Abstract
Purpose: Rapid depletion of white blood cells, platelets, and reticulocytes are hallmarks of hematopoietic injury of acute radiation syndrome (H-ARS) and, if left untreated, can lead to severe health consequences including death. While the granulocyte colony stimulating factors (G-CSF) filgrastim (Neupogen®), pegfilgrastim (Neulasta®), and sargramostim (Leukine®) are approved to increase survival in patients exposed to a myelosuppressive dose of radiation, no medical countermeasure is currently available for treatment of the thrombocytopenia that also results following radiation exposure. Romiplostim (Nplate®), a thrombopoietin receptor agonist, is the first FDA-approved thrombopoiesis-stimulating protein for the treatment of low platelet (PLT) counts in adults with chronic immune thrombocytopenia. Herein, we present the results of an analysis in mice of romiplostim as a medical countermeasure to improve survival and PLT recovery following acute radiation.
Materials and methods: Male and female C57BL/6J mice (11 − 12 weeks of age, n = 21/sex/group) were total body irradiated (TBI) with 6.8 Gy X-rays that reduces 30-day survival to 30% (LD70/30). Vehicle, romiplostim, and/or pegfilgrastim were administered subcutaneously beginning 24 h after TBI for 1–5 days. Evaluation parameters included 30-day survival, pharmacokinetics, and hematology.
Results: Full or maximal efficacy with an ∼40% increase in survival was achieved after a single 30 µg/kg dose of romiplostim. No further survival benefit was seen with higher (100 µg/kg) or more frequent dosing (3 or 5 once daily doses at 30 µg/kg) of romiplostim or combined treatment with pegfilgrastim. Pharmacodynamic analysis revealed that the platelet nadir was not as low and recovery was faster in the irradiated mice treated with romiplostim when compared with irradiated control animals (Day 8 versus 10 nadir; Day 22 versus 29 recovery to near baseline). Platelet volume also increased more rapidly after romiplostim injection. Kinetic profiles of other hematology parameters were similar between TBI romiplostim-treated and control mice. Peak serum levels of romiplostim in TBI mice occurred 4 − 24 h (Tmax) after injection with a t1/2 of ∼24 h. Cmax values were at ∼6 ng/ml after 30 µg/kg ± TBI and ∼200 ng/ml after 300 µg/kg. A 10-fold higher romiplostim dose increased the AUClast values by ∼35-fold.
Conclusion: A single injection of romiplostim administered 24 h after TBI is a promising radiation medical countermeasure that dramatically increased survival, with or without pegfilgrastim, and hastened PLT recovery in mice.
Introduction
Large penetrating doses of total body ionizing radiation, such as those that could occur during radiological accidents or terrorism, results in well understood acute effects on the hematopoietic system. This hematopoietic subsyndrome of acute radiation syndrome (H-ARS) is characterized by dose-dependent bone marrow destruction leading to rapid depletion of white blood cells, platelets, and reticulocytes (i.e. lymphocytopenia, neutropenia, thrombocytopenia, and anemia) (Singh and Seed Citation2017). Such damage to the hematopoietic system can lead to lethality from infections, hemorrhages, and poor wound healing. The granulocyte colony stimulating factors (G-CSF) filgrastim (Neupogen®) and pegfilgrastim (Neulasta®) and the granulocyte–macrophage colony stimulating factor (GM-CSF) sargramostim (Leukine®) have recently been approved by the US Food and Drug Administration to increase survival in patients acutely exposed to myelosuppressive doses of radiation (NEUPOGEN® (filgrastim) [product monograph]: Thousand Oaks, CA: Amgen Inc; NEULASTA® (pegfilgrastim) [product monograph]: Thousand Oaks, CA: Amgen Inc; LEUKINE® (sargramostim) [product monograph]: Bridgewater, NJ: Sanofi-Aventis US Inc). These three myeloid cytokines are currently residing in the United States Strategic National Stockpile for emergency use in the event of a radiological incident (U.S. Department of Health and Human Services Press Office Citation2016; National Library of Medicine et al. Citation2018). These three approved cytokines address post-radiation neutropenia (low neutrophil counts) and the increased risk of infection and febrile neutropenia (Waselenko et al. Citation2004; Singh et al. Citation2015) but do not address thrombocytopenia (low platelet counts) and excessive bleeding that also occurs in H-ARS. Thus, a radiation medical countermeasure to address thrombocytopenia would likely have a positive effect on H-ARS patients when administered alone or in combination with one of the G(M)-CSFs.
Romiplostim (Nplate®) is a thrombopoietin (TPO) receptor agonist (i.e., a thrombopoietin mimetic) approved to treat low platelet counts in adults with chronic immune thrombocytopenia (ITP), an autoimmune disease characterized by low platelet counts (NPLATE® (romiplostim) [product monograph]: Thousand Oaks, CA: Amgen Inc). A recent report using romiplostim as a successful ‘on demand therapy’ for emergency management of severe ITP with life-threating bleeding further confirms the usefulness of romiplostim in life-threating bleeding situations (Gellens et al. Citation2017).
Romiplostim is a recombinant fusion protein that binds the TPO receptor (c-Mpl) to stimulate megakaryocyte-mediated production of platelets activating the same downstream signaling cascade as endogenous TPO (Broudy and Lin Citation2004; Provan and Newland Citation2007). Romiplostim has no sequence homology to endogenous TPO thereby reducing the potential for neutralizing antibody production which halted the clinical development of earlier recombinant TPO mimetics (Li et al. Citation2001). Structurally, romiplostim is a peptibody molecule meaning it has two copies of a peptide domain that binds and activates the TPO receptor and two copies of a human immunoglobulin Fc carrier domain that increases the molecule’s circulating half-life (Molineux and Newland Citation2010).
Our evaluation of romiplostim as a potential radiation medical countermeasure to increase survival in an X-ray irradiated mouse model of H-ARS, with and without co-administration of pegfilgrastim, is presented herein. The pharmacodynamic and pharmacokinetic profile of romiplostim in irradiated mice is also presented.
Materials and methods
Animals
Male and female C57BL/6J mice (Jackson Laboratory, Sacramento, CA) aged 11–12 weeks at study start were housed at an American Association for Accreditation of Laboratory Animal Care, international-accredited facility. All research protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Males were single housed due to the aggressive nature of this strain. Females were housed ≤ 3 per cage. Housing rooms were maintained on a 12-h light/dark cycle at 68 − 79 °F, 30 − 70% relative humidity. Food (Envigo Tekland Chow #2018C) and purified, reverse osmosis water were provided ad libitum. Supplemental water-softened chow and HydroGel® (ClearH2O®, Westbrook, ME) were provided to all mice beginning 4 days after radiation. Mice were randomized to treatment groups based on body weight 1–3 days before irradiation. Clinical observations were scored on a three-point scale (slight, moderate, extreme) and were performed daily with an afternoon observation performed for any mouse with a morning finding scored as moderate or extreme. Body weights were determined at least 3× weekly with increasing measurement frequency as body weight loss occurred (i.e. once and twice daily when body weight loss reached ≥ 15% and ≥ 20%, respectively, of the pre-irradiation weight).
Radiation and dosimetry
Total body irradiation was performed using a Pantak HF320 X-ray unit to deliver a single 6.8 (LD70/30) or 6.5 Gy (LD30/30) dose to mice placed into individual plastic animal box holders on a rotating Plexiglas platform (to ensure dose uniformity at ∼1 rpm) located 45 cm centered vertically below the exposure beam. Up to six mice were irradiated at a time on Day 1 with each irradiation session occurring between 7:15 and 11:15 am to minimize circadian effects (Williams et al. Citation2010; Plett et al. Citation2012). Exposure energy settings were 230 kVp, 5 mA, with 2 mm aluminium filtration that provided a dose rate of 1.09 Gy/min.
A PTW-UNIDOS electrometer connected to a controlled universal field class Farmer-type ionization chamber [PTW Freiburg GmbH; calibrated annually by an accredited laboratory (K&S Associates, Inc Nashville, TN)] was used to determine the delivered dose. Pre-exposure sessions to evaluate and confirm the energy and dose rate were performed with the chamber probe placed sequentially in all six positions of the box-holder base in an orientation to mimic the mid-body location of the mouse. The average of these six dose rate readings taken immediately prior to each exposure session was used to calculate the exposure duration time. Stability of the dose rate was then confirmed at the end of each session. The pre-session dose rate remained stable at 1.09 ± 0.03 Gy/min over the 15-month period in which the 5 studies described herein were conducted.
Dose formulation
Dose formulations were prepared on the day of use in amber glass vials, maintained on wet ice, and used within 4 h of preparation. Romiplostim (Nplate®, lyophilized powder in vial, stored refrigerated and protected from light; Amgen, Thousand Oaks, CA) was reconstituted at 500 µg/ml with sterile water for injection, USP (Fresenius Kabi) and then diluted with vehicle [sterile saline for injection, USP (Hospira, Rocky Mount, NC)] to obtain dose formulations of 0.6, 2, 6, 20 and 60 µg/ml. Pegfilgrastim (Neulasta®, 10 mg/ml, stored refrigerated and protected from light; Amgen) was diluted to 60 µg/ml with the vehicle 5% dextrose [generated from 50% dextrose for injection, USP (Hospira) and sterile water for injection]. Mixing was by gentle, repeated, and round-trip inversions.
Dose regimen
Test and control articles were administered by subcutaneous (sc) injection to the scapular region 24 − 26 h post-irradiation (Day 1) at 5.0 ml/kg based on the Day-1 pre-irradiation body weight of each mouse. Romiplostim was tested at 3, 10, 30, 100, and 300 µg/kg. Pegfilgrastim was tested at 300 µg/kg as reported in other studies (Farese et al. Citation2012; Chua et al. Citation2014; Hankey et al. Citation2015). For repeat dose administration, 30 µg/kg romiplostim was injected every 24 ± 2 h on Days 1 − 3 or 1 − 5 with saline administered as the control article such that all animals received a total of 5 once daily injections.
Euthanasia criteria
Early removal of an animal (unscheduled euthanasia, moribund sacrifice) occurred when body weight loss was ≥ 30% relative to Day-1 or when at least three abnormal clinical observations occurred with at least two of those findings being scored as moderate or extreme. Along with weight loss, abnormalities in postural adjustments, appearance, behavior, response to stimuli, physiological functions, or ambulation were clinical observations used in the early removal criteria (e.g. hunched posture, ruffled fur, hypoactivity, hypothermia, eye discharge, squinting, swelling, dehydration, thinness, dyspnea, ataxia, and tremors). Findings observed but not included in the early removal criteria included alopecia, eschar formation, and skin and fur discoloration.
Survival analysis
Three separate studies with 21 mice/sex/treatment group/study were performed to evaluate 30-day survival. Kaplan–Meier survival analysis used StataSE 14.1 software combining data from both sexes (n = 42/group) and combining animals that were found dead with those that were sacrificed in moribund condition. Log rank tests of survivor function used the Stata STS test to determine statistical significance. Data was plotted using GraphPad Prism 6.07 (La Jolla, CA).
Blood collection and hematology
For pharmacodynamic (hematology) analysis, 4 − 6 mice/sex/dose were pre-assigned to collection timepoints. Blood was collected from isoflurane anesthetized mice via the retro-orbital sinus a maximum of two times per animal at prescheduled times with the final collection being terminal and the collections spaced to minimize survival bias and the stress of multiple collections. Collection cohorts were Days 4 + 8, Days 6 + 10, Days 12 + 18, Days 14 + 25, and Days 22 + 29 such that those in the first cohort of mice were bled before any mortality was expected, and those in the last cohort were bled after the critical survival period had passed. An additional four males and four females non-irradiated, non-injected mice were bled on Day 4 as baseline control samples. Standard hematology parameters were analysed using a Siemens ADVIA® 2120 Hematology System. Data from both sexes were combined with the sample size varying from 1 to 11/group/timepoint because of early moribundity especially in the control and low dose groups. Homogeneity of variance across groups was tested for platelet count using the Stata robar test with linear regression analysis and Benjamini–Hochberg correction for multiple comparisons (False Discovery Rates) and was plotted using Prism 6.07.
Bio- and pharmacokinetic analysis
Blood was collected at terminal sacrifice from 3 mice/sex/dose level at 1, 4, 12, 24, 48, 72, and 96 h after romiplostim administration; processed to serum; and quantified by enzyme-linked immunosorbent assay (ELISA; Syneos Health, Princeton, NJ) using a pair of proprietary capture and detection antibodies and standard blocking, washing, and detection procedures. The assay range was 0.270 ng/ml (lower limit of quantification, LLOQ) to 10.9 ng/ml (upper limit of quantification, ULOQ). The assay was performed 14 times; method reproducibility was demonstrated during incurred sample reanalysis using 31% of the total sample size. Pharmacokinetic parameters and constants using non-compartmental modeling were determined using Phoenix® WinNonlin® v6.3 software (Certara, Princeton, NJ).
Results
Survival benefit of romiplostim in a mouse model of H-ARS
The ability of a single sc administered dose of romiplostim to provide a 30-day survival benefit in a mouse model of H-ARS was investigated. In this model, romiplostim was administered 24 h after the mice received a total body irradiation (TBI) dose that was targeted, based on a yearly institutional lethality profile, to provide 70% lethality over a period of 30 days (LD70/30). Radiation doses of 6.5 Gy for LD30/30 and 6.8 Gy for LD70/30 were based on results from 30-day survival studies conducted at our institution over the past 3 years with combined sexes and the same IACUC approved early removal criteria. The animal numbers used in each of these studies (21/sex/group) were designed to have 80% power (20% probability of making a type II error) to detect with statistical significance a 30% survival benefit between groups using combined sexes (e.g. 30% survival in the control group versus 60% survival in the treatment group).
After a single sc injection of 30 or 100 µg/kg romiplostim on Day 1 (24 h post-irradiation), 57 and 52% of the mice survived to Day 30, respectively, compared with 17% survival in the TBI control mice that received a saline injection instead of romiplostim ( and ). This increase in survival was statistically significant (p ≤ .02, n = 42 per group comprised of 21 males and 21 females). Lower romiplostim dose levels (3 and 10 µg/kg) resulted in 41 and 38% of the mice surviving to Day 30 yet did not show a statistically significant survival benefit over controls. In other words, a single 30 or 100 µg/kg dose of romiplostim provided the same ∼40% increase in 30-day survival compared with controls, whereas lower dose levels provided a more modest ∼20% increase that did not reach the level of statistical significance.
Figure 1. Kaplan–Meier 30-day survival analysis of single and repeat dose romiplostim administration. (A) A single dose of romiplostim 24 h post-irradiation at 30 or 100 μg/kg provided a statistically significant (*p ≤ .01) and similar survival benefit compared with control mice injected with saline. Administration of 3 or 10 μg/kg romiplostim provided a more modest survival increase and did not reach the level of statistical significance. (B) Administration of 30 μg/kg romiplostim for 3 or 5 consecutive days provided the same statistically significant survival benefit over saline administration as did a single romiplostim administration.
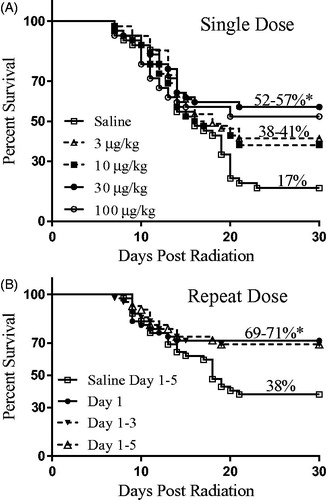
Table 1. 30-Day survival summary.
Consistent with our H-ARS model, survival was higher in each of the female dose groups compared with the males in this study and in the repeat dose study described below. Moreover, although these studies were not powered to detect differences using data from a single sex, statistically significant increases in survival compared with sex-matched controls were seen for both males and females following administration of 30 µg/kg romiplostim ().
Repeat dose romiplostim provided no further survival benefit
The survival benefit provided by three and five consecutive once daily doses of 30 µg/kg romiplostim was also investigated. As shown in and , administration of romiplostim on Days 1 − 3 or Days 1 − 5 provided no additional survival benefit when compared with a single Day 1 romiplostim injection. Specifically, one, three, or five injections of romiplostim beginning 24 h post-irradiation each provided a statistically significant (p ≤ .01) 31 − 33% survival benefit when compared with TBI mice that received only saline injections on Days 1 − 5.
Overall survival levels were higher in this repeat dose study than those seen in the single dose study for both the saline control mice (38 versus 17% survival) and the 30 µg/kg single dose romiplostim-treated mice (71 versus 57% survival). However, the romiplostim survival benefit was similar in these two studies (33 and 40% improvement) and shown to be efficacious in both sexes. Thus, the difference in control group survival may be due to study-to-study variability and/or increased hydration from five daily doses of saline, both of which are known to be confounding variables in mouse ARS survival studies (Booth et al. Citation2012; Plett et al. Citation2012; Plett et al. Citation2015).
Romiplostim plus pegfilgrastim provided no further survival benefit
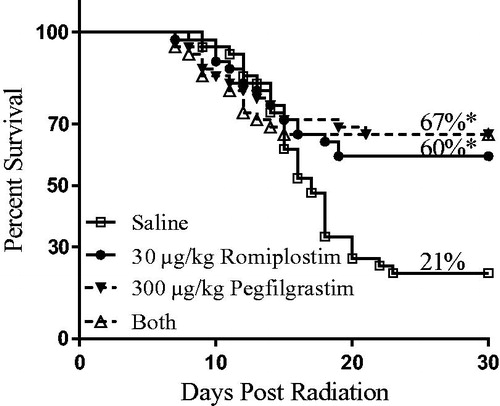
Co-administration of romiplostim and pegfilgrastim was investigated to determine if the combination would provide an additional survival benefit given that these two pharmaceuticals promote differentiation and proliferation of different cell types—platelets by romiplostim (a TPO mimetic) and neutrophils by pegfilgrastim (pegylated G-CSF). A single administration 24 h post-irradiation of 30 μg/kg romiplostim, 300 μg/kg pegfilgrastim, or both 30 μg/kg romiplostim and 300 μg/kg pegfilgrastim provided a similar and statistically significant (p ≤ .01) ∼40% survival benefit compared with mice injected with only saline (, ). Specifically, 60% of TBI mice survived to Day 30 when given romiplostim, 67% of TBI mice survived to Day 30 when given pegfilgrastim alone or pegfilgrastim plus romiplostim, yet only 21% of TBI mice survived to Day 30 when given a saline injection.
Figure 2. Kaplan–Meier 30-day survival analysis of single dose romiplostim and/or pegfilgrastim administration. A single administration 24 h post-irradiation of 30 μg/kg romiplostim, 300 μg/kg pegfilgrastim, or both 30 μg/kg romiplostim and 300 μg/kg pegfilgrastim provided a similar and statistically significant (*p < .01) survival benefit compared with mice injected with saline.
Pharmacodynamics of romiplostim following irradiation
The pharmacodynamic effects of romiplostim on platelets and other hematology parameters were examined in TBI mice that received 0, 3, 30, or 300 µg/kg romiplostim 24 h after a LD30/30 irradiation. A lower level of irradiation (6.5 Gy, LD30/30) was used in this experiment than that used in the survival experiments (6.8 Gy, LD70/30) presented above because (1) hematopoietic injury occurs across a wide range of exposure levels including relatively low levels of radiation (Williams et al. Citation2010), (2) TBI mice become more susceptible to lethality following blood collection (Plett et al. Citation2012), and (3) we wanted to have sufficient numbers of surviving mice at the later post-dose time points for a robust analysis. Because romiplostim boosts platelet levels in humans and animals (Wang B et al. Citation2004; Krzyzanski et al. Citation2013) and boosts survival in irradiated mice as shown above, it was important to investigate if and to what extent romiplostim was affecting platelets in this H-ARS mouse model.
Platelet count
As shown in , platelet levels rapidly dropped after 4 days post-irradiation reaching a nadir in saline injected, irradiated mice on Day 10. Mice that received 30 or 300 µg/kg romiplostim 24 h post-irradiation, had an even more rapid drop in platelet levels post-irradiation reaching a nadir on Day 8 instead of Day 10. Although the drop occurred faster in the romiplostim-treated higher dose level groups, it was less severe reaching a Day 8 nadir after 300 µg/kg romiplostim of 22,778 ± 8843 platelets/µl blood (n = 9, 5 males and 4 females) compared with a Day 10 nadir in the saline injected group of 13,375 ± 2722 platelets/µl blood (n = 8; 4 males and 4 females). Moreover, in the 30 and 300 µg/kg romiplostim-treated groups, platelets returned to near baseline levels (≥400,000 platelets/µl blood) one week earlier (Days 22 versus 29) than that seen in the saline control group. These differences in platelet levels between irradiated romiplostim-treated and saline control mice were statistically significant (p ≤ .002 with Benjamini–Hochberg False Discovery Rates of ≤ 4%) from Days 6 to 22 for the 300 µg/kg group and from Days 6 to 10 and on Day 22 for the 30 µg/kg group (note: the 30 µg/kg group also differed with statistical significance from the saline group on Days 12 − 18 although the p value was higher at ≤.03). The number of mice in the saline and 3 µg/kg groups that survived past Day 14 were limited due to the effects of radiation plus the added stress/trauma of blood collection with none of the mice in the 3 µg/kg romiplostim group surviving past Day 25 (n = 2, 5, 1, and 3 on Days 18, 22, 25, and 29, respectively, for the saline group compared with n = 6 − 11/day throughout for the 30 and 300 µg/kg dose groups). The survival effects of radiation with and without romiplostim and independent of the confounding effects of blood collection could be assessed for this study using the cohort of 6 males and 5 − 6 females per group that was preassigned to collection on Day 22 + 29. Survival on Day 22 in this small cohort of 11 − 12 mice/group that had not been previously bled was 42%, 0%, 82%, and 100% in the 0, 3, 30, and 300 µg/kg romiplostim dose groups, respectively. Although the sample size was small, these results show yet again the survival benefit provided by romiplostim and confirm a radiation dose level greater than the targeted LD30 (i.e. LD58 for the saline control group and LD100 for the 3 µg/kg group).
Figure 3. Platelet count and volume after romiplostim administration in TBI mice. (A) When romiplostim was administered 24 h post-irradiation at 30 and 300 µg/kg, platelet levels dropped faster and returned to near baseline levels sooner compared with irradiated mice injected with saline or 3 µg/kg romiplostim (nadir Day 8 versus Day 10; recovery Day 22 versus Day 29). Statistically significant differences in platelet count were observed during Days 8 − 22 for the 30 and 300 µg/kg groups when compared with the saline controls. (B) Mean platelet volume was larger with statistical significance (arrows) on Days 6 and 8 in TBI mice that received 30 and 300 µg/kg romiplostim than it was in the TBI saline control mice. Non-irradiated untreated control mice (*n = 8) were analysed on Day 4 to provide baseline values; data from male and female mice were combined to determine the group mean ± SD at each time point.
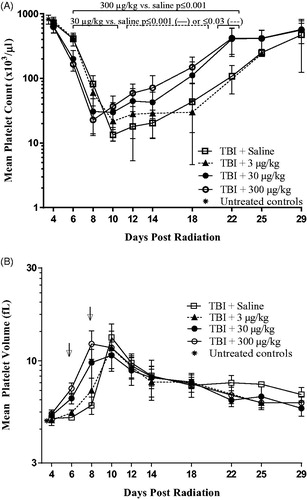
In summary, without romiplostim, platelet levels plateaued at or near the Day 10 nadir for a few days before returning to baseline by ∼ Day 29 in the few survivors whereas with a single Day 1 injection of romiplostim at 30 or 300 µg/kg, there was an earlier return to baseline by ∼ Day 22 with many survivors yet still a plateau at the Day 8 nadir in the 30 µg/kg dose group. Moreover, when 3 µg/kg romiplostim was administered, a level that was too low to promote a statistically significant survival benefit, platelet levels followed a similar pattern to that seen in the saline control mice (i.e. Day 10 nadir, plateau, and late recovery toward baseline).
Mean platelet volume
As shown in , mean platelet volume (MPV) increased after TBI in all groups, including the saline control group, with similar maximum values achieved ∼10 days post-irradiation [mean values of 10.7 − 13.2 femtoliters (fL) with standard deviations (SD) of 1.2 − 2.3 and n = 7 − 9/group]. However, the increase in MPV began and peaked sooner in TBI mice that also received higher doses of romiplostim. Specifically, on Days 6 and 8 post-irradiation, MPV was greater with statistical significance (p ≤ .01) in mice that received 30 or 300 µg/kg romiplostim 24 h after TBI than in the TBI control mice, and peak platelet size was achieved 2 days earlier after 300 µg/kg romiplostim than in the other groups (Days 8 versus 10).
Other hematology parameters
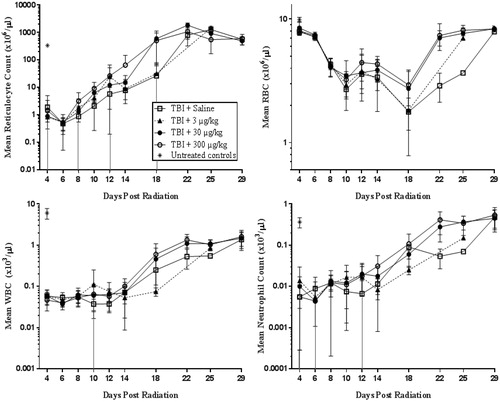
In contrast to the effects of romiplostim on platelets, romiplostim had no notable effect on the level of other hematology parameters such as reticulocytes, red blood cells, white blood cells (WBC), and neutrophils in the irradiated mice (). However, TBI alone affected these parameters, with large decreases in reticulocytes, white blood cells, and neutrophils seen on Day 4 in each of the TBI groups compared with untreated, non-irradiated control mice. Similar to previous results from Plett et al., the rapid decrease in these other blood cell types following TBI occurred a few days earlier than the platelet level decrease, and the effects of radiation on red blood cells was of a much smaller magnitude and similarly had a delayed onset (Plett et al. Citation2012).
Figure 4. The levels of other hematology parameters (reticulocyte, red blood cell, white blood cell, and neutrophil counts) in TBI plus romiplostim-treated mice were not notably different from TBI saline control mice although these parameters were affected by radiation. Non-irradiated untreated control mice (*) were analysed on Day 4 to provide baseline values; data from male and female mice were combined to determine the group mean ± SD at each time point.
Pharmacokinetics of romiplostim following irradiation
To eventually inform a romiplostim dose regimen to treat humans acutely exposed to myelosuppressive doses of radiation, the pharmacokinetics of romiplostim in irradiated animals should be compared with the already well characterized pharmacokinetics in non-irradiated animals and healthy human subjects (Wang et al. Citation2004; Wang et al. Citation2010; Krzyzanski et al. Citation2013). Thus, we used an ELISA to determine the serum concentrations of romiplostim after a single subcutaneous injection of 3, 30, or 300 µg/kg in male and female irradiated mice and in non-irradiated mice that received 30 µg/kg romiplostim. Serum romiplostim levels in all mice injected with 3 µg/kg were less than the assay lower limit of quantification (LLOQ = 0.270 ng/ml or 0.401 ng/ml depending on the assay date). Serum levels of romiplostim were also below the LLOQ after 30 µg/kg romiplostim in all three mice at the following time points post-injection: 1 h in irradiated and non-irradiated males and females; 72 and 96 h in non-irradiated males; and 48 and 72 h in non-irradiated females (but measurable in 1 of 3 non-irradiated females at 96 h). As shown in , the serum concentration after 30 µg/kg administration with and without irradiation were similar. Serum from animals treated with the highest romiplostim dose (300 µg/kg) showed markedly higher levels than those seen after injection of the 30 µg/kg dose.
Figure 5. Serum concentrations of romiplostim after a single sc administration 24 h after irradiation (solid line) and in non-irradiated (dashed line) male and female mice. (▲) 30 µg/kg romiplostim, no TBI; (●) 30 µg/kg romiplostim + TBI; (■) 300 µg/kg romiplostim + TBI. Error bars indicate SD of the mean. N = 3 except when values were < LLOQ [n = 1 for non-irradiated (▲) males at 24 h and females at 96 h; n = 2 for non-irradiated (▲) males at 48 h and 30 µg/kg romiplostim + TBI (●) males at 4 and 24 h]. Values < LLOQ not shown and not included in the mean.
![Figure 5. Serum concentrations of romiplostim after a single sc administration 24 h after irradiation (solid line) and in non-irradiated (dashed line) male and female mice. (▲) 30 µg/kg romiplostim, no TBI; (●) 30 µg/kg romiplostim + TBI; (■) 300 µg/kg romiplostim + TBI. Error bars indicate SD of the mean. N = 3 except when values were < LLOQ [n = 1 for non-irradiated (▲) males at 24 h and females at 96 h; n = 2 for non-irradiated (▲) males at 48 h and 30 µg/kg romiplostim + TBI (●) males at 4 and 24 h]. Values < LLOQ not shown and not included in the mean.](/cms/asset/0125d994-7b16-4536-be58-c2e6387993de/irab_a_1605465_f0005_b.jpg)
The pharmacokinetics of romiplostim were determined using the serum drug level data and are presented in . The Tmax was 12 and 24 h, for males and females, respectively, after a 30 µg/kg dose with or without irradiation and resulted in mean Cmax values of about 5–7 ng/ml. The Tmax was earlier at 4 h for males and 12 h for females after the 300 μg/kg dose and resulted in mean Cmax values of 201 and 184 ng/ml for males and females, respectively. Romiplostim was eliminated with t1/2 of 24.3–29.9 h. Given the small sample size and with some samples below the LLOQ in the 30 µg/kg dose groups, it remains unclear if there are sex-based differences in clearance. The differences in mean AUClast values between the irradiated and non-irradiated 30 µg/kg dose groups were likely the result of individual animal variability rather than due to radiation exposure. Increasing the dose of romiplostim by 10-fold from 30 to 300 µg/kg resulted in circulating serum drug levels that were 30- to 40-fold higher. In other words, exposure based on AUClast increased with dose level, although the ∼35-fold increase in exposure was higher than the 10-fold increase in the amount of romiplostim that was administered.
Table 2. Pharmacokinetic parameters of romiplostim subcutaneously administered to irradiated and non-irradiated mice.
Discussion
The results presented herein demonstrate that a single subcutaneous dose of romiplostim administered 24 h after a lethal, total body dose of X-ray radiation increased 30-day survival in mice and hastened platelet recovery. Although G(M)-CSFs have received regulatory approval to increase survival and treat neutropenia post-irradiation, romiplostim is a promising, much needed medical countermeasure because thrombocytopenia contributes to the increased risk of lethal bleeding in acute radiation syndrome. Indeed, Stickney et al. (Citation2007) reported that the duration of thrombocytopenia more accurately predicted mortality in irradiated rhesus monkeys than the duration of neutropenia, suggesting that thrombocytopenia may be more clinically relevant to survival in H-ARS than has been previously recognized.
The survival benefit provided by a single dose of romiplostim to irradiated mice presented herein is consistent with and extends recently published results (Yamaguchi et al. Citation2018). Yamaguchi et al. tested romiplostim following 137Cs γ-ray irradiation in only female mice with administration by the intraperitoneal route instead of the more clinically relevant sc route. Like us, they showed that 1, 3 and 5 once daily doses of romiplostim were all effective. However, in our studies which delivered 30 µg/kg romiplostim beginning 24 h post-irradiation, there was no further increase in survival following repeat dose administration while their optimal dose regimen included administration for three consecutive days at 50 µg/kg 2 h post-irradiation. Although they showed that administration 2 h post-irradiation was more effective than waiting 24 h post-irradiation, it is unlikely that romiplostim could be administered earlier than 24 h following radiation exposure during a mass casualty event. Furthermore, the ability to promote survival after only a single sc administration is highly attractive for logistical considerations given the large number of people that might be exposed following a radiologic incident. Their result that waiting 48 h post-irradiation to administer romiplostim provided no survival benefit is worrisome for practical use but worthy of further exploration and was not examined in our investigation.
In the studies described herein, survival was almost always higher in the females than the males of each dose group. This sex difference is a general feature of the H-ARS model and not attributed to different effects of romiplostim on males versus females. Using the same 6.8 Gy dose for both sexes consistently results in more males than females meeting the early removal criteria. Although the studies were not powered for single sex analysis, it is curious that survival in males was equal to or greater than that in females only in the pegfilgrastim-treated animals and always lower in males than females in the control and romiplostim-treated animals. This potential sex difference is worthy of further investigation.
As predicted, a single administration of romiplostim decreased the severity and duration of thrombocytopenia in lethally TBI mice in a dose dependent manner. Curiously, irradiated mice administered efficacious levels of romiplostim (30 and 300 µg/kg) had a quicker drop in circulating platelet levels than the irradiated control mice. Four days post-TBI, platelet levels were similar across groups and at levels seen in non-irradiated animals after which the drop in platelet levels from Days 4 to 6 appeared to be particularly steep in the romiplostim treated mice compared to those that received saline. It remains unknown how romiplostim might be causing this initial more rapid drop in platelets, but it is encouraging that platelet levels reach a shallower nadir in the high dose romiplostim-treated group followed by a robust and quicker increase to near baseline levels than that seen in the controls.
The observation of a quicker increase in MPV after romiplostim administration in TBI mice is consistent with results from non-irradiated healthy mice administered TPO or TPO mimetics (Levin et al. Citation1982; Daw et al. Citation1998). For example, administration of PEG-rmMGDF (a pegylated, truncated, recombinant form of TPO) to C57BL/6J mice increased MPV relative to controls with peak values 4 days post-injection followed by a return to baseline ∼2 days later (Daw et al. Citation1998). Increases in platelet size are also seen in disease-states associated with increased platelet turnover (e.g. in patients with immune thrombocytopenia, recovering after chemotherapy, or with severe arterial disease) (Handtke et al. Citation2018). Thus, the quicker increase in MPV following romiplostim administration in irradiated mice compared with irradiated control mice is supporting evidence that romiplostim is acting similarly in irradiated and non-irradiated mice to speed up the release of platelets from the bone marrow into circulation.
We showed there was no further survival benefit to lethally irradiated mice when a single dose of romiplostim, which acts to address thrombocytopenia, is combined with a single dose of pegfilgrastim, which acts to address neutropenia. However, we acknowledge that this experiment did not closely examine potential changes in hematopoietic cell lineage in these co-administered mice and that a mechanistic analysis following co-administration would be important to understand if there are short or long-term benefits (or detriments) to co-administration. Hirouchi et al. showed 100% 30-day survival in lethally irradiated mice after G-CSF, erythropoietin, and romiplostim were co-administered each for 5 days beginning 2 h post-irradiation compared with 63% survival when romiplostim was withheld from the dosing regimen (Hirouchi et al. Citation2015). These investigators saw no significant difference in the composition of the peripheral blood or bone marrow progenitor cells between these two groups although they only interrogated surviving mice on Day 30 which is after the recovery from both thrombocytopenia and neutropenia. Thus, testing the efficacy, performing concurrent hematology assessments, and monitoring for hemorrhages following administration of romiplostim, alone and in combination with G(M)-CSFs, in a larger animal model, such as non-human primates, would be a critical next step in the development of romiplostim for eventual clinical use for H-ARS.
In summary, our data indicate that romiplostim is a promising radiation countermeasure that dramatically increased survival and hastened platelet recovery in irradiated mice after a single sc injection delivered 24 h post-irradiation.
Acknowledgements
The authors thank Raquica Butler and her team at Syneos Health for the bioanalytical analysis of romiplostim in serum.
Disclosure statement
The authors from SRI International, D.I Bunin, J. Bakke, C.E. Green, H.S. Javitz and P.Y. Chang, report no conflicts of interest. M. Fielden is an Amgen, Inc. employee and stockholder.
Additional information
Funding
Notes on contributors
Deborah I. Bunin
Deborah I. Bunin, Director Molecular Toxicology at SRI Biosciences, conducts non-clinical safety toxicology and efficacy studies to advance radiation medical countermeasures, vaccines, biologics, and other therapeutics toward regulatory approval.
James Bakke
James Bakke, Research Scientist/Study Director at SRI Biosciences, conducts radiation model development, efficacy studies to advance radiation medical countermeasures and non-clinical safety toxicology of small molecule drugs, vaccines, biologics and medical devices toward regulatory approval.
Carol E. Green
Carol E. Green, Senior Director, Toxicology and Pharmacokinetics at SRI Biosciences, manages preclinical development programs, interprets toxicity data results, and performs pharmacokinetic and toxicokinetic data analysis.
Harold S. Javitz
Harold S. Javitz, Senior Biostatistician at SRI conducts statistical analyses for toxicological studies, efficacy studies of radiation medical countermeasures, medical devices for screening for radiation exposure, efficacy and cost analyses for smoking cessation interventions, and sleep studies.
Mark Fielden
Mark Fielden, Scientific Director, Comparative Biology and Safety Sciences, at Amgen, conducts nonclinical safety toxicology studies to support clinical development and approval of therapeutics.
Polly Y. Chang
Polly Y. Chang, received her Ph.D. in Biophysics from the University of California, Berkeley. She is currently the Senior Director of Molecular and Genetic Toxicology at SRI International and is actively engaged in radiation biology research coupled with product development of medical countermeasures for radiation protection.
References
- Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. 2012. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 103:383–399.
- Broudy VC, Lin NL. 2004. AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl. Cytokine. 25:52–60.
- Chua HL, Plett PA, Sampson CH, Katz BP, Carnathan GW, MacVittie TJ, Lenden K, Orschell CM. 2014. Survival efficacy of the PEGylated G-CSFs Maxy-G34 and neulasta in a mouse model of lethal H-ARS, and residual bone marrow damage in treated survivors. Health Phys. 106:21–38.
- Daw NC, Arnold JT, Abushullaih BA, Stenberg PE, White MM, Jayawardene D, Srivastava DK, Jackson CW. 1998. A single intravenous dose of murine megakaryocyte growth and development factor potently stimulates platelet production, challenging the necessity for daily administration. Blood. 91:466–474.
- Farese AM, Cohen MV, Stead RB, Jackson W, 3rd, Macvittie TJ. 2012. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat Res. 178:403–413.
- Gellens R, Habchi S, Freppel S, Couret D, Iacobelli S. 2017. Romiplostim for the emergency management of severe immune thrombocytopenia with intracerebral hemorrhage. Front Neurol. 8:737.
- Handtke S, Steil L, Greinacher A, Thiele T. 2018. Toward the relevance of platelet subpopulations for transfusion medicine. Front Med (Lausanne). 5:17.
- Hankey KG, Farese AM, Blaauw EC, Gibbs AM, Smith CP, Katz BP, Tong Y, Prado KL, MacVittie TJ. 2015. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat Res. 183:643–655.
- Hirouchi T, Ito K, Nakano M, Monzen S, Yoshino H, Chiba M, Hazawa M, Nakano A, Ishikawa J, Yamaguchi M, et al. 2015. Mitigative effects of a combination of multiple pharmaceutical drugs on the survival of mice exposed to lethal ionizing radiation. Curr Pharm Biotechnol. 17:190–199.
- Krzyzanski W, Sutjandra L, Perez-Ruixo JJ, Sloey B, Chow AT, Wang YM. 2013. Pharmacokinetic and pharmacodynamic modeling of romiplostim in animals. Pharm Res. 30:655–669.
- LEUKINE® (sargramostim) [product monograph]: Bridgewater, NJ: Sanofi-Aventis US Inc.
- Levin J, Levin FC, Hull DF, 3rd, Penington DG. 1982. The effects of thrombopoietin on megakaryocyte-cfc, megakaryocytes, and thrombopoiesis: with studies of ploidy and platelet size. Blood. 60:989–998.
- Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, Kuter DJ. 2001. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 98:3241–3248.
- Molineux G, Newland A. 2010. Development of romiplostim for the treatment of patients with chronic immune thrombocytopenia: from bench to bedside. Br J Haematol. 150:9–20.
- National Library of Medicine, National Institues of Health, U.S. Department of Health and Human Services, Radiation Emergency Medical Management. 2018. Myeloid Cytokines for Acute Exposure to Myelosuppressive Doses of Radiation (Hematopoietic Subsyndrome of ARS). ; [updated June 30, 2018; accessed January 18, 2019]. https://www.remm.nlm.gov/cytokines.htm.
- NEULASTA® (pegfilgrastim) [product monograph]: Thousand Oaks, CA: Amgen Inc.
- NEUPOGEN® (filgrastim) [product monograph]: Thousand Oaks, CA: Amgen Inc.
- NPLATE® (romiplostim) [product monograph]: Thousand Oaks, CA: Amgen Inc.
- Plett PA, Sampson CH, Chua HL, Jackson W, Vemula S, Sellamuthu R, Fisher A, Feng H, Wu T, MacVittie TJ, et al. 2015. The H-ARS dose response relationship (DRR): validation and variables. Health Phys. 109:391–398.
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, et al. 2012. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 103:343–355.
- Provan D, Newland A. 2007. Romiplostim. Drugs Fut. 32:781–787.
- Singh VK, Newman VL, Seed TM. 2015. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine. 71:22–37.
- Singh VK, Seed TM. 2017. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int J Radiat Biol. 93:851–869.
- Stickney DR, Dowding C, Authier S, Garsd A, Onizuka-Handa N, Reading C, Frincke JM. 2007. 5-androstenediol improves survival in clinically unsupported rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol. 7:500–505.
- U.S. Department of Health and Human Services Press Office. 2016. HHS enhances nation's health preparedness for radiological threats. ; [updated October 6, 2016; accessed January 18, 2019]. https://wayback.archive-it.org/3926/20170128052606/https://www.hhs.gov/about/news/2016/10/06/hhs-enhances-nation-s-health-preparedness-radiological-threats.html.
- Wang B, Nichol JL, Sullivan JT. 2004. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther. 76:628–638.
- Wang YM, Krzyzanski W, Doshi S, Xiao JJ, Perez-Ruixo JJ, Chow AT. 2010. Pharmacodynamics-mediated drug disposition (PDMDD) and precursor pool lifespan model for single dose of romiplostim in healthy subjects. AAPS J. 12:729–740.
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, et al. 2004. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 140:1037–1051.
- Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason KA, Medhora MM, et al. 2010. Animal models for medical countermeasures to radiation exposure. Radiat Res. 173:557–578.
- Yamaguchi M, Hirouchi T, Yokoyama K, Nishiyama A, Murakami S, Kashiwakura I. 2018. The thrombopoietin mimetic romiplostim leads to the complete rescue of mice exposed to lethal ionizing radiation. Sci Rep. 8:10659.
