Abstract
Purpose: To evaluate the efficacy of boron neutron capture therapy (BNCT) for a heterotopic U87 glioblastoma model in SCID mice using boron phenylalanine (BPA), sodium borocaptate (BSH) and liposomal BSH as boron compounds at a unique, accelerator-based neutron source.
Materials and methods: Glioblastoma models were obtained by subcutaneous implantation of U87 cells in the right thighs of SCID mice before administration of 350 mg/kg of BPA (BPA-group), 100 mg/kg of BSH (BSH-group) or 100 mg/kg of BSH in PEGylated liposomes (liposomal BSH-group) into the retroorbital sinus. Liposomes were prepared by reverse-phase evaporation. Neutron irradiation was carried out at a proton accelerator with a lithium target developed for BNCT at the Budker Institute of Nuclear Physics, Novosibirsk, Russian Federation. A proton beam current integral of 3 mA/h and energy of 2.05 MeV were used for neutron generation.
Results: Boron compound accumulation in tumor tissues at the beginning of irradiation was higher in the BPA group, followed by the Liposomal BSH and BSH groups. Tumor growth was significantly slower in all irradiated mice from the 7th day after BNCT compared to untreated controls (p < .05). Tumor growth in all treated groups showed no large variation, apart from the Irradiation only group and the BPA group on the 7th day after BNCT. The overall trend of tumor growth was clear and the differences between treatment groups became significant from the 50th day after BNCT. Tumor growth was significantly slower in the Liposomal BSH group compared to the Irradiation only group on the 50th (p = .012), 53rd (p = .005), and the 57th (p = .021) days after treatment. Tumor growth in the Liposomal BSH group was significantly different from that in the BPA group on the 53rd day after BNCT (p = .021) and in the BSH group on the 50th (p = .024), 53rd (p = .015), and 57th (p = .038) days after BNCT. Skin reactions in the form of erosions and ulcers in the tumor area developed in treated as well as untreated animals with further formation of fistulas and necrotic decay cavities in most irradiated mice.
Conclusions: We observed a tendency of BNCT at the accelerator-based neutron source to reduce or suspend the growth of human glioblastoma in immunodeficient animals. Liposomal BSH showed better long-term results compared to BPA and non-liposomal BSH. Further modifications in liposomal boron delivery are being studied to improve treatment outcomes.
Introduction
Human glioblastoma is the most common of all glial tumors, being the most malignant and one of the most radioresistant (Ostrom et al. Citation2014). The standard treatment for glioblastoma is a combination of surgical resection and adjuvant radiotherapy and chemotherapy (Kobyakov et al. Citation2016). However, despite advances in modern therapies and complex, multidisciplinary treatments, median overall survival is only about 15 months after diagnosis (Zanders et al. Citation2019).
The causes of glioblastoma resistance to various methods of treatment are uncontrolled cell proliferation, dysregulation of apoptosis, severe tumor invasion and angiogenesis (Byvaltsev et al. Citation2015).
To combat this resistance, boron neutron capture therapy (BNCT) is a promising direction in the treatment of brain tumors. It was designed to be binary, first proposed by Locher (Citation1936), and it is based on the interaction of two relatively harmless components: a 10B-nucleus and a thermal neutron. Selective accumulation of 10B inside tumor cells and subsequent irradiation with epithermal neutrons results in destruction of only tumor cells. At the same time, surrounding healthy cells are not exposed to the damaging effects of radiation mainly due to lesser boron accumulation (Locher Citation1936). Clinical trials conducted at nuclear reactors showed the efficacy of BNCT in treatment of brain tumors, including glioblastomas, as well as in soft tissue tumors, tumors of parenchymal organs and skin cancer (Iarullina et al. Citation2015; Barth et al. Citation2018).
Neutrons in a narrow energy range (near the upper boundary of the epithermal range, from 0.5 eV to 30 keV) with a high flux density of 109 cm−2 s−1 are necessary for BNCT, and, until recently, the only neutron sources meeting these required parameters were specialized or remodeled nuclear reactors (Sauerwein et al. Citation2012). However, construction of accelerators that could be used to conduct BNCT started in the 1990s (Anderson et al. 1994) and, to date, several prototypes of purpose-built installations have already been created in different countries (Taskaev et al. Citation2018; Taskaev Citation2019). The advantage of using an accelerator when conducting BNCT is the high content of epithermal neutrons in the irradiation beam (Kanygin et al. 2015) which makes it possible to achieve maximum therapeutic effects with minimal negative effects of radiation. One of the most advanced BNCT installations exists at the Budker Institute of Nuclear Physics in Novosibirsk Science City, which is based on a tandem accelerator with vacuum insulation and a lithium target (Taskaev Citation2015). Due to its unique construction, a stationary proton beam with an energy of 2 MeV and a current of up to 5 mA was obtained at the facility (Ivanov et al. Citation2016), sufficient to achieve successful in vitro and in vivo BNCT results (Zaidi et al. Citation2017; Sato et al. Citation2018).
For successful BNCT, a compound enriched in the 10B isotope is required, which needs to be accumulated in tumor tissue at concentrations greater than 20 µg/g, with a concentration ratio of 3:1 (tumor to normal tissue) or higher during the irradiation procedure (Sauerwein et al. Citation2012). Currently, sodium borocaptate (BSH) and boron phenylalanine (BPA) are the 10B compounds most widely used in clinical trials (Sauerwein et al. Citation2012). However, these drugs, as shown by numerous studies, do not have sufficient selectivity and studies on development of new 10B compounds capable of selective accumulation in tumor tissues are being carried out worldwide in a two-pronged approach. On the one hand, development of new boron-containing drugs with suitable BNCT characteristics are ongoing while, on the other hand, technologies are being developed for targeted delivery of boron-containing compounds that can pass through the blood brain barrier (BBB) (Barth et al. 2018).
To this end, this study investigates the efficacy of BNCT for human U87 glioblastoma using a heterotopic (subcutaneous) tumor model in SCID mice at an accelerator-based neutron source with liposomal BSH delivery in comparison with its free form and BPA.
Materials and methods
Laboratory animals
SCID mice aged 8–12 weeks (SHO-PrkdcscidHrhr) and under SPF status were used in this study. The experimental animals were kept in individually ventilated cages by family groups of 2–5 individuals in the SPF Vivarium of the Institute of Cytology and Genetics of the SB RAS at a temperature of 22–26 °C, at a relative humidity of 30–60% and a light/dark mode of 14/10 with dawn at 01:00. Food and water were provided to animals ad libitum.
Tumor cells
U87 human glioblastoma cells were obtained from the ‘Center for Genetic Resources of Laboratory Animals’ of the Institute of Cytology and Genetics of the Russian Academy of Sciences, Novosibirsk, Russian Federation. Cells were cultivated in DMEM/F12 (1:1) (Biolot, St.-Petersburg, Russia) supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and gentamicin 50 µg/ml (Dalkhimpharm, Khabarovsk, Russia) at 37°С and 5% CO2.
Tumor model preparation
Three weeks prior to irradiation experiments, the tumor cells were trypsinized, centrifuged and carefully resuspended in serum-free medium at a concentration of 100 000 cells/1 µl. This cell suspension was injected subcutaneously in the right thighs of experimental animals in a volume of 100 µl (10 million cells per animal) to seed solid U87 glioblastoma tumors.
Boron compounds
BPA and BSH (Katchem, Prague, Czech Republic), as well as the PEGylated liposomal form of BSH, were used as boron compounds. To prepare the BPA solution, fructose was used in molar excess according to the protocol by Rossini et al. (Citation2015). Liposomes were obtained by phase inversion followed by extrusion with the lipid phase of DSPC, DSPE-PEG, and CH used in a 2:1:0.11 molar ratio (Maruyama et al. Citation2004). One gram of this lipid mixture was dissolved in 22 ml of a solution consisting of chloroform and diethyl ether in an equal volume ratio. After that, 8 ml of a 125 mM BSH aqueous solution was added dropwise to the resulting solution, and the resulting emulsion was sonicated for 1 min. Then, the emulsion was placed in a rotary evaporator and the organic solvents were removed for 60 min under a working vacuum at a temperature of 30 °C. To obtain liposomes with a uniform size distribution, the obtained gel was extruded through a polycarbonate membrane with a pore size of 100 nm using an extruder (Lipex Biomembranes, Vancouver, BC, Canada) at 60 °C. Unencapsulated free BSH was removed by ultracentrifugation at 200,000×g for 20 min at 4 °C (Optima MAX-XP; Beckman Coulter) and the suspension was resuspended in saline. The size and charge of the obtained particles were measured using a ZetasizerNano ZS90 (Malvern Panalytical Ltd., Malvern, UK).
Boron compound biodistribution
All boron compounds were injected intravenously into the retroorbital sinus (Yardeni et al. Citation2011). To assess boron distribution in tissues, animals were divided into 3 groups, each receiving either BSH at a dose of 100 mg/kg, BPA at a dose of 350 mg/kg, or Liposomal BSH at a dose of 100 mg/kg. The animals were sacrificed at different time intervals after compound injection (1, 2, 3, and 4 h for BSH and BPA; 24 h for liposomal BSH). Blood, tumor, brain, kidney and liver tissues were sampled and processed as previously reported (Zaboronok et al. Citation2015) and analyzed for boron concentration using an iCAP6500 ICP optical emission spectrometer (ThermoFisher Scientific, Inc., Waltham, MA, USA.).
Irradiation experiments
For irradiation experiments, animals were divided into 5 groups: the 1st group received BSH at a dose of 100 mg/kg 2 h before irradiation (Sauerwein et al. Citation2012); the 2nd group received BPA at a dose of 350 mg/kg 1.5 h before irradiation (Carpano et al. Citation2015); group 3 received a liposomal form of BSH at a dose of 100 mg/kg 24 h before irradiation (Barth et al. Citation2018); the 4th group of mice was irradiated without the administration of boron compounds; and the 5th group was the untreated control that was not exposed to radiation or boron compounds.
Irradiation was carried out 3 weeks after orthotopic xenotransplantation of U87 human glioblastoma cells to mice thighs when tumor volumes reached 90–110 mm3 (Kanygin et al. Citation2017). Tumor sizes were monitored using high-field magnetic resonance (MR) imaging (11.7 T, BioSpec 117/16 USR, Bruker, Billerica, MA, USA) by directly estimating the linear dimensions of the tumor using T1- and T2-weighted MR imaging techniques. The final tumor size values were calculated using Paravision 5.0 software (Bruker, Billerica, MA, USA) based on contrast differences between the surrounding tissues and the tumor.
To carry out the irradiation procedure, the animals were anesthetized by sequential, intraperitoneal administration of medetomidine hydrochloride (Domitor, Orion Pharma, Moscow, Russia) and tiletamine hydrochloride (Zoletil, Virbac Sante Animale, Carros, France).
For irradiation, the animals were transported to the accelerator-based neutron source at the Budker Institute of Nuclear Physics in a thermally insulated container with ventilation. The source of epithermal neutrons included an electrostatic tandem accelerator with vacuum insulation, a lithium neutron-generating target, and a neutron beam shaping assembly (Bayanov et al. Citation2006; Zaidi et al. Citation2017). Detailed parameters of the neutron-producing accelerator are described previously (Kasatov et al. Citation2016; Zaidi et al. Citation2018). The accelerator is designed to deliver an epithermal neutron beam for clinical use in humans. Therefore, to irradiate mice, a special plexiglass moderator was positioned between the target and the mice to ensure maximum thermal neutron density in the tumor area.
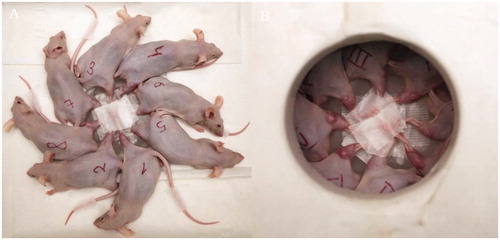
The mice were placed radially in a 7.5% lithium polyethylene container with fixation of the right thigh to the center of the container (). The lid of the container covered their bodies, exposing only the leg with the tumor, which reduced radiation exposure to non-target areas. The container was located at a distance of 0.5 cm from the target with 7.4 cm of plexiglass attached. The animals were irradiated for 90 min as a single irradiation by a neutron beam with the following accelerator operation parameters: proton energy – 2.05 MeV, current integral – 3 mAh.
Figure 1. Mice were placed radially with fixation of the right thigh to the center of the container (A). The lid of the container covered their bodies, leaving the leg with the tumor exposed (B) thereby reducing radiation exposure to the main body.
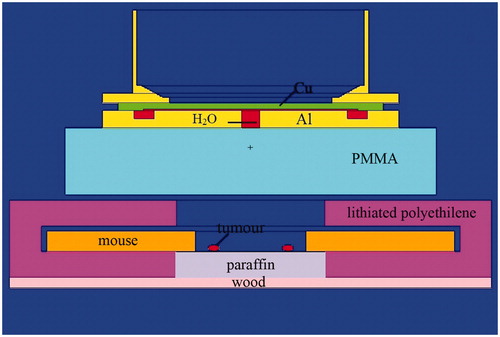
The experimental model was compiled and the doses were calculated using the NMC code to simulate the transport of particles by the Monte Carlo method (Brednikhin et al. Citation2012). When calculating the irradiation doses for each dose component, we used the kerma coefficients introduced by Goorley et al. (Citation2002). The compound biological effectiveness (CBE) for healthy tissues was considered as 1.35 and −3.8 for the tumor tissue (Hiratsuka and Fukuda Citation2012). In the experimental model (), the mice were set as a hollow cylinder of water with an outer and an inner radius of 11 and 3 cm, a height of 2 cm and an assumed mass of 26 g. The density of the cylinder that modeled the mice was calculated taking into account its mass and volume. Tumors were defined as water spheres with a radius of 0.3 cm at a distance of 2 cm from the center of the box.
Figure 2. Model of the experiment. The mice were set as a hollow cylinder of water with an outer and an inner radius of 11 and 3 cm, a height of 2 cm and an assumed mass of 26 g. The density of the cylinder that modeled the mice was calculated taking into account its mass and volume. Tumors were defined as water spheres with a radius of 0.3 cm at a distance of 2 cm from the center of the box. PMMA: Poly(methyl methacrylate), or plexiglass.
After irradiation, the animals were kept at the SPF-Vivarium of the Institute of Cytology and Genetics of the SB RAS with initial family groups of 2–5 individuals. The condition of the mice was recorded daily. In particular, changes in skin, motor activity, behavior, and body weight were evaluated. During observation, the tumor volume was measured with a caliper 3 times a week with an interval of 1–2 days and calculated using the formula: V = length × (width2/2).
A dynamic assessment of tumor growth was performed. Due to development of ulcers, fistulas and decay cavities in the tumor area, in some cases tumor volume measurement was technically impossible. For ethical reasons, mice with a tumor volume of 3 cm3 or more were euthanized. Thus, the tumor growth comparison is shown from the day of irradiation until the 29th day for the untreated control group, until the 57th day for all irradiated mice, and with the data of at least 4 animals from each group. On Day 65 after BNCT, all animals underwent planned euthanasia. The experiments were performed in compliance with the relevant directives of the European Community (86/609/EEC).
Statistical analysis
Statistical analysis of experimental data was performed using SPSS Statistics Version 26 (IBM Corporation, Armonk, NY, USA) and STATISTICA 8 software (Dell, Round Rock, TX, USA). The differences between the experimental groups were analyzed using repeated measures analysis of variance (RMANOVA) with Bonferroni post hoc testing and the non-parametric Mann–Whitney test. The tumor volume ratios to the initial size are given as means ± SEs. p < .05 was considered as statistically significant.
Results
Boron accumulation
Boron biodistribution over time after compound injection is shown in . Boron accumulation in tumor tissues at the beginning of irradiation was higher in the BPA group, followed by the Liposomal BSH and Non-liposomal BSH groups.
Table 1. Boron (10B) biodistribution in tissues after boron compound injection.
Absorbed doses
Boron doses are shown in . Boron accumulation determined the distribution of boron doses in tissues. Additionally, according to the results of calculations and without taking into account the boron dose, the tumor and the leg received 5.3 Gy-Eq and the body of the mouse during this time received 1.7 Gy-Eq.
Table 2. Boron-related absorbed doses (Gy-Eq) in tissues after irradiation. In addition to boron doses, the tumor and the leg received 5.3 Gy-Eq and the body of the mice during that time received 1.7 Gy-Eq.
Tumor growth
Tumor growth was analyzed as tumor size ratio to the initial size in all irradiated groups and the untreated control group from irradiation day until the 29th day (). Tumor growth was significantly slower in Irradiation only, BPA, and BSH groups on the 5th day after BNCT compared to the control group (#p < .05). Tumor growth was significantly slower in all irradiated mice from the 7th day after BNCT compared to untreated controls (*p < .05; **p < .01). In general, the differences in tumor growth in all treated groups during the first month after the irradiation was insignificant, apart from the Irradiation only group and the BPA group on the 7th day after BNCT (&p < .05). All p-values were calculated by RMANOVA.
Figure 3. Dynamics of subcutaneous U87 tumor volume ratios to the initial size in animals from different groups during the first 29 days (A) and during 31–57 days (B) after irradiation. Data are presented as means ± SEs. The difference in tumor volume ratios between the control group and irradiation only, BPA, and BSH groups, #p < .05; between the irradiation only and the BPA groups, &p < .05; and differences between the control group and all irradiated groups from the 7th day after BNCT, *p < .05, **p < .01 (A). Differences in tumor volume ratios between the irradiation only and the liposomal BSH groups, *p < .05, **p < .01; between the liposomal and non-liposomal BSH groups, #p < .05; and the difference between the BPA and the liposomal BSH groups, &p = .021 (B). p-values by RMANOVA.
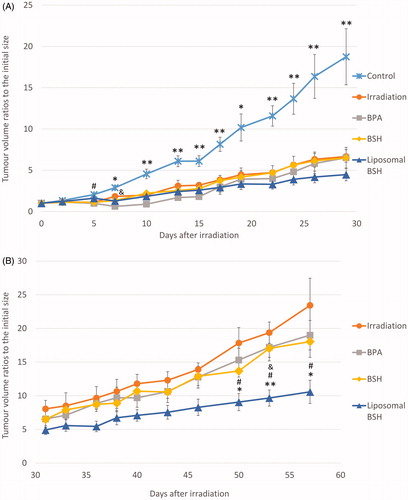
On , tumor growth ratios to the initial size in all irradiated groups from the 31st till 57th day after BNCT are shown. Though the overall tendency of tumor growth was clear, the differences between the treatment groups became significant from the 50th day after BNCT. Tumor growth was significantly slower in the Liposomal BSH group compared to the Irradiation only group on the 50th (p = .012), 53rd (p = .005), and the 57th (p = .021) days after treatment (on , *p < .05; **p < .01). Tumor growth in the Liposomal BSH group was significantly different from that in the BPA group on the 53rd day after BNCT (& p = .021) and differed from the tumor growth in the BSH group on the 50th (p = .024), 53rd (p = .015), and 57th (p = .038) days after BNCT (on , #p < .05). All p-values were calculated by RMANOVA.
Skin reactions with and without irradiation
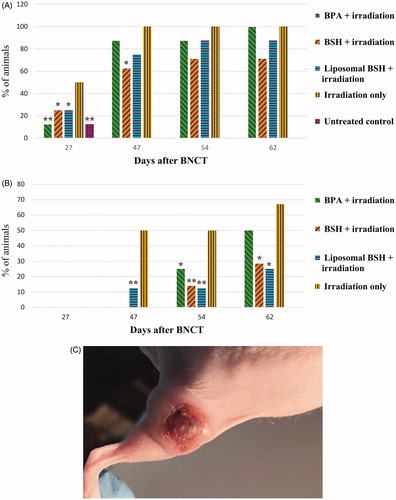
Starting from the 27th day, skin reactions in the form of erosions (loss of epidermis without an associated loss of dermis; Yancey and Lawley Citation2018) and ulcers (sores on the skin accompanied by the disintegration of tissue and complete loss of the epidermis with a break in the basement membrane; Suliman et al. Citation2017) in the tumor area developed in most treated as well as in untreated animals (). Reaching their maximum manifestation 40–45 days post-BNCT, erosions and ulcers further developed into fistulas (here: abnormal connections or passageways between the inner parts of the tumors and the skin surface (modified from Garefalakis et al. Citation2017), and decay cavities in the tumor area in most irradiated animals resulting from tumor tissue necrosis (). Skin erosions, ulcers and fistulas occurred only in the tumor area while the skin on other areas of the irradiated legs, as well as shielded parts of the body, was intact (). Apart from these side effects, the overall clinical progression of the animals was uneventful.
Figure 4. The presence of an ulcer in the tumor area (A), *p ≤ .05, **p ≤ .01 – significant differences with respect to the animals irradiated without boron compounds, chi-square test; the presence of fistulas and decay cavities (B), *p ≤ .01, **p ≤ .001 – significant differences with respect to the animals irradiated without boron compounds, chi-square test, photo of ulcer in the tumor area (C). Statistical significance by the Mann–Whitney test.
Discussion
In all groups of irradiated animals, a decrease in tumor size and a slowdown of tumor growth were noted in contrast to the untreated non-irradiated controls up to 29 days after irradiation. Maximum tumor growth was observed in untreated animals without irradiation, where tumors in all animals exceeded the volume of 3 cm3, and therefore that group was withdrawn from the experiment at the 30th day after irradiation. The average increase in tumor volume in the treated groups during the first month of observation after irradiation was approximately 3 times lower compared to the untreated controls whereas, in the second month after irradiation, more prominent tumor growth was observed in all treated groups.
In general, the tumor growth suppression effect observed in the current study was consistent with the results of previous in vitro studies (conducted at the same accelerator at the Budker Institute of Nuclear Physics) which showed a significant suppressive effect of BNCT on the growth of U251 human glioma cells (Zaidi et al. Citation2017), T98-G, CHO-1K, and V-79 tumor cells incubated with BPA (Sato et al. Citation2018). In many respects, this is similar to an in vivo study of the growth of a subcutaneously implanted U87 tumor in Nu/J mice after BNCT (Kanygin et al. Citation2019).
In a previous study by Kanygin et al. (Citation2019), 100 mg/kg of BSH was injected in the retroorbital sinus of Nu/J mice bearing subcutaneous U87 human glioma 1.5 h before neutron irradiation. This resulted in a demonstrable therapeutic effect of BNCT as significant tumor growth suppression occurred in the treatment group compared to untreated controls (as observed from the 4th day after irradiation) with a 30% survival increase in the BSH-treated group.
The results of the current study showed that, compared to the BPA and BSH groups, tumor growth was significantly suppressed in the Liposomal BSH group at the end of the observation period. Additionally, apart from the Liposomal BSH group, tumor growth in the other two treatment groups varied only slightly from the Irradiation only group, possibly because of a relatively low accumulation of non-liposomal BSH in the tumor tissue and possible wash out of BPA from the tumor along the course of BNCT. Liposomal BSH demonstrated a comparatively better tumor growth suppression most likely due to prolonged boron retention in the tumor tissue, although a more extensive study should be performed to maximize the effect of liposomal delivery. Regarding BPA wash out, in a previous human clinical trial, continuous BPA injection was used during irradiation to stabilize the therapeutic boron concentration in tumor tissue during the entire BNCT course (Watanabe et al. Citation2016).
In some studies, boron concentration in animal tumors after boron compound administration was higher than in our experiments. Iida-Onishi et al. (Citation2009), evaluating the biological effects of BNCT for the human malignant meningioma cell line IOMM-Lee, published data on BPA accumulation in subcutaneous U87 glioma in BALB/c nu/nu mice showing an increase in tumor boron concentration in a dose-dependent manner with values of 3.20 ± 1.00, 7.24 ± 1.16 and 13.25 + 2.20 µg/g at 2.5 h after tail vein injections of 125, 250 and 375 mg/kg of BPA, respectively. In a study by Sun et al. (Citation2016), polyamido amine (PAMAM) dendrimer carriers encapsulating BSH were conjugated with a CD133 antibody and tested in vitro and in vivo. BSH administration was used as a control and tumor boron concentration 1 h after tail vein BSH injection was 21.2 ± 1.9 µg/g in CD133+ and 20.8 ± 3.4 µg/g in CD133- SU-2 glioma xenografts. The lack of a reliable BNCT effect in our BSH group may be due to insufficient accumulation within the tumor as well as a short deposition time within the tumor cells (). In multiple reported studies with variants of tumor models, quite contradictory results on the accumulation of BSH in the tumor have been shown and BSH often quickly washed out of tumors after administration (Sauerwein et al. Citation2012; Barth et al. Citation2018).
Larger boron compound doses injected prior to irradiation or continuous infusions, which might pose technical difficulties in small animals, may have improved boron accumulation and corresponding tumor suppression effects in contrast to the single boron compound injections we used. Joel et al. (Citation1999) studied the effect of dose and infusion time on BPA delivery in 9 L gliosarcoma-bearing Fisher 344 rats and showed that BPA total dose increases from 250 to 1000 mg/kg, administered intravenously over a 2-h infusion period, resulted in an increase in tumor boron concentration from ∼30 to ∼70 µg 10B/g, with a constant T:B boron concentration ratio of about 3.7:1. Similarly, extending the infusion time from 2 to 6 h, at a constant dose-rate of 125 mg BPA/kg/h, resulted in an increase in tumor boron concentration from ∼30 to ∼80 µg 10B/g, while, again, maintaining a constant T:B ratio of about 3.7:1. In contrast, intracarotid infusion of BPA for 1 h at a dose rate of 125 mg BPA/kg resulted in an increase in the tumor boron concentration from ∼26 to ∼38 µg 10B/g with a corresponding increase in the T:B ratio from 3.5:1 to 5.0:1.
Advanced liposomal delivery studied by different BNCT scientific groups has also shown the increase in the tumor boron concentration compared to non-liposomal compounds. Maruyama et al. (Citation2004) studied BSH-containing transferrin (TF)-conjugated polyethyleneglycol liposomes that could maintain tumor boron concentration over 30 ppm for at least 72 h after injection. This treatment was superior to PEG liposomes, bare liposomes and free BSH, resulting in tumor growth suppression and improved long-term survival of Colon 26 tumor-bearing mice after tail vein injections at 5 and 20 mg 10B/kg before nuclear reactor-based irradiation with 2 × 1012 neutrons/cm2 for 37 min. Nakamura (2008) showed boron concentrations of 8.8 ppm in tumor tissue 30 h after tail vein injection of BSH-liposomes in EMT6 tumor bearing BALB/c mice with higher tumor boron concentrations after i.v. administration of more advanced liposome-based compounds. Kueffer et al. (2013) studied unilamellar liposomes, developed using an equimolar mixture of cholesterol and 1,2-distearoyl-sn-glycero-3-phosphocholine that incorporate Na3[1-(2′-B10H9)-2-NH3B10H8] in the aqueous interior and K[nido-7-CH3(CH2)15-7,8-C2B9H11] in the bilayer. After two identical tail vein injections given 24 h apart in female BALB/c mice bearing flank EMT6 tumors, tumor boron levels exceeded 67 µg/g in tumors 54 h after the initial injection, showing significant suppression of tumor growth over control mice without liposome injection after irradiation with 1.6 × 1012 thermal neutrons/cm2 for 30 min. Spermidinium closo-dodecaborate-encapsulating liposomes were developed by Tachikawa et al. (Citation2014) and showed maximum mouse Colon 26 tumor boron concentrations of 202.7 and 82.4 ppm achieved 36 h after tail vein injection at doses of 100 and 30 mg 10B/kg, respectively. This was superior to Na2BSH- and Na2[B12H11NH3]-encapsulating liposomes in tumor growth control and animal survival after irradiation with 1.3–2.2 × 1012 neutrons/cm2 for 50 min at a nuclear reactor.
A recent study by Luderer et al. (Citation2019) was devoted to thermally sensitive liposomes for improving boron delivery and mainly focused on differences in accumulation rather than the amount of delivered boron. The authors developed an animal model of athymic nude mice containing bilateral D54 glioma tumors on their right and left flank (2 tumors/mouse). After 5 min of pre-hyperthermia treatment, mice were tail vein injected with an equimolar BPA-f free drug, BPA-f TSL, or BPA-f non-TSL. The left side tumor was exposed to hyperthermia (42 °C) for an additional 30 min post-injection while the right side tumor served as a 37 °C control. A similar boron content was observed in the hyperthermic (216 ± 70 ppb) and normothermic (341 ± 88 ppb) treated tumors after delivery of BPA-f as a free drug. In contrast, BPA-f TSL showed an 8.4-fold higher boron accumulation (692 ± 99 ppb) in the hyperthermic treated tumor compared to the normothermic tumor (82 ± 20 ppb). Non-TSL BPA-f administration resulted in no detectable signals at normothermic temperatures and a low signal of only 104 ± 41 ppb in the hyperthermic tumor.
In comparison to these studies, our BSH-containing pegylated liposomes, in spite of a lower tumor boron concentration, showed better long-term results compared to BPA and non-liposomal BSH, albeit only at the end of the 2-month follow-up period. With boron delivered by liposomal BSH, a longer follow-up period was needed to verify the BNCT effect. One explanation of the confirmed BNCT effect in case of liposomes is the higher chance of localizing boron compounds inside tumor cells. In this case, the effect of BNCT is strengthened even with relatively low overall tumor boron concentrations compared to non-liposomal compounds. This may be due to non-liposomal compound concentration in the intercellular space, resulting in lesser therapeutic effect even with higher overall tumor boron concentrations.
The injection site we used was probably atypical for BNCT experiments, and, to the best of our knowledge, previous researchers used mainly tail vein injections prior to neutron irradiation. The injection site could also therefore influence drug accumulation and corresponding tumor growth suppression. In an early study by Coderre et al. (Citation1990), BPA was administrated in single per os (intragastric) doses and showed significant accumulation of boron in subcutaneous KHJJ murine mammary tumors in BALB/c mice, subcutaneous and intracranial GS-91 rat gliomas in F-344 rats, and the human U-87 MG glioma xenografts in nude mice. Regarding the boron compounds biodistribution and further irradiation studies, we can also state, that we do not irradiate same animals that we take tissue samples from, therefore, conditions of irradiation experiments, including stress related to transportation, anesthesia and possible temperature fluctuations might play a role in some discrepancy in boron accumulation and further irradiation effects. Larger animal groups and more extensive biodistribution experiments are necessary to verify conformity in the results, which is out of the scope of the present study. The tumor size reduction in animals irradiated without boron compounds was apparently due to the presence of a component of fast neutrons and gamma radiation in the beam which has a destructive effect on tumor cells. However, this component was benign to normal tissues, as we have previously shown that the flux of epithermal neutrons obtained at the accelerator does not affect the viability of laboratory animals at therapeutic doses and does not lead to any significant damage in vital organs (Kanygin et al. Citation2017).
The appearance of erosions and ulcers in the tumor areas in treated and untreated animals and further formation of fistulous passages in most irradiated animals with longer follow-up period could be due to tissue-specific apoptosis, which is possible with large tumor volumes and subcutaneous localization. The skin reactions that we observed in irradiated mice could develop not only due to boron-related tissue damage during neutron capture reactions, but also due to the effect of ionizing radiation on tumor tissue itself, assuming higher susceptibility of constantly dividing tumor cells to ionizing radiation. Therefore, skin damage was obvious in both groups as tumor necrosis may have initiated damaging immune cascades. In all cases, skin erosions, ulcers and fistulas occurred only in the tumor area and skin on other areas of the irradiated legs, as well as shielded parts of the body, was intact, showing no effect of irradiation. Thus, we believe that skin-related sequelae from treatments were mainly due to the specific decay/necrosis of tumor tissue rather than direct skin damage. This phenomenon requires further and detailed investigations to delineate the exact mechanisms for the observed effect.
Due to shielding of mice bodies, no major complications in vital organs were observed, and the overall condition of the mice, apart from the tumor growth and the abovementioned tumor site reaction, was uneventful.
In the current study we aimed to reveal the possibilities of an accelerator-based neutron source for BNCT animal experiments with the desire to spur further advancement to clinical trials. However, adjustments in compounds (dosages/administrations) as well as modifications and advancements of the accelerator-based neutron source itself are important future aims to realize this goal.
Conclusion
We observed a tendency of BNCT at the accelerator-based neutron source to reduce or suspend the growth of human glioblastoma in immunodeficient animals. Liposomal BSH showed better long-term results compared to BPA and non-liposomal BSH, though only at the end of the 2-month follow-up period. Further modifications in liposomal boron delivery are being studied to improve treatment outcomes. In vivo studies have also demonstrated the safety of the neutron beam. Thus, an accelerator-based neutron source specifically designed for BNCT with its unique ability to create a fairly uniform generation of accelerated epithermal neutrons will undoubtedly provide opportunities for more extensive studies.
Acknowledgements
The authors are grateful to A.N. Makarov, I.M. Schudlo, D.A. Kasatov, Ya.A. Kolesnikov, E.O. Sokolova, A.M. Koshkarev, and T.A. Bykov, Budker Insitute of Nuclear Physics, for generation of neutrons.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Evgenii Zavjalov
Evgenii Zavjalov, Ph.D., graduated from the Novosibirsk State University and obtained a Ph.D. degree at the Institute of Systematics and Ecology of Animals Siberian Branch of the Russian Academy of Science. At present: head of the Department of Genetic Resources of Laboratory Animals of the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Science and the Senior Researcher at the Laboratory of Boron Neutron Capture Therapy (BNCT), Faculty of Physics, Novosibirsk State University since 2015.
Alexander Zaboronok
Alexander Zaboronok, M.D., Ph.D. in medicine, graduated from the Belarussian State Medical University, certified neurosurgeon in Belarus and Russian Federation, obtained a Ph.D. degree at the University of Tsukuba, Assistant Professor of the Department of Neurosurgery, the University of Tsukuba, Senior Researcher at the Laboratory of Boron Neutron Capture Therapy (BNCT), Faculty of Physics, Novosibirsk State University, member of the University of Tsukuba BNCT research group since 2013, member of the international collaborative BNCT research group since 2015.
Vladimir Kanygin
Vladimir Kanygin, M.D., Ph.D., graduated from the Novosibirsk State Medical University, certified neurosurgeon, neurooncologist and radiologist in Russian Federation, Associate Professor of the Department of Neurosurgery of the Novosibirsk State Medical University, Head of the Laboratory of Medical and Biological Problems of BNCT at the Novosibirsk State University, Chief Researcher at Novosibirsk State University, member of the international BNCT collaborative research group since 2013.
Anna Kasatova
Anna Kasatova, M.D., graduated from Kemerovo State Medical University, certified neurosurgeon in Novosibirsk, Russian Federation, Postgraduate at Neurosurgery Department, Irkutsk State Medical University, Junior Researcher at the Laboratory of Boron Neutron Capture Therapy (BNCT), Faculty of Physics, Novosibirsk State University, Junior Researcher at the Budker Institute of Nuclear Physics of Siberian Branch Russian Academy of Sciences.
Aleksandr Kichigin
Aleksandr Kichigin, M.D., graduated from Novosibirsk State Medical University, certified neurosurgeon, radiologist in Russian Federation, Graduate student Department of neurosurgery and innovation medicine Irkutsk State Medical University, Junior Researcher at the Laboratory of Boron Neutron Capture Therapy (BNCT), Faculty of Physics, Novosibirsk State University.
Rinat Mukhamadiyarov
Rinat Mukhamadiyarov, Ph.D. in Biophysics, graduated from Tomsk State University, obtained Ph.D. degree at the Institute of Biophysics Siberian Branch of RAS, Assistant Professor of the Department of Pharmacognosy, Kemerovo State Medical University, Head of the Department of Department of Pharmacognosy, Kemerovo State Medical University, Senior Researcher at the Laboratory of Boron Neutron Capture Therapy the Budker Institute of Nuclear Physics since 2014, member of the international collaborative BNCT research group since 2018. Senior Researcher at the Laboratory for Vascular Biology, Department of Experimental Medicine, Research Institute for Complex Issues of Cardiovascular Diseases.
Ivan Razumov
Ivan Razumov, Ph.D., D.Sc., in biology, graduated from Gorky State University, obtained Ph.D. and D.Sc., degrees at the State Research Center of Virology and Biotechnology ‘Vector’, Koltsovo, Novosibirsk region, Russia, Senior Researcher of the Laboratory of Genetics laboratory animals, the Institute of Cytology and Genetics, SB RAS, Novosibirsk, Russia and Senior Researcher at the Laboratory of Boron Neutron Capture Therapy (BNCT), Faculty of Physics, Novosibirsk State University, Novosibirsk, Russia.
Tatiana Sycheva
Tatiana Sycheva, Master in Physics, graduated from Novosibirsk State Technical University, research engineer at Budker Institute of Nuclear Physics at the Laboratory of Boron Neutron Capture Therapy (BNCT).
Bryan J. Mathis
Bryan J. Mathis, M.S., Ph.D., graduated from the Univ. of South Carolina School of Medicine with a Ph.D. in Biomedical Sciences (Concentration: Cardiovascular Immunology). Assistant Professor of the Faculty of Medicine, Univ. of Tsukuba with specialty in science writing, immunology, cancer, cardiovascular necrosis and international collaboration. Member of International Collaborative BNCT research team since 2017.
Sakura Eri B. Maezono
Sakura Eri B. Maezono, B.S.c. in Biology, graduated from the University of Tsukuba. Currently a Graduate student in Human Biology, School of Integrative and Global Majors and at the Hayashi Laboratory of International Institute for Integrative Sleep (WPI-IIIS), the University of Tsukuba.
Akira Matsumura
Akira Matsumura, M.D., Ph.D., Dr. Med., graduated from the University of Tsukuba, certified neurosurgeon in Japan, obtained a Ph.D. degree at the University of Tsukuba (Japan) and Dr. of Medicine at the University of Göttingen (Germany), Professor of the Department of Neurosurgery and Head of the BNCT project of the University of Tsukuba, Vice President of the International Society for Neutron Capture Therapy and member of the Japan-Russia collaborative BNCT research group.
Sergey Taskaev
Sergey Taskaev, D.Sc., graduated from Novosibirsk State University, obtained Ph.D. and D.Sc. degrees at the Budker Institute of Nuclear Physics, Leading Researcher at the Budker Institute of Nuclear Physics, Head of BNCT Lab. at Novosibirsk State University. Author and coauthor of more than 200 scientific publications, including one monography and 16 patents.
References
- Anderson O, Alpen E, Kwan J, Wells R, DeVries G, Faltens A, Reginato L. 1994. ESQ-focused 2.5 MeV DC accelerator for BNCT. In: Suller VP, Petit-Jean-Genaz C, editors. EPAC 94. Proceedings of the 4th European Particle Accelerator Conference; 27 Jun–1 Jul. London (England): World Scientific Pub; p. 2619–2621.
- Barth RF, Mi P, Yang W. 2018. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun (Lond). 38:35.
- Barth RF, Zhang Z, Liu T. 2018. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun (Lond). 38:36.
- Bayanov B, Belov V, Taskaev S. 2006. Neutron producing target for accelerator based neutron capture therapy. J Phys: Conf Ser. 41:460–465.
- Brednikhin SA, Lezhnin SI, Frolov SA, Yurov DM. 2012. [NMC code for statistical modeling of neutron transport in fissionable media. Validation and applications]. Moscow: IBRAE. Russian Academy of Sciences, Institute of Problems of Safety, Development of Atomic Energy. No. IBRAE-2012-04.
- Byvaltsev VA, Stepanov IA, Belykh EG, Kanygin VV, Kichigin AI. 2015. Molecular biology of high-grade gliomas. Sib Med J. 133:5–9. Russian.
- Carpano M, Perona M, Rodriguez C, Nievas S, Olivera M, Santa Cruz GA, Brandizzi D, Cabrini R, Pisarev M, Juvenal GJ, et al. 2015. Experimental studies of boronophenylalanine ((10)BPA) biodistribution for the individual application of boron neutron capture therapy (BNCT) for malignant melanoma treatment. Int J Radiat Oncol Biol Phys. 93:344–352.
- Coderre JA, Glass JD, Fairchild RG, Micca PL, Fand I, Joel DD. 1990. Selective delivery of boron by the melanin precursor analogue p-boronophenylalanine to tumors other than melanoma. Cancer Res. 50:138–141.
- Garefalakis M, Hickey M, Johnson N. 2017. Gynecological morbidity. In: Quah SR, Cockerham WC, editors. International encyclopedia of public health. 2nd ed. Oxford (UK): Academic Press. p. 342–353.
- Goorley JT, Kiger WS 3rd, Zamenhof RG. 2002. Reference dosimetry calculations for neutron capture therapy with comparison of analytical and voxel models. Med Phys. 29:145–156.
- Hiratsuka J, Fukuda H. 2012. Malignant melanoma. In: Sauerwein WAG, Wittig A, Moss R, Nakagawa Y, editors. Neutron capture therapy. principles and applications. Berlin: Springer-Verlag; p. 433–448.
- Iarullina AI, Kanygin VV, Kichigin AI, Zhdanova MG, Muhamadiyarov RA, SYu T. 2015. Treatment of brain tumours by the method of boron-neutron capture therapy: difficulties and modern solutions. Pac Med J. 4:4–10. Russian.
- Iida-Onishi K, Kawabata S, Miyata S, Masabuchi T, Doi A, Yokoyama K, Kuroiwa T, Ono K, Kumada H, Kirihata M, et al. 2009. Evaluation of the biological effects of boron neutron capture therapy for the humanmalignant meningioma cell line IOMM-Lee. Bull Osaka Med Coll. 55:9–19.
- Ivanov AA, Kasatov DA, Koshkarev AM, Makarov AN, Ostreinov YM, Sorokin IN, Taskaev SY, Shchudlo IM. 2016. Obtaining a proton beam with 5-mA current in a tandem accelerator with vacuum insulation. Tech Phys Lett. 42:608–611.
- Joel DD, Coderre JA, Micca PL, Nawrocky MM. 1999. Effect of dose and infusion time on the delivery of p-boronophenylalanine for neutron capture therapy. J Neurooncol. 41:213–221.
- Kanygin V, Kichigin A, Krivoshapkin A, Taskaev S. 2017. Prospectives of boron-neutron capture therapy of malignant brain tumours. AIP Conf Proc. 1882:020030.
- Kanygin VV, Kichigin AI, Gubanova NV, SYu T. 2015. Opportunities for boron-neutron capture therapy in the treatment of malignant brain tumours. Herald of Radiology and Radiology. 6:36–42. Russian.
- Kanygin VV, Zavyalov EL, Simonovich AE, Kasatova AI, Kichigin AI, Razumov IA, Taskaev S. 2019. [Boron neutron capture therapy of human glioblastoma on in vivo tumour models]. Modern Problems of Science and Education. 1:19. Russian.
- Kasatov D, Koshkarev A, Kuznetsov A, Makarov A, Ostreinov Y, Shchudlo I, Sorokin I, Sycheva T, Taskaev S, Zaidi L. 2016. The accelerator neutron source for boron neutron capture therapy. J Phys Conf Ser. 769:012064.
- Kobyakov GL, Bekyashev AKh, Golanov AV, Konovalov AN, Naskhletashvili DR, Potapov AA, Rzayev DA, Ryzhova MV, Smolin AV, Trunin YYu. 2016. [Practical recommendations on the medicinal treatment of primary tumours of the central nervous system]. Malignant Tumours. 4:64–84. Russian.
- Kueffer PJ, Maitz CA, Khan AA, Schuster SA, Shlyakhtina NI, Jalisatgi SS, Brockman JD, Nigg DW, Hawthorne MF. 2013. Boron neutron capture therapy demonstrated in mice bearing EMT6 tumors following selective delivery of boron by rationally designed liposomes. Proc Natl Acad Sci USA. 110:6512–6517.
- Locher G. 1936. Biological effects and therapeutic possibilities of neutrons. Am J Roentgenol Radium Ther. 36:1–13.
- Luderer MJ, Muz B, Alhallak K, Sun J, Wasden K, Guenthner N, de la Puente P, Federico C, Azab AK. 2019. Thermal sensitive liposomes improve delivery of boronated agents for boron neutron capture therapy. Pharm Res. 36:144.
- Maruyama K, Ishida O, Kasaoka S, Takizawa T, Utoguchi N, Shinohara A, Chiba M, Kobayashi H, Eriguchi M, Yanagie H. 2004. Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumours by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT). J Control Release. 98:195–207.
- Nakamura H. 2008. Liposomal boron delivery system for neutron capture therapy. Yakugaku Zasshi. 128:193–208. Japanese)
- Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, et al. 2014. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 16:896–913.
- Rossini AE, Dagrosa MA, Portu A, Saint Martin G, Thorp S, Casal M, Navarro A, Juvenal GJ, Pisarev MA. 2015. Assessment of biological effectiveness of boron neutron capture therapy in primary and metastatic melanoma cell lines. Int J Radiat Biol. 91:81–89.
- Sato E, Zaboronok A, Yamamoto T, Nakai K, Taskaev S, Volkova O, Mechetina L, Taranin A, Kanygin V, Isobe T, et al. 2018. Radiobiological response of U251MG, CHO-K1 and V79 cell lines to accelerator-based boron neutron capture therapy. J Radiat Res. 59:101–107.
- Sauerwein WAG, Wittig A, Moss R, Nakagawa Y, editors. 2012. Neutron capture therapy. Principles and applications. Berlin: Springer-Verlag.
- Suliman YA, Bruni C, Johnson SR, Praino E, Alemam M, Borazan N, Cometi L, Myers B, Khanna D, Allanore Y, et al. 2017. Defining skin ulcers in systemic sclerosis: systematic literature review and proposed World Scleroderma Foundation (WSF) definition. J Scleroderma Relat Disord. 2:115–120.
- Sun T, Li Y, Huang Y, Zhang Z, Yang W, Du Z, Zhou Y. 2016. Targeting glioma stem cells enhances anti-tumor effect of boron neutron capture therapy. Oncotarget. 7:43095–43108.
- Tachikawa S, Miyoshi T, Koganei H, El-Zaria ME, Vinas C, Suzuki M, Ono K, Nakamura H. 2014. Spermidinium closo-dodecaborate-encapsulating liposomes as efficient boron delivery vehicles for neutron capture therapy. Chem Commun. 50:12325–12328.
- Taskaev S. 2015. Accelerator based epithermal neutron source. Phys Part Nuclei. 46:956–990.
- Taskaev S. 2019. Development of an accelerator-based epithermal neutron source for boron neutron capture therapy. Phys Part Nuclei. 50:569–575.
- Taskaev S, Kanygin V, Byvaltsev V, Zaboronok A, Volkova O, Mechetina L, Taranin A, Kichigin A, Iarullina A, Eliseenko I, et al. 2018. Opportunities for using an accelerator-based epithermal neutron source for boron neutron capture therapy. Biomed Eng. 52:73–76.
- Watanabe T, Hattori Y, Ohta Y, Ishimura M, Nakagawa Y, Sanada Y, Tanaka H, Fukutani S, Masunaga SI, Hiraoka M, et al. 2016. Comparison of the pharmacokinetics between L-BPA and L-FBPA using the same administration dose and protocol: a validation study for the theranostic approach using [18F]-L-FBPA positron emission tomography in boron neutron capture therapy. BMC Cancer. 16:859.
- Yancey KB, Lawley TJ. 2018. Approach to the patient with a skin disorder. In Jameson JL, Fauci A, Kasper D, Hauser S, Longo D, Loscalzo J, editors. Harrison’s principles of internal medicine. 20th ed. New York: McGraw-Hill Education. Part 2, Cardinal Manifestations and Presentation of Diseases. Section 8, Alterations in the Skin; p. 325.
- Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. 2011. Retro-orbital injections in mice. Lab Anim (NY). 40:155–160.
- Zaboronok A, Yamamoto T, Nakai K, Yoshida F, Uspenskii S, Selyanin M, Zelenetskii A, Matsumura A. 2015. Hyaluronic acid as a potential boron carrier for BNCT: Preliminary evaluation. Appl Radiat Isot. 106:181–184.
- Zaidi L, Belgaid M, Taskaev S, Khelifi R. 2018. Beam shaping assembly design of 7Li(p,n)7Be neutron source for boron neutron capture therapy of deep-seated tumour. Appl Radiat Isot. 139:316–324.
- Zaidi L, Kashaeva EA, Lezhnin SI, Malyshkin GN, Samarin SI, Sycheva TV, Taskaev SYu, Frolov SA. 2017. Neutron-beam-shaping assembly for boron neutron-capture therapy. Phys Atom Nuclei. 80:60–66.
- Zanders ED, Svensson F, Bailey DS. 2019. Therapy for glioblastoma: is it working? Drug Discov Today. 24:1193–1201.
