Abstract
Free Cu species in soils is a key issue to its bioavailability. However, predictive models for Cu speciation across a wide range of soils were still unavailable. In this study, Cu speciation in 34 contaminated soil samples were investigated via analytical technique and predictive models. The results showed that most of free Cu2+ was underestimated when using default log KCuFA and 65% active fulvic acid as inputs in models of WHAM VI and NICA-Donnan. The best prediction was found when using either adjusted active fulvic acid from 10% to 125% for WHAM VI or from 15% to 65% for NICA-Donnan model with the RMSE < 0.32 and r2 > 0.96. In contrast, NICA-Donnan demonstrated a slightly stronger binding for Cu than WHAM VI due to extra 26% of samples was underestimated. This work presents a comprehensive database of Cu speciation and an effective attempt of free Cu2+ prediction in a wide range of Chinese soils.
1. Introduction
It is well verified that the total metal concentration in soils is not a good indicator for its toxicity [Citation1,2] which is mainly controlled by the metal in soil solution [Citation3]. In solution systems, the free metal ion was hypothesized to control toxicity response of organisms [Citation4]. It is relatively easy to determine the free metal speciation in fresh waters by analytical techniques or chemical models considering that properties of humic substances are comparatively stable and easily measureable [Citation5]. In contrast, metal speciation in soils is more difficult to be confirmed due to the complexity, mainly in nature and sources of soil organic carbon (SOC) [Citation6,7].
Copper (Cu) as an important trace element has been studied carefully in aquatic and terrestrial systems around the world. Also, it is one of the most affinity metals to humic substances compared with others [Citation8–10]. It was reported that humic complexed form accounted for up to 99.9% of total Cu in solution [Citation3,8,11] indicating that Cu speciation is extremely sensitive to humic substances heterogeneity in quantity and quality [Citation12,13]. The dissolved organic matter (DOM) is a complex mixture of reactive molecules and its reactive part is dominated by humic substances, comprising humic acids (HA), fulvic acids (FA) and hydrophilic acid [Citation8], and its effect on Cu speciation in soil systems are still unclear.
Free Cu2+ activity is considered as an important factor on evaluation of Cu toxicity [Citation3,4,14] and could be determined via electrochemical analytical technique [Citation15–17] or chemical model prediction. Given the intrinsic differences among models, the most reliable and well-tested chemical speciation models are recognized as WHAM (V-VII) [Citation18,19] and NICA-Donnan [Citation20] which were developed to describe the electrostatic and specific interactions between the charged humic substances and ions. Although both of models are considered as providing good fit to most data, but due to their different formulations, their predictions do not necessarily converge [Citation21]. Also, a key question about evaluation of chemical speciation models is: how well do model predictions compare against speciation analytical technique? The uncertainties using these models are whether humic substances considered in the modelling are truly representative of the DOM in solution systems.
In the present study, Cu speciation was investigated based on a wide range of true soil pore water samples across China due to Chinese soils ranged widely in soil physicochemical properties [Citation22] and Cu speciation as well as its partition in these soils are scarcely reported. The Visual MINTEQ incorporated with NICA-Donnan [Citation23] and WHAM VI were used to estimate Cu speciation in soil pore waters and compare with results from a cupric ion-selective electrode (Cu-ISE) technique. The aim of this study is to find a good predictable model for free Cu2+ activity in Chinese soils and further applicability to the Cu risk assessment in terrestrial systems. It is very meaningful to undertake this exploration in Chinese soils not only because of the severely increasing pollution situation in China [Citation24] but also presenting a comprehensive database of Cu speciation and modelling worldwide.
2. Materials and methods
2.1. Soil samples
Seventeen soil samples from multiple agricultural locations across China were collected. The bulk soil properties were measured and displayed in Table . Total concentrations of Cu in soils were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES; Spectro Flame Modula, Spectro), following digestion with aqua regia [Citation25]. Soil pH was determined in a mixture of 1:5 soil:water (w/v). Total concentrations of carbon were determined using a LECO combustion analyzer (CNS 2000). The cation exchange capacity (CEC) was measured using unbuffered silver-thiourea method. Two contaminated Cu concentrations for each soil were obtained through adding designed Cu chloride (CuCl2) amounts. The concentrations selected were referenced to barley root elongation assay in 17 Chinese soils with toxicity thresholds ranging from EC5 (5% inhibition concentration) to EC90 (90% inhibition concentration) reported by Li et al. [Citation26], and a lower and higher inhibition concentrations of Cu to barley root elongation were adopted for each soil, respectively. All Cu contaminated soil samples were air-dried, sieved to 2 mm plastic screen and kept six months in room temperature before use.
Table 1. Soil solid-phase properties.
2.2. Extraction and analysis of soil pore water
Soil pore-water was extracted for contaminated soils using the method of Thibault and Sheppard [Citation27]. The soils were rewetted to maximum water holding capacity (MWHC) with Milli-Q deionized water and incubated for 24 h at 25 °C in 25-ml filtration tubes. Soils were then centrifuged at 3500 RCF for 45 min with the filtration tube inside a 50-ml centrifuge tube that contained a small spacer in the bottom [Citation1]. Extracted solutions were then centrifuged at 12,500 RCF for 45 min and filtered through a 0.45 μm filter. The total number of filtration tubes required generally varied from 10 to 15, giving a total volume of 60–100 ml, which was combined and then split into two duplicate samples for Cu-ISE and subsequent analysis. The extracted solutions were stored at 4 °C prior to analysis. The analysis of the solutions included dissolved organic carbon (DOC) (FormacsTOC/TNAnalyser), major cation concentration by ICP-OES [Citation25], and major inorganic anion concentrations (i.e. CO32−, Cl−, NO3− and SO42−) by ion chromatography (IC; Dionex4000i, AS9-HC column).
2.3. Measurement of free Cu2+ activity in pore water
The free Cu2+ activity was determined with an Orion 94–29 Cu-ISE combination electrode with an AgS/CuS double junction reference electrode at 20 °C. The CuS crystal of the cupric electrode was polished for 2 min with 2 grades of alumina (Al2O3) (first polished with 0.3 μm followed by the 0.05 μm) before use. The electrode was conditioned in a 500 ppb Cu standard (approximate pH 4) overnight before measurements for improving electrode sensitivity. The Cu-ISE calibration solutions contained 10 of different Cu concentrations as follows: 5 ml of Cu(NO3)2 (1 mmol L−1), 5 ml of iminodiacetic acid (0.01 mol L−1), 5 ml of potassium acid phthalate (KHC8H4O4) (0.025 mol L−1), 5 ml of KNO3 (0.1 mol L−1) in each standard, 10 of various volume of NaOH (0.02 mol L−1) (i.e. 1, 4, 6, 8, 9, 9.2, 9.3, 9.4, 9.7 and 10 mL) and corresponding Milli-Q water for a total volume of 50 mL to obtain 10 different concentrations of Cu standards with gradually decreased pH (between 9.3 and 4.3). The 10 standard solutions for the calibration were deemed sufficient to get a representative linear regression equation with r2 ≥ 0.99, and prepared the previous day to allow chemical equilibration overnight.
The free Cu2+ activities in pore waters were measured immediately after extraction, and in order of increasing free Cu2+ ion for better reproducibility and faster analysis. The pH was measured using micro electrode pH (Thermo Fisher Scientific Inc., MA, USA) [Citation28]. The electrode potential (EP, mV) determined by Cu-ISE was then plotted against free Cu2+ activity (expressed as logarithm-transformed base) calculated by Visual MINTEQ which was particularly added stability constants of iminodiacetic acid according to Rachou et al. [Citation16]. This showed a liner relationship with the following regression equation.The slope of the electrode response obtained in the calibration (28.6 mV/free Cu2+) was close to the theoretical Nernstian slope of 29.08 at 20 °C.
2.4. Calculation of Cu speciation by models
2.4.1. WHAM VI
The program was used to calculate free Cu2+ activities in soil pore water using measured parameters of pH, DOC concentration (g L−1), total soluble cations (i.e. Cu, Ca, Mg, K and Na) and anions (i.e. Cl−, ,
, and
) as inputs. Default parameters for active proton and metal-binding by fulvic acid (FA, 50% C) were used and 100% DOM in soil solutions was defined as FA. In order to improve the prediction of free Cu2+ activity, reasonable assumptions on DOM active fraction (%AFA) in models and binding constants of FA to Cu (log K) were taken, which was actually a recalibration of the model localization [Citation29]. The best agreement between measured and predicted free Cu2+ activity, expressed as log (free Cu2+ activity) was obtained according to the minimal root mean squared error (RMSE). The 65% AFA (35% FA as inert) as a starting value [Citation30] for modelling, the FA input (g L−1) for running the models was thus obtained by DOC × 2 × %AFA and dissolved cations and anions were expressed in total molar concentration (mol L−1). As colloidal Fe(OH)3 and Al(OH)3 can easily pass 0.45 μm filters, which result in measured ‘dissolved’ Fe and Al higher than the true condition that can interact with DOM and other ligands [Citation12,30], consequently, free Fe3+ and Al3+ activities were used as input and estimated from the solubility constants of colloidal Al(OH)3 (log Ksol = 8.5) and Fe(OH)3 (log Ksol = 3.0) at 20 °C [Citation31]. The temperature of 293 K and the atmospheric partial pressure of CO2 (PCO2) of 10−3.5 atm were used in models.
2.4.2. NICA-Donnan model
To ensure consistency, thermodynamic data (for inorganic metal complexes) from MINTEQA2 version 4 (http://www.epa.gov/ceampubl/mmedia/minteq/supple1.pdf) was used in databases of both humic substances. The temperature and PCO2was used the same values in the two models. The default stability constants and 65% AFA were also used as the start values for Cu speciation computation. Similar to WHAM VI, the %AFA and stability constants were adjusted to get the best agreement between measured and predicted free Cu2+ activity.
3. Results
3.1. Soil solid-phase properties
Selected soil solid-phase properties were shown in Table . The analytical data showed that there was a wide variation in soil characteristics. The soil pHs ranged from 4.93 to 8.90 (70% of soil with pH > 7.0), soil organic carbon content (SOC) from 0.60 to 4.28%, CEC from 4.77 to 29.5 cmol+ kg−1, and clay content from 10 to 66%. The Cu concentration varied from 31 to 1485 mg kg−1 with 48-fold difference (Table ).
Table 2. Soil pore water properties.
3.2. Soil pore water properties and Cu distribution
Selected soil pore water properties were listed in Table . Soil pore water pH varied from 4.39 to 8.07. The concentrations of DOC ranged from 102 to 765 mg L−1 with an average of 299 mg L−1. Total concentrations of soluble Cu changed within two orders of magnitude, from 1.10 to 48.9 μmol L−1 for 34 samples. The free Cu2+ activity (log (free Cu2+)) varied from −11.5 to −5.30 by up to six orders of magnitude. The percentage of free Cu2+ activity to total soluble Cu in solution was diverse from a value of <0.1 to 39.8% with an average of 2.73%. Most of fractions were less than 0.5% except for a number of acidic soils with soil pore water pH < 6.5.
The data in Figure showed that total soluble Cu in soil pore water was correlated significantly to total Cu concentrations in soil with r2 value of 0.55 and DOC in soil pore water to a lesser extent with r2 of 0.45. The stepwise regression analysis showed that the combined the factors of total Cu concentrations in soils and DOC contents in soil pore water could explain 63% variances of soluble Cu in soil pore water (Equation 1 in Table ).
Figure 1. Relationships between (a) soluble Cu in pore water and total Cu in soil. (b) soluble Cu and DOC in pore water.
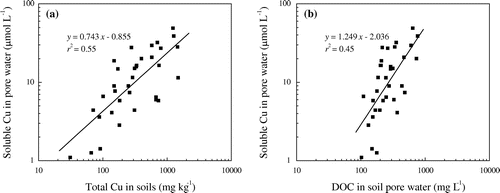
Table 3. The regression equations between Cu speciation and soil or soil pore-water properties.
The distribution of Cu between the solid and aqueous phases was described by a partition coefficient, Kd (solid phase concentration/solution phase concentration) [Citation32] were calculated and shown in Table . The range of Kd values varied from 126 to 2064 L kg−1. The regression analysis between Kd and soil or soil pore water properties based on logarithmic transferred data showed that dissolved Cu content and CEC were two important factor affecting Kd with an r2 value of 0.42 (Equation 3 in Table ) and it was difficult to find a better correlation while took all soil pHs into consideration. However, there was an improved empirical prediction on Kd using total Cu concentrations in soil, CEC and DOC concentration when only analyzed the soils with pH > 7.2 (Equation 4 in Table ). As soluble Cu was controlled by total Cu concentrations in soil and DOC in soil pore water, therefore, it was basically consistent for prediction on Kd for Equations 3 and 4.
3.3. Empirical prediction of free Cu2+activity using soil or soil pore water properties
The free Cu2+ activities were influenced mainly by two factors: soil pore water pH and total Cu concentration (Equations 5 and 6, Table ). The regressions in Table explained reasonable proportions (65%) of the variability in free Cu2+. Other soil parameters, including SOM, CEC and clay content were excluded in explaining the variability in free Cu2+. The percentage of free Cu2+ to total soluble Cu was negatively related to soil pore water pH with r2 of 0.64 (Equation 7 in Table ), which explained why the soils with low pH values had a higher percentage of free Cu2+ in pore water (3.64–39.8%). Also, Lofts et al. [Citation33] showed a strong relationship between log (free Cu2+ activity) and pH, which is similar to the results in our study. These results from Sauvé et al. [Citation34] (c.f. log (free Cu2+) = −1.40 pH + 1.70 log (total Cu) – 3.42, r2 = 0.85) and Vulkan et al. [Citation14] (c.f. log (free Cu2+) = −1.79 pH + 1.47 log (total Cu) + 0.53, r2 = 0.89) also confirmed the effect of pH and total Cu content on free Cu2+, despite a slight difference on coefficients of equations.
3.4. Prediction of Cu speciation using models
3.4.1. WHAM VI
The agreement between calculated and measured free Cu2+ activity was evaluated using match degree of corresponding log (free Cu2+ activity) values due to free Cu2+ activity varied up to 6 orders of magnitude in soil pore water and almost 80% of free Cu2+ activity accounted for less than 0.5% of total soluble Cu. As the data shown in Figure (a), when 65% of AFA and default binding constants log KCuFA as input values, 56% of free Cu2+ activity in soil pore water were severely underestimated by model especially for soils with lower pH values. In order to get the better agreement of free Cu2+ activity, adjusting AFA ratio between 10 and 30% for these 19 samples underrated and maintaining 65% AFA for the other 15 samples, the regression between measured and calculated free Cu2+ activity was significantly improved with decreased RMSE 0.42 and increased r2 value of 0.94. Also, there were 4 in 34 samples (i.e. S7 and S11) overestimated by model for free Cu2+ activity (see Figure (a)), enhancing the AFA from 65 to 100% or 125% for the four samples could benefit the fit further (Table ). The best agreement between measured and predicted free Cu2+ activity was achieved using adjusted AFA ratio ranging from 10 to 125% for all samples with the smallest RMSE 0.23 and the highest r2 of 0.98 (see Figure (b) and Table ). Generally, it was found that %AFA was positively correlated to soil pore water pH despite a few exceptions. It varied from 10 to 30% (15% on average) for samples with pore water pH < 6.5, from 10 to 65% (36% on average) for samples with pore water pH 6.5–7.5, from 30 to 125% (69% on average) for samples with pore water pH > 7.5. For the three samples using 125% AFA as inputs for modelling, free Cu2+ activity was all lower than 10−10 mol L−1.
Figure 2. Comparison of WHAM VI calculated free Cu2+ ion and measured free Cu2+ ion in 34 samples of soil pore water. Free Cu2+ activity was predicted with WHAM VI using either (a) 65% AFA and default log KCuFA, (b) adjusted AFA rate from 10% to 125% and default log KCuFA, (c) 65% AFA and adjusted log KCuFA from 1.0 to 2.3.
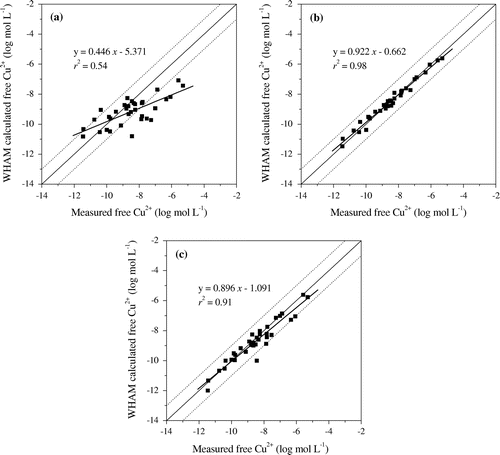
Table 4. Summary of the parameterization of WHAM 6 and NICA-Donnan parameters based on goodness-of-fit of measured free Cu2+ activity data.
Meanwhile, in order to weaken the binding of Cu to DOM for samples undervalued, log KCuFA was decreased from 2.1 to 1.0 and to intensify Cu affinity to DOM for samples overvalued, log KCuFA was increased from 2.1 to 2.3, the correlation between estimated and measured free Cu2+ activity also improved significantly with RMSE 0.49 and r2 0.91 (Figure (c) and Table ). Nevertheless, there was no consistent match between predicted and measured free Cu2+ activity on premise of adjusting site heterogeneity (ΔLK2) from 1.4 to 2.34.
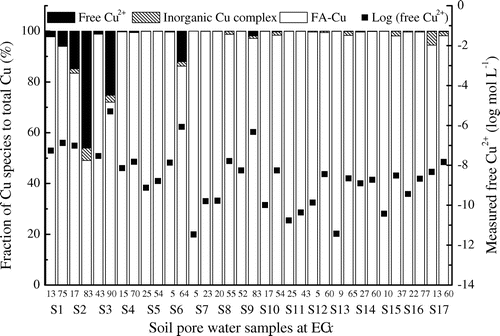
Due to contaminated concentrations of soils at between toxicity thresholds EC5 and EC90 according to Cu inhibition in barley root elongation, the fractions of Cu species to total soluble Cu were calculated by WHAM VI using the optimized AFA rate (10–125%) to analyze the impact of free Cu2+ concentration and its percentage on ECx (see Figure ). It was found that Cu bound to DOM was the predominant species (>98%) in the majority of soil pore waters obtained in the present study except for a number of low-pH soils (i.e. S1, S2, S3 and S6). The percentage of FA-Cu across 34 samples was insignificantly correlated to Cu inhibition to barley root elongation. In Figure , inhibition rate of barley root elongation at 5 and 90%, the log (free Cu2+) reached the maximum −5.3 (S3) and the minimum −11.5 (S7) mol L−1, respectively. The correlative relationship between inhibition rate (%) of barley root elongation and log (free Cu2+ activity) or percentage of free Cu2+ concentration showed that these two factors could explain variances of inhibition rate (%) by 43% and 15%, respectively.
Figure 3. The proportions of Cu species calculated by WHAM 6 and measured free Cu2+ activity (log mol L−1) in soil pore water for 34 samples at toxicity thresholds of ECx (x ranged from 5 to 90). A lower and higher inhibition concentrations of Cu to barley root elongation were adopted for each soil, respectively.
3.4.2. NICA-Donnan model
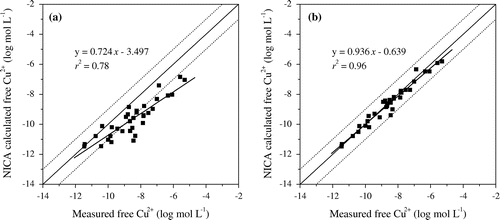
Similarly to WHAM VI, free Cu2+ activity was underestimated for 82% of samples (Figure (a)) by NICA-Donnan model when using 65% AFA and default constants as inputs. Decreasing %AFA to 30% for samples underestimated, the prediction was improved significantly (see Table ), but there were still some values far away from the 1:1 line. To continue to decrease %AFA to 15% for the 47% of samples with larger RMSE, the regression was extremely improved with the smallest RMSE 0.32 and the highest r2 0.96 (Figure (b)). Differently to WHAM VI, adjustment of binding constant of log KCuFA2 could not benefit the improvement on prediction of free Cu2+ activity to a significant level. Across the adjusted %AFA of 34 samples for the best fit, it was 15% for samples with pH < 7.0, and 15–65% (35% on average) for samples with pH > 7.0.
Figure 4. Comparison of NICA-Donnan calculated free Cu2+ ion and measured free Cu2+ ion in the 34 samples of soil pore water. Free Cu2+ activity was predicted with NICA-Donnan using either (a) 65% AFA and default log KCuFA2, (b) adjusted AFA rate from 15% to 65% and default log KCuFA2.
4. Discussion
4.1. Distribution of Cu in soil pore water and soil solid
It is showed in Table that soluble Cu was only correlative to total Cu in soil and DOC in soil pore water, which is partly similar to the result from Lamb et al. [Citation35] (c.f. log (soluble Cu) = −0.0261 log (total Cu in soil) + 0.254 pH + 0.635 log (SOC) − 2.813, r2 = 0.72) because pH was excluded from the regression equation in our study. Howbeit, Luo et al. [Citation36] also concluded that only SOC in soil could control soluble Cu content in soil and pH played a negligible effect on it. Similarly, all of these results confirmed the influence of SOC (or expressed as DOC) on soluble Cu concentrations in soils.
For solid-solution distribution of Cu, it was very difficult to obtain a linear relationship between Kd and soil pore water pH across 34 samples (Table ), which is dissimilar to studies from Sauvé et al. [Citation32] (c.f. log (Kd) = 0.21 pH – 0.51 log (SOM) + 1.75) and Vulkan et al. [Citation14] (c.f. log (Kd) = 0.34 pH – 0.58 log (DOC) + 1.74) due to pH as the top important factor to Kd in their studies. However, Luo et al. [Citation36] (c.f. log (Kd) = 1.04 log (total Cu) – 0.91 log (SOC) + 0.70, r2 = 0.57) and Lamb et al. [Citation35] (Kd of Cu was only correlated with DOC, r2 = 0.72) also determined pH was not an indispensible factor to Cu distribution, which is generally consistent to the results in our study. Also, Antoniadis and Golia [Citation37] expressed that Kd of Cu was significantly related to soil CEC instead of soil pH and SOC due to soil samples were strongly wreathed, given that CEC was also an effective predictor on Kd of Cu in our study (see Equations 3 and 4 in Table ), therefore, Cu distribution is supposed to be strongly soil-dependent.
4.2. Prediction of free Cu2+ activity using models
For both of models, the best prediction on free Cu2+ was obtained only when adjusting AFA rate to the bigger values with increase of soil pore water pH, which is possibly due to competition between H+ and Cu2+ ion for DOM binding sites resulting in most of DOM in acidic soils inefficient [Citation38,39]. The free Cu2+ activity at ECx varied by more than four orders of magnitude among the soils tested and was related to Cu concentration in tomato shoots with r2 of 0.41 [Citation3], which is quite similar to these results in our study. Although there was a significant correlation between free Cu2+ concentration and its toxicity to barley root elongation, however, free Cu2+ activity alone was not a good predictor of plant toxicity due to it only explained approximately 50% of variance of toxicity. Given most of soluble Cu as DOM-complexed form in soil pore water, presumably, a small proportion of Cu-organic complexes could be toxic along with free Cu2+. Guo et al. [Citation40] found that weakly bound Cu-organic complexes, such as malate-complexed Cu, was nearly 0.5-fold toxic to plant in contrast to free Cu2+ ion; and strongly bound Cu-organic complexes, such as EDTA-complexed Cu was completely nontoxic. Due to affinity of Cu to EDTA, malate and FA was 20.49 (log K), 4.53 (in Visual MINTEQ), and 2.16 (in WHAM VII), therefore, a small amount of FA-bound Cu could be possibly toxic to barley root elongation to explain another half variances of toxicity response. As for how much proportion of DOM-complexed Cu could contribute to its toxicity to plant should be further considered.
Dissolved Al and Fe concentrations in soil pore water were lower than the limit of detection of 0.25 mg L−1 and 0.5 mg L−1, respectively, except S1 and S2 with total soluble Al ranging from 0.58 to 1.06 mg L−1. Due to dissolved Al3+ could be considered as a critical parameter in free metal speciation [Citation41] and most of Al in Ferrosol with pH < 4.5 existed as free Al3+ ion [Citation42], the Cu2+ speciation was recalculated for both S1 and S2 using measured Al concentrations instead of calculated Al3+ activity from Al(OH)3 colloid as inputs in WHAM VI. The results showed that free Cu2+ activity derived from measured Al was approximately 0.36 order of magnitude closer to measured values when using adjusted %AFA (10–30%) as active DOM. Thus, the effect of Al3+ ion on free Cu2+ species in acidic S1 and S2 should be considered carefully. It was reported that for samples with >99% of Cu as DOM complex, the fraction of Cu species associated with AlO(OH) was negligible [Citation11]. In our study, most of samples with high percentage of FA-bound Cu (>98%), hence, impacts of Al and Fe on prediction of Cu speciation are extremely limited.
A large number of studies adopted between 50% and 100% AFA as assumption of active DOM to compute free Cu2+ concentration in solution [Citation14,30,43–45], it was changeable depending on DOM nature and quantity in systems. Amery et al. [Citation44] found that 65% AFA in WHAM VI could fit measured FA by UV-absorbance at 254 nm to a great extent, but Stockdale et al. [Citation17] concluded that 65% AFA in WHAM VII could cause free Cu2+ more than one order of magnitude higher than predicted values for 38% of samples. Vulkan et al. [Citation14] found that 69% AFA used in WHAM VI could ensure predicted free Cu2+ in soil solution closer to measured values, but this ratio was only consistent to that for alkaline soils, not acidic soils in our study. Some studies also used certain %HA as active DOM [Citation10,46] to predict Cu speciation. For example, Zhu and Guéguen [Citation11] adjusted FA:HA ratio from 1:0 to 1:1 to compute free Cu2+ concentration via WHAM VII, and found a very small effect on it when Cu bound to DOM was > 99%. However, Ponthieu et al. [Citation10] concluded that FA:HA ratio ranging from 1:0 to 0:1 influenced up to an order of magnitude of free Cu2+ concentration due to HA with log CuHA (2.38) complexed more Cu than FA with log CuFA (2.16). In our study, assuming HA in WHAM VI as active DOM instead of FA at the best optimized %AFA (10–125%), the predicted free Cu2+ activity was approximately 1.6 orders of magnitude lower than measured values even for these samples with higher proportion of organic complexes. Given that HA proportion in soil solutions was quite low [Citation8], hence, assuming FA as active DOM for modelling rather than HA is more realistic in this study.
In our study, the frequently-used ratio of 65% AFA generally caused the underprediction of free Cu2+ for both of models, and 10–125% AFA for WHAM VI and 15–65% for NICA-Donnan were the optimized ratio for best agreement of free Cu2+ activity. Groenenberg et al. [Citation47] found the humic substances fractions of DOM in the soil solution varied between 14% and 63% depending on the samples. Ren et al. [Citation8] also reported only 16–42% of DOM was humic substances and consisted mainly of FA as the most crucial affecting factor for Cu speciation. These results are very similar to those from NICA-Donnan predictions in this study. As to 125% AFA as input in WHAM VI for the three samples with measured free Cu2+ activity < 10−10.4 mol L−1, it was possibly related to either high pHs (7.77 and 7.79) for 2 samples and lower total soluble Cu (2.84 μmol L−1) for 1 sample. The model did not predict any concentrations to be below 10−13 mol L−1, but measured values could be as low as 10−16 mol L−1 [Citation19], adjusting %AFA to a range (>100%) could be a recalibration of the model intrinsically for the sample with quite low free Cu2+ activity. Besides, Amery et al. [Citation44] estimated that active DOM ranged from 28% to 152% in soil solutions in a soil profile collected over time and Djae et al. [Citation45] found that free Cu2+ speciation in 55 soil solutions was mainly overestimated when using default constant and 65% AFA in WHAM VII, and using an optimized AFA percentage (35–215%) and log KCuFA (1.84–2.46) could make the fit better. In this study, the adjusted AFA (10–125%) and binding constant log KCuFA (1.0–2.3) in WHAM VI also ensured the prediction maximally optimized. Differently, the parameters were mainly adjusted downward in our study but upward in study of Djae et al. [Citation45] from the start value of 65% AFA and default constants for the better estimation.
However, the difference in optimization of parameters in these models was connected to nature/heterogeneity of samples along with various analytical issues but more likely attributed to the DOM isolation procedure. The DOM affinity for metals could be affected by extraction procedures and soil pre-treatment methods [Citation48]. In this study, DOC concentrations (300 mg L−1 on average) were approximately 3-times higher than the values (100 mg L−1 on average) in study of Nolan et al. [Citation13] using different soil pre-treatment methods despite similar SOC variations. Meanwhile, Nolan et al. [Citation1] also found that predicted free Cu2+ by model using 70% AFA for Australian soils was underestimated about three orders of magnitude compared with measured results when adopted similar DOC extraction procedure (217 mg L−1 on average) to the present study. One-month soil incubation could decrease DOC concentration by 2–3 times and elevated its affinity for metals by 1–1.5-fold in contrast to 4 days’ incubation [Citation44]. All of soils were incubated only 24 h before extraction in our study, which probably accounted for the high DOC concentrations but low affinity capacity for Cu in soil pore water.
Generally, free Cu2+ prediction is more complicated than other trace element, e.g. Zn, Ni, Cd, and Co [Citation7,17,19] due to a large proportion (up to 99.9%) of soluble Cu was DOM-complexed form [Citation11]. The DOM nature is extremely crucial to Cu speciation and how to select a reliable technique to measure its nature is another issue. Amery et al. [Citation44] studied that specific UV-absorbance of DOM at 254 nm could represent aromaticity of DOM, and was positive proportionally to %AFA. Ahmed et al. [Citation21] used the three-dimensional fluorescence excitation emission matrix spectroscopy to evaluate the fractions of FA and HA in DOM and found using these fractions improved model predictability in contrast to using conventional 65% AFA assumption. Ren et al. [Citation8] adopted a rapid DAX-8 resin technique to fractionate DOM mainly into FA and HA, and found that measured FA concentration incorporated with NICA-Donnan to calculate free Cu2+ could fit the measured values much better than an assumed FA ratio. Further, the selection of analytical techniques for free Cu2+ activity could play a role on its modelling correction especially for samples with low [Cu]/[DOC] ratios [Citation5,19,21]. Tipping and Lofts [Citation19] described that Donnan membrane technique was more consistent to the model prediction than voltammetric technique (overrated) and ion exchange column method (underrated). However, Stockdale et al. [Citation17] assessed that voltammetry yielded the greatest fraction of agreement for Cu2+ activity than ISE technique and the competitive ligand method. There are substantial variations among different analysis techniques but no systematic bias from the model was observed across techniques.
4.3. Comparison between methods
Although both of models obtained excellent fit after adjusting %AFA or binding constant, there was a slight difference on free Cu2+ predictability for them. The NICA-Donnan model caused slightly higher bias for free Cu2+ prediction when using 65% AFA and default constants as input values for all samples in this study. Also, NICA-Donnan appeared to predict much stronger binding of DOC to Cu2+ than WHAM VI, because more than three-quarter samples were underestimated for NICA-Donnan and only half samples for WHAM VI when using 65% AFA as input. The AFA ratio was 125% as the maximum in WHAM VI but 65% in NICA-Donnan model to fulfill the prediction. Weng et al. [Citation30] compared free Cu2+ activity obtained with WHAM VI and NICA-Donnan with a variation of DOM composition, and concluded that WHAM VI was slightly better than NICA-Donnan with lower RMSE. Ahmed et al. [Citation21] found that NICA-Donnan showed 2-times higher RMSE values for free Cu2+ prediction than WHAM VII especially for samples with low [Cu]/[DOM] ratio. Sierra et al. [Citation49] confirmed that WHAM VII was especially practical for Cu speciation calculation where chemical speciation was driven basically for organic matter.
Besides, the difference in free Cu2+ prediction was further analyzed when using 65% AFA and default constant as inputs, it was less than 0.7 orders of magnitude between two models for the samples with pH <7.7, and close to one order of magnitude for samples with pH >7.7. Similarly, Ponthieu et al. [Citation10] evaluated the impact of DOM composition in Cu speciation in 36 calcareous soil solutions (pH 7.0–8.4) using WHAM VI and NICA-Donnan, and difference of free Cu2+ predicted by two models was enlarged with increase of pH. The difference of free Cu2+ prediction might be related to the distinction of intrinsic property in these two humic substances models. The NICA-Donnan model uses a bimodal, continuous distribution of affinities for protons and metal ions, whereas WHAM VI is based on a discrete set of sites, and phenolic contents for the two models are also negatively correlated [Citation18,20]. As a whole, predictability of models for Cu speciation is strongly dependent on humic substances nature and quantity in soil solution.
5. Conclusion
The Cu concentrations and free Cu2+ activities in pore waters for 34 Chinese soil samples were measured in order to determine its controlling factors and predictive models. Total Cu in soils and DOC in soil pore water were two key properties explaining soluble Cu content in soil pore water and its distribution. The free Cu2+ activity in soil pore water was controlled mainly by pore water pH and total Cu concentrations.
The predictability of WHAM VI and NICA-Donnan models for free Cu2+ was evaluated, which indicated that both models overestimated combination of DOM and Cu resulting in the lower free Cu2+ activities. While using varied AFA ratio from 10% to 125% for WHAM VI and from 15% to 65% for NICA-Donnan these two models provided preferable estimates of free Cu2+ activity with r2 > 0.96. Also, NICA-Donnan model showed a slightly stronger affinity for Cu2+ in pore water than WHAM VI considering that extra 26% of samples were undervalued on free Cu2+ activity. The impact of the composition of the DOM on the free Cu2+ prediction by models could vary up to two orders of magnitude depending on what ratio of AFA was used to represent DOM. Despite DOM nature was not measured by analytical techniques in this study, this work made a good attempt on Cu speciation prediction in a wide range of Chinese soils.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Natural Science Foundation of China [grant number 41401361]; the project of Research on Migration/Transformation and Safety Threshold of Heavy Metals in Farmland Systems [grant number 2016YFD0800406]; the Natural Science Foundation of Liaoning Province [grant number 2015020584]; and The High-level Leading Talent Introduction Program of GDAS.
Acknowledgements
The authors thank the financial support by the Natural Science Foundation of China (41401361), the project of Research on Migration/Transformation and Safety Threshold of Heavy Metals in Farmland Systems (2016YFD0800406), the Natural Science Foundation of Liaoning Province (2015020584) and The High-level Leading Talent Introduction Program of GDAS. Also the authors thank national long-term soil experimental stations in China for soil collection and Mike McLaughlin for his support and Cathy Fiebiger in CSIRO for technical assistance.
References
- Nolan AL, McLaughlin MJ, Mason SD. Chemical speciation of Zn, Cd, Cu, and Pb in pore waters of agricultural and contaminated soils using Donnan dialysis. Environ Sci Technol. 2003;37:90–98.10.1021/es025966k
- Li B, Zhang HT, Ma YB, et al. Relationships between soil properties and toxicity of copper and nickel to bok choy and tomato in Chinese soils. Environ Toxicol Chem. 2013;32:2372–2378.10.1002/etc.v32.10
- Zhao FJ, Rooney CP, Zhang H, et al. Comparison of soil solution speciation and diffusive gradients in thin-films measurement as an indicator of copper bioavailability to plants. Environ Toxicol Chem. 2006;25:733–742.10.1897/04-603R.1
- Lofts S, Criel P, Janssen CR, et al. Modelling the effects of copper on soil organisms and processes using the free ion approach: towards a multi-species toxicity model. Environ Pollut. 2013;178:244–253.10.1016/j.envpol.2013.03.015
- Unsworth ER, Warnken KW, Zhang H, et al. Model predictions of metal speciation in freshwaters compared to measurements by in situ techniques. Environ Sci Technol. 2006;40:1942–1949.10.1021/es051246c
- Benedetti MF, Van Riemsdijk WH, Koopal LK, et al. Metal ion binding by natural organic matter: from the model to the field. Geochim Cosmochim Acta. 1996;60:2503–2513.10.1016/0016-7037(96)00113-5
- Weng LP, Temminghoff EJM, Lofts S, et al. Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ Sci Technol. 2002;36:4804–4810.10.1021/es0200084
- Ren ZL, Tella M, Bravin MN, et al. Effect of dissolved organic matter composition on metal speciation in soil solutions. Chem Geol. 2015;398:61–69.10.1016/j.chemgeo.2015.01.020
- Pourret O, Lange B, Houben D, et al. Modeling of cobalt and copper speciation in metalliferous soils from Katanga (Democratic Republic of Congo). J Geochem Exploit. 2015;149:87–96.10.1016/j.gexplo.2014.11.011
- Ponthieu M, Pourret O, Marin B, et al. Evaluation of the impact of organic matter composition on metal speciation in calcareous soil solution: comparison of model VI and NICA-Donnan. J Geochem Exploit. 2016;165:1–7.10.1016/j.gexplo.2016.01.017
- Zhu Y, Guéguen C. Evaluation of free/labile concentrations of trace metals in Athabasca oil sands region streams (Alberta, Canada) using diffusive gradient in thin films and a thermodynamic equilibrium model. Environ Pollut. 2016;219:1140–1147.10.1016/j.envpol.2016.09.018
- Van Laer L, Smolders E, Degryse F, et al. Speciation of nickel in surface waters measured with the Donnan membrane technique. Anal Chim Acta. 2006;578:195–202.10.1016/j.aca.2006.06.070
- Nolan AL, Ma YB, Lombi E, et al. speciation and isotopic exchangeability of nickel in soil solution. J Environ Qual. 2009;38:485–492.10.2134/jeq2006.0275
- Vulkan R, Zhao FJ, Barbosa-Jefferson V, et al. Copper speciation and impacts on bacterial biosensors in the pore water of copper-contaminated soils. Environ Sci Technol. 2000;34:5115–5121.10.1021/es0000910
- Sauvé S, McBride MB, Hendershot WH. Ion-selective electrode measurements of copper(II) activity in contaminated soils. Arch Environ Contam Toxicol. 1995;29:373–379.10.1007/BF00212503
- Rachou J, Gagnon C, Sauvé S. Use of an ion-selective electrode for free copper measurements in low salinity and low ionic strength matrices. Environ Chem. 2007;4:90–97.10.1071/EN06036
- Stockdale A, Tipping E, Lofts S. Dissolved trace metal speciation in estuarine and coastal waters: comparison of WHAM/Model VII predictions with analytical results. Environ Toxicol Chem. 2015;34:53–63.10.1002/etc.2789
- Tipping E. Humic ion binding model VI: an improved description of the interactions of protons and metal ions with humic substances. Aquat Geochem. 1998;4:3–47.10.1023/A:1009627214459
- Lofts S, Tipping E. Assessing WHAM/model VII against field measurements of free metal ion concentrations: model performance and the role of uncertainty in parameters and inputs. Environ Chem. 2011;8:501–516.10.1071/EN11049
- Milne CJ, Kinniburgh DG, van Riemsdijk WH, et al. Generic NICA−Donnan model parameters for metal-ion binding by humic substances. Environ Sci Technol. 2003;37:958–971.10.1021/es0258879
- Ahmed IAM, Hamilton-Taylor J, Bieroza M, et al. Improving and testing geochemical speciation predictions of metal ions in natural waters. Water Res. 2014;67:276–291.10.1016/j.watres.2014.09.004
- Xiong Y, Li QK. The soils of China. 2nd ed. Beijing: Science Press; 1987. Chinese.
- Gustafsson JP. Visual MINTEQ, ver 2.61. Stockholm: Royal Institute of Technology, Department of Land and Water Resources Engineering; 2009. Available from: http://www.lwr.kth.se/English/OurSoftware/vminteq/
- Ministry of Environmental Protection of the People’s Republic of China. A national survey of soil pollution bulletin. Beijing; 2014. Chinese.
- Zarcinas BA, McLaughlin MJ, Smart MK. The effect of acid digestion technique on the performance of nebulization systems used in inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal. 1996;27:1331–1354.10.1080/00103629609369636
- Li B, Ma YB, McLaughlin MJ, et al. Influences of soil properties and leaching on copper toxicity to barley root elongation. Environ Toxicol Chem. 2010;29:1–8.
- Thibault DH, Sheppard MI. A disposable system for soil pore-water extraction by centrifugation. Commun Soil Sci Plant Anal. 1992;23:1629–1641.10.1080/00103629209368692
- Rayment GE, Higginson FR. Ion-exchange properties. In: Rayment GE, Higginson Fr, editors. Australian laboratory handbook of soil and water chemical methods. Melbourne: Inkata; 1992. p. 137–194.
- Cheng T, Schamphelaere KAC, Lofts S, et al. Measurement and computation of zinc binding to natural dissolved organic matter in European surface waters. Anal Chim Acta. 2005;542:230–239.10.1016/j.aca.2005.03.053
- Weng LP, Temminghoff EJM, van Riemsdijk WH. Aluminum speciation in natural waters: measurement using Donnan membrane technique and modeling using NICA-Donnan. Water Res. 2002;36:4215–4226.10.1016/S0043-1354(02)00166-5
- Oorts K, Bronckaers H, Smolders E. discrepancy of the microbial response to elevated copper between freshly spiked and long-term contaminated soils. Environ Toxicol Chem. 2006;25:845–853.10.1897/04-673R.1
- Sauvé S, Hendershot W, Allen HE. Solid-solution partitioning of metals in contaminated soils: dependence on pH, total metal burden, and organic matter. Environ Sci Technol. 2000;34:1125–1131.10.1021/es9907764
- Lofts S, Spurgeon DJ, Svendsen C, et al. Deriving soil critical limits for Cu, Zn, Cd, and Pb: a method based on free ion concentrations. Environ Sci Technol. 2004;38(13):3623–3631.10.1021/es030155 h
- Sauvé S, McBride MB, Norvell WA, et al. Copper solubility and speciation of in situ contaminated soils: effects of copper level, pH and organic matter. Water Air Soil Pollut. 1997;100:133–149.10.1023/A:1018312109677
- Lamb DT, Ming H, Megharaj M, et al. Heavy metal (Cu, Zn, Cd and Pb) partitioning and bioaccessibility in uncontaminated and long-term contaminated soils. J Hazard Mater. 2009;171:1150–1158.10.1016/j.jhazmat.2009.06.124
- Luo XS, Zhou DM, Liu XH, et al. Solid/solution partitioning and speciation of heavy metals in the contaminated agricultural soils around a copper mine in eastern Nanjing city, China. J Hazard Mater A. 2006;131:19–27.10.1016/j.jhazmat.2005.09.033
- Antoniadis V, Golia EE. Sorption of Cu and Zn in low organic matter-soils as influenced by soil properties and by the degree of soil weathering. Chemosphere. 2015;138:364–369.10.1016/j.chemosphere.2015.06.037
- Ritchie JD, Perdue EM. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim Cosmochim Acta. 2003;67:85–96.10.1016/S0016-7037(02)01044-X
- Daldoul G, Souissi R, Souissi F, et al. Assessment and mobility of heavy metals in carbonated soils contaminated by old mine tailings in North Tunisia. J Afr Earth Sci. 2015;110(110):150–159.10.1016/j.jafrearsci.2015.06.004
- Guo XY, Ma YB, Wang XD, et al. Re-evaluating the effects of organic ligands on copper toxicity to barley root elongation in culture solution. Chemical Speciation Bioavailability. 2010;22:51–59.10.3184/095422910X12632121425090
- Han S, Naito W, Hanai Y, et al. Evaluation of trace metals bioavailability in Japanese river waters using DGT and a chemical equilibrium model. Water Res. 2013;47:4880–4892.10.1016/j.watres.2013.05.025
- Xu RK, Ji GL. Influence of pH on dissolution of aluminum in acid soils and the distribution of aluminum ion species. Acta Pedologica Sinica. 1998;35:162–171. Chinese.
- Almås AR, Lombnaes P, Sogn TA, et al. Speciation of Cd and Zn in contaminated soils assessed by DGT-DIFS, and WHAM/Model VI in relation to uptake by spinach and ryegrass. Chemosphere. 2006;62:1647–1655.10.1016/j.chemosphere.2005.06.020
- Amery F, Degryse F, Degeling W, et al. The copper-mobilizing-potential of dissolved organic matter in soils varies 10-Fold depending on soil incubation and extraction procedures. Environ Sci Technol. 2007;41:2277–2281.10.1021/es062166r
- Djae T, Bravin MN, Garnier C, et al. Parameterizing the binding properties of dissolved organic matter with default values skews the prediction of copper solution speciation and ecotoxicity in soil. Environ Toxicol Chem. 2017;36:898–905.10.1002/etc.v36.4
- Xu J, Tan W, Xiong J, et al. Copper binding to soil fulvic and humic acids: NICA-Donnan modeling and conditional affinity spectra. J Colloid Interface Sci. 2016;473:141–151.10.1016/j.jcis.2016.03.066
- Groenenberg JE, Koopmans GF, Comans RNJ. Uncertainty analysis of the nonideal competitive adsorption−donnan model: effects of dissolved organic matter variability on predicted metal speciation in soil solution. Environ Sci Technol. 2010;44:1340–1346.10.1021/es902615w
- Kalbitz K, Solinger S, Park JH, et al. Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci. 2000;165:277–304.10.1097/00010694-200004000-00001
- Sierra J, Roig N, Giménez Papiol G, et al. Prediction of the bioavailability of potentially toxic elements in freshwaters. Comparison between speciation models and passive samplers. Sci Total Environ. 2017;605–606:211–218.10.1016/j.scitotenv.2017.06.136
