration during storage: effect of temperature. Org. Geochem. 2001;32:755–758.10.1016/S0146-6380(01)00039-0
- Yu L-P, Yan X-P. Factors affecting the stability of inorganic and methylmercury during sample storage. TrAC Trends Anal. Chem. 2003;22:245–253.10.1016/S0165-9936(03)00407-2
- Braaten HF, de Wit HA, Fjeld E, et al. Environmental factors influencing mercury speciation in Subarctic and Boreal lakes. Sci. Total Environ. 2014;476–477:336–345.10.1016/j.scitotenv.2014.01.030
- Rajabi HR, Shamsipur M, Zahedi MM, et al. On-line flow injection solid phase extraction using imprinted polymeric nanobeads for the preconcentration and determination of mercury ions. Chem. Eng. J. 2015;259:330–337.10.1016/j.cej.2014.08.025
- Chen C, Peng M, Hou X, et al. Improved hollow fiber supported liquid–liquid–liquid membrane microextraction for speciation of inorganic and organic mercury by capillary electrophoresis. Anal. Methods. 2013;5:1185–1191.10.1039/c2ay26214b
- Brombach C-C, Chen B, Corns WT, et al. Methylmercury in water samples at the pg/L level by online preconcentration liquid chromatography cold vapor-atomic fluorescence spectrometry. Spectrochim. Acta, Part B. 2015;105:103–108.10.1016/j.sab.2014.09.014
- Escudero LB, Olsina RA, Wuilloud RG. Polymer-supported ionic liquid solid phase extraction for trace inorganic and organic mercury determination in water samples by flow injection-cold vapor atomic absorption spectrometry. Talanta. 2013;116:133–140.10.1016/j.talanta.2013.05.001
- Wang W, Chen M, Chen X, et al. Thiol-rich polyhedral oligomeric silsesquioxane as a novel adsorbent for mercury adsorption and speciation. Chem. Eng. J. 2014;242:62–68.10.1016/j.cej.2013.12.063
- Li B-H. Rapid speciation analysis of mercury by short column capillary electrophoresis on-line coupled with inductively coupled plasma mass spectrometry. Anal. Methods. 2011;3:116–121.10.1039/C0AY00480D
- Xiang G, Li L, Jiang X, et al. Thiol-modified magnetic silica sorbent for the determination of trace mercury in environmental water samples coupled with cold vapor atomic absorption spectrometry. Anal. Lett. 2013;46:706–716.10.1080/00032719.2012.726679
- Guzmán-Mar JL, Hinojosa-Reyes L, Serra AM, et al. Applicability of multisyringe chromatography coupled to cold-vapor atomic fluorescence spectrometry for mercury speciation analysis. Anal. Chim. Acta. 2011;708:11–18.10.1016/j.aca.2011.09.037
- Song X, Ye M, Tang X, et al. Ionic liquids dispersive liquid–liquid microextraction and HPLC-atomic fluorescence spectrometric determination of mercury species in environmental waters. J. Sep. Sci. 2013;36:414–420.10.1002/jssc.201200571
- Sarafraz-Yazdi A, Fatehyan E, Amiri A. Determination of mercury in real water samples using in situ derivatization followed by sol–gel–solid-phase microextraction with gas chromatography-flame ionization detection. J. Chromatogr. Sci. 2014;52:81–87.
- Kim E, Noh S, Lee Y-G, et al. Mercury and methylmercury flux estimation and sediment distribution in an industrialized urban bay. Mar. Chem. 2014;158:59–68.
- Leopold K, Foulkes M, Worsfold PJ. Preconcentration techniques for the determination of mercury species in natural waters. TrAC Trends Anal. Chem. 2009;28:426–435.10.1016/j.trac.2009.02.004
- Pelcová P, Dočekalová H, Kleckerová A. Development of the diffusive gradient in thin films technique for the measurement of labile mercury species in waters. Anal. Chim. Acta. 2014;819:42–48.10.1016/j.aca.2014.02.013
- Fernández-Gómez C, Dimock B, Hintelmann H, et al. Development of the DGT technique for Hg measurement in water: comparison of three different types of samplers in laboratory assays. Chemosphere. 2011;85:1452–1457.10.1016/j.chemosphere.2011.07.080
- Pelcová P, Dočekalová H, Kleckerová A. Determination of mercury species by the diffusive gradient in thin film technique and liquid chromatography–atomic fluorescence spectrometry after microwave extraction. Anal. Chim. Acta. 2015;866:21–26.10.1016/j.aca.2015.01.043
- Zhang T, Kucharzyk KH, Kim B, et al. Net methylation of mercury in estuarine sediment microcosms amended with dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environ. Sci. Technol. 2014;48:9133–9141.10.1021/es500336j
- Avramescu ML, Zhu J, Yumvihoze E, et al. Simplified sample preparation procedure for measuring isotope-enriched methylmercury by gas chromatography and inductively coupled plasma mass spectrometry. Environ. Toxicol. Chem. 2010;29:1256–1262.
- Zhang YR, Wang RQ, Xue T, et al. Effects of soil properties and flooding on the mobility and transformation of mercury in a temperate riparian wetland. Soil Sediment Contam.: Int. J. 2015;24:191–205.
- Terzano R, Santoro A, Spagnuolo M, et al. Solving mercury (Hg) speciation in soil samples by synchrotron X-ray microspectroscopic techniques. Environ. Pollut. 2010;158:2702–2709.10.1016/j.envpol.2010.04.016
- Hojdová M, Rohovec J, Chrastný V, et al. The influence of sample drying procedures on mercury concentrations analyzed in soils. Bull. Environ. Contam. Toxicol. 2015;94:570–576.10.1007/s00128-015-1521-9
- Gao Z, Ma X. Speciation analysis of mercury in water samples using dispersive liquid–liquid microextraction combined with high-performance liquid chromatography. Anal. Chim. Acta. 2011;702:50–55.10.1016/j.aca.2011.06.019
- Spietelun A, Marcinkowski Ł, de la Guardia M, et al. Green aspects, developments and perspectives of liquid phase microextraction techniques. Talanta. 2014;119:34–45.10.1016/j.talanta.2013.10.050
- Stanisz E, Werner J, Matusiewicz H. Mercury species determination by task specific ionic liquid-based ultrasound-assisted dispersive liquid–liquid microextraction combined with cold vapour generation atomic absorption spectrometry. Microchem. J. 2013;110:28–35.10.1016/j.microc.2013.01.006
- Andruch V, Burdel M, Kocúrová L, et al. Application of ultrasonic irradiation and vortex agitation in solvent microextraction. TrAC Trends Anal. Chem. 2013;49:1–19.10.1016/j.trac.2013.02.006
- Leng G, Chen W, Wang Y. Speciation analysis of mercury in sediments using ionic-liquid-based vortex-assisted liquid–liquid microextraction combined with high-performance liquid chromatography and cold vapor atomic fluorescence spectrometry. J. Sep. Sci. 2015;38:2684–2691.10.1002/jssc.v38.15
- Asensio-Ramos M, Ravelo-Pérez LM, González-Curbelo MA, et al. Liquid phase microextraction applications in food analysis. J. Chromatogr. A. 2011;1218:7415–7437.10.1016/j.chroma.2011.05.096
- Moreno F, García-Barrera T, Gómez-Ariza JL. Simultaneous speciation and preconcentration of ultra trace concentrations of mercury and selenium species in environmental and biological samples by hollow fiber liquid phase microextraction prior to high performance liquid chromatography coupled to inductively coupled plasma mass spectrometry. J. Chromatogr. A. 2013;1300:43–50.10.1016/j.chroma.2013.02.083
- Jeannot MA, Przyjazny A, Kokosa JM. Single drop microextraction – development, applications and future trends. J. Chromatogr. A. 2010;1217:2326–2336.10.1016/j.chroma.2009.10.089
- Xiong X, Qi X, Liu J, et al. Comparison of modifiers for mercury speciation in water by solid phase extraction and high performance liquid chromatography–atomic fluorescence spectrometry. Anal. Lett. 2014;47:2417–2430.10.1080/00032719.2014.910667
- Trujillo SI, Alonso EV, Pavón JMC, et al. Use of a new enrichment nanosorbent for speciation of mercury by FI-CV-ICP-MS. J. Anal. At. Spectrom. 2015;30:2429–2440.10.1039/C5JA00335K
- Ma S, He M, Chen B, et al. Magnetic solid phase extraction coupled with inductively coupled plasma mass spectrometry for the speciation of mercury in environmental water and human hair samples. Talanta. 2016;146:93–99.10.1016/j.talanta.2015.08.036
- Rodríguez-Reino MP, Rodríguez-Fernández R, Peña-Vázquez E, et al. Mercury speciation in seawater by liquid chromatography–inductively coupled plasma-mass spectrometry following solid phase extraction pre-concentration by using an ionic imprinted polymer based on methyl–mercury–phenobarbital interaction. J. Chromatogr. A. 2015;1391:9–17.10.1016/j.chroma.2015.02.068
- Yordanova T, Dakova I, Balashev K, et al. Polymeric ion-imprinted nanoparticles for mercury speciation in surface waters. Microchem. J. 2014;113:42–47.10.1016/j.microc.2013.11.008
- Gao Y, Yang W, Zheng C, et al. On-line preconcentration and in situ photochemical vapor generation in coiled reactor for speciation analysis of mercury and methylmercury by atomic fluorescence spectrometry. J. Anal. At. Spectrom. 2011;26:126–132.10.1039/C0JA00137F
- Chen ML, Ma HJ, Zhang SQ, et al. Mercury speciation with L-cysteine functionalized cellulose fibre as adsorbent by atomic fluorescence spectrometry. J. Anal. At. Spectrom. 2011;26:613–617.10.1039/c0ja00185f
- Carneado S, Peró-Gascón R, Ibáñez-Palomino C, et al. Mercury(ii) and methylmercury determination in water by liquid chromatography hyphenated to cold vapour atomic fluorescence spectrometry after online short-column preconcentration. Anal. Methods. 2015;7:2699–2706.10.1039/C4AY02929A
- Pietilä H, Perämäki P, Piispanen J, et al. Determination of low methylmercury concentrations in peat soil samples by isotope dilution GC-ICP-MS using distillation and solvent extraction methods. Chemosphere. 2015;124:47–53.10.1016/j.chemosphere.2014.11.001
- Pirrone N, Sharif A, Monperrus M, et al, Determination of methyl mercury and inorganic mercury in natural waters at the pgL−1 level: intercomparison between PT-GC-Pyr-AFS and GC-ICP-MS using ethylation or propylation derivatization. E3S Web of Conferences. 2013;1. 09001. Available from: http://www.e3s-conferences.org/articles/e3sconf/pdf/2013/01/e3sconf_ichm13_09001.pdf
- Jagtap R, Krikowa F, Maher W, et al. Measurement of methyl mercury (I) and mercury (II) in fish tissues and sediments by HPLC-ICPMS and HPLC-HGAAS. Talanta. 2011;85:49–55.10.1016/j.talanta.2011.03.022
- Nevado JJ, Martin-Doimeadios RC, Krupp EM, et al. Comparison of gas chromatographic hyphenated techniques for mercury speciation analysis. J. Chromatogr. A. 2011;1218:4545–4551.10.1016/j.chroma.2011.05.036
- Kadlecova M, Daye M, Ouddane B. Improvement in determination of methylmercury in sediments by headspace trap gas chromatography and atomic fluorescence spectrometry after organic extraction and aqueous phase ethylation. Anal. Lett. 2014;47:697–706.10.1080/00032719.2013.848364
- Gao Y, De Craemer S, Baeyens W. A novel method for the determination of dissolved methylmercury concentrations using diffusive gradients in thin films technique. Talanta. 2014;120:470–474.10.1016/j.talanta.2013.12.023
- Kim Y-H, Kim K-H, Yoon H-O, et al. The application of gas chromatography-time-of-flight mass spectrometry to the analysis of monomethyl mercury at sub-picogram levels. Microchem. J. 2013;110:107–112.10.1016/j.microc.2013.03.002
- Timerbaev AR. Element speciation analysis using capillary electrophoresis: twenty years of development and applications. Chem. Rev. 2013;113:778–812.10.1021/cr300199v
- Li P, Zhang X, Hu B. Phase transfer membrane supported liquid–liquid–liquid microextraction combined with large volume sample injection capillary electrophoresis–ultraviolet detection for the speciation of inorganic and organic mercury. J. Chromatogr. A. 2011;1218:9414–9421.10.1016/j.chroma.2011.10.071
- Yin Y, Liu Y, Liu J, et al. Determination of methylmercury and inorganic mercury by volatile species generation-flameless/flame atomization-atomic fluorescence spectrometry without chromatographic separation. Anal. Methods. 2012;4:1122–1125.10.1039/c2ay05886c
- Xu F, Kou L, Jia J, et al. Metal-organic frameworks of zeolitic imidazolate framework-7 and zeolitic imidazolate framework-60 for fast mercury and methylmercury speciation analysis. Anal. Chim. Acta. 2013;804:240–245.10.1016/j.aca.2013.09.058
- Guerrini L, Rodriguez-Loureiro I, Correa-Duarte MA, et al. Chemical speciation of heavy metals by surface-enhanced Raman scattering spectroscopy: identification and quantification of inorganic- and methyl-mercury in water. Nanoscale. 2014;6:8368–8375.10.1039/c4nr01464b
- Chen L, Li J, Chen L. Colorimetric detection of mercury species based on functionalized gold nanoparticles. ACS Appl. Mater. Interfaces. 2014;6:15897–15904.10.1021/am503531c
- Zhang Z, Zhang B, Qian X, et al. Simultaneous quantification of Hg(2+) and MeHg(+) in aqueous media with a single fluorescent probe by multiplexing in the time domain. Anal. Chem. 2014;86:11919–11924.10.1021/ac503900w
- Wei Q, Nagi R, Sadeghi K, et al. Detection and spatial mapping of mercury contamination in water samples using a smart-phone. ACS Nano. 2014;8:1121–1129.10.1021/nn406571t
- Reis AT, Coelho JP, Rodrigues SM, et al. Development and validation of a simple thermo-desorption technique for mercury speciation in soils and sediments. Talanta. 2012;99:363–368.10.1016/j.talanta.2012.05.065
- Yin Y, Chen B, Mao Y, et al. Possible alkylation of inorganic Hg(II) by photochemical processes in the environment. Chemosphere. 2012;88:8–16.10.1016/j.chemosphere.2012.01.006
- Lusilao-Makiese JG, Tessier E, Amouroux D, et al. Seasonal distribution and speciation of mercury in a gold mining area, north-west province. S. Afr., Toxicol. Environ. Chem. 2014;96:387–402.
- Bendicho C, Lavilla I, Pena-Pereira F, et al. Green chemistry in analytical atomic spectrometry: a review. J. Anal. At. Spectrom. 2012;27:1831.10.1039/c2ja30214d