Abstract
Purpose
The concept of the adverse outcome pathway (AOP) has recently gained significant attention as to its potential for incorporation of mechanistic biological information into the assessment of adverse health outcomes following ionizing radiation (IR) exposure. This work is an account of the activities of an international expert group formed specifically to develop an AOP for IR-induced leukemia. Group discussions were held during dedicated sessions at the international AOP workshop jointly organized by the MELODI (Multidisciplinary European Low Dose Initiative) and the ALLIANCE (European Radioecology Alliance) associations to consolidate knowledge into a number of biological key events causally linked by key event relationships and connecting a molecular initiating event with the adverse outcome. Further knowledge review to generate a weight of evidence support for the Key Event Relationships (KERs) was undertaken using a systematic review approach.
Conclusions
An AOP for IR-induced acute myeloid leukemia was proposed and submitted for review to the OECD-curated AOP-wiki (aopwiki.org). The systematic review identified over 500 studies that link IR, as a stressor, to leukemia, as an adverse outcome. Knowledge gap identification, although requiring a substantial effort via systematic review of literature, appears to be one of the major added values of the AOP concept. Further work, both within this leukemia AOP working group and other similar working groups, is warranted and is anticipated to produce highly demanded products for the radiation protection research community.
Introduction
Leukemia
Epidemiological studies consistently show that human populations exposed to ionizing radiation are at an increased risk of developing leukemia. In the late 1940s, an increase in the incidence of leukemia was the first late effect that was observed among the atomic bomb survivors in Hiroshima and Nagasaki (Folley et al. Citation1952). The Life Span Study of atomic bomb survivors is, to this day, an extremely important source of information for the system of radiation protection. The exposure regime for the atomic bomb survivors, however, is very different from exposure scenarios relevant for radiation protection: the atomic bomb survivors were exposed to acute, moderate-to-high doses of radiation, whereas for radiation protection purposes, protracted, low doses are more relevant. In this light, the study of exposed workers, such as the INWORKS study of French, American and British workers in the nuclear industry, are important complements to the life span study. The INWORKS study demonstrated an association between radiation dose and risk of leukemia for workers chronically exposed to low doses of radiation. The magnitude of the risk is similar to that for the acutely exposed A-bomb survivors of low doses (Hsu et al. Citation2013; Leuraud et al. Citation2015), and the results of the INWORKS study do not indicate the existence of a threshold dose below which no risk exists, strengthening the scientific basis for the current system of radiation protection. Uncertainties associated with the effects at low doses, however, remain large, and epidemiological studies would have to be of a formidable scale to reduce these further. A thorough understanding and description of the mechanisms involved in radioleukaemogenesis therefore is essential to complement epidemiological studies in low dose, low dose-rate settings.
AOP
The Adverse Outcome Pathway (AOP) framework can be viewed as a way of consolidating knowledge on biological mechanisms behind a specific health adverse outcome (AO), typically a disease, as a result of exposure to a stressor. It takes the form of measurable key events (KE) that are causally linked by key event relationships (KER), starting from a very first interaction of a stressor with a biological system, molecular initiating event (MIE), that eventually lead to an AO. The concept has been developed by the Organization for Economic Cooperation and Development (OECD) and tailored predominantly to the effects of chemical agents, capitalizing on numerous advantages that it can bring: from the identification of potentially hazardous agents among a plethora of new substances humans come into contact with, to the reduction of animal use for safety testing, and to regulatory decision making (Vinken Citation2013).
Given these various benefits and marked successes of the AOP concept in both human and environmental chemical toxicology research, the concept has recently gained the attention of the radiation protection research and regulatory communities (NCRP Citation2020). Among the major drivers and motivators of such attention are the need for convergence and integration of the knowledge in radiobiological and epidemiological domains, as well as the capacity of the AOP framework to identify knowledge gaps by utilizing a systematic approach of knowledge screening and organization within a given AOP. In addition, AOP development can foster and stimulate international research and policy cooperation and collaboration due to the diverse skill sets required and disciplines involved. Since the concept is new to the radiation protection research community it is important to promote active communication and sharing of relevant experience in order to facilitate the development of AOPs to radiation-induced health effects (Chauhan et al. Citation2021; Citation2022).
MELODI and ALLIANCE workshop on AOP development
On 12–16 April 2021 a virtual AOP workshop was organized by the Institut de Radioprotection et de Sureté Nucléaire (IRSN) under the joint auspices of the MELODI (Multidisciplinary European Low Dose Initiative) and the ALLIANCE (European Radioecology Alliance) associations with the overall objective to enhance awareness of and trigger interest in the AOP concept among those involved in radioprotection research. To achieve this objective, several groups of experts were brought together, and five topical sessions were organized. Major highlights of this workshop are described in a subsequent section. Of note, four working groups were formed at the AOP workshop aiming to develop specific AOPs for four radiation-induced outcomes: (1) cardiovascular disease (human domain); (2) reproduction effect (environmental domain); (3) leukemia (human domain); (4) neurodevelopmental disease (environmental domain).
The coauthors of this article are members of the Leukemia working group. The objective of the group is to develop an AOP for radiation-induced leukemia, specifically acute myeloid leukemia (AML). Correspondingly, the purpose of this manuscript is to describe the organization of this effort, the systematic approach chosen and implemented, and to report the current status of the work and the proposed draft of the AOP to radiation-induced AML.
Leukemia and ionizing radiation in mouse models
To understand the mechanisms of radiation leukaemogenesis, animal models and more specifically mouse models have proven to be extremely valuable. Although several mouse strains were described as prone to radiation-induced leukemia i.e. RF, SJL/J, and the C3H/He strains, the CBA inbred mouse strain (sub-strains CBA/H, CBA/Ca and CBA/Cne (Rithidech et al. Citation1999) has been the primary model to study radiation-induced leukemia and more particularly radiation-induced Acute Myeloid Leukemia (rAML).
Major pathway
The CBA strain has a very low background AML incidence (<1/1000), the induction level of rAML in mice exposed to an acute whole-body X-rays 3 Gy dose is ∼20%, and rAML cases have clear histopathological similarities with human myeloid leukemia (Major Citation1979). Interestingly, the rAML induction rate has decreased in more recent studies, presumably due to more stringent mouse husbandry conditions making chronic inflammation that can promote myelopoiesis and eventually leukemogenesis less likely. Mechanistically, early after in vivo exposure (as early as 24 hours), bone marrow cells carrying interstitial chromosome 2 aberrations can be detected in 100% of the mice (Bouffler et al. Citation1997). This partial deletion of one chromosome 2 homologue is the first characteristic molecular event in murine rAML (more than 80% of cases) irrespective of the radiation quality (Peng et al. Citation2009; Brown et al. Citation2015). The minimal deleted region contains the mouse tumor suppressor Sfpi1 gene (PU.1 in humans) which encodes a transcription factor essential for myeloid cell development in the bone marrow compartment (Alexander et al. Citation1995; Silver et al. Citation1999; Cook et al. Citation2004; Brown et al. Citation2011; Olme et al. Citation2013; Citation2013). Over time, an increasing number of mice show clonal expansion of bone marrow cells with chromosome 2 aberrations reaching approximatively ∼50%. In addition to this deletion, in most rAML cases (68–86% of rAML cases), a specific point mutation in the 5th exon of the second Sfpi1/PU.1 homologue is found in the amino acid residue arginine 235 (R235) of the DNA binding domain of the protein PU.1 (Cook et al. Citation2004; O'Brien et al. Citation2020).
Minor pathways
In rAML cases where no chromosome 2 aberrations can be found, alternative pathways exclusive from each other have been identified with mutations in FLT3 (FLT3-ITD, 4% of cases) or KRAS (KRAS G12, 3% of cases) (Finnon et al. Citation2012; O’Brien et al. Citation2020) and a newly identified pathway neither requiring chromosome 2 deletion nor Sfpi1/PU.1 R235 mutation, but rather a significant increase in Sfpi1 DNA methylation with reduction of Sfpi1/PU.1 transcriptional expression (O’Brien et al. Citation2020). Of interest, the cases of Flt3-ITD clearly appear to have a sex bias with all cases found in females (Finnon et al. Citation2012; O'Brien et al. Citation2020). However, these represent minor pathways and the major pathway consists of the chromosome 2 deletion with a Sfpi1/PU.1 R235 mutation occurring predominantly in male mice. No additional driver mutation has been so far captured between the occurrence of the initiating event (chromosome 2 deletion) and the second hit (R235), during the latency for full rAML (9–18 months) in ∼20% of the irradiated mice.
Using an engineered CBA mouse model carrying a fluorescent marker on chromosome 2, located inside the minimum deleted region, pre-leukaemic cells were monitored in vivo and confirmed this sequence of molecular events in radiation leukaemogenesis. Moreover, the isolation of pre-leukemic hematopoietic stem and progenitor cells irradiated in vivo at different time-points following radiation exposure, allowed the detection of the presence of Sfpi1/PU.1 point mutations within a subpopulation of these preleukemic cells which expand rapidly after detection hence identifying the ‘target’ cells in radiation induced AML (Finnon et al. Citation2012; Olme et al. Citation2013). Importantly, a sex related difference in the phenotype of the preleukemic cells and leukemia, suggests a sex specific imbalance in the radiation-induced leukemic target cell. These findings fit well with the classic two-hit model involving a first irreversible mutational hit (interstitial deletion of one Sfpi1/PU.1 gene on chromosome 2) creating a pool of pre-leukaemic cells that acquire a growth advantage but which are not fully malignant and still able to produce fully differentiated descendant cells. Subsequently, following a relatively long period of time (over months up to 18 months), one or more intermediate cells acquire a second mutational hit (the point mutation in the remaining Sfpi1 copy), resulting in the formation of a fully leukaemic cell which undergoes further clonal expansion and eventually develops into rAML (Dekkers et al. Citation2011; Gault et al. Citation2019; Stouten et al. Citation2021).
Leukemia and ionizing radiation in humans
In humans, the exposure to ionizing radiation increases the incidence of AML, which has been reported in Japanese atomic bombing survivors, as well as cancer patients treated with radiotherapy. Therapy-related and more specifically radiotherapy associated AML (RT-AML) is a well-recognized potential complication of cytotoxic therapy for the treatment of a primary cancer. A predisposition to RT-AML has been detected demonstrating that repair of DNA double-strand breaks can affect susceptibility to RT-AML (Darakhshan et al. Citation2006; Patel et al. Citation2016). Regarding the relevance of the CBA mouse model to humans, although PU.1 is rarely directly mutated in human primary and RT-AML, the gene is commonly downregulated through indirect mechanisms (Verbiest et al. Citation2015) suggesting that the same pathway is targeted.
Driver mutations can be detected years before diagnosis in patients with blood malignancies by monitoring clonal evolutionary dynamics in relatively ‘’simple’’ genetic structures (i.e. harboring less mutations than most adult solid tumours) such as AML (Cheek and Naxerova Citation2022). Epigenetic drivers may be involved as well to further modulate pre-leukaemic clone growth rates but a long-time interval between the acquisition of the first driver event and AML diagnosis is confirmed and fits well with the rAML reported in mice (Verbiest et al. Citation2018).
These early driver mutations in long-lived hematopoietic stem cells may represent an ‘’acquired’’ susceptibility which can increase the risk of developing AML if exposed to radiation (Gomolka et al. Citation2020) which can be modulated by oxidative stress, metabolism and diet (Karabulutoglu et al. Citation2019; Gomolka et al. Citation2020; Karabulutoglu et al. Citation2021). Numerous lines of evidence suggest that otherwise healthy individuals can harbor clonal mutations in the stem cell compartment which associate strongly with future haematological malignancies (Wong et al. Citation2015; Anglesio et al. Citation2017; Loh et al. Citation2018). Although no acquired radiosusceptibility biomarkers are currently under development, the somatic genetic landscape cannot be ignored as a source of inherent radiosusceptibility. Increased understanding in this area, particularly with regards to other tissue types, will determine the utility of mosaic radiosusceptibility as an avenue of exploration.
In human AML multiple genes, classified in 8 functional categories have been identified which participate in the pathogenesis of the disease and thus can be considered as driver mutations. Their relevance is reflected by the fact that two or more of these driver mutations are present in 86% of AML patients (Dohner et al. Citation2015). Importantly, several of these mutations are present in some healthy individuals, in which mutated hematopoietic stem and progenitor cells (HSPC) are able to self-renew and differentiate and in most individuals never acquire malignant features. This phenomenon is called clonal hematopoiesis of indeterminate potential. Similarly, the frequency of gene mutations characteristic for AML increases also with age leading to age-related clonal hematopoiesis (Steensma Citation2018).
The presence of these mutated HSPC clones, also called leukemia initiating clones, can be considered as a pre-leukemic condition (Genovese et al. Citation2014; Jaiswal et al. Citation2014). In order to develop into a malignant clone, the cooperation of mutated genes or generation of fusion genes is needed. It should be emphasized, however, that a relatively low number of driver mutations and one or two additional so-called ‘cooperative’ mutations are enough for a pre-leukemic clone to acquire a malignant phenotype (Ding et al. Citation2012). Another important characteristic is that most patients at the time of diagnosis display clonal heterogeneity (Bullinger et al. Citation2017).
Thus, the development of the pre-leukemic or leukemia-initiating clones can be regarded as a first hit in the pathogenesis of the disease and subsequent events (regarded as ‘second hit’) are needed for eventual clinical development of AML.
Ionizing radiation may act as a ‘first hit’ or in a promotional role
IR has the potential to induce new DNA damage and to lead to the development of pre-leukemic clones and thus can serve as the first hit event. Though, within the context of radiation leukaemogenesis after radiotherapy, IR most probably also plays a promotional role, by selecting a preexistent mutant HSPC clone (McNerney et al. Citation2017). This hypothesis is supported by observations that clonal hematopoiesis in radiotherapy-treated patients increased to 25% (compared to 1% found in control individuals) (Coombs et al. Citation2017). Whether clonal selection is random, or IR preferentially targets selection of clones with certain mutations is very unclear from the literature. Some data indicate that RT-AML has a mutational spectrum similar to de novo induced AML, while other data indicate that certain cytogenetic changes (such as deletions in chromosomes 5 and 7) and mutations (such as TP53 mutation) are more frequent, similar to AML developing after chemotherapy with alkylating agents (Pedersen-Bjergaard et al. Citation1993; Christiansen et al. Citation2001; Smith et al. Citation2003). Additionally, radiation was shown to promote clones with certain oncogenic mutations in a mouse model of T-lymphocytic leukemia (Marusyk et al. Citation2009).
Mutational signatures in rAML: data from chernobyl
Several studies compared AML mutational spectra of patients who were exposed to various doses of IR during the Chernobyl accident and patients in whom no IR exposure history was recalled. Alterations to the AML1 gene is a frequent genetic lesion in AML. Most often the t(8,21) translocation results in a fusion gene between the AML1 and ETO (Lo Coco et al. Citation1997). Another characteristic gene rearrangement affects the MLL gene, which has been described both in spontaneous and therapy-related AML (Super et al. Citation1993). Klymenko et al. reported that AML1/ETO translocations were significantly less frequent, while MLL gene translocations were absent in AML patients previously exposed to IR during the Chernobyl accident compared to patients developing spontaneous leukaemias (Klymenko et al. Citation2005; Citation2005). Poluben et al. (Poluben et al. Citation2019) investigated the genetic profile of patients exposed at Chernobyl developing multiple myeloproliferative neoplasms (including AML) and found that the frequency of certain characteristic driver mutations occurred at different frequencies compared to non-exposed patients. The frequency of JAK2 mutation decreased, that of the CALR gene increased, while triple negative cases lacking mutations in JAK2, MPL and CALR genes also increased. Furthermore, the authors identified several novel mutations in triple negative patients (affecting the ATM, EZH2 and SUZ12 genes), which might be regarded as novel driver mutations (Poluben et al. Citation2019). While none of these studies could identify a specific mutation or other genetic damage that can be considered as a marker of IR-induced leukemia, in general there was agreement that the mutational spectra of spontaneous and IR-related AMLs are different.
The promotional role of IR in AML pathogenesis is evident at the level of the bone marrow microenvironment as well. For example, IR induces senescence in various cellular components, which in turn secrete multiple pro-inflammatory molecules as part of their ‘senescence-associated secretory phenotype’. Chronic inflammation contributes to the selection and expansion of the malignant clone (Wang et al. Citation2011; Citation2020). It is thus evident that rAML pathogenesis shares multiple mechanistic similarities to spontaneous AML, and that IR is a significant risk driver that can promote the same (key) events occurring in spontaneous leukaemogenesis. This information may therefore be used in the development of the AOP.
The development of the AOP concept in radiation protection research
Why is the radioprotection community interested in AOPs?
Risk estimates for radiation induced cancer and more recently, non-cancer diseases are mainly derived from epidemiological data. However, there are high uncertainties for risk estimations at acutely delivered doses below 100 mGy or at low dose rates (<5 mGy/h) (NCRP Citation2012) since data are almost exclusively extrapolated from medium and high dose exposures. Although very large epidemiological studies have been initiated in the field of low dose research, such as the One Million U.S. Workers and Veterans Study of Low-Dose Radiation Health Effects [MWS; also called Million Persons Study (MPS)] (Bouville et al. Citation2015; Boice et al. Citation2022), the International Nuclear Workers Study (INWORKS), the European pooled study of radiation-induced cancer from pediatric computed tomography (EPI-CT), or other low-dose pooling studies, it remains highly desirable to integrate biological data and mechanisms to support these epidemiology-derived risk estimates. Important information about biological mechanisms acting at low doses and dose rates and their relation to potentially induced adverse effects are derived from radiobiological experiments, animal studies or human molecular epidemiological studies that focus on bioindicators (biomarkers of disease) for radiation induced adverse effects. AOP models can provide structured and systematically evaluated, biologically derived information from the first initiating event and subsequent key events triggering the adverse outcome, such as radiation induced leukemia. A study by the National Council on Radiation Protection and Measurements (NCRP) titled 'Approaches for Integrating Information from Radiation Biology and Epidemiology to Enhance Low-Dose Health Risk Assessment’ was begun in November 2017 and published in 2020. A major aim was to suggest how to integrate and combine data from radiation biology studies with those from epidemiological studies to develop Biologically-Based Dose-Response (BBDR) models. Data are presented and summarized in NCRP Report No. 186 on how radiation biology data and epidemiological data together can enhance Low-Dose health risk assessment (NCRP Citation2020). At the European Radiation Protection Week in Stockholm 2019, a special session was dedicated to introduce AOPs to the radiation research community by Olivier Laurent and others. Here it was outlined how molecular epidemiological studies can contribute to identify bioindicators for adverse health effects and how biologically derived models can integrate new data. A major contribution of the AOP concept in the field of radiation protection is clearly to collect and validate accurate and highly structured information necessary for risk assessment (Chauhan et al. Citation2019; Citation2021; Preston et al. Citation2021). In fact, the UNSCEAR 2020/2021 Report on ‘Biological mechanisms relevant on the inference of cancer risks on low-dose and low-dose-rate radiation’ stated clearly that ‘Across the world, systematic reviews are increasingly becoming the basis for these [risk] assessments’ (UNSCEAR Citation2021).
Establishing an AOP implementation group
In June 2021, the Radiation and Chemical (Rad/Chem) AOP joint topical group was established. The group became part of the existing initiatives within the High-Level Group on Low Dose Research (HLG-LDR) which is overseen by the OECD Nuclear Energy Agency’s (NEA) Committee on Radiation Protection and Public Health (CRPPH). A major task of the joint AOP topical group is to enhance implementation of AOPs into hazard and risk assessment. Therefore, this AOP joint topical group set up global workshops and international initiatives, following the MELODI/ALLIANCE AOP workshop in April 2021, to intensify discussion, identify research gaps and exchange ideas between chemical and radiation risk assessment and on incorporating the AOP framework in research and regulation.
At the workshop, it was acknowledged that an AOP is not a tool to directly assess risk, although it can facilitate this task. Also, the participating experts agreed there are certain differences in the underlying motivation and needs between the development of AOPs for chemical toxicity and radiation effects. Thus, the motivators for chemical toxicity AOP development include the identification of new potentially hazardous substances and concurrently minimizing animal testing, which would have little application to radiation AOP development (Vinken Citation2013). Yet, two features of the AOP framework that are common between the radiation and chemotoxicity domains and very valuable are the ability to identify knowledge gaps and, hence, research priorities, and the potential to build knowledge for the evaluation of co-exposure effects within the concept of the exposome (Wild Citation2005).
Current state of radiation AOPs
Since 2012, when OECD launched a new program for the evaluation of toxicity and human health hazards from chemical exposures using the AOP analytical construct, 736 stressors have been included in the AOP-Wiki database (assessed on 28 February 2022). The vast majority of the stressors in those AOPs are chemicals, with only 11 AOP having links or reference to IR (). These AOPs describe various adverse outcomes, such as lung cancer, breast cancer, chromosomal aberrations and mutations, apoptosis or population decline. Only one AOP entitled ‘Deposition of energy leading to lung cancer’ has been approved by the Extended Advisory Group on Molecular Screening and Toxicogenomics (EAGMST) review process. This AOP was developed and submitted for review in 2019. Collectively, KEs that are related to ionizing radiation are: deposition of energy, increase in RONS (reactive oxygen and nitrogen species), up-regulation of reactive oxygen species, chronic reactive oxygen species, increase in DNA damage, increase in oxidative damage, increase in strand breaks, increase in chromosomal aberrations and mutations, inadequate DNA repair, increase in cell proliferation and altered meiotic chromosome dynamics.
Table 1. The list of AOPs from AOP-wiki (aopwiki.org) that contain ionizing radiation as a stressor (assessed on 28 February 2022).
AOPs aim to provide a mechanistic understanding on the toxicologic action of agents on different levels of organization, from molecule to organism or even populations. This is why the definition of the first molecular initiating event leading to the adverse outcome is crucial and should be stressor agnostic. ‘A molecular initiating event (MIE) is the initial interaction between a molecule and a biomolecule or biosystem that can be causally linked to an outcome via a pathway’, this definition was suggested by Allen et al. (Allen et al. Citation2014) to include different fields of sciences not limited to toxicology. As mentioned before, choosing a MIE for IR as a stressor presented certain difficulties and required substantial discussions, including with OECD experts involved in the AOP program. In contrast to chemical stressors where MIEs rely on (bio)chemical modification of molecules, the primary event occurring in cells upon exposure to IR has a physical nature – deposition of energy into the matter along the photon or particle track. Although this event would not be easily applicable to chemical stressors, it brings one important benefit specific to the IR exposure scenarios - it can be related to various adverse outcomes in a quantitative manner since deposition of energy is directly related to dose of radiation. Therefore, in line with Chauhan et al. (Citation2021), the Leukemia working group decided to use 'Deposition of energy’ as the MIE in the AOP to rAML.
The MELODI/ALLIANCE workshop and the initiation of the leukaemia AOP working group
The objectives of the program for the MELODI/ALLIANCE AOP April 2021workshop were to facilitate, through international multidisciplinary collaboration, the practical aspects of developing radiation effects AOPs, the exchange of experience and observations, and eventually a submission of AOPs on four different adverse outcomes to OECD for review and inclusion into the AOP-wiki (aopwiki.org). We, therefore, describe in the ensuing sections, the activities of the Leukemia working group during the workshop, along with the approach that was selected and the current results.
Activities during the AOP workshop
The option of participation in a working group was included in the workshop registration form; thus, the working groups were formed on a voluntary basis prior to the workshop. On the first day and after the first session that included general introductory and educational presentations by the OECD experts involved in the AOP activities, short introductions of the working groups and the objectives were made by group leads. The experts that formed the leukemia working group represented the following expertise areas: radiobiology, epidemiology, immunology, medicine, and modeling. None of the working group members had previous expertise developing AOPs. One member had previously participated in an OECD-guided 3-day training on AOP development. From the second day of the workshop, the working groups’ activities consisted of three daily sessions of 1.5 h each and one final session of 3 h. An OECD expert was present at least during the first session on Day 1 – to present an overall workflow recommendation, to guide the initial discussion and to help define the group’s next steps forward. Key guiding questions for developing an AOP were reviewed including:
What regulatory questions will the AOP support? Knowledge gaps, test understanding of mechanisms, co-exposure scenarios, and data consolidation?
What is the exposure scenario of interest? (Medical testing or treatment, Environmental scenarios, Occupational hazards, Ecotoxicological scenarios, Cosmic rays, etc.)
What is the proposed AO, and is there sufficient evidence from epidemiological data to substantiate the causal link between the stressor and the AO? Are there controversies and inconsistencies in the literature surrounding these data? Is this AO relevant across different species?
Are there systematic reviews and available empirical data to support this AO? What is the main important evidence? What type of evidence predominates?
To help identify the target organs relevant to the AOP, identify the IR exposure type (External, Internal, Mixed)
Are there mimics of the stressor?
What is the understanding of the mechanism to the AO? Are there important modulators or countermeasures to the AO?
List potential KE/KERs that are essential to achieve the AO of interest? Are there feedback loops to these KERs?
To help identify if the AOP will be networked, identify if it will involve multiple stressors and/or multiple AOs
Is life stage/age/sex an important modulator of the AO?
Do we need focused sessions on each KER?
It was acknowledged that whereas producing a draft outline of the AOP in the four days of discussions within the AOP workshop was achievable, a considerable follow-up effort would be required for completing the AOP according to the guidelines and recommendations of OECD. That would include, among other major tasks, generation of knowledge database and weight of evidence mapping. These major tasks converge on the overall added value of the AOP as seen by this group: knowledge consolidation and knowledge gap identification. The group discussed and agreed that the AOP being developed would be qualitative, rather than quantitative, given the lack of expertise in AOP development and uncertainties that exist in the assessment of cancer risks upon exposure to low-dose IR. It did not take the group long time to agree that the focus should not be made exclusively on a low-dose region and all knowledge that causally links IR with leukemia should be included in the review of evidence. Similarly, the dose-rate and radiation quality factors were considered in terms of how they can affect the selection of an MIE and the first few KEs. It is worth noting that this initial discussion of exposure scenarios/quality intertwined with the discussion of an MIE and it was not a trivial task to arrive at a consolidated and reasonable solution, even with the help of an OECD coach on AOP. Indeed, whereas the issue of dose-rate has an analogy in chemo-toxicological AOPs, the issue of radiation quality does not seem to have a counterpart. It was decided that the best path forward was to consider all evidence, irrespective of exposure dose-rate and radiation quality, and later on, at the level of extraction of data for individual KEs, consider whether adjustments would be needed. The ensuing elaboration resulted in the selection of energy deposition as the MIE. This MIE may seem to contradict the OECD recommendation to use MIEs that are agnostic to a stressor. However, being specific to ionizing radiation, but not chemicals, deposition of energy is agnostic to radiation quality in general terms, partially satisfying the requirement. Another advantage of this MIE is the perceived ease of implementation into quantification since it is a physical and measurable event that has a direct relevance to radiation dose. However, discussions continue about the optimal MIE for radiation AOPs within several radiation AOP communities and interest groups.
Next, some discussion time was devoted to a potential target group. It was deemed most reasonable to focus on leukemia in the adult population without sex specificity. This seemed to represent various cohorts of the general public that are exposed to environmental, accidental or medical radiation, as well as workers exposed occupationally. Published literature appears to favor this selection and it was hoped that strong supportive evidence would permit elaboration of the AOP. The identification of the target group as adults helped the group to narrow down on a specific disease as an AO. It is known that AML is the most common subtype of leukemia in adults, comprising about 80% of all acute leukemia cases in this target group (Shallis et al. Citation2019). It is also common in infants, but not children (Shallis et al. Citation2019). Chronic myeloid leukemia is also present in the adult population, albeit with a much lower frequency (Siegel et al. Citation2019). Therefore, it was reasonable to anticipate that the data causally linking IR exposure with AML would be the most robust in terms of volume and statistical power in epidemiological studies, and more mechanistic insight would therefore be anticipated from radiobiological studies. Indeed, a ground-breaking study of the INWORKS cohort of nuclear energy workers in three countries reported the highest rate of deaths due to AML among the four different leukemia subtypes (Leuraud et al. Citation2015). In summary, the leukemia working group agreed that the proposed AOP will aim to consolidate available literature causally linking IR exposure to AML through a deposition of energy as the MIE and through multiple KEs, with no specificity as to the dose/dose-rate value or radiation quality, and applicable to an adult mammalian organism without sex specificity.
Review of aopwiki
On Day 2, the group reviewed and discussed AOPs available in AOP-wiki (aopwiki.org) that were relevant to leukemia as an AO. Only one AOP contained ‘leukaemia’ in its title (AOP #202). This AOP however dealt with infant leukemia and thus was not relevant to the AOP under development by the topical working group. Also, 8 other AOPs were identified that contained ‘leukaemia’ elsewhere within the various fields of an AOP webpage. However, only one AOP (AOP #272) had IR as a stressor and thus presenting certain interest with respect to the AOP to leukemia. A few others contained certain KEs of relevance: AOP # 293, 294 and 296 dealt with reactive oxygen and nitrogen species (RONS) and relevant DNA damage in connection with chromosomal aberrations and mutations or breast cancer. It was determined that some of the KEs from those AOPs present interest for a potential use in the AOP to leukemia, particularly as early KEs following the MIE of energy deposition. A more detailed account of existing AOPS of relevance to IR is given in the next subsection.
The next step for the group was to arrive at a consensus that subsequent discussion of KEs leading to AML should take the form of a top-down approach. This approach was based on a long-standing, classical mechanistic view of AML wherein the disease requires only a few genetic mutations that turn a normal myeloblast into a malignant cell that expands clonally (Dekkers et al. Citation2011; Stouten et al. Citation2021). Therefore, a KE leading to the AO AML would be a clonal expansion of the undifferentiated myeloid progenitor cells. This KE represents a net result of several other biological mechanisms and changes occurring both at the tissue and cellular level. The group discussed those and created a draft list of changes and/or processes that could qualify as KEs. The resulting list included:
Suppression of the immune system that would allow the pre-neoplastic cells to avoid the immune suppression/surveillance (Fleenor et al. Citation2010; Henry et al. Citation2011; Camacho et al. Citation2021)
Cell proliferation of a mutant cell(s) (Larrue et al. Citation2019)
Formation of senescent cells in the bone marrow niche that creates a pro-inflammatory microenvironment due to secreted pro-inflammatory molecules (senescence-associated secretory phenotype, SASP) (Sabin and Anderson Citation2011; Bai et al. Citation2020)
IR-induced changes and processes that would be specific to earlier responses and operating at cellular and molecular levels that could be considered as hypothetical KEs were:
Formation of various DNA lesions directly from photons or particle tracks or indirectly from reactive oxygen and nitrogen species (Riley Citation1994; Rothkamm et al. Citation2003)
Fixation of a fraction of those DNA lesions as mutations as a result of inaccurate or incomplete repair (Rassool et al. Citation2007)
These processes were deemed essential for a mechanistic description of pathogenesis leading to AML following IR exposure. The ensuing discussions focused on formulation of specific KEs based on the described processes and their alignment into an AOP-like diagram with various KE relationships (KER).
Discussions of Day 3 and 4 focused on further elaboration of the sequence of the KEs, KERs and the identification of potential feedback loops. The group highlighted that DNA damage can be induced both directly and indirectly, via the production of ROS. Furthermore, ROS are known to be able to cause cell senescence, which can contribute to alterations in the microenvironment, mostly due to pro-inflammatory molecules secreted by senescent cells (Zhao et al. Citation2020; Takasugi et al. Citation2022). Such tissue alteration, if sustained for a sufficiently long period, can further lead to immune system dysregulation and inability to exert its surveillance and suppression of the clonal expansion of transformed cells (Wodnar-Filipowicz and Kalberer Citation2007). It was agreed that this crosstalk between the cellular and tissue effects, and the remodeling of the microenvironment, either directly by ROS formation or via senescence, is a plausible candidate for inclusion into the AOP to leukemia. A mouse study by Hepburn et al. (Citation1987) showed that rAML cells that were serially passaged through multiple syngeneic recipients grew faster compared to original rAML cells, supporting the role of the microenvironment in increasing the aggressiveness of leukaemic cells. At the same time, an earlier transplantation study revealed no effect of microenvironment on the growth properties of the rAML cells in this CBA-H mouse model (Meldrum and Mole Citation1982). The group therefore acknowledged that causal evidence linking these events to AML was limited and required further exploration and literature analysis. Nonetheless, relevant KEs should be included in the hypothetical draft AOP as a branch parallel to the one associated with the formation of gene mutations directly from IR-induced DNA damage (). As discussed later in this article, it was anticipated that review and analysis of the literature using a systematic approach and data extraction would allow improved understanding of the extent of empirical support for the role of the senescence-immune system axis in the pathogenesis of IR-induced AML.
Draft of a hypothetical AOP to AML
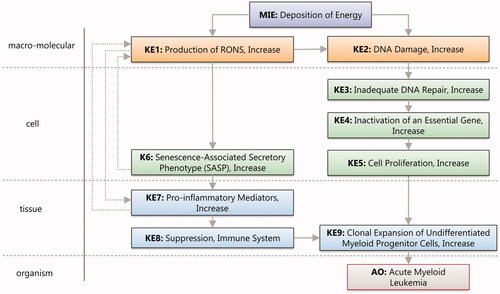
A proposed title of the AOP was ‘Deposition of energy by ionizing radiation leading to acute myeloid leukemia (AML)’ which reflects two main components, the stressor and the AO. This AOP describes the chain of events where exposure to ionizing radiation leads to acute myeloid leukemia (AML).
The molecular initiating event (MIE) for IR as a stressor is ‘Deposition of energy’ (Alloni et al. Citation2014). A single photon or a particle of ionizing radiation may cause multiple ionization events within a cell and interact with both water and biomolecules. Energy depositing onto water causes production of reactive oxygen species that by chemical reaction leads to formation of reactive nitrogen species. Therefore, the KE1 was called ‘Production of RONS, Increase’ (RONS stands for Reactive Oxygen and Nitrogen Species). These radicals contribute to two important branches leading to the development of AML (). The first branch starts with the second key event (KE2), ‘DNA Damage, Increase’ and includes DNA damage produced by both RONS (KE1) and energy deposition (MIE) (Goodhead Citation1994; Lomax et al. Citation2013). Depending on the extent and complexity of the damage, cells activate a number of defence mechanisms such as DNA Damage Response (DDR). However, DNA damage repair can be error prone, so that inadequate DNA repair (KE3) leads to accumulation of mutations. Such mutations or chromosomal aberrations can affect or inactivate critical genes (KE4) (Genovese et al. Citation2014; Jaiswal et al. Citation2014) within hematopoietic stem cells (HSCs). Increased proliferation (KE5) of the affected HSC further leads to the formation of preleukemic myoblast cells that are capable of clonal expansion (KE5) (Verbiest et al. Citation2018).
The second branch that starts with KE1 describes the formation of the senescence-associated secretory phenotype or SASP (KE6) due to increased cellular RONS (Hellmich et al. Citation2020), and includes a positive feedback loop wherein SASP can stimulate the generation of additional RONS. Similarly, the production of proinflammatory mediator cytokines and chemokines (KE7) can be caused by RONS. The combined effects of KE1, KE6 and KE7 lead to the establishment of a senescent bone marrow microenvironment that further leads to immune suppression (KE8). Finally, the suppressed immune system (KE8) favors clonal expansion of the undifferentiated myeloid progenitor cells (KE9), which ultimately leads to the manifestation of AML (AO) (Genovese et al. Citation2014; Jaiswal et al. Citation2014).
The OECD guidelines and the AOP principles state that an AOP is a living document, so that refinement and revision is not only possible, but also welcome after submission to the AOP-wiki (aopwiki.org). This very much applies to this specific hypothetical AOP which has been registered on the OECD web site as AOP number 432 and presented in . As described next, the group decided to use a systematic approach to consolidate supporting literature and to build a quantitative weight of evidence matrix. It is anticipated that this will result in a refinement and revision of current KEs, as well as potential suggestions for new KEs and KERs.
A systematic approach to literature screening
Although a hypothetical draft AOP to IR-induced leukemia has been generated, using the existing expertise and skills of the members of the leukemia working group, as well as a preliminary narrative literature screen, further work must focus on generation of weight of evidence for each of the KEs and their KERs. It is not uncommon that this subsequent review and literature analysis is done in a narrative manner. Alternatively, a systematic review offers a number of advantages. The most obvious is the avoidance of reviewer bias by following a defined protocol with a question/hypothesis statement and a set of inclusion and exclusion criteria that are based on the question being addressed. Additionally, the workflow is transparent and reproducible, and results in a data extraction stage. Lastly, the systematic review is often capable of identifying studies that are suitable for meta-analysis, thus making quantitative evaluations possible. The systematic review approach has been increasingly used for reviews in biomedical literature, and many of its benefits are immediately applicable to the literature review for development of AOPs. It is not surprising then that the research community involved in the advancing the area of radiation AOPs turned their attention to systematic review of literature as a valuable approach in the development of AOPs (Kozbenko et al. Citation2022).
As discussed above, the hypothetical AOP to rAML contains KEs and KERs that require further review and validation. Indeed, whereas the epidemiologic evidence connecting IR exposure to AML is strong (Leuraud et al. Citation2015; Juliusson et al. Citation2021), and the mechanistic evidence linking the MIE to KE1 through KE5 and KE5 to KE9 is strong for both human and murine AML models, the evidence for the secondary branch connecting KE1->KE6-8 may not be as strong. Indeed, for non-radiation induced AML, support for KE8 is compelling (Khaldoyanidi et al. Citation2021), whereas suppression of the immune system has not been thoroughly investigated with respect to radiation leukaemogenesis, and as such represents one of the central knowledge gaps of the proposed AOP. It is anticipated that this will be addressed in the ongoing systematic review of literature that this working group is engaged. Below are details of this ongoing systematic literature screen and the current status is described.
The systematic approach consists of two phases. In phase 1, the literature search is carried out to identify all experimental studies that link the MIE (IR exposure in our case, since deposition of energy is a hallmark of IR exposure) to the AO, AML. The returned references are screened by title and abstract first, and then by full text – using the inclusion and exclusion criteria. These criteria are formulated based on the PEO statement (Population, Exposure, Outcome). The data is then extracted from the final list of included references focusing on the ability to support existing KEs and to identify new KEs. Importantly, this approach allows the development of a weight of evidence to support information and evidence mapping. Thus, knowledge gaps can be further identified and verified in a more systematic and convincing manner. In phase 2, more literature searches are carried out, this time to identify supporting literature for each KE pair and corresponding KER in a non-stressor specific manner. In other words, evidence supporting KE->KE8 in non-radiation spontaneous leukaemogenesis, that would be missed in the phase 1 screen, will be identified and used for the AOP.
The inclusion criteria, based on the PEO statement were:
Population: human, adults, epidemiological, laboratory animal, ex vivo, in vivo, in vitro.
Exposure: all types of ionizing radiation (high and low LET).
Outcomes: leukemia, acute myeloid leukemia.
The exclusion criteria were:
Type of publication: reviews, editorials, commentaries, etc.
Population: human radiotherapy patients with leukemia, leukemia cell lines, cancer cell lines, trans-generational (i.e. parental exposures).
Exposure: non-ionizing radiation, IR as leukemia treatment, IR combined with chemotherapy.
Outcome: any non-AML leukemia explicitly mentioned as focus of study, children leukemia.
Language and availability: studies not in English, French, Russian or German (spoken by the working group members), without abstracts or full texts available.
Three major biomedical literature databases (PubMed, Web of Science and Scopus) were searched using the queries shown in ; no limitation by date was used.
Table 2. List of literature databases used and corresponding search terms.
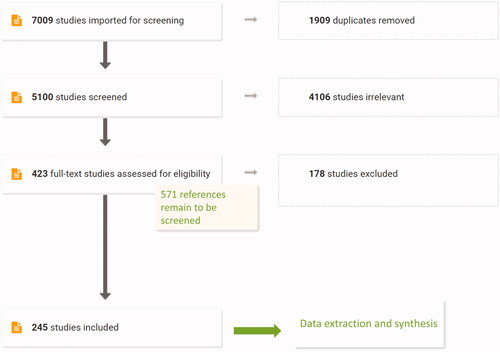
Resulting references were uploaded to Covidence online systematic review tool (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia; available at www.covidence.org) and subsequent screening was performed using this tool which was specifically designed for the systematic review process by biomedical experts and thus incorporates multiple features that greatly facilitate the review process, including data extraction (Babineau Citation2014). In total, our searches resulted in 7009 references, with 1909 being duplicates. After removal of duplicates, 5100 studies were screened by title and abstract and 4106 studies were excluded. The resulting 994 studies went to full text review and currently, 423 out of 994 studies were screened and 245 included in the final stage of data extraction. Based on this stage, it is anticipated that the full text of the remaining 571 references will allow approximately 330 additional studies into the data extraction stage, making it a total of about 575 studies. This number is large and is indicative of a substantial empirical support of the overall connection of IR to AML. It remains to be seen how this substantive evidence will eventually be distributed over the KEs included in the hypothetical AOP to radiation-induced leukemia ().
Future steps
The future activities will primarily focus on the completion of the systematic review and screen processes within the phase 1 and 2 of the proposed systematic AOP development workflow. The objectives will be mapping of evidence to generate the weight of evidence (WOE) support for the proposed AOP and the concomitant identification and validation of knowledge gaps and identification of new KEs and KERs. The systematic approach chosen by this group, although undoubtedly demanding in terms of effort and time, seems to be the best path forward for achieving a transparent review and consolidation of the existing knowledge on the radiation-induced AML. A special attention will be made to reviewing the knowledge related to tissue microenvironment and the immune system dysregulation in AML to validate and potentially revise the corresponding KEs in the presented AOP. A crosstalk between various processes and levels, e.g. cellular, tissue and organism, should be examined for a potential to be included into the AOP as feedback loops. For example, AML cells can modify themselves the microenvironment to suppress the anti-cancer immunity (Yasinska et al. Citation2018). Additionally, low-dose IR is known to modify the function of the immune system in a manner that is not predictable from the effects of high doses, with stimulatory effects being one of the outcomes. Whether these types of responses can affect the process of leukaemogenesis upon low-dose exposures relevant to human exposure scenarios (e.g. <100 mGy) and how it should be reflected in the AOP remains to be determined. Lastly, recent advances in the mechanistic understanding of the leukaemogenesis in general, e.g. the role of mutations preceding the IR exposure (Rubner et al. Citation2012), epigenetic reprogramming (Karlic et al. Citation2014), or clonal heterogeneity (Schuringa and Bonifer Citation2020) deserve a thorough account in the ensuing literature screen and review to examine whether respective KEs should be introduced in the AOP.
Conclusions
The concept of the AOP has promise as an analytical construct that can mechanistically represent biological processes of a developing adverse health outcome following exposure to ionizing radiation (IR). Leukemia is a widely recognized cancer type that can be caused by human exposure to IR and it is therefore a perfect candidate AO to develop a case example of an AOP with IR as a stressor. This working group was formed specifically to to do so, and the group was able to register a proposed AOP for review to the OECD-curated AOP-wiki database (aopwiki.org). A preliminary literature search was performed using a systematic review software program that followed the publishing requirements for a meta-analysis. There is a need for a further, more robust literature review to generate supportive weight of evidence information for all proposed KER. This next step of a more time-intensive, systematic approach to complete the literature review is anticipated to produce a consensus on robust weight of evidence information, along with the identification of knowledge gaps, and potentially new KEs and KERs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Dmitry Klokov
Dmitry Klokov, Ph.D., is a head of the Laboratory of Experimental Radiotoxicology and Radiobiology (LRTOX), Institute of Radioprotection and Nuclear Safety (IRSN), Fontenay-aux-Roses, France. He also holds an Adjunct Professor position at the Department of Biochemistry, Microbiology and Immunology of University of Ottawa. Previously, Dmitry Klokov has led a low-dose radiobiology program at the Canadian Nuclear Laboratories. His research interests include various domains within the field of low-dose radiation effects, with an overarching goal to understand mechanisms of early genotoxic and molecular responses and how they may contribute to long-term health outcomes, such as cancer, cardiovascular and neurological pathologies, and transgenerational effects. Dmitry Klokov has also been involved in various international initiatives in low-dose radiobiological research and cooperation, including those coordinated by UNSCEAR, NEA, MELODI, DOE and others.
Kimberly Applegate
Kimberly Applegate, M.D., M.S., is a member of NCRP Council and ICRP Main Commission as chair of Committee 3, focusing on radiation protection research and policy in medicine. Dr. Applegate is a retired professor of radiology and pediatrics from the University of Kentucky in Lexington. Dr. Applegate’s policy, education, and research work has contributed to documentation and awareness of the variation in care and improvement in the imaging justification and optimization processes, and health outcomes.
Christophe Badie
Christophe Badie, Ph.D., is a biologist who carried out his PhD in Radiobiology-Radiopathology at Gustave Roussy Institute in Villejuif near Paris. In 2005 he became the leader of the Cancer Mechanisms and Biomarkers group in the Radiation Effects department, Radiation Chemical and Environmental Hazards of UK Health Security agency near Oxford. He is working on the development of biomarkers, mainly using human blood as source of information. He has also developed several mouse models to get insights into the molecular mechanisms of radiation leukaemogenesis. He has significant involvement in radiation research in Europe, MELODI (European Multidisciplinary European Low Dose Risk Research Initiative). He is in the scientific committee of the International Association of Biological and EPR Radiation Dosimetry (IABERD), RENEB (European network of biological dosimetry, Realizing the European Network of Bio dosimetry). He is also a member of a NATO RTG group and a member of the ICRP C1 committee.
Dag Anders Brede
Dag Anders Brede, Ph.D., is a professor of Radioecology at the Faculty of Environmental Sciences and Natural Resource Management/Norwegian University of Life Sciences (NMBU). He is a principal investigator at the Center for Environmental Radioactivity (CERAD CoE) and co-lead on CERAD research on biological effects of ionizing radiation and species radiosensitivity.
Fieke Dekkers
Fieke Dekkers, Ph.D. is a mathematician and senior scientist working in the field of radiation research at the National Institute for Public Health and the Environment in the Netherlands and at the Department of Mathematics at Utrecht University, both in the Netherlands. Her research interest includes mathematical modeling of longs term health effects of exposure to ionizing radiation. She is secretary of the European low-dose platform MELODI and a member of the Belgian UNSCEAR delegation.
Melis Karabulutoglu
Melis Karabulutoglu joined Cancer Mechanisms and Biomarkers group as a D.Phil. student (January 2018) under supervision of Dr. Christophe Badie and Dr. Mark A Hill on a joint degree programme with the University of Oxford. Her PhD project focuses on investigating the mechanisms of radiation leukaemogenesis, characterization of hematopoietic stem cells and modulation of risk.
Eric Andreas Rutten
Eric Andreas Rutten is a D.Phil. student in the Department of Oncology, University of Oxford, working in the Cancer Mechanisms and Biomarkers Group at the United Kingdom Health Security Agency (UKHSA) on the role of extracellular vesicles in the development of radiation-induced acute myeloid leukaemias. Eric previously did his BSc and MRes in Imperial College London in the field of Biochemistry and Molecular and Cellular Bioscience. His research interests include the development of pre-cancer biomarkers for early risk assessment and preventative therapy, the use of extracellular vesicles as biomarkers or as therapeutic agents for cancers, as well as personalized treatment regimens for patients based on genetic and epigenetic markers and evolving biomarker landscape.
Katalin Lumniczky
Katalin Lumniczky M.D., Ph.D. is the head of the Radiation Medicine Unit, Department of Radiobiology and Radiohygiene, National Public Health, Budapest, Hungary. She is a radiation biologist, her main research interest is focused on understanding radiation-induced non-targeted effects, radiation-induced immune alterations and the impact of radiation on intercellular signaling.
Maria Gomolka
Maria Gomolka, Ph.D., is a biologist and senior scientist in the Radiation Biology working group of the Federal Office for Radiation Protection, Oberschleissheim, Germany. She focuses on biological radiation reactions, radiation sensitivity, biobanking of radiation exposed individuals and biomarker development for radiation protection. She is and has been involved in various international and national collaborative platforms and projects, such as MELODI, RadoNorm, Concert and DoReMi with a special focus on low-dose radiation research.
References
- Alexander BJ, Rasko JE, Morahan G, Cook WD. 1995. Gene deletion explains both in vivo and in vitro generated chromosome 2 aberrations associated with murine myeloid leukemia. Leukemia. 9(12):2009–2015.
- Allen TE, Goodman JM, Gutsell S, Russell PJ. 2014. Defining molecular initiating events in the adverse outcome pathway framework for risk. Chem Res Toxicol. 27(12):2100–2112.
- Alloni D, Cutaia C, Mariotti L, Friedland W, Ottolenghi A. 2014. Modeling dose deposition and DNA damage due to low-energy β(-) emitters. Radiat Res. 182(3):322–330.
- Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noe M, Horlings HM, Lum A, Jones S, Senz J, Seckin T, et al. 2017. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. 376(19):1835–1848.
- Babineau J. 2014. Product review: covidence (systematic review software). J Can Health Libr Assoc. 35(2):68–71.
- Bai J, Wang Y, Wang J, Zhai J, He F, Zhu G. 2020. Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling. Am J Physiol Cell Physiol. 318(5):C1005–C1017.
- Boice JD, Jr., Cohen SS, Mumma MT, Ellis ED. 2022. The Million Person Study, whence it came and why. Int J Radiat Biol. 98(4):537–550.
- Bouffler SD, Meijne EI, Morris DJ, Papworth D. 1997. Chromosome 2 hypersensitivity and clonal development in murine radiation acute myeloid leukaemia. Int J Radiat Biol. 72(2):181–189.
- Bouville A, Toohey RE, Boice JD, Jr., Beck HL, Dauer LT, Eckerman KF, Hagemeyer D, Leggett RW, Mumma MT, Napier B, et al. 2015. Dose reconstruction for the million worker study: status and guidelines. Health Phys. 108(2):206–220.
- Brown NL, Finnon R, Bulman RA, Finnon P, Moody J, Bouffler SD, Badie C. 2011. Sfpi1/PU.1 mutations in mouse radiation-induced acute myeloid leukaemias affect mRNA and protein abundance and associate with disrupted transcription. Leuk Res. 35(1):126–132.
- Brown N, Finnon R, Manning G, Bouffler S, Badie C. 2015. Influence of radiation quality on mouse chromosome 2 deletions in radiation-induced acute myeloid leukaemia. Mutat Res Genet Toxicol Environ Mutagen. 793:48–54.
- Bullinger L, Dohner K, Dohner H. 2017. Genomics of acute myeloid leukemia diagnosis and pathways. J Clin Oncol. 35(9):934–946.
- Camacho V, Kuznetsova V, Welner RS. 2021. Inflammatory cytokines shape an altered immune response during myeloid malignancies. Front Immunol. 12:772408.
- Chauhan V, Beaton D, Hamada N, Wilkins R, Burtt J, Leblanc J, Cool D, Garnier-Laplace J, Laurier D, Le Y, et al. 2022. Adverse outcome pathway: a path toward better data consolidation and global co-ordination of radiation research. Int J Radiat Biol. 1–10.
- Chauhan V, Hamada N, Monceau V, Ebrahimian T, Adam N, Wilkins RC, Sebastian S, Patel ZS, Huff JL, Simonetto C, et al. 2021. Expert consultation is vital for adverse outcome pathway development: a case example of cardiovascular effects of ionizing radiation. Int J Radiat Biol. 97(11):1516–1525.
- Chauhan V, Said Z, Daka J, Sadi B, Bijlani D, Marchetti F, Beaton D, Gaw A, Li C, Burtt J, et al. 2019. Is there a role for the adverse outcome pathway framework to support radiation protection? Int J Radiat Biol. 95(2):225–232.
- Cheek DM, Naxerova K. 2022. Mapping the long road to cancer. Cell. 185(6):939–940.
- Christiansen DH, Andersen MK, Pedersen-Bjergaard J. 2001. Mutations with loss of heterozygosity of p53 are common in therapy-related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. JCO. 19(5):1405–1413.
- Cook WD, McCaw BJ, Herring C, John DL, Foote SJ, Nutt SL, Adams JM. 2004. PU.1 is a suppressor of myeloid leukemia, inactivated in mice by gene deletion and mutation of its DNA binding domain. Blood. 104(12):3437–3444.
- Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, Hyman DM, Solit DB, Robson ME, Baselga J, et al. 2017. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 21(3):374–382 e374.
- Darakhshan F, Badie C, Moody J, Coster M, Finnon R, Finnon P, Edwards AA, Szluinska M, Skidmore CJ, Yoshida K, et al. 2006. Evidence for complex multigenic inheritance of radiation AML susceptibility in mice revealed using a surrogate phenotypic assay. Carcinogenesis. 27(2):311–318.
- Dekkers F, Bijwaard H, Bouffler S, Ellender M, Huiskamp R, Kowalczuk C, Meijne E, Sutmuller M. 2011. A two-mutation model of radiation-induced acute myeloid leukemia using historical mouse data. Radiat Environ Biophys. 50(1):37–45.
- Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan MD, et al. 2012. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 481(7382):506–510.
- Dohner H, Weisdorf DJ, Bloomfield CD. 2015. Acute myeloid leukemia. N Engl J Med. 373(12):1136–1152.
- Finnon R, Brown N, Moody J, Badie C, Olme CH, Huiskamp R, Meijne E, Sutmuller M, Rosemann M, Bouffler SD. 2012. Flt3-ITD mutations in a mouse model of radiation-induced acute myeloid leukaemia. Leukemia. 26(6):1445–1446.
- Fleenor CJ, Marusyk A, DeGregori J. 2010. Ionizing radiation and hematopoietic malignancies: altering the adaptive landscape. Cell Cycle. 9(15):3005–3011.
- Folley JH, Borges W, Yamawaki T. 1952. Incidence of leukemia in survivors of the atomic bomb in Hiroshima and Nagasaki, Japan. Am J Med. 13(3):311–321.
- Gault N, Verbiest T, Badie C, Romeo PH, Bouffler S. 2019. Hematopoietic stem and progenitor cell responses to low radiation doses - implications for leukemia risk. Int J Radiat Biol. 95(7):892–899.
- Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, et al. 2014. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 371(26):2477–2487.
- Gomolka M, Blyth B, Bourguignon M, Badie C, Schmitz A, Talbot C, Hoeschen C, Salomaa S. 2020. Potential screening assays for individual radiation sensitivity and susceptibility and their current validation state. Int J Radiat Biol. 96(3):280–296.
- Goodhead DT. 1994. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol. 65(1):7–17.
- Hellmich C, Moore JA, Bowles KM, Rushworth SA. 2020. Bone marrow senescence and the microenvironment of hematological malignancies. Front Oncol. 10:230.
- Henry CJ, Marusyk A, DeGregori J. 2011. Aging-associated changes in hematopoiesis and leukemogenesis: what’s the connection? Aging (Albany NY). 3(6):643–656.
- Hepburn M, Doherty I, Briscoe C, Riches A. 1987. Transplantation and morphological studies of primary and passaged murine radiation-induced myeloid leukaemias. Leuk Res. 11(11):1001–1009.
- Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, Kimura A, Kamada N, Dohy H, Tomonaga M, et al. 2013. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat Res. 179(3):361–382.
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. 2014. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 371(26):2488–2498.
- Juliusson G, Lehmann S, Lazarevic V. 2021. Epidemiology and etiology of AML. In: Röllig C, Ossenkoppele GJ, editors. Acute myeloid leukemia. Cham: Springer International Publishing; p. 1–22.
- Karabulutoglu M, Finnon R, Cruz-Garcia L, Hill MA, Badie C. 2021. Oxidative stress and x-ray exposure levels-dependent survival and metabolic changes in murine HSPCs. Antioxidants (Basel). 11(1):11.
- Karabulutoglu M, Finnon R, Imaoka T, Friedl AA, Badie C. 2019. Influence of diet and metabolism on hematopoietic stem cells and leukemia development following ionizing radiation exposure. Int J Radiat Biol. 95(4):452–479.
- Karlic H, Herrmann H, Varga F, Thaler R, Reitermaier R, Spitzer S, Ghanim V, Blatt K, Sperr WR, Valent P, et al. 2014. The role of epigenetics in the regulation of apoptosis in myelodysplastic syndromes and acute myeloid leukemia. Crit Rev Oncol Hematol. 90(1):1–16.
- Khaldoyanidi S, Nagorsen D, Stein A, Ossenkoppele G, Subklewe M. 2021. Immune biology of acute myeloid leukemia: implications for immunotherapy. J Clin Oncol. 39(5):419–432.
- Klymenko SV, Bink K, Trott KR, Bebeshko VG, Bazyka DA, Dmytrenko IV, Abramenko IV, Bilous NI, Zitzelsberger H, Misurin AV, et al. 2005. MLL gene alterations in radiation-associated acute myeloid leukemia. Exp Oncol. 27(1):71–75.
- Klymenko S, Trott K, Atkinson M, Bink K, Bebeshko V, Bazyka D, Dmytrenko I, Abramenko I, Bilous N, Misurin A, et al. 2005. Aml1 gene rearrangements and mutations in radiation-associated acute myeloid leukemia and myelodysplastic syndromes. J Radiat Res. 46(2):249–255.
- Kozbenko T, Adam N, Lai V, Sandhu S, Kuan J, Flores D, Appleby M, Parker H, Hocking R, Tsaioun K, et al. 2022. Deploying elements of scoping review methods for Adverse Outcome Pathway development: a space travel case example. Int J Radiat Biol. Submitted. 1–12.
- Larrue C, Heydt Q, Saland E, Boutzen H, Kaoma T, Sarry JE, Joffre C, Recher C. 2019. Oncogenic KIT mutations induce STAT3-dependent autophagy to support cell proliferation in acute myeloid leukemia. Oncogenesis. 8(8):39.
- Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O'Hagan JA, Hamra GB, Haylock R, Laurier D, Moissonnier M, et al. 2015. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2(7):e276–e281.
- Lo Coco F, Pisegna S, Diverio D. 1997. The AML1 gene: a transcription factor involved in the pathogenesis of myeloid and lymphoid leukemias. Haematologica. 82(3):364–370.
- Loh PR, Genovese G, Handsaker RE, Finucane HK, Reshef YA, Palamara PF, Birmann BM, Talkowski ME, Bakhoum SF, McCarroll SA, et al. 2018. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature. 559(7714):350–355.
- Lomax ME, Folkes LK, O'Neill P. 2013. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol). 25(10):578–585.
- Major IR. 1979. Induction of myeloid leukaemia by whole-body single exposure of CBA male mice to x-rays. Br J Cancer. 40(6):903–913.
- Marusyk A, Casas-Selves M, Henry CJ, Zaberezhnyy V, Klawitter J, Christians U, DeGregori J. 2009. Irradiation alters selection for oncogenic mutations in hematopoietic progenitors. Cancer Res. 69(18):7262–7269.
- McNerney ME, Godley LA, Le Beau MM. 2017. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 17(9):513–527.
- Meldrum RA, Mole RH. 1982. Radiation-induced myeloid leukaemia in CBA/H mice: a non-immunogenic malignant disease in syngeneic mice. Br J Cancer. 45(3):403–412.
- NCRP Report 171. 2012. Uncertainties in the estimation of radiation risks and probability of disease causation. Bethesda (MD): NCRP.
- NCRP Report 186. 2020. Approaches for integrating information from radiation biology and epidemology to enhance low-dose health risk assessments. Bethesda (MD): NCRP.
- O'Brien G, Cruz-Garcia L, Zyla J, Brown N, Finnon R, Polanska J, Badie C. 2020. Kras mutations and PU.1 promoter methylation are new pathways in murine radiation-induced AML. Carcinogenesis. 41(8):1104–1112.
- Olme CH, Brown N, Finnon R, Bouffler SD, Badie C. 2013. Frequency of acute myeloid leukaemia-associated mouse chromosome 2 deletions in X-ray exposed immature haematopoietic progenitors and stem cells. Mutat Res. 756(1–2):119–126.
- Olme CH, Finnon R, Brown N, Kabacik S, Bouffler SD, Badie C. 2013. Live cell detection of chromosome 2 deletion and Sfpi1/PU1 loss in radiation-induced mouse acute myeloid leukaemia. Leuk Res. 37(10):1374–1382.
- Patel A, Anderson J, Kraft D, Finnon R, Finnon P, Scudamore CL, Manning G, Bulman R, Brown N, Bouffler S, et al. 2016. The influence of the CTIP polymorphism, Q418P, on homologous recombination and predisposition to radiation-induced tumorigenesis (mainly rAML) in mice. Radiat Res. 186(6):638–649.
- Pedersen-Bjergaard J, Philip P, Larsen SO, Andersson M, Daugaard G, Ersboll J, Hansen SW, Hou-Jensen K, Nielsen D, Sigsgaard TC. 1993. Therapy-related myelodysplasia and acute myeloid leukemia. Cytogenetic characteristics of 115 consecutive cases and risk in seven cohorts of patients treated intensively for malignant diseases in the Copenhagen series. Leukemia. 7(12):1975–1986.
- Peng Y, Brown N, Finnon R, Warner CL, Liu X, Genik PC, Callan MA, Ray FA, Borak TB, Badie C, et al. 2009. Radiation leukemogenesis in mice: loss of PU.1 on chromosome 2 in CBA and C57BL/6 mice after irradiation with 1 GeV/nucleon 56Fe ions, X rays or gamma rays. Part I. Experimental observations. Radiat Res. 171(4):474–483.
- Poluben L, Puligandla M, Neuberg D, Bryke CR, Hsu Y, Shumeiko O, Yuan X, Voznesensky O, Pihan G, Adam M, et al. 2019. Characteristics of myeloproliferative neoplasms in patients exposed to ionizing radiation following the Chernobyl nuclear accident. Am J Hematol. 94(1):62–73.
- Preston RJ, Ruhm W, Azzam EI, Boice JD, Bouffler S, Held KD, Little MP, Shore RE, Shuryak I, Weil MM. 2021. Adverse outcome pathways, key events, and radiation risk assessment. Int J Radiat Biol. 97(6):804–814.
- Rassool FV, Gaymes TJ, Omidvar N, Brady N, Beurlet S, Pla M, Reboul M, Lea N, Chomienne C, Thomas NS, et al. 2007. Reactive oxygen species, DNA damage, and error-prone repair: a model for genomic instability with progression in myeloid leukemia? Cancer Research. 67(18):8762–8771.
- Riley PA. 1994. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 65(1):27–33.
- Rithidech KN, Cronkite EP, Bond VP. 1999. Advantages of the CBA mouse in leukemogenesis research. Blood Cells Mol Dis. 25(1):38–45.
- Rothkamm K, Kruger I, Thompson LH, Lobrich M. 2003. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 23(16):5706–5715.
- Rubner Y, Wunderlich R, Ruhle PF, Kulzer L, Werthmoller N, Frey B, Weiss EM, Keilholz L, Fietkau R, Gaipl US. 2012. How does ionizing irradiation contribute to the induction of anti-tumor immunity? Front Oncol. 2:75.
- Sabin RJ, Anderson RM. 2011. Cellular Senescence - its role in cancer and the response to ionizing radiation. Genome Integr. 2(1):7.
- Schuringa JJ, Bonifer C. 2020. Dissecting clonal heterogeneity in AML. Cancer Cell. 38(6):782–784.
- Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. 2019. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 36:70–87.
- Siegel RL, Miller KD, Jemal A. 2019. Cancer statistics, 2019. CA A Cancer J Clin. 69(1):7–34.
- Silver A, Moody J, Dunford R, Clark D, Ganz S, Bulman R, Bouffler S, Finnon P, Meijne E, Huiskamp R, et al. 1999. Molecular mapping of chromosome 2 deletions in murine radiation-induced AML localizes a putative tumor suppressor gene to a 1.0 cM region homologous to human chromosome segment 11p11-12. Genes Chromosom Cancer. 24(2):95–104.
- Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, Vardiman JW, Rowley JD, Larson RA. 2003. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 102(1):43–52.
- Steensma DP. 2018. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2(22):3404–3410.
- Stouten S, Verduyn Lunel S, Finnon R, Badie C, Dekkers F. 2021. Modeling low-dose radiation-induced acute myeloid leukemia in male CBA/H mice. Radiat Environ Biophys. 60(1):49–60.
- Super HJ, McCabe NR, Thirman MJ, Larson RA, Le Beau MM, Pedersen-Bjergaard J, Philip P, Diaz MO, Rowley JD. 1993. Rearrangements of the MLL gene in therapy-related acute myeloid leukemia in patients previously treated with agents targeting DNA-topoisomerase II. Blood. 82(12):3705–3711.
- Takasugi M, Yoshida Y, Hara E, Ohtani N. 2022. The role of cellular senescence and SASP in tumour microenvironment. Febs J.
- UNSCEAR 2021. Annex C. Biological mechanisms relevant for the inference of cancer risks from low-dose and low-dose-rate radiation UNSCEAR 2020/2021 report. Vienna: UNSCEAR.
- Verbiest T, Bouffler S, Nutt SL, Badie C. 2015. PU.1 downregulation in murine radiation-induced acute myeloid leukaemia (AML): from molecular mechanism to human AML. Carcinogenesis. 36(4):413–419.
- Verbiest T, Finnon R, Brown N, Cruz-Garcia L, Finnon P, O'Brien G, Ross E, Bouffler S, Scudamore CL, Badie C. 2018. Tracking preleukemic cells in vivo to reveal the sequence of molecular events in radiation leukemogenesis. Leukemia. 32(6):1435–1444.
- Vinken M. 2013. The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology. 312:158–165.
- Wang B, Kohli J, Demaria M. 2020. Senescent cells in cancer therapy: friends or foes? Trends Cancer. 6(10):838–857.
- Wang Y, Liu L, Zhou D. 2011. Inhibition of p38 MAPK attenuates ionizing radiation-induced hematopoietic cell senescence and residual bone marrow injury. Radiat Res. 176(6):743–752.
- Wild CP. 2005. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 14(8):1847–1850.
- Wodnar-Filipowicz A, Kalberer CP. 2007. Function of natural killer cells in immune defence against human leukaemia. Swiss Med Wkly. 137 Suppl 155(Suppl 155):25S–30S.
- Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, Lamprecht TL, Shen D, Hundal J, Fulton RS, et al. 2015. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 518(7540):552–555.
- Yasinska IM, Goncalves Silva I, Sakhnevych S, Gibbs BF, Raap U, Fasler-Kan E, Sumbayev VV. 2018. Biochemical mechanisms implemented by human acute myeloid leukemia cells to suppress host immune surveillance. Cell Mol Immunol. 15(11):989–991.
- Zhao Y, Shao Q, Peng G. 2020. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol Immunol. 17(1):27–35.