Abstract
Purpose: Radiation biology is a branch of the radiation research field which focuses on studying radiation effects in cells and organisms. Radiation can be used in biological investigations for two, mutually non-exclusive reasons: (1) to study biological processes by perturbing their functioning (qualitative approach) and (2) to assess consequences of radiation-induced damage (quantitative approach). While the former approach has a basic research character, the latter has an applied character that is driven by needs of medical applications and radiological protection. Radiation protection biology is defined in the sense of the second approach. The aim of the article is to provide a historical review of how radiation protection biology developed and how it influences radiological protection.
Conclusions: While radiobiological investigations started immediately after the discovery of X-rays, the qualitative approach dominated until the end of World War II. After 1945, the nuclear weapons race and nuclear energy programs initiated quantitative radiobiological research. Radiation protection biology does not provide results from which radiation risks can be directly derived. Rather, it provides data that is necessary for understanding the nature of risks. Most recent years have seen, especially in Europe, a growing interest in coordinated studies on the effects of low radiation doses.
Introduction
The starting dates of some scientific fields are difficult to define. A good example is astronomy. People have always been observing movements of celestial objects, so how can we say when the observations turned from amateurish to scientific? The situation is different with the radiation research field. It started precisely on 8 November 1895 when Wilhelm Conrad Röntgen discovered X-rays (Röntgen Citation1895; Farmelo Citation1995).
The importance of Röntgen’s discovery for the field of medical diagnostics was realized immediately. By early 1896, X-rays started to be widely used for probing the human body (Rowland Citation1896; Roberts Citation1897; Farmelo Citation1995). There was no reason to assume that radiation exposure was associated with any harm. Indeed, how could harm arise from an agent that could not be appreciated by the senses? X-rays were regarded as ‘invisible light’, a concept created by Röntgen himself, who tested it by looking directly into the X-ray beam (Röntgen Citation1895). Moreover, radiation was proclaimed to be the source of energy which was responsible for driving biochemical reactions in the body. This belief was boosted by the discovery of radioactivity by Henrie Becquerel and Marie and Pierre Curie along with the recognition that waters in many health spas were radioactive (Macklis Citation1996).
Although injuries in the form of skin burns and tissue necrosis became apparent within the first few years after discovery of radiation and radioactivity (Codman Citation1902; Pitkin Citation1904; Martland Citation1929; Martland and Humphries Citation1929; Lambert Citation2001), it was believed that radiation is safe provided that the exposure is kept below a level which causes injuries (Walker Citation2000). Moreover, it was widely believed that the cause of the injuries was not radiation itself, but such phenomena as static electricity or individual sensitivity (Lambert Citation2001). Also, at a time when the concept of the gene was fuzzy (Summers Citation2011), there was no reason to assume that radiation could cause effects at the DNA level which we today refer to as stochastic (Hulse et al. Citation1982). Consequently, there was no interest and no need for research directed at understanding the biological mechanisms of radiation action from the perspective of radiological protection. Radiation biology was a dynamic branch of science during the first 40 years of the 20th century (Spear Citation1953), but the incentive for pursing experiments was the general fascination with radiation and its ability to deposit energy in a well-defined, spatiotemporal setting at a microscale dimension (Sloan and Fogel Citation2011a). The situation has dramatically changed after World War II, following the development and testing of nuclear weapons and the realization of exposure of humans and the environment to man-made radiation. Ever since, the rationale for carrying out radiation biology research is seen in the demand for a good understanding of qualitative and quantitative radiation effects, especially following exposure to low doses (Salomaa et al. Citation2017).
A number of excellent reviews describing achievements and developments in radiation biology have been published in recent years (Bedford and Dewey Citation2002; Preston Citation2004; Boss et al. Citation2014; Streffer Citation2015; Nakamura Citation2019), so what new insights are presented in the current review? Our approach was not to summarize scientific achievements in the field of radiation biology but to highlight research carried out in the context of radiological protection of people. How and to what extent was radiation biology—then and now—driven by the needs of radiological protection? Which radiobiological findings influenced radiation protection regulations? How were needs for more knowledge in the field of radiation biology identified and what strategies were chosen to answer them? We focus on experimental radiation biology, largely leaving aside the fields of radiation epidemiology and therapeutic use of radiation. We also do not discuss radiobiological research on non-human biota and the environment. The review is written from a chronological viewpoint but also contains personal deliberations based on a long-term involvement in the field.
The early years (1895–1945)
Observations of skin reactions leading to the concept of the tolerance dose
Röntgen’s discovery of radiation triggered its enthusiastic use to treat nearly any kind of malady or discomfort ().
Figure 1. Swedish advertisement for the use of radium. The text runs: ‘Apply radium. Radium is increasingly applied by medical science all over the world to treat, among others gout, sciatica, rheumatism, nerve pain, upset metabolism, kidney disease, anemia, senescence-associated weakness and much more. Seek advice with your medic! Radium drives away pain, facilitates metabolism, increases appetite, eases digestion, improves blood production, calms the nerves, removes the toxic uric acid from the body and prevents age-related weakness’. Source: Allers Familj Journal No. 10, 5 March 1924.

Radiation as a medical intervention was especially promoted by electrotherapists who were already experienced in treating a panoply of maladies with weak electric currents and now realized the chance of expanding their activity (Knight and Wilson Citation1996). X-rays could equally well be applied to treat lung tuberculosis, lupus vulgaris, epilepsy and superficial cancer. It is important to stress that the decision to use radiation was not based on any scientific, biological or medical investigations. Evidence-based medicine was born only in the second half of the 20th century. It was based on the fascination with radiation and the success of treatment was judged based on individual patients without any proper controls or statistical analyses which are used today. Publications described individual cases and conclusions were based on ‘clinical judgment’ (Knight and Wilson Citation1996). Tage Sjögren and Thor Stenbeck from Stockholm are said to be the first to successfully use X-rays to treat a case of skin cancer in 1899 (Spear Citation1953). They delivered over 100 treatments in the course of 9 months. Three years earlier the Viennese dermatologist Leopold Freund used daily X-ray irradiation during 3 weeks to remove a hairy nevus from the skin of a girl (Thames and Hendry Citation1987). In both cases, the rationale to apply the therapeutic dose of X-rays in fractions was not based on any biological considerations but resulted simply from the low power of their X-ray machines, necessitating repeated exposures. Treatment was guided by day-to-day observations of patient response (Thames and Hendry Citation1987). During the following years, two schools of radiotherapy emerged: the German school which favored increasing the dose per fraction and reducing the duration of treatment and the French school which favored low dose per fraction and long treatment time. Both approaches were based on technical developments of radiation source: while Siemens produced increasingly powerful X-ray tubes, the French relied on radium discovered by the Curies, the low activity of which made fractionated irradiation necessary. Interestingly, Bergonié and Tribondeau, the two French pioneer investigators of the impact of proliferation on cellular radiosensitivity, favored the German school (Vogin and Foray Citation2013). The superiority of fractionating was demonstrated in 1927 by C. Regaud and R. Ferroux who performed fractionation experiments on testes of mammals and demonstrated that the best therapeutic effect (measured as sterilization of the animals) with minimal side effects (measured as damage to the scrotum skin) was achieved under fractionated irradiation (Thames Citation1988).
Detrimental effects of radiation in the form of skin necrosis (mainly on hands) were discovered very soon after discovery of X-rays and in the form of cancers some years later (Knight and Wilson Citation1996). The mechanisms of the effects were not understood and many professionals actually questioned that the effects were related to the action of radiation. Nevertheless, individual physicians and professional societies recommended shielding of radiation sources, keeping distance and reducing the time of exposure. The unofficial bodies had no statutory powers, but their recommendations helped in reducing the extent of harm (Spear Citation1953). The first international recommendations on radiological protection were published in 1928 by the newly formed International X-Ray and Radium Protection Committee (IXRPC) which was later to become the International Commission on Radiological Protection (ICRP) (Clarke and Valentin Citation2009). It generally recommended using shielding, reducing time of exposure and maximizing the distance to a radiation source (International X-Ray and Radium Protection Committee Citation1928). No dosimetric recommendations were given except that ‘an operator should place himself as remote as practicable from the X-ray tube. It should not be possible for a well-rested eye of normal acuity to detect in the dark appreciable fluorescence of a screen placed in the permanent position of the operator’. This recommendation was based on common sense and not on any biological evidence or physical measurements of dose.
Skin erythema was the most common radiation effect observed in patients and radiologists. In the absence of any reliable physical dosimetry, the ‘erythema dose’ was the most popular measure of radiation. Hospital staff calibrated their X-rays machines according to the irradiation time that it required to induce erythema. Most often, a human thigh would be exposed at a given distance, voltage and current until the skin began to redden. Not surprisingly, the first dose limit, or the permissible dose, was based on the erythema dose. In 1924, Arthur Mutscheller reasoned that 0.1 of an erythema dose received in the period of a month was safe. One year later Rolf Sievert judged that people received between 0.001 and 0.0001 of an erythema dose naturally per year. He decided that it was safe to be exposed to 0.1 of an erythema dose per year. This equals to ca 0.01 per month. In 1930, Alfred Barcley and Sydney Cox judged that it was safe to receive 0.08 of an erythema dose per year. Again, the estimates were based on out of the gut judgment and not on any biological or epidemiological observations (Caufield Citation1989). When the unit of international röntgen (r) was introduced (Taylor Citation1958), it became common to assume that an erythema dose equalled to 600 r. Consequently, the 1934 recommendations of the IXRPC stated that ‘under satisfactory working conditions a person in normal health can tolerate exposure to X rays to an extent of about 0.2 international röntgens (r) per day’ (International X-ray and Radium Protection Commission, Citation1934). Roughly, this equals to 0.1 of an erythema dose per year and remained the valid permissible dose until the 1950ties.
Biological investigations
Fascination with ionizing radiation lead biologists to investigate its effect on animals and plants but the choice of doses and of the studied material was largely haphazard. Also, the obtained results were largely qualitative. Nevertheless, as described by Spear (Citation1953), many facts of fundamental importance were discovered such as the variability in radiosensitivity in cells of various tissues, the sparing effect of fractionation and reduced dose rate, and latency period between irradiation and manifestation of effects. Due to lack of standardized dosimetry during the first 25 years of the 20th century, the results were of little value for radiological protection (Spear Citation1953). The situation did not improve even after the method of measuring ionization in air was suggested (Russ Citation1922) and approved by the International Commission on Radiological Units and Measurements (ICRU) as the international dose unit roentgen (r) (Taylor Citation1958). The reason for this was that biologists did not see the need for precise dosimetry and seldom collaborated with radiation physicists. An exception was the field of genetics, where, as described below, the unique spatiotemporal characteristics of the interaction of radiation with matter made it an ideal tool to study the structure of the gene.
Biophysical and genetic investigations
There is no doubt that a radiobiological discovery of great importance for radiological protection was the observation by H.J. Muller that X-rays induce mutations in Drosophila melanogaster (Muller Citation1927). This discovery, confirmed and expanded by others (Timofeeff-Ressovsky et al. Citation1935), played an important role in the adoption of the linear non-threshold (LNT) approach for radiological protection. From today’s perspective, where we are strongly concerned with understanding and quantifying health effects of ionizing radiation, it is intuitive to assume that the same incentive drove Muller to carry out his experiments. The reality is, however, quite different. Geneticists in the 1920ties were highly interested in elucidating the nature of the gene. The leading geneticist T.H. Morgan was of the opinion that genes were theoretical entities whose existence was inferred from experimental data, but which were not material particles (Sloan and Fogel Citation2011b). This view was not shared by his student H.J. Muller, who believed in the material nature of genes. The disagreement and personal frictions led Muller to break with Morgan’s group and pursue his own line of investigations which aimed at examining mutations as ‘position effects’ in the gene matter (Sloan and Fogel Citation2011b). Starting from analyzing spontaneous mutations, Muller than moved on to test the effect of X-rays on the frequency of mutations. Why X-rays? Here, it should be reconciled that during the early years of the 20th century it was realized that radiation is particulate and that it could be used to explore animate matter in the same way as it was used to explore inanimate matter (Summers Citation2011). Of great importance were two developments: (1) the usage of roentgen (r) as the unit of dose which allowed a direct interpretation of the dose as number of ion pairs or clusters of ion pairs produced by radiation particles in a confined space and (2) the development of an approach by Crowther where cellular structures are considered as targets to be inactivated by radiation particles (Summers Citation2011). This approach which was later called the target theory was further developed by others (Summers Citation2011) and played an important role in developing an early model of the gene structure and of mutations which was laid out in the seminal ‘three-man paper’ by Timofeeff-Ressovsky et al. in 1935 (Sloan and Fogel Citation2011a). The paper was ground-breaking not only from the perspective of genetics, but also because it was the first interdisciplinary investigation involving a geneticist, a biophysicist and a quantum physicist. It inspired Erwin Schrödinger in his lectures on what is life (Schrödinger Citation1946) and is regarded as the beginning of molecular biology (Summers Citation2011).
The three-man paper is also remarkable because it states that (1) the dose response for mutations is linear and that there is no reason to assume the existence of a threshold of dose below which no mutations are induced and (2) there is no dose rate effect for the frequency of mutations. These observations are discussed by the authors in relation to the size and structure of a gene and mechanisms of mutagenesis. As will be discussed below, it was only after World War II that they became relevant for considerations of radiation risk in radiological protection.
Beneficial biological effects of low radiation doses and the exposure limit to radium
The discovery of radiation by Röntgen in 1895 and following discovery of radioactivity by Henrie Becquerel and Marie and Pierre Curie (Macklis Citation1996) coincided with advances in inorganic chemistry and in biochemistry (Hunter Citation2000). The identification of enzymes as catalysts made it possible to understand how biological reactions are catalyzed but left the question open regarding the vital energy which was responsible for building order in biological systems. The natural aspect of radioactivity coming from the earth element radium sparked the belief that radiation may be the vital force driving biochemical reactions. This belief was reinforced by the discovery that waters in many spas were radioactive which, in turn, initiated the use of radiation as a cure for any disease (Macklis Citation1996) (). Harmful effects of radiation were believed to be restricted to high doses and beneficial action of low radiation doses was confirmed by results of often poorly designed biological experiments on a variety of species and endpoints demonstrating stimulatory effects (Luckey Citation1980; Luckey and Lawrence Citation2006). A change in the perception of risk associated with radiation exposure occurred during early 1930ties, but not due to more careful biological experiments. Rather, it resulted from the widely reported death of Eben Byers in 1932 (Macklis Citation1993) and of numerous radium dial painters caused by radium ingestion (Clark Citation1997). Especially the latter events demonstrated that internal exposure to fairly low activities of radium was dangerous and should be prevented. However, what level of radium exposure cold be tolerated by an organism was not known. In order to find this out, researchers from the Massachusetts Institute of Technology were requested in 1936 by the Food and Drug Administration of U.S.A. to carry out experiments with rats to determine how much radium should be allowed in such consumer products as creams, toothpaste or contraceptive vaginal jellies (Caufield Citation1989). However, rats were soon found to be several hundred times more resistant to radium than humans. Therefore, further studies focused on humans who were occupationally or medically exposed to radium. Based on analyzing 27 human cases it was concluded that ingestion of <0.5 mCi (∼18 MBq) caused no harmful effects in the body. Indeed injuries were observed in those people who ingested more than 1.2 mCi (∼44 MBq). It was then recommended by the National Bureau of Standards that occupational exposure to radium be stopped when the radium activity in a worker’s body exceeded 0.1 mCi (ca 4 MBq) (Caufield Citation1989). Similarly as with the tolerance dose of external exposure, the internal exposure limit to radium was not based on any sound biological or epidemiological analyses but rather on out of the gut feeling.
The post-atomic bomb years (1945–today)
The development and detonation of atomic bombs in Hiroshima and Nagasaki in August 1945 opened a new era of both peaceful and military application of nuclear technology. The nuclear arms race that followed lead to a large number of atmospheric nuclear tests resulting in wide-spread radioactive contamination of the environment and, consequently, exposure of people to low doses of radiation (Pravaile Citation2014). The desire to better understand health effects of exposure to radioisotopes from the fallout triggered financial support for dedicated radiobiological studies. Thus, compared to the pre-World War II era and notwithstanding research aiming at the medical use of radiation, the focus of radiation biology turned towards understanding mechanisms of radiation effects from the perspective of assessing health risks. A number of large-scale animal studies were initiated where consequences of external and internal exposure to various radiations and radionuclides were analyzed (Haley et al. Citation2011). Focus was placed on the effects of dose, dose rate, and fractionation on life shortening, tumorigenesis and genetic changes. The results, together with pre-war studies on the genetic effects of radiation in Drosophila convinced some scientists, notably Herman Muller, that there is no safe dose of radiation and that atmospheric atomic bomb testing must stop. In U.S.A., this opinion was rejected by employees of the governmental Atomic Energy Commission, set up to promote the development of nuclear technology, who were convinced that the nuclear tests were safe (Hamblin Citation2007). In order to solve the issue, an independent study on the Biological Effects of Atomic Radiation (BEAR) was initiated under the auspices of the National Academy of Sciences (NAS). In U.K., a similar study was started under the auspices of the British Medical Research Council. Despite some disagreements regarding the assessment of genetic risks, both studies reached similar conclusions that the genetic effects of radiation increase linearly with dose and no threshold of dose exists. The reports were published back to back in June 1956 (Hamblin Citation2007). The BEAR committee was later renamed to National Academy of Sciences Advisory Committee on the Biological Effects of Ionizing Radiation (BEIR) and since 1972 published seven reports on biological and health effects of ionizing radiation.
The ICRP did not meet between 1937 and 1950 (Clarke and Valentin Citation2009), so no recommendations on internal exposure limits were given until 5 years after World War II. In its 1950 recommendations, the ICRP states that the commission ‘is not in a position to make firm recommendations regarding the maximum permissible amounts of radioactive isotopes that may be taken into, or retained in the body’ (ICRP Citation1951). Nevertheless, based on maximum permissible exposures to radioactive isotopes for occupational workers used in U.S.A., Canada and U.K., exposure limits were given for radium, plutonium, strontium, polonium, tritium, carbon 14, sodium 15, phosphor 32, cobalt 60, and iodine 131 (ICRP Citation1951). Although the ICRP does not specify the source of data for the limits, it can be assumed that they, at least partly, came from the extensive animal experiments (Haley et al. Citation2011). However, it must be mentioned that some of the knowledge must also have come from human radiation experiments which were commonly carried out between the years 1944 and 1974 in U.S.A. and other nuclear program states (Advisory Committee on Human Radiation Experiments, Citation1995). Medical patients, prisoners, soldiers and children were intentionally exposed to radiation and isotopes, often without their knowledge or consent. Human experiments were not unique to the radiation field but were in line with the ethical standards (or the lack of such) at the time. Also, the process of acquiring scientific results from the Life Span Study of Hiroshima and Nagasaki survivors during the first 20 years of the program carried out by the Atomic Bomb Casualty Commission (ABCC) was criticized for low ethical standards in that survivors were treated as Guinea pigs (Beatty Citation1993).
In 1955, due to growing world-wide concerns about the health impacts of atomic tests, the General Assembly of the United Nations established the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) to collect and evaluate information on the levels and effects of ionizing radiation (http://www.unscear.org). The first session was held from 14 to 23 March 1956 in New York. Ever since, representatives of UNSCEAR members meet once per year to discuss the available scientific data on sources and effects of ionizing radiation and approve regular reports which are submitted to the UN General Assembly. A complete list of reports and annexes is given in Supplementary Table S1, where reports addressing radiation biology are highlighted. All reports can be downloaded from http://www.unscear.org (publications and are herein only identified by their publication year and not listed in the references). It is worthwhile to look at the full list because it reflects the areas of research in the field of radiation biology which were and are regarded as relevant for radiological protection. Indeed, in setting up radiation protection recommendations, the ICRP heavily relies on UNSCEAR reports as the source of scientific knowledge (Clarke and Valentin Citation2009; Preston Citation2017).
Before discussing the content of the UNSCEAR reports, it is interesting to note that the three annexes of the first report that deal with biological effects were to more than 90% based on studies published after World War II. A notable exception are the genetic studies of Herman Muller and others on gene mutations in the fruit fly. This confirms that radiation biology studies carried out before World War II were not directed at tackling problems of radiological protection.
The first two UNSCEAR reports contain annexes related to ‘fundamental radiobiology’. In a way, the reading of these annexes today is a humbling lesson because the key elements of radiobiology are already all discussed: linear energy transfer (LET), relative biological effectiveness (RBE), threshold and non-threshold dose effect relationships, dose rate effects (referred to as time intensity factor), oxygen effect, radioprotectors, radiosensitizers, factors influencing radiosensitivity of cells and organisms, adaptive responses and bystander effects (referred to as secondary effects). Both reports categorize radiation effects according to a system not used today: (1) somatic effects which are limited to the irradiated organism and include both tissue and cancer effects and (2) genetic effects which are limited to descendants of cells (hereditary effects). Later reports focused more specifically on issues relevant for radiological protection: cancer effects (1964, 1972, 1977, 1988, 1993, 2010), somatic effects in the sense of tissue reactions and non-cancer effects (1969, 1972, 1977, 1986, 1988, 1993, 2006, 2010), and genetic or hereditary effects (1966, 1972, 1977, 1982, 1986, 1988, 1993, 2001, 2010). Also, separate reports were published on chromosomal and DNA damage (1969, 2000) combined effects of radiation and other agents (reports 1982 and 2000), effects on non-human biota (1996, 2008), biological effects of internal emitters (2016), and effects of low (1994, 2000) and high (1988) doses of radiation.
How and to what extent did the achieved results influence ICRP’s recommendations for radiological protection? After 1950 recommendations were published in 1954, 1958, 1966, 1977, 1990 and 2007 (Clarke and Valentin Citation2009). However, anyone expecting a direct impact of radiobiological results on radiation protection standards will be disappointed. As pointed out by Preston, radiobiology research has not been used to any great extent in the development of radiation risk estimates (Preston Citation2017). How could they, given the major problem of extrapolating results from cell or animal experiments to man. Not surprisingly, radiation risk factors and dose limits are mainly derived from epidemiology studies, in particular those from the Life Span Study (LSS) of the Japan atomic bomb survivors (Clarke and Valentin Citation2009). The difficulty of extrapolating risk factors from laboratory studies to man was realized quite early (Mole Citation1957; ICRP Citation1965) and discussed extensively for studies on genetic effects of radiation, where, in fact, no germinal mutations or transmissible genetic diseases induced by ionizing radiation in humans have been identified (Sankaranarayanan Citation1993; Sankaranarayanan and Chakraborty Citation2000). Radiation biology contributes to radiological protection not by delivering risk estimates, but by providing mechanistic information about the action of radiation on cells and organisms. Pertinent examples of relevant radiobiological studies are investigations on the shape of the dose response curve (Belli et al. Citation2015) and on the value of the dose and dose rate effectiveness factor (DDREF) (Ruhm et al. Citation2015). This input is essential for inferring risks from epidemiological approaches which are observational studies that heavily rely on radiobiological knowledge for establishing causal relationships from correlations. Moreover, understanding the mechanisms and dose response relationships of radiation effects is essential for providing reliable expertize to the society abut risks of radiation and optimal levels of protection (Wojcik et al. Citation2018).
Since the 1970ties, citizens of countries with advanced economies began to be increasingly aware of health and environmental risks associated with modern technologies such as nuclear power (Beck Citation1986; Harremoes Citation2001). The concern was boosted by the nuclear accidents at Three Mile Island, Chernobyl and Fukushima Daaichi. This, together with the growing use of radiation in medical diagnostics, initiated efforts at national and international levels to support and coordinate radiobiological research with the aim to better understand health effects of low radiation doses. Such research programs were started in several countries (Fukunaga et al. Citation2017; Brooks Citation2018; Cho et al. Citation2019; Wang et al. Citation2018). In the European Union (EU), radiation protection research is currently being reorganized in an attempt to combine EU funds and national funds from EU member states in an effort to increase the available financial capacity for a better understanding of biological and health effects of low dose radiation (Ruhm et al. Citation2018). The history of this effort is concisely described below.
Low-dose research program in the European Union
European research projects addressing health risks from manmade and/or natural radiation sources have been ongoing since World War II to improve our understanding of the effects of radiation on human health and on the environment. Projects were funded by national grants and programs. The European radiation protection research program was initiated in 1957 by the European Atomic Energy Community (EAEC, usually referred to as ‘Euratom’) and initially agreed in Rome by France, Italy, the Federal Republic of Germany, Netherland, Belgium and Luxemburg. Article 30 of the treaty postulates that ‘basic standards shall be laid down within the Community for the protection of the health of workers and the general public against the dangers arising from ionizing radiations’. In 1984 the European Commission introduced comprehensive science and technology ‘frame work’ programs (FP) to support European scientific and technological developments. These FP ran over periods of 3–5 years and the current FP (named Horizon 2020) will end in 2020. Research in the field of radiological protection is funded by the Euratom Fission program.
The high level expert group
During the FP6 program (2002–2006), discussions were initiated between Euratom officials and representatives of several national authorities for nuclear safety to develop strategies to strengthen radiation protection research in Europe with a focus on effects on health from low doses of ionizing radiation. Views were expressed that after many decades of national and Euratom funding, the impact of radiobiological research on the reduction of uncertainties in risk assessment in the wide area of radiological protection had been limited. As a result of these discussions the European Commission, and several EU Member States (France, Finland, Germany, Italy, and U.K.) established in 2008 a ‘high level and expert group’ (HLEG) in order to explore means to take advantage of scientific progress in biology and propose a strategy how the rapid developments in biology could be used to reduce uncertainties in several key questions described below. The HLEG members were assisted by experts from the research community to identify research priorities and training needs. The report was published in 2009 (HLEG Citation2009).
A brief summary of the HLEG report is presented below including some personal views on the process and the results. The objectives of the group were:
To formulate and agree the policy goals to be addressed by low dose risk research.
To develop a strategic research agenda and road map for such research in Europe.
To specify the essential elements of and next steps for establishing a sustainable operational framework for low dose risk research in Europe.
Key policy questions addressed were ‘How robust is the current system of radiation protection and risk assessment, given its uncertainties? How can it be improved for delivering intended levels of protection of the population from occupational, environmental and medical exposures to ionizing radiation?’ The report identified six specific key issues requiring investigation to challenge the policy question above
The shape of dose-response for cancer.
Tissue sensitivities for cancer induction.
Individual variability in cancer risk.
The effects of radiation quality (type).
Risks from internal radiation exposure.
Risks of, and dose response relationships for, non-cancer diseases and hereditary effects.
Each of the key questions were assessed from the point of state of the art and challenges. Summaries of discussions and conclusions on each key question were presented as figures. In order to show the reasoning of the HLEG members, the figure summarizing indicative research directions on the shape of the dose response () is discussed in more detail.
Figure 2. Indicative research directions to address issues on the shape of the dose response relationship and tissue sensitivities for cancer. Solid boxes indicate that experimental studies have to be combined with modelling studies. The dashed-line box implies that experimental and modelling studies should be carried out concurrently and the dotted-line box denotes epidemiological studies. Based on (HLEG Citation2009).
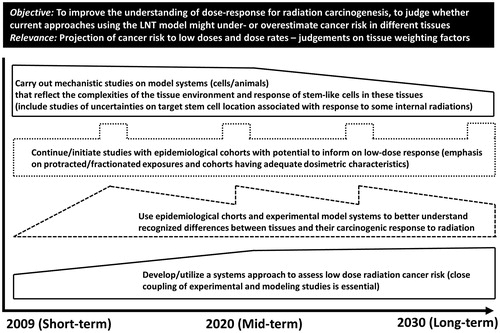
The shapes of boxes in the figure designate potential research efforts with indications of changes over time. Solid boxes indicate that experimental studies have to be combined with modelling studies. The dashed-line box implies that experimental and modelling studies should be carried out concurrently and the dotted-line box denotes epidemiological studies.
The HLEG report emphasized that the key questions addressed would require significant efforts in time and funding to be solved. The period outlined predicted that several decades of low dose research would be needed before answers to the policy questions could be based on new scientific results. Furthermore, the needs of investments in basic research during most of this time was emphasized in order to reach a mechanistic understanding of the processes that underlie the causes of uncertainties behind the six key questions.
Beside the key questions, the HLEG report also contains a proposal to set up an trans-national European organization ‘capable of ensuring appropriate governance of research in this field, in the pursuit of a long term shared vision: uniting the programs of the various funding bodies and research organizations, thus ensuring long term research funding in accordance with an agreed strategic research agenda (SRA); interfacing with the many stakeholders, in particular regulatory bodies and the broader scientific community; overseeing investments in key infrastructures, as well as knowledge management, training and education. For this purpose, it is proposed to set up a new European Platform, to be named MELODI: Multidisciplinary European LOw Dose Initiative’.
The initiative to include in the HLEG report the proposal to form the MELODI platform was taken by representatives of national authorities from France, Germany, Italy, Finland, U.K., and representatives of Euratom. The expert advisory group was involved solely in assessing the research strategies.
MELODI
MELODI was initiated in 2009 by signing a letter of intent by five national organizations from France (CEA, IRSN), Italy (ISS), Germany (BfS), and Finland (STUK) (http://www.melodi-online.eu/) and founded in 2010 as a registered French association. At the start MELODI had 15 member organizations that by 2018 had increased to 44. The history and progress of MELODI has been reviewed in several articles (Belli et al. Citation2011, Citation2015; Salomaa et al. Citation2017; Repussard Citation2018).
The overall aims of MELODI follow closely the proposals laid out in the HLEG report (HLEG Citation2009). Importantly, because MELODI was set up to coordinate low dose research in Europe, it was emphasized that MELODI must ensure that its future SRA and road map are very clearly and visibly separated from potential self-serving interests of the members. Also, any future FP calls suggested by MELODI to the EC must not be influenced by or tailored to suit the partners, but are driven only by the true policy and scientific goals and are equally open to the full scientific community (Belli et al. Citation2011).
DOREMI, OPERRA and CONCERT
What followed were 3 large integrative research projects DOREMI (http://www.melodi-online.eu/DoReMi/home.html), OPERRA (http://www.melodi-online.eu/operra.html), and CONCERT (http://www.concert-h2020.eu). All three projects were based on actions aiming at attracting researchers and national funding bodies to the field of low dose radiation and to strengthen the integration of the European research community on the basis of a European SRA. While DOREMI (finished in 2015) was a Network of Excellence (NoE) and OPERRA (finished in 2017) was a Coordinated Support Action (CSA), CONCERT (will finish in 2020) is a European Joint Program (EJP) which is based on the principle of co-funding, linking national funding to EC funding.
The creation of MELODI sparked the formation of a network between other EU platforms in order to integrate activities in the field of radiation protection research i.e. dosimetry [EURADOS, http://www.eurados.org (Ruhm et al. Citation2016)], radioecology (ALLIANCE, http://www.er-alliance.org), emergency preparedness (NERIS, http://www.eu-neris.net), RENEB [http://www.reneb.net, (Kulka et al. Citation2017)] and radiation protection in medicine (EURAMED). Each platform has developed its own SRA. Moreover, within the CONCERT program a work package, including all the platforms, aims to develop a joint roadmap across the different disciplines that can serve as guide for national as well as Euratom programs to optimize the use of resources and to join the efforts to tackle the challenges identified for radiation protection the joint SRAs of the platforms.
HLEG report and lessons learned
The HLEG report and the activities of the platforms together with the efforts to develop a joint radiation protection research program have provided a new base for radiation protection research in Europe. It is premature to draw any conclusions about the outcome in terms of the national funding programs and if those have adopted to the priorities set by the SRAs as this remains to be evaluated. Nevertheless, the influence of the last ten years of development in terms of strategies for low dose research in Europe on the Euratom programs starts to be visible, the funding has been more evenly distributed between the different scientific priorities of the platforms, medical radiation protection issues has become one of the priority areas for funding and focus on education and training as well as on programs for infrastructures has increased. It will take several years before a meaningful evolution of the European low dose program can be performed and the effects on the research achievements, long term needs of national competence in member states of Europe and integration of different academic disciplines in the universities needed for future research activities can be evaluated. The work and planning of the future research activities will have to be transparent, focused on long term strategies and including the needs of all European member states.
Conclusions
Although investigations of biological effects of radiation started shortly after the discovery of radiation in 1895, radiation protection biology started only after the initiation of the atomic bomb programs in 1945. Radiation protection biology does not provide direct data on radiation risks to humans. Such results come from epidemiological studies. However, and not less importantly, it contributes to radiological protection of people by providing qualitative and quantitative information about the action of radiation on cells and organisms such as the shape of the dose response curve, dose rate effects and mechanisms of individual sensitivity to radiation. This input is essential for inferring risks from epidemiological approaches and for providing reliable expertize to the society abut risks of radiation and optimal levels of protection. We would like to conclude this article with a quotation from the first UNSCEAR report (1958, annex G) which is still highly relevant for radiation protection biology today: ‘radiobiology is not a science in itself; it is but an applied science and it rests entirely on our knowledge of the great principles of biology which cannot be studied independently of one another. The understanding of some aspects may at times progress more rapidly than that of others, but in the long run all these have to be integrated into one harmonious picture. The problem is not merely to push forward the study of genetics or of carcinogenesis, because it is obvious that these problems are dependent on most other aspects of cell physiology. Our ignorance of fundamental biology (taken in its widest possible sense) is undoubtedly the major factor limiting our understanding of radiation effects on man’.
Wojcik_Supplemmentary_Table_1.docx
Download MS Word (16.1 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
Notes on contributors
Andrzej Wojcik
Andrzej Wojcik, PhD, is professor of radiation biology at the Stockholm University. He focuses on studying the cellular effects of radiation, with special focus on combined exposure to radiations of different qualities. He is also interested in developing and validating cytogenetic tools for biological dosimetry.
Mats Harms-Ringdahl
Mats Harms-Ringdahl, PhD, is professor emeritus of radiation biology at the Stockholm University. His long-term research focus was on biological effects of low dose and low dose rate exposure on cells and animals. He was a member of the High Level Expert Group on European Low Dose Risk Research and for several years served as member of the MELODI management team.
References
- Advisory committee on human radiation experiments. 1995. Final report. Washington: Government Printing Office.
- Beatty J. 1993. Scientific collaboration, internationalism, and diplomacy: the case of the atomic bomb casualty commission. J Hist Biol. 26:205–231.
- Beck U. 1986. Risikogesellschaft. Auf dem Weg in eine andere Moderne. Frankfurt am Main: Suhrkamp Verlag.
- Bedford JS, Dewey WC. 2002. Radiation Research Society. 1952-2002. Historical and current highlights in radiation biology: has anything important been learned by irradiating cells? Radiat Res. 158:251–291.
- Belli M, Salomaa S, Ottolenghi A. 2011. MELODI: the ‘Multidisciplinary European Low-Dose Initiative’. Radiat Prot Dosimetry. 143:330–334.
- Belli M, Tabocchini MA, Jourdain JR, Salomaa S, Repussard J. 2015. The European initiative on low-dose risk research: from the HLEG to MELODI. Radiat Prot Dosimetry. 166:178–181.
- Boss MK, Bristow R, Dewhirst MW. 2014. Linking the history of radiation biology to the hallmarks of cancer. Radiat Res. 181:561–577.
- Brooks AL. 2018. Low dose radiation: the history of the U.S. Department of Energy research program. Washington: Washington State University Press.
- Caufield C. 1989. Multiple exposures: chronicles of the radiation age. Chicago (IL): The University of Chicago Press.
- Cho K, Imaoka T, Klokov D, Paunesku T, Salomaa S, Birschwilks M, Bouffler S, Brooks AL, Hei TK, Iwasaki T, et al. 2019. Funding for radiation research: past, present and future. Int J Radiat Biol. 2:1–25. doi: 10.1080/09553002.2018.1558303.
- Clark C. 1997. Radium girls: women and industrial health reform, 1910-1935. Chapel Hill (NC): The University of North Carolina Press.
- Clarke RH, Valentin J. 2009. The history of ICRP and the evolution of its policies. Ann ICRP. 109:75–110.
- Codman EA. 1902. A study of the cases of accidental X-ray burns hitherto recorded. Reprinted from Philadelphia Medical Journal. March 8.
- Farmelo G. 1995. The discovery of X-rays. Scient Am. 273:68–73.
- Fukunaga H, Yokoya A, Taki Y, Prise KM. 2017. Radiobiological implications of Fukushima nuclear accident for personalized medical approach. Tohoku J Exp Med. 242:77–81.
- Haley B, Wang Q, Wanzer B, Vogt S, Finney L, Yang PL, Paunesku T, Woloschak G. 2011. Past and future work on radiobiology mega-studies: a case study at Argonne National Laboratory. Health Phys. 100:613–621.
- Hamblin JD. 2007. ‘A dispassionate and objective effort’: negotiating the first study on the biological effects of atomic radiation. J Hist Biol. 40:147–177.
- Harremoes P. 2001. Late lessons from early warnings: the precautionary principle 1896-2000. Copenhagen: European Environmental Agency.
- [HLEG] High Level and Expert Group. 2009. Report of high level expert group on European low dose risk research. http://www.hleg.de/fr.pdf.
- Hulse EV, Path FRC, Mole RH. 1982. Reflections on the terms stochastic and non-stochastic as currently used in radiological protection. Br J Radiol. 55:321–324.
- Hunter GK. 2000. Vital forces: the discovery of the molecular basis of life. San Diego (CA): Academic Press.
- ICRP. 1951. International recommendations on radiological protection. Revised by the International Commission on Radiological Protection at the Sixth International Congress of Radiology, London, July 1950. Br J Radiol. 56:431–439.
- ICRP. 1965. The evaluation of risks from radiation. ICRP Publications 8. Oxford: Pergamon.
- International X-Ray and Radium Protection Committee. 1928. International recommendations for X-ray and radium protection on the proposal of the Radio-Physics Section adopted by the Second International Congress of Radiology in Stockholm, July 27th, 1928.
- International X-Ray and Radium Protection Commission. 1934. International recommendations for X-ray and radium protection, revised by the International X-ray and Radium Protection Commission at the fourth International Congress of Radiology, Zürich, July 1934.
- Knight N, Wilson JF. 1996. Chapter 1, The early years of radiation therapy. In: Gagliardi R, Wilson FJ, editors. A history of radiological sciences: oncology. Reston: Radiology Centennial; p. 1–20.
- Kulka U, Abend M, Ainsbury E, Badie C, Barquinero J, Barrios L, Beinke C, et al. 2017. RENEB – Running the European Network of biological dosimetry and physical retrospective dosimetry. Int J Radiat Biol. 93:2–14.
- Lambert B. 2001. Radiation: early warnings; late effects. In: Harremoes P, Gee D, MacGavin M, Stirling A, Keys J, Wynne B, Guedes Vaz S, editors. Late lessons from early warnings: the precautionary principle 1896–2000. Copenhagen: European Environmental Agency.
- Luckey TD. 1980. Hormesis with ionizing radiation. Boca Raton, FL: CRC Press.
- Luckey TD, Lawrence KS. 2006. Radiation hormesis: the good, the bad, and the ugly. Dose Resp. 4:169–190.
- Macklis RM. 1993. The great radium scandal. Sci Am. 8:94–99.
- Macklis RM. 1996. Chapter 11, Radiomedical fraud and popular perceptions of radiation. In: Gagliardi R, Wilson FJ, editors. A history of radiological sciences: oncology. Reston: Radiology Centennial; p. 277–292.
- Martland HS. 1929. Radium poisoning. Monthly Labor Rev. 28:20–95.
- Martland HS, Humphries RE. 1929. Osteogenic sarcoma in dial painters using luminous paint. Arch Pathol. 7:406–417.
- Mole RH. 1957. Shortening of life by chronic irradiation: the experimental facts. Nature. 180:456–460.
- Muller HJ. 1927. Artificial transmutation of the gene. Science. 66:87.
- Nakamura N. 2019. History of radiation genetics: light and darkness. Int J Radiat Biol. 24:1–16. doi:10.1080/09553002.2019.1572251.
- Pitkin JT. 1904. Dangers to the X-ray operator. Am X-Ray J. 14:9.
- Pravaile R. 2014. Nuclear weapons tests and environmental consequences: a global perspective. AMBIO. 43:729–744.
- Preston RJ. 2004. Radiation biology: concepts for radiation protection. Health Phys. 87:3–14.
- Preston RJ. 2017. International organizations, risk assessment and research-why, what and how. Mutat Res. 806:75–80.
- Repussard J. 2018. Understanding low dose radiation exposure effects: MELODI’s views on developing international cooperation. Int J Radiat Biol. 29:1–9. doi:10.1080/09553002.2018.1547439.
- Roberts H. 1897. The American X-Ray Journal. Am X-Ray J. 1:1.
- Röntgen WC. 1895. Über eine neue Art von Strahlen. Sitzungsberichte der Physikalisch-Medizinischen Gesellschaft zu Würzburg Jahrgang. 132–149.
- Rowland S. 1896. Preface. Arch Clin Skiagr. 1:3–4.
- Ruhm W, Friedl AA, Wojcik A. 2018. Coordinated radiation protection research in Europe: is it the beginning of a new era? Radiat Environ Biophys. 57:1–4.
- Ruhm W, Woloschak GE, Shore RE, Azizova TV, Grosche B, Niwa O, Akiba S, Ono T, Suzuki K, Iwasaki T, et al. 2015. Dose and dose-rate effects of ionizing radiation: a discussion in the light of radiological protection. Radiat Environ Biophy. 54:379–401.
- Ruhm W, Fantuzzi E, Harrison R, Schuhmacher H, Vanhavere F, Alves J, Bottollier-Depois JF, Fattibene P, Knezevic Z, Lopez MA, et al. 2016. EURADOS strategic research agenda: vision for dosimetry of ionising radiation. Radiat Prot Dosimetry. 168(2):223–234.
- Russ S. 1922. The measurement of X-ray intensity and the necessity for an international method. Proc Phys Soc London. 35:5D.
- Salomaa S, Jourdain JR, Kreuzer M, Jung T, Repussard J. 2017. Multidisciplinary European low dose initiative: an update of the MELODI program. Int J Radiat Biol. 93:1035–1039.
- Sankaranarayanan K. 1993. Ionizing radiation, genetic risk estimation and molecular biology: impact and inferences. Trends Genet. 9:79–84.
- Sankaranarayanan K, Chakraborty R. 2000. Ionizing radiation and genetic risks. XII. The concept of ‘potential recoverability correction factor’ (PRCF) and its use for predicting the risk of radiation-inducible genetic disease in human live births. Mutat Res. 453:129–181.
- Schrödinger E. 1946. What is life? Cambridge: Cambridge University Press.
- Sloan PR, Fogel B. 2011a. Creating a physical biology. The threee-man paper and early molecular biology. Chicago (IL): The University of Chicago Press.
- Sloan PR, Fogel B. 2011b. Introduction. In: Sloan PR, Fogel B, editors. Creating a physical biology. The three-man paper and early molecular biology. Chicago (IL): The University of Chicago Press.
- Spear FG. 1953. Radiations and living cells. New York: John Wiley and Sons.
- Streffer C. 2015. An update on the mechanisms and pathophysiological consequences of genomic instability with focus on ionizing radiation. Res Rep Biol. 6:225–233.
- Summers WC. 2011. Physics and genes. In: Sloan PR, Fogel B, editors. Creating a physical biology. The three-man paper and early molecular biology. Chicago (IL): The University of Chicago Press.
- Taylor LS. 1958. History of the international commission on radiological units and measurements (ICRU). Health Phys. 1:306–314.
- Thames HD. 1988. Early fractionation methods and the origins of the NSD concept. Acta Oncol. 27:89–103.
- Thames HD, Hendry JH. 1987. Fractonation in radiotherapy. London: Taylor & Francis.
- Timofeeff-Ressovsky NW, Zimmer KG, Delbrück M. 1935. Über die Natur der Genmutation und der Genstruktur. Nachrichten Von Der Gesellschaft Der Wissenschaften zu Göttingen Biologie. 13:189–245.
- Vogin G, Foray N. 2013. The law of Bergonie and Tribondeau: a nice formula for a first approximation. Int J Radiat Biol. 89:2–8.
- Walker JS. 2000. Permissible dose: a history of radiation protection in the twentieth century. London: University of California Press.
- Wang Y, Bannister LA, Sebastian S, Le Y, Ismail Y, Didychuk C, Richardson RB, Flegal F, Paterson L, Causey P, et al. 2018. Low-dose radiobiology program at Canadian nuclear laboratories: past, present and future. Int J Radiat Biol. 24:1–11. doi:10.1080/09553002.2018.1562252.
- Wojcik A, Hamza K, Lundegård I, Enghag M, Haglund K, Arvanitis L, Schenk L. 2018. Educating about radiation risks in high schools: towards improved public understanding of the complexity of low-dose radiation health effects. Radiat Environ Biophy. 58:13–20.
