Abstract
Botulinum toxin type A (BoNT-A) was first isolated in 1946, and since then, several formulations have been developed and widely used to treat wrinkles by inducing muscle paralysis. This multicenter, double-blind, randomized, parallel-group, active-controlled phase 3 clinical trial was designed to evaluate the efficacy and safety of a newly developed BoNT-A formulation, BMI2006, in improving moderate to severe glabellar wrinkles and to compare with existing onabotulinumtoxin A (OBoNT) injections. A total of 276 subjects were enrolled and received 20 units of the randomized material, which was intramuscularly injected into five different locations on the forehead. The primary endpoint, assessed at 4 weeks, showed no statistically significant difference in the improvement rate of glabellar wrinkles between the two groups, with BMI2006 demonstrating non-inferiority to comparator BoNT-A. Secondary endpoints, evaluated by both treating investigators and independent investigators, also exhibited similar improvement rates throughout the study period. Both groups reported high levels of satisfaction with no statistical difference between the two groups. Safety evaluations indicated mild and transient adverse events, with no serious reactions observed. In conclusion, BMI2006 is an effective and safe BoNT-A for treating glabellar wrinkles with an expected duration of action between 8 and 12 weeks.
Introduction
Botulism is a condition caused by a neurotoxin produced by bacteria known as Clostridium botulinum. It results in severe muscle paralysis in the human body. Historically, it was recognized as a form of food poisoning, often associated with the consumption of spoiled sausages, hence the name “botulism,” derived from the Latin word “botulus,” meaning sausage. The discovery of the involvement of the bacterium Clostridium botulinum in botulism dates back to 1895. It was found that this pathogen played a crucial role in the development of botulism. The symptoms of botulism are not caused by the bacterium itself but by the toxin it produces. Botulinum toxin has gained significant attention as a biological weapon, leading to increased research on toxin isolation. Botulinum toxin is classified into seven different serotypes (A to G) and four toxin types (I to IV) based on serum and toxin characteristics [Citation1,Citation2].
In 1979, the US FDA granted limited approval for the use of Botulinum toxin type A (BoNT-A), in the treatment of strabismus, marking its first application in humans. Subsequently, BoNT-A received FDA approval in 1989 for the treatment of blepharospasm, strabismus, and hemifacial spasm. For the first time for cosmetic purpose, BoNT-A was approved by FDA for the treatment of moderate to severe glabella lines. As of now, cosmetic indications approved by the FDA are moderate to severe frown lines between the eyebrows (glabellar lines), moderate to severe crows’ feet lines, and moderate to severe forehead lines [Citation3,Citation4].
BoNT-A is widely accepted as a treatment for improving the glabellar lines, and several formulations of BoNT-A have been approved for this purpose. Glabellar lines, also known as frown lines, are wrinkles that appear between the eyebrows. These lines develop due to the contraction of facial muscles, including the corrugator, depressor supercilii, and procerus muscles. The actions of BoNT-A in improving glabellar wrinkles are achieved by inhibiting the release of the neurotransmitter acetylcholine from the presynaptic nerve terminal, thereby preventing facial muscle contraction. Previous clinical studies have demonstrated that BoNT-A is highly effective and has a good safety profile for correcting glabellar lines [Citation5–7].
This clinical trial is a phase 3 study designed to evaluate the efficacy and safety of BMI2006, a newly developed BoNT-A formulation. It aims to compare the effectiveness and safety of BMI2006 with existing onabotulinumtoxin A injections in adult patients who seek improvement in moderate to severe glabellar wrinkles.
Methods
Study design
This clinical trial was designed as a multicenter, double-blind, randomized, parallel-group, active-controlled phase III study and conducted at three tertiary university hospitals in Seoul, the Republic of Korea. The trial focused on adult patients with moderate to severe glabellar wrinkles. The study was approved by the hospital’s institutional review board (IRB no. 2020-006-442) and registered in ClinicalTrials.gov (NCT05531968). Written informed consent was obtained from all subjects. Those who voluntarily agreed to participate in the clinical trial and were qualified based on the selection and exclusion criteria were randomly assigned to either the test group (BMI2006 injection group) or the control group (existing comparator BoNT-A injection group) in a 1:1 ratio. As a comparator drug, onabotulinumtoxin A (Botox®, Allergan, Inc., Irvine, CA, USA), which approved by the FDA for moderate to severe glabellar lines, was used. BMI2006 was newly developed BoNT-A (onabotulinumtoxin A), which was produced from the same strain of Clostridium botulinum type A with identical genotype as the comparator BoNT-A, and it consists of toxin protein of the same molecular weight. The formulation consists of Clostridium botulinum type A toxin, human serum albumin, and sodium chloride.
Participants who were assigned random numbers received a clinical trial drug, totaling 20 units, administered injections of 0.1 ml each per site (4 U/0.1 ml) through intramuscular (IM) injection into five locations on the forehead. Subsequently, the efficacy and safety of the drugs were evaluated over a period of 16 weeks, with visits to the facility occurring at 4-week intervals (4 weeks, 8 weeks, 12 weeks, and 16 weeks).
In this clinical trial, we evaluated using the Facial Wrinkle Scale (FWS) 4-point scale, referencing the photo guidelines for assessing glabellar lines. The 4-point scale is as follows: At rest, 3 point (severe: wrinkles are very apparent), 2 point (moderate: wrinkles are apparent), 1 point (mild: wrinkles are slightly visible) and 0 point (none: no wrinkles are visible). When the forehead was fully furrowed, 3 point (severe: wrinkles are clearly observed and the base of the deepest wrinkle is not visible from the surface), 2 point (moderate: wrinkles are clearly observed and the base of the deepest wrinkle is visible from the surface), 1 point (mild: wrinkles are observed), and 0 point (none: no wrinkles are observed). In the classification of indications for FDA-approved BoNT for glabella lines, a 4-point scale is utilized, as well as in other phase 3 clinical trials of BoNTs for glabella lines [Citation4,Citation6,Citation7]. Consequently, for this particular clinical trial, a photo-guideline 4-point FWS score was developed and validated for use, and subsequently implemented in the trial.
Patient population
The inclusion criteria were as follows: 1) individuals aged 19 to 65, both male and female; 2) participants whose forehead wrinkles, as judged by the investigator during the screening and randomization processed, scored 2 or 3 points (indicating moderate to severe wrinkles on FWS 4-point scale) when the forehead was fully furrowed; 3) individuals who, after receiving a detailed explanation about this clinical trial, understood the information, voluntarily decided to participate, and provided written consent.
The exclusion criteria were as follows: 1) individuals with systemic neuromuscular junction disorders, such as severe myasthenia gravis, Lambert-Eaton syndrome, and amyotrophic lateral sclerosis; 2) those with facial nerve paralysis or a history of eyelid surgery; 3) significant facial asymmetry; 4) individuals for whom physical improvement of glabellar wrinkles was difficult, such as cases where wrinkles do not flatten when pressed with fingers; 5) participants who have taken drugs with systemic neuromuscular blocking effects within the 4 weeks prior to screening; 6) individuals who have received anticoagulants, antiplatelet agents, or systemic NSAIDs within 7 days prior to receiving the investigational drug; 7) those with skin infections, skin conditions, scars, or abnormalities at the injection site; 8) individuals who have undergone specific procedures on the glabellar area (including the forehead) within the last 12 months; 9) individuals who have received botulinum toxin preparations within 6 months before screening; 10) pregnant or lactating individuals; 11) individuals with allergies or sensitivities to the investigational drug or its components.
Treatments
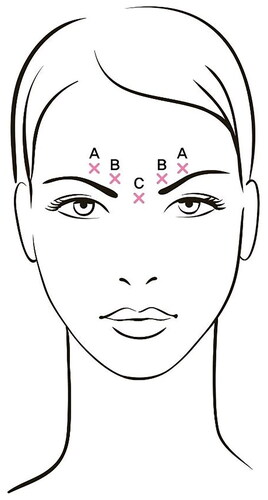
The dosages and administration methods for the investigational drug and the control drug were the same. The botulinum toxins 100 U were diluted with 0.9% sterile saline solution to a volume of 2.5 ml and administered through intramuscular injection with a 30-gauge needle into five locations on the glabellar area: A) the middle of the corrugator supercilii muscle (each eyebrow line muscle), 4 U (0.1 ml) for each side; B) the medial corrugator supercilii muscle (inner corner of each eyebrow line muscle), 4 U (0.1 ml) for each side; and C) the procerus muscle (between the eyebrows), 4 U (0.1 ml). This resulted in a total injection volume of 0.5 ml, corresponding to 20 U, for both the investigational and control drugs ().
Figure 1. A total of 20 units/0.5 ml of botulinum toxin A was injected intramuscularly in 0.1 ml at 5 points. (A) Middle of the corrugator supercilii muscle (each eyebrow line muscle). (B) Medial corrugator supercilii muscle (inner corner of each eyebrow line muscle). (C) Procerus muscle (between the eyebrows).
To reduce the risk of complications associated with eyelid drooping, especially in participants with prominent eyebrow depressor muscles, injections near the levator palpebrae superioris muscle (the muscle responsible for raising the upper eyelid) were avoided. When administering injections to the inner corner of the eyebrow line muscles and the central eyebrow area, a distance of least 1 cm was maintained from the eye and position the injection just below the browbone. Caution was exercised to avoid injecting into blood vessels. To prevent leakage below the edge of the eye, the thumb or index finger was firmly placed below the eye’s edge before injecting. During the injection process, the needle was oriented upward toward the middle, and the injection volume was measured accurately.
Efficacy assessments
The treating investigators assessed the glabellar wrinkles of subjects in both their resting (static) and expressive (dynamic) states using a 4-point Facial Wrinkle Scale, following the photo guidelines for glabellar wrinkle evaluation at baseline (0 week), 4 weeks, 8 weeks, and 12 weeks after treatment. The scale ratings were as follows: 0, no wrinkles; 1, mild wrinkles; 2, moderate wrinkles; and 3, severe wrinkles. Three independent dermatologist investigators assessed the glabellar wrinkles of subjects in both their resting and expression states using standardized digital photographs at each visit. These evaluators independently assessed the subject’s photos without exchanging opinions. If two primary evaluators agreed on their assessment results, those results were recorded. However, if the two primary evaluators had differing assessments, the assessment results of the third evaluator were used as the final assessment score for the subject’s glabellar wrinkles. An improvement was defined as an assessment score for glabellar wrinkles showing “an improvement of two points or more compared to the baseline.”
At each visit after treatment, the participants evaluated their current level of improvement in glabellar wrinkles compared to the baseline. The improvement scores were as follows: +4, completely improved (100% improvement); +3, significantly improved (75% improvement); +2 moderately improved (50% improvement); +1, slightly improved (25% improvement); 0, no change; −1, slightly worsened; −2, moderately worsened; −3, significantly worsened; and −4, completely worsened. An improvement for glabellar wrinkles was defined as an improvement score of +2, +3, or +4 points.
At each visit after treatment, the participants evaluated their current level of satisfaction with regards to glabellar wrinkles in comparison to the baseline. The satisfaction scores were as follows: 7, very satisfied; 6, satisfied; 5, slightly satisfied; 4, neither satisfied nor dissatisfied; 3, slightly dissatisfied; 2, dissatisfied; and 1, very dissatisfied. A satisfactory outcome for glabellar wrinkles was defined as a satisfaction score of 6 or 7 points.
The primary endpoint was the improvement rate of glabellar wrinkles, which was assessed by the treating investigators at 4 weeks after treatment (during expression). The secondary endpoints were as follows: 1) the improvement rate of glabellar wrinkles assessed by treating investigators at 8, 12, and 16 weeks after treatment (during expression); 2) the improvement rate of glabellar wrinkles assessed by treating investigators at 4, 8, 12, and 16 weeks after treatment (at rest); 3) the improvement rate of glabellar wrinkles assessed by independent investigators at 4, 8, 12, and 16 weeks after treatment (during expression); 4) the improvement rate of glabellar wrinkles assessed by independent investigators at 4, 8, 12, and 16 weeks after treatment (at rest); 5) the improvement rate of glabellar wrinkles assessed by subjects at 4, 8, 12, and 16 weeks after treatment; and 6) the satisfaction of subjects at 4, 8, 12, and 16 weeks after treatment.
Safety assessments
For safety evaluation, all adverse events that occurred during the clinical trial period were observed and monitored. Adverse events were evaluated according to the Severity Criteria for Adverse Drug Reactions provided by the Korea Institute of Drug Safety and Risk Management [Citation8]. Accordingly, severe adverse events were defined as those that render the subject unable to perform normal activities of daily living. Hematological and urine tests were conducted before treatment, as well as at 4 and 16 weeks after treatment. Vital signs and physical examinations were performed during each visit.
Neutralizing antibody formation tests were conducted both before and after treatment and the frequency of changes in neutralizing antibody levels (positive/negative) from the pretreatment screening to the end-of-treatment is presented for each treatment group. A mixture of human serum and Clostiridum botulinum toxin type A was intraperitoneally administered to ICR mice, and then we observed the mortality to determine whether neutralizing antibodies were produced.
Statistical analysis
The data obtained from the subjects in this clinical trial were categorized into three sets: Safety Set, Full Analysis Set (FAS), and Per-Protocol Set (PPS). For efficacy evaluations, PPS was used as the primary analysis group, and FAS was utilized as the supplementary analysis group. Safety evaluations were conducted on the Safety Set. Efficacy assessments in this clinical trial were conducted following the “Intention-To-Treat Principle,” while safety assessments were based on the actual administered treatment. All statistical tests were performed using a two-tailed test at a significance level (α) of 5%. However, non-inferiority tests were conducted as one-sided tests at a significance level of 2.5%, using the lower bound of the 95% two-sided confidence interval.
All statistical analyses were performed using SAS® (Version 9.4 or higher). Statistical values including the mean, standard deviation, percentile, and confidence intervals with decimal places were rounded to the nearest hundredth. P-values were reported up to four decimal places, and those lower than .0001 were indicated as “<.0001.”
Results
Demographics
Out of the 278 individuals who consented to participate in this clinical trial, 2 were excluded based on the results of the screening test. As a result, a total of 276 participants were randomly assigned either to the BMI2006 injection group (test group; 139 participants) or to the comparator BoNT-A injection group (control group; 137 participants). All 276 participants in this clinical trial received a single intramuscular injection of 20 U (0.5 ml), diluted in 0.9% sterile saline to a total volume of 2.5 ml. The injection was administered to five sites in the glabellar area, with two sites in the center and inner part of each eyebrow and one site in the frown line, using a 30 G needle.
In the test group, there were 27 male participants (19.42%) and 112 female participants (80.58%), while in the control group, there were 22 male participants (16.06%) and 115 female participants (83.94%). The average age of the test group was 46.90 ± 9.14 years, with participants ranging from a minimum age of 19 to a maximum age of 65 years. In the control group, the average age was 46.47 ± 9.31 years, with participants ranging from a minimum age of 20 to a maximum age of 65 years. The difference in demographics, including sex, age, weight, and height between the two groups was not statistically significant.
Primary end point
At 4 weeks after treatment, the test group showed a 78.83% (108 out of 137 participants) improvement rate, while the control group had a 83.09% (113 out of 136 participants) improvement rate. The difference in the “improvement rate of glabellar wrinkles as assessed by treating investigators at 4 weeks after treatment (during expression)” between the two groups was not statistically significant (p = .3706) ().
Table 1. The improvement rate of glabellar wrinkles at rest and expression assessed by investigators.
Furthermore, the difference in improvement rates between the two groups (test group – control group) was −4.26%, and the lower bound of the two-sided 95% confidence interval (CI) was −13.56%. Since the lower bound of the CI exceeded the predefined non-inferiority margin of −15%, it can be concluded that the test group demonstrated non-inferiority compared to the control group.
Secondary end points
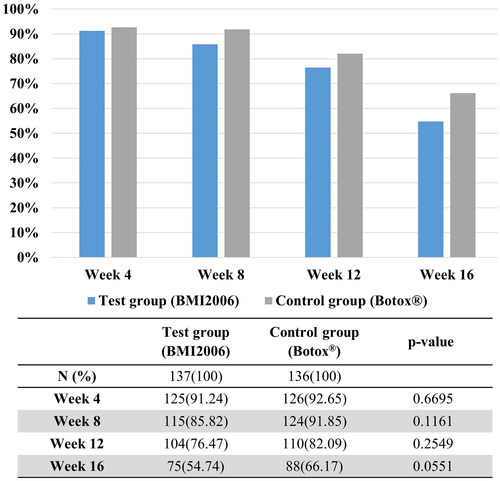
At 8 weeks after treatment (during the expression state), the test group showed a 66.42% (89 out of 134 participants) improvement rate, while the control group had a 65.93% (89 out of 135 participants) improvement rate. The difference in the ‘improvement rate of glabellar wrinkles as assessed by treating investigators at each visit after treatment’ was not statistically significant between the two groups. The details are shown in , and clinical photographs of representative subjects are presented in .
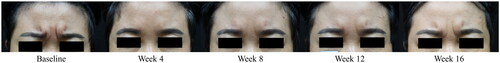
Figure 2. Representative photographs of changes in glabellar wrinkles before and after BMI 2006 injection. Improvement peaked at 4 weeks and gradually decreased over time but remained better than baseline.
In the assessment performed by independent investigators, the test group showed an 83.94% (115 out of 137 participants) improvement rate, while the control group had an 83.09% (113 out of 136 participants) improvement rate at 4 weeks after treatment. The difference in the “improvement rate of glabellar wrinkles as assessed by independent investigators at 4 weeks after treatment (during expression)” between the two groups was not statistically significant (p = .8493; ). In the assessment of glabellar wrinkle scale by independent investigators, no statistically significant difference was found in the improvement rate of glabellar wrinkles between the two groups at rest or expression states during each visit. The details are shown in .
Table 2. The improvement rate of glabellar wrinkles at rest and expression assessed by independent investigators.
The improvement rate of glabellar wrinkles assessed by the subjects was more than 90% at 4, 8, and 12 weeks after treatment, with the majority of subjects evaluating the improvement as at least “moderate” (). There was no statistically significant difference between the two groups in the improvement rate as assessed by the subject.
Figure 3. The percentage of glabellar wrinkles improved by 90% or more as assessed by the subject. There was no statistically significant difference between the two groups at any time point.
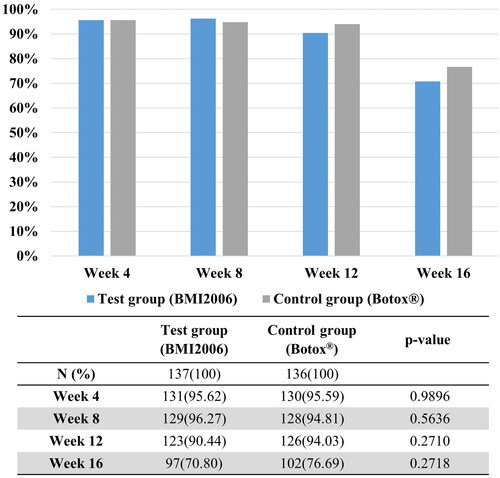
At 4 weeks after treatment, the percentage of participants satisfied with the improvement effect in the test group was 91.37% (127 out of 139 participants), while in the control group, it was 92.70% (127 out of 137 participants). By the 16-week time point, the percentage of satisfied participants in the test group was 54.74% (75 out of 137 participants), whereas in the control group, it was 66.17% (88 out of 133 participants). Throughout the entire follow-up period, more than half of the participants evaluated their satisfaction levels as “satisfied” or “very satisfied” (). There was no statistically significant difference between the two groups in satisfaction scores as assessed by the participants. At 16 weeks, the greatest difference was observed; however, it was still not statistically significant.
Safety evaluation
At each visit, adverse events were assessed, and an analysis of adverse drug reactions that could not be ruled out as having a causal relationship with the investigational drug was conducted. The results showed that in the test group, the incidence of adverse reactions was 8.63% (12 out of 139 participants), while in the control group, it was 5.84% (8 out of 137 participants). None of these adverse reactions were classified as severe. The most common adverse reactions were pyrexia, headache, injection site pain, and paresthesia. These adverse reactions were mostly mild and transient. Laboratory examinations, including complete blood cell count, liver function and renal function tests, coagulation tests, and urine analysis, mostly showed normal or clinically insignificant abnormalities at screening, 4 weeks and 16 weeks after treatment.
The analysis of the frequency of changes in neutralizing antibody levels from before (baseline, screening) to after (at 16 weeks) the administration of the investigational drug showed that in the control group, one participant (0.75%) exhibited a change from negative to positive in the formation of neutralizing antibodies. However, in all other participants from both groups, the test results for neutralizing antibody formation remained negative before and after administration. For subjects in the control group who exhibited neutralizing antibody formation, the efficacy was assessed using glabellar wrinkles severity scores. The primary efficacy evaluation results showed a change in severity score from severe to mild. In addition, there were no adverse events or safety issues related to this neutralizing antibody formation.
Discussion
Interest in facial wrinkle correction continues to rise, and BoNT-A has long been widely used for the improvement of facial wrinkles. In this phase 3 clinical study targeting moderate to severe glabellar wrinkles, the primary efficacy evaluation variable was assessed at the time of maximum effect. The test group showed a 78.83% improvement rate, while the control group showed an 83.09% improvement rate. The difference in the “improvement rate of glabellar wrinkles as assessed by treating investigators at 4 weeks after treatment (during expression)” was not statistically significant between the two groups. Furthermore, the test group demonstrated non-inferiority compared to the control group. The majority of phase 3 clinical trials, including those conducted for glabellar lines of BoNT-A (used as comparator drug in this study) undergoing FDA approval indication, utilized a 4-point scale for wrinkle assessment [Citation4,Citation6,Citation7,Citation9]. Additionally, there was previous report of phase 3 clinical trials employing a 5-point scale by adding “very severe” [Citation10]. Using the 4-point scale, three dermatologists underwent validation and training according to the photoguidelines for wrinkle grading. Through blinded evaluations, they were able to assess statistical differences.
The evaluation of the improvement rate of the BMI2006 in the clinical trial, specifically in relation to the improvement in glabellar lines at the 12-week time point as assessed by both the treating and independent investigators, shows a somewhat diminishing trend. Consequently, the effective duration of the BMI2006 for the clinical trial in treating moderate to severe glabellar wrinkles is considered to be between 8 to 12 weeks. Typically, the duration of action of botulinum toxin preparations is approximately 3 to 4 months, and the BMI2006 did not exhibit a significant difference in duration of action [Citation6,Citation11].
There have been previous study reports with longer-term follow-up than our study. A phase 3, double-blind, randomized, placebo-controlled study evaluated the efficacy of abobotulinumtoxin A on moderate to severe glabellar lines for up to six months. Similar to this study, efficacy evaluation was conducted using a 4-point scale and a responder was defined as a patient with severity grade 0 or 1 at maximum frown. On day 29, the proportion of responders was highest at 88.3%, and this effect was maintained up to 34.0% by day 113. The proportions of responders on day 148 and day 183 were 17.1% and 4.9%, respectively, indicating a significant decrease in efficacy as the study progressed from 4 to 6 months [Citation12]. Other phase 3 clinical studies evaluating the efficacy of BONT-A for moderate to severe glabellar lines also confirmed a decrease in efficacy from day 90 to day 150, consistent with the observed decline in efficacy [Citation11,Citation13].
Regarding the formation of neutralizing antibodies, it was confirmed that antibody formation occurred in 0.75% (1 participant) of the participants in this study. However, the efficacy of botulinum toxin was confirmed in one subject who neutralizing antibodies were detected. Therefore, further research is needed to determine the effectiveness of repeated treatments in patients who have previously developed neutralizing antibodies during BoNT-A treatment for wrinkles. There were previous large-scale phase III study results have confirmed the formation of neutralizing antibodies after repeated administrations of BoNT-A for the treatment of glabellar lines. In previous phase 3 study, a total of 768 participants received six repeated treatments over 17 months, and it was reported that none of the participants developed neutralizing antibodies to BoNT-A [Citation14].
In conclusion, BMI2006, administered at a single injection of 20 U, is comparable to preexisting BoNT-A 20 U in reducing glabellar wrinkles in patients with moderate to severe glabellar wrinkles. This assessment was made over a period of 16 weeks. Based on these positive findings regarding safety and efficacy, it is anticipated that BMI2006 can be widely utilized for the improvement of various types of facial wrinkles.
Ethics approval
The study was approved by the hospital’s institutional review board (IRB no. 2020-006-442) and registered in ClinicalTrials.gov (NCT05531968). All participants signed a photo release consent form authorizing the reproduction and distribution of any images collected during the study.
Disclosure statement
Joo-Sun Son and Eun-Kyoung Kim are employed at BMI KOREA CO., LTD. The other authors report there are no competing interests to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author, KBJ, upon reasonable request.
Additional information
Funding
References
- Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. Dermatol Surg Oncol. 1992;18:1.
- Carruthers A, Carruthers J. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin. Dermatol Surg. 1998;24(11):1189–8. doi: 10.1111/j.1524-4725.1998.tb04097.x.
- Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43(2 Pt. 1):249–259. doi: 10.1067/mjd.2000.105567.
- Won CH, Lee HM, Lee WS, et al. Efficacy and safety of a novel botulinum toxin type a product for the treatment of moderate to severe glabellar lines: a randomized, double-blind, active-controlled multicenter study. Dermatol Surg. 2013;39(1 Pt 2):171–178. doi: 10.1111/dsu.12072.
- Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285(8):28.
- Won CH, Kim HK, Kim BJ, et al. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxin A in the treatment of glabellar lines: a multicenter, randomized, double‐blind, active‐controlled study. Int J Dermatol. 2015;54(2):227–234. doi: 10.1111/ijd.12627.
- Kim BJ, Kwon HH, Park SY, et al. Double‐blind, randomized non‐inferiority trial of a novel botulinum toxin a processed from the strain CBFC 26, compared with onabotulinumtoxin A in the treatment of glabellar lines. J Eur Acad Dermatol Venereol. 2014;28(12):1761–1767. doi: 10.1111/jdv.12408.
- Korea Institute of Drug Safety & Risk Management. Adverse drug reaction assessment report. 1 ed. Korea Institute of Drug Safety & Risk Management. 2013:60–63.
- Wu Y, Zhao G, Li H, et al. Botulinum toxin type a for the treatment of glabellar lines in Chinese: a double-blind, randomized, placebo-controlled study. Dermatol Surg. 2010;36(1):102–108. doi: 10.1111/j.1524-4725.2009.01390.x.
- Kerscher M, Rzany B, Prager W, et al. Efficacy and safety of IncobotulinumtoxinA in the treatment of upper facial lines: results from a randomized, double-blind, Placebo-Controlled, phase III study. Dermatol Surg. 2015;41(10):1149–1157. doi: 10.1097/DSS.0000000000000450.
- Beer KR, Shamban AT, Avelar RL, et al. Efficacy and safety of prabotulinumtoxinA for the treatment of glabellar lines in adult subjects: results from 2 identical phase III studies. Dermatol Surg. 2019;45(11):1381–1393. doi: 10.1097/DSS.0000000000001903.
- Ascher B, Rzany B, Kestemont P, et al. Liquid formulation of AbobotulinumtoxinA: a 6-month, phase 3, double-blind, randomized, placebo-controlled study of a single treatment, ready-to-use toxin for moderate-to-severe glabellar lines. Aesthet Surg J. 2020;40(1):93–104. doi: 10.1093/asj/sjz003.
- Rzany BJ, Ascher B, Avelar RL, et al. A multicenter, randomized, double-blind, placebo-controlled, single-dose, phase III, non-inferiority study comparing PrabotulinumtoxinA and OnabotulinumtoxinA for the treatment of moderate to severe glabellar lines in adult patients. Aesthet Surg J. 2020;40(4):413–429.
- Monheit GD, Cohen JL; Reloxin Investigational Group. Long-term safety of repeated administrations of a new formulation of botulinum toxin type a in the treatment of glabellar lines: interim analysis from an open-label extension study. J Am Acad Dermatol. 2009;61:421–425.