Abstract
Purpose
Ionizing radiation causes various types of DNA damage e.g. single strand breaks (SSB) and double strand breaks (DSB), whereby the SSB/DSB ratio is shifted toward the DSB with increasing LET. For the DNA-incorporated Auger electron emitter Iodine-125 a SSB/DSB ratio of 5.4:1 is calculated based on computer simulations. In the presented work the SSB/DSB ratio of DNA-incorporated Iodine-125 was experimentally determined and compared to external homogenous γ-irradiation.
Materials and methods
Iodine-125-iododeoxyuridine (I-125-UdR) was incorporated into the DNA of SCL-II cells and cells were subsequently frozen for decay accumulation. Accordingly, external γ-irradiation (Cs-137) experiments were performed in frozen cells. After exposure the neutral or alkaline Comet Assay was performed to quantify DSB or DSB and SSB, respectively. Automated quantification of the comets was performed using the Olive Tail Moment (Metafer CometScan; MetaSystems). Calculation of absorbed dose for Auger electrons on cellular level is extremely biased due to the exclusive DNA localization of I-125-UdR. To avoid dose calculation the γ-H2AX assay was used in order to allow the comparison of the Comet Assay data between both investigated radiation qualities.
Results
For low-LET γ-radiation, a SSB/DSB ratio of 10:1 was determined. In contrast, a lower SSB/DSB ratio of 6:1 was induced by DNA-incorporated Iodine-125 which compares very well to the calculated values of Pomplun and co-authors
Conclusion
DNA-incorporated Iodine-125 induces a high-LET type DNA damage pattern in respect to SSB/DSB ratio.
Introduction
Ionizing radiation induce various types of DNA lesions e.g. single strand breaks (SSB) and double strand breaks (DSB), whereas the DSB is the most critical DNA lesion in respect to cell survival. Moreover, the ratio between SSB and DSB is shifted toward the DSB with increasing LET (Christensen et al. Citation1972). The emission of low-energy short-ranged Auger electrons (AE) can be regarded as high-LET radiation (Kassis and Adelstein Citation2005; Balagurumoorthy et al. Citation2008; Boyd et al. Citation2008) because the Auger electrons deposit a large amount of energy in a very small volume around the decay site.
For the DNA-associated Auger Electron Emitter (AEE) Iodine-125, a high-LET type DNA damage pattern in respect to SSB/DSB ratio was calculated based on computer simulations (Pomplun et al. Citation1996). To proof experimentally whether Iodine-125 show a high-LET type SSB/DSB ratio, the amount of DSB and SSB induction was examined using the neutral and the alkaline comet assay in SCL-II cells after exposure to the DNA-associated AEE Iodine-125-iododeoxyuridine (I-125-UdR) in comparison with homogenous, external γ-irradiation (Singh et al. 1988). In our study, the alkaline comet assay was used at pH 10 to avoid an overestimation of SSB formation by AP sites and oxidative base lesions which can be transformed into artificial SSB at pH > 12 (Kohn Citation1991; Tice et al. Citation2000; Luke et al. Citation2010).
The calculation of the absorbed dose for AE is strongly biased due to the exclusive DNA localization of I-125-UdR and, therefore, the inhomogeneous energy deposition caused by the low energy short-ranged AE. To avoid dose calculation the γ-H2AX assay as a biomarker for DSB induction was used in order to compare the Comet Assay data after exposure to I-125-UdR and γ-rays.
Materials and methods
Cell line and culture conditions
The SCL-II cell line (squamous carcinoma cell line II) is derived from a squamous epithelium carcinoma of a 91-year-old male patient (Tilgen et al. Citation1983). The cells grow adherent with unlimited proliferation capacity in minimum essential medium Eagle (MEM, PAA Laboratories GmbH, Cölbe, Germany) with l-glutamine (0.292 g/l), supplemented with 16% fetal bovine serum (FBS, PAA Laboratories GmbH) in a humidified incubator with a 5% CO2 atmosphere at 37 °C as previously described (Kriehuber et al. Citation2004).
Exposure to I-125-UdR
3 × 106 cells per cell culture flask (TPP, 25 cm2, Techno Plastic Products AG, Trasadingen, Switzerland) were seeded and incubated with 10 ml MEM (PAA) overnight. Different activities (0–2 kBq/ml) of I-125-UdR (Perkin-Elmer GmbH, Rodgau, Germany) were added to the cell culture medium and cells were incubated for 24 h. Cells were then washed twice in phosphate buffered saline (PBS, PAA Laboratories GmbH), trypsinized, resuspended in freezing medium (FBS + 10% dimethyl sulfoxide), aliquoted in cryogenic vials (VWR International, Darmstadt, Germany) and stored at −150 °C for decay accumulation up to 4,100 decays per cell. Prior to storage, the amount of incorporated I-125-UdR was determined by activity measurement using a gamma counter (1480 Gamma Counter, Perkin Elmer). Cultures without I-125-UdR, but treated identically, served as controls.
γ-Irradiation of SCL-II cells
Prior to irradiation cells were trypsinized, resuspended in freezing medium and stored in cryogenic vials at −150 °C until irradiation. During the irradiation process, the vials were kept on dry ice. To achieve similar exposure conditions when compared to the I-125-UdR experiments, cells were irradiated in frozen state with external γ-radiation up to 40 Gy (≈ 60 min) using a Cs-137 irradiation unit (0.6 MeV, 0.64 Gy/min, Gammacell 40; Atomic Energy of Canada Limited, Mississauga, Canada). Non-irradiated controls were treated in parallel with the identical protocol.
γ-H2AX-immunocytochemistry and quantification by flow cytometry
For the γ-H2AX Assay aliquots of the same cells as for the Comet Assay were used. Cells were thawed, washed with PBS, resuspended with MEM medium supplemented with 16% of FBS and incubated for 30 minutes at 37 °C. Cells were then washed with PBS and fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, UK) for 20 minutes. After washing the cells twice with PBS, cells were permeabilized with 1% Triton X-100 (Roth, Karlsruhe, Germany) in PBS for 20 min at RT. After washing with TBP buffer (1% bovine serum albumin (BSA), 0.2% Triton X-100 (Sigma-Aldrich, Steinheim, Germany) in PBS) the cells were resuspended in 7.5% goat serum (PAA Laboratories) in PBS for 1 h at RT. Cells were washed twice with TBP buffer followed by an incubation with TBP buffer containing anti-phospho-histone H2AX (Ser-139) mouse monoclonal antibody (mAb) (1:700, Merck KGaA, Darmstadt, Germany) for 1 h at RT. Subsequently, the cells were washed twice with TBP buffer followed by an incubation with TBP buffer containing Alexa Fluor 488 goat anti-mouse IgG (H + L) highly cross-adsorbed (1:700, ThermoFischer Scientific, Massachusetts, USA) for 45 min at RT in the dark. Afterwards, cells were washed with TBP buffer and were resuspended in PBS prior to analysis. Cells were analyzed by flow cytometry assessing the γ-H2AX signal intensity as a function of the DNA content as previously described (Unverricht-Yeboah et al. Citation2018). Additional images of the cells with γ-H2AX foci were taken by a fluorescence microscope (Axio Observer Z1 LSM 700, Zeiss) equipped with a 63× Plan-APOCHROMAT 63×/1.4 oil DTC objective (Zeiss) and a camera (AxioCam MRm, Zeiss).
Comet assay
The Comet Assay was performed by a modified protocol of Singh et al. (1988) and Ostling and Johanson (Citation1984). After exposure to I-125-UdR or γ-radiation, cells were thawed, washed twice in ice-cold phosphate buffered saline (PBS, PAA Laboratories GmbH). After cell counting, the cells were resuspended with ice cold PBS (105 cells/ml) and mixed 1:10 with 37 °C low melting agarose (Trevigen Inc., Gaithersburg, Maryland, United States of America). After adding 50 μl of this cell suspension to each well of the CometSlides™ (Trevigen), the slides were incubated for 20 min at 4 °C.
Alkaline comet assay
Triton X-100 (1:100) and DMSO (1:10) were freshly added to the alkaline lysis solution (2.5 M NaCl, 100 mM Na3EDTA, 10 mM Tris Base, pH 10). For cell lysis, the slides with embedded cells were submerged in the alkaline lysis solution and incubated for 20–24 h at 4 °C. The slides were washed twice with 4 °C cold alkaline electrophoresis buffer (300 mM NaOH, 1 mM Na3EDTA) and then incubated for 40 min with alkaline electrophoresis buffer at 4 °C. Then, electrophoresis was done at 1 V/cm for 30 min on ice in fresh alkaline electrophoresis buffer. Slides were then rinsed for 10 min in neutralization buffer (0.4 M Tris Base, pH 7.5) and incubated for 5 min in 70% ethanol. After drying for 10–15 min at 37 °C, the DNA was stained with propidium iodide (PI, 0.5 µg/ml in distilled water) for 30 min in the dark at RT. After rinsing the slides gently with water and drying by 37 °C, the slides with the embedded lysed cells were analyzed.
Neutral comet assay
The neutral lysis solution (pH 8, CometAssay® Lysis Solution, Trevigen Inc.) was cooled down to 4 °C before cell lysis. For the lysis step, slides were submerged in the neutral lysis solution and incubated for 20–24 h at 4 °C. After washing the slides for 30 min with cold neutral electrophoresis buffer (0.1 M Tris Base, 0.3 M sodium acetate, pH 9; Merck KGaA, Darmstadt, Germany) at 4 °C, electrophoresis was done at 1 V/cm for 45 min on ice in fresh neutral electrophoresis buffer. Subsequently, slides were rinsed in precipitation buffer (43.3 ml of 95% ethanol, 6.7 ml of Ammonium Acetate solution (5.78 g NH4Ac in 10 ml distilled water)) for 30 min at RT followed by an incubation in 70% ethanol for 30 min. After drying for 10–15 min at 37 °C, the DNA was stained with propidium iodide (PI, 0.5 µg/ml in distilled water) for 30 min at RT. After rinsing the slides gently with water and drying at 37 °C, the slides with the embedded lysed cells were analyzed.
Comet analysis
The slides were scanned with a fluorescence microscope (Axio Imager.Z2, Zeiss GmbH, Göttingen, Germany) equipped with an EC Plan-NEOFLUAR 20×/0.5 objective (Zeiss) and a camera (MetaSystems Hard & Software GmbH, Altlussheim, Germany). The fluorescence signals of at least 150 cells per data point were measured and the Olive Tail Moment (OTM) was calculated by the software Metafer (MetaSystems). The OTM is defined as the product of the distance between the center of gravity of the comet head and the center of gravity of the tail and the percentage of DNA in tail compared to total fluorescence intensity. Additional images of the cells with comets were taken by a fluorescence microscope (Axio Observer Z1 LSM 700, Zeiss) with a 63× Plan-APOCHROMAT 63×/1.4 oil DTC objective (Zeiss) and a camera (AxioCam MRm, Zeiss).
Calculation of the SSB/DSB ratios
After normalizing the data to the respective controls, the OTM values were displayed as a function of the γ-H2AX signal intensity. After fitting the regression curves using EXCEL software, the SSB/DSB ratio was calculated. The slope of the alkaline comet data (SSB and DSB) was first subtracted from the slope of the neutral comet data (DSB), which then only represented the SSB. This value was then divided by the slope of the neutral Comet data to calculate the SSB/DSB ratio.
Results
Quantification of γ-H2AX signal intensity after exposure to I-125-UdR and γ-radiation
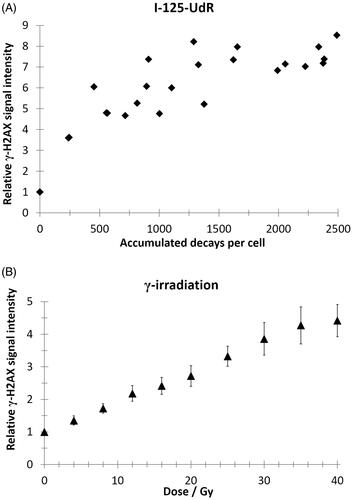
Phosphorylation of histone H2AX (γ-H2AX) occurs at sites flanking DNA DSB after exposure to ionizing radiation (Rogakou et al. Citation1998). The γ-H2AX induction is a biomarker for DSB induction and allows the determination of DNA damage after exposure to I-125-UdR or γ-radiation. To avoid dose calculation for Auger electrons on cellular level, which is extremely biased due to the exclusive DNA localization of I-125-UdR, the γ-H2AX assay was used in order to allow the comparison of the Comet Assay data between both investigated radiation based on DSB induction. When comparing single foci after exposure to I-125-UdR and γ-radiation microscopically, it seems that the foci sizes and intensities were similar and the foci were homogenously distributed over the cells (). However, the foci were overlapping at higher accumulated I-125 decays or γ-ray doses, which prevented counting of single discrete foci (). To overcome these limitations, flow cytometry was chosen to quantify the increase of the mean γ-H2AX signal intensity of single irradiated cells normalized to the respective control cells. The relative γ-H2AX intensity after γ-irradiation increased dose-dependent in a linear manner. Between 8 and 40 Gy the increase is significantly different compared to the control (). After exposure to I-125-UdR the quantification of the relative γ-H2AX signal intensity showed on average an increase with increasing numbers of accumulated decays per cell as well ().
Figure 1. Representative images of γ-H2AX immuno-stained SCL-II cells after exposure to γ-radiation or I-125-UdR taken by a confocal laser scanning microscope. Exposure was carried out in frozen cells. Cells were PFA-fixed 30 min after thawing and subsequently immuno-stained (Anti-phospho-histone H2AX (139) clone JBW301; Alexa Fluor 488 goat anti-mouse IgG (H + L); DAPI).
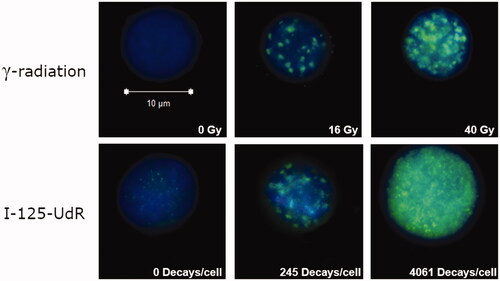
Figure 2. Quantification of the mean γ-H2AX signal intensity by flow cytometry (>10,000 cells/events per data point) as a function of accumulated decays per cell after I-125-UdR exposure (A) or as a function of radiation dose after γ-irradiation (B). Values are normalized to respective controls. In the dose range from 8–40 Gy the relative γ-H2AX signal intensity increased significantly after γ-irradiation when compared to non-irradiated controls (p < .05).
Combination of the comet assay and γ-H2AX assay data
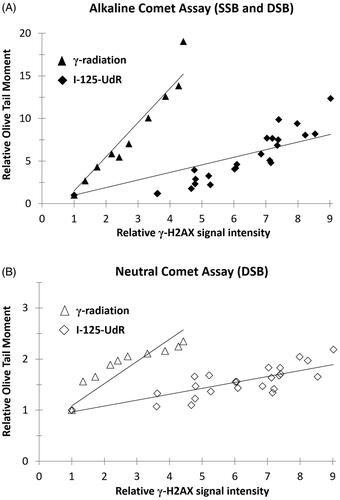
In order to compare the Comet Assay data between both radiation qualities, the relative Olive Tail Moment (OTM) was displayed as a function of the relative γ-H2AX signal intensity. Both, the OTM and the γ-H2AX signal intensity were previously normalized to the respective controls. The comparison of the regression curves of the alkaline or neutral Comet Assay data showed at the same γ-H2AX signal intensity a 3.5-fold or 4-fold higher increase of the OTM values after γ-irradiation when compared to I-125-UdR exposure ().
Figure 3. The relative Olive Tail Moment data of the alkaline Comet Assay (A) or neutral Comet Assay (B) are shown as a function of the relative γ-H2AX signal intensity after exposure to I-125-UdR and γ-radiation.
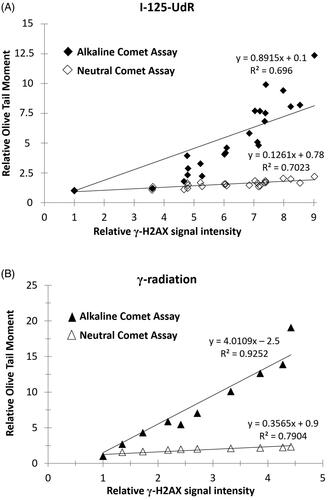
The SSB/DSB ratios for each radiation quality were calculated using the slopes of the regression curves of the alkaline and neutral Comet Assay data in . A SSB/DSB ratio of 6:1 was determined for the DNA-incorporated Auger electron emitter Iodine-125 whereas for γ-rays a higher SSB/DSB ratio of 10:1 was calculated.
Figure 4. The relative Olive Tail Moment data of the alkaline and neutral Comet Assay are shown as a function of the relative γ-H2AX signal intensity after exposure to I-125-UdR (A) or γ-radiation (B). The SSB/DSB ratios were calculated from the slopes of the regression curves showing a SSB/DSB ratio of 6:1 for DNA-incorporated Auger electron emitter Iodine-125 and a SSB/DSB ratio of 10:1 for external γ-irradiation, respectively.
Discussion
The comet assay was developed by Ostling and Johanson (Citation1984) and is an established method of detecting DNA damage. The neutral comet assay is mostly used to detect double strand DNA breaks, whereas the alkaline comet assay is more sensitive for smaller amounts of DNA damage, including single and double strand DNA breaks (Singh et al. 1988). Even though the comet assay has many advantages, it has limitations regarding specificity and sensitivity i.e. the assay has a limited dynamic range. If very small DNA fragments occur, measuring their signal is problematic. However, when only a few DNA breaks are present, the DNA fragments remain too large to migrate and damage cannot be detected. As more breaks are introduced, a measurable fraction of the DNA will begin to migrate and average tail length increases (Olive and Durand Citation2005). For this reason we have chosen a wide dose range up to rather high radiation doses. The specificity of the comet assay with regard to the detection of DNA damage is also limited. Because DNA fragments are generally detected by the neutral and alkaline comet assay, the apoptosis-associated DNA fragmentation can also be detected, which has already been used by several groups (Olive et al. Citation1993; Gopalakrishna and Khar Citation1995; Godard et al. Citation1999; Kizilian et al. Citation1999). However, apoptosis induction and, foremost, execution takes a certain amount of time, which is why it is assumed that only the late stages marked by DNA cleavage but not the early stages of apoptosis can be detected by the comet assay (Choucroun et al. Citation2001; Wilkins et al. Citation2002). Therefore, in our study, radiation-induced apoptosis can be excluded, as the cells were irradiated in frozen state and were lysed shortly after thawing. Thus, the time was too short to execute apoptosis.
For low-LET γ-radiation, a SSB/DSB ratio of 10:1 was determined in SCL-II cells. In contrast, DNA-incorporated Iodine-125 induced a much lower SSB/DSB ratio of 6:1, which is in very good accordance with the calculated SSB/DSB ratio of 5.4:1 for I-125-UdR based on computer simulation by Pomplun et al. (Citation1996). DNA-associated Iodine-125 seems to be far more efficient in the DSB production than low-LET radiation. This can be explained by the high energy deposition at the decay site of Iodine-125 which will generate multiple strand breaks in the DNA double helix in the close vicinity of the AEE. Thus, closely opposed SSB convert easily into a DSB. In addition, the induction of closely opposed SSB is very likely for high-LET particle radiation as well, as a large amount of ionizing events occur along the track in a rather small volume. Thus, the SSB/DSB ratio shifts toward the DSB with increasing LET (Christensen et al. Citation1972) resulting in a low SSB/DSB ratio for high-LET radiation. Therefore, the low SSB/DSB ratio of I-125-UdR shown here experimentally, lead to the conclusion that DNA-incorporated Iodine-125 is an efficient inducer of DSB and, hence, showing rather high-LET type DNA damage pattern with respect to the SSB/DSB ratio. These high-LET type biological effects of the AEE Iodine-125 are compatible with its physical properties. The emission of Auger electrons is, therefore, accordingly regarded as high-LET radiation (Kassis and Adelstein Citation2005; Balagurumoorthy et al. Citation2008; Boyd et al. Citation2008) as low energy short-ranged Auger Electrons deposit a large amount of energy in a very small volume around the decay site.
The neutral and alkaline Comet Assay was performed to quantify DSB or DSB and SSB, respectively. In both assays, the OTM increased much more pronounced after γ-irradiation when compared to I-125-UdR exposure (). A very likely explanation for this finding is that I-125 incorporated into the DNA double helix as I-125-UdR cause mainly complex DNA lesions (Kassis et al. Citation1987; Pomplun et al. Citation2002) with several SSB and DSB around the decay site, which then result in ultra-small DNA fragments after the Comet Assay treatment. These ultra-small DNA fragments are not accordingly detected in the Comet Assay as they migrate too far in the agarose gel and cannot be discriminated well from the PI fluorescence background signal. In contrast, ionizing events induced by γ-rays are more homogenously distributed over the whole genomic DNA (Ward Citation1988) generating rather large DNA fragments, which will migrate only for short distances in the Comet assay and can be accordingly quantified. Thus, the Comet Assay seem to generally underestimate the true number of SSB and DSB after I-125-UdR exposure.
The calculation of the SSB/DSB ratio induced by Iodine-125 incorporated into the DNA double helix as I-125-UdR published by Pomplun et al. (Citation1996) was, for the first time, confirmed by experimental data.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
Notes on contributors
Marcus Unverricht-Yeboah
Marcus Unverricht-Yeboah, Dr. rer. nat., Dipl.-Biol., is Postdoctoral Researcher in the Radiation Biology Laboratory at Forschungszentrum Jülich, Jülich Germany.
Kathrin Holtmann
Kathrin Holtmann, M.Sc. in Nuclear Applications, B.Sc. in Applied Chemistry, is former Research Student in the Radiation Biology Laboratory at Forschungszentrum Jülich and, recently, project engineer for Dismantling, Radiation Protection Measurements and Software Systems at Brenk Systemplanung GmbH, Aachen, Germany.
Ralf Kriehuber
Ralf Kriehuber, Dr. rer. nat., Dipl.-Biol., M.Sc. in Radiation Biology, is Research Scientist and Head of the Radiation Biology Laboratory at Forschungszentrum Jülich, Jülich, Germany.
References
- Balagurumoorthy P, Chen K, Adelstein SJ, Kassis AI. 2008. Auger electron-induced double-strand breaks depend on DNA topology. Radiat Res. 170:70–82.
- Boyd M, Sorensen A, McCluskey AG, Mairs RJ. 2008. Radiation quality-dependent bystander effects elicited by targeted radionuclides. J Pharm Pharmacol. 60:951–958.
- Choucroun P, Gillet D, Dorange G, Sawicki B, Dewitte J. 2001. Comet assay and early apoptosis. Mutat Res. 478:89–96.
- Christensen RC, Tobias C, Taylor W. 1972. Heavy-ion-induced single- and double-strand breaks in phiX-174 replicative form DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 22:457–477.
- Godard T, Deslandes E, Lebailly P, Vigreux C, Poulain L, Sichel F, Poul JM, Gauduchon P. 1999. Comet assay and DNA flow cytometry analysis of staurosporine‐induced apoptosis. Cytometry. 36:117–122.
- Gopalakrishna P, Khar A. 1995. Comet assay to measure DNA damage in apoptotic cells. J Biochem Biophys Methods. 30:69–73.
- Kassis AI, Adelstein SJ. 2005. Radiobiologic principles in radionuclide therapy. J Nucl Med. 46:4S–12S.
- Kassis A, Sastry K, Adelstein S. 1987. Kinetics of uptake, retention, and radiotoxicity of 125IUdR in mammalian cells: implications of localized energy deposition by Auger processes. Radiat Res. 109:78–89.
- Kizilian N, Wilkins R, Reinhardt P, Ferrarotto C, McLean J, McNamee J. 1999. Silver-stained comet assay for detection of apoptosis. Biotechniques. 27:926–930.
- Kohn KW. 1991. Principles and practice of DNA filter elution. Pharmacol Ther. 49:55–77.
- Kriehuber R, Riedling M, Simkó M, Weiss DG. 2004. Cytotoxicity, genotoxicity and intracellular distribution of the Auger electron emitter (65)Zn in two human cell lines. Radiat Environ Biophys. 43:15–22.
- Luke AM, Chastain PD, Pachkowski BF, Afonin V, Takeda S, Kaufman DG, Swenberg JA, Nakamura J. 2010. Accumulation of true single strand breaks and AP sites in base excision repair deficient cells. Mutat Res. 694:65–71.
- Olive PL, Durand RE. 2005. Heterogeneity in DNA damage using the comet assay. Cytometry Part A. 66:1–8.
- Olive PL, Frazer G, Banáth JP, Banath JP. 1993. Radiation-induced apoptosis measured in TK6 human B lymphoblast cells using the comet assay. Radiat Res. 136:130–136.
- Ostling O, Johanson KJ. 1984. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 123:291–298.
- Pomplun E, Terrissol M, Demonchy M. 1996. Modelling of initial events and chemical behaviour of species induced in DNA units by Auger electrons from 125I, 123I and carbon. Acta Oncol. 35:857–862.
- Pomplun E, Terrissol M, Hille R. 2002. Ratio of complex double strand break damage induced by 125IUdR and 123IUdR correlates with experimental in vitro cell killing effectiveness. Radiat Prot Dosimetry. 99:81–82.
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 273:5858–5868.
- Singh NP, McCoy MT, Tice RR, Schneider EL. 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 175:184–191.
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. 2000. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 35:206–221.
- Tilgen W, Boukamp P, Breitkreutz D, Dzarlieva RT, Engstner M, Haag D, Fusenig NE. 1983. Preservation of morphological, functional, and karyotypic traits during long-term culture and in vivo passage of two human skin squamous cell carcinomas. Cancer Res. 43:5995–6011.
- Unverricht-Yeboah M, Giesen U, Kriehuber R. 2018. Comparative gene expression analysis after exposure to 123I-iododeoxyuridine, gamma- and alpha-radiation-potential biomarkers for the discrimination of radiation qualities. J Radiat Res. 59:411–429.
- Ward JF. 1988. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 35;95–125.
- Wilkins RC, Kutzner B, Truong M, Sanchez‐Dardon J, McLean J. 2002. Analysis of radiation‐induced apoptosis in human lymphocytes: Flow cytometry using Annexin V and propidium iodide versus the neutral comet assay. Cytometry. 48:14–19.
