Abstract
Purpose
Although the adverse health risks associated with low-dose radiation (LDR) are highly debated, relevant data on neuronal function following chronic LDR exposure are still lacking.
Materials and Methods
To confirm the effect of chronic LDR on the progression of Alzheimer’s disease (AD), we investigated changes in behavior and neuroinflammation after radiation exposure in wild-type (WT) and 5xFAD (TG) mice, an animal model of AD. WT and TG mice, classified by genotyping, were exposed to low-dose-rate radiation for 112 days, with cumulative doses of 0, 0.1, and 0.3 Gy, then evaluated using the open-field and Y-maze behavioral function tests. Changes in the levels of APP processing- and neuroinflammation-related genes were also investigated.
Results
No apparent change was evident in either non-spatial memory function or locomotor activity, as examined by the Y-maze and open field tests, respectively. Although chronic LDR did not affect the levels of APP processing, gliosis (Iba1 and GFAP), or inflammatory cytokines (IL-1β, IL-6, and TNF-α), the levels of IFN-γ were significantly downregulated in TG mice following LDR exposure. In an additional analysis, we examined the genes related to IFN signaling and found that the levels of interferon induced transmembrane protein 3 (IFITM3) were decreased significantly in TG mice following LDR with 0.1 or 0.3 Gy.
Conclusions
Therefore, this study revealed the possibility that LDR could affect the progression of AD, which may be associated with decreased IFN-related signaling, especially IFITM3. Our findings suggest that further studies are required regarding the potential role of LDR in the progression of AD.
1. Introduction
Ionizing radiation can be used for various medical purposes, including therapeutic radiology (tumor treatment) or diagnostic imaging (X-ray or CT scan); however, many people are concerned about receiving radiation, even with low-dose radiation (LDR). In addition, exposure to LDR from previous nuclear accidents or airline travel has increased public concern regarding the critical effects of radiation exposure, even though radiation exposure during medical treatment is below the permissible level. Numerous preclinical studies have demonstrated that exposure to high-dose radiation (HDR) is detrimental to the central nervous system (CNS). Cranial radiation with 10 Gy leads to abnormalities in behavioral functions, including learning and memory (Raber et al. Citation2004; Son et al. Citation2016), depression (Son et al. Citation2014), and anxiety (Lee, Kim et al. Citation2022). The expression profiles of genes within the brain may differ depending on the dose of ionizing radiation (Yin et al. Citation2003). However, to date, few behavioral studies have been published on behavior following LDR.
Alzheimer’s disease (AD) is a well-known neurodegenerative disease and the leading cause of dementia. It has been suggested that deposition of amyloid beta (Aβ) and/or accumulation of hyperphosphorylated tau protein may play a role in the pathogenesis of AD (d‘Errico and Meyer-Luehmann Citation2020). However, the underlying pathological mechanism of AD has not yet been identified. Although many researchers are trying to develop effective therapeutics or medications for AD, there is currently no cure (Long and Holtzman Citation2019). Recently, lecanemab, a amyloid beta-directed monoclonal antibody, was approved for AD with mild cognitive impairment (van Dyck et al. Citation2023). However this drug delays the progression of AD, not a cure, therefore, development of mechanism-based therapies for AD is still needed. Radiation exposure is thought to have negative effects on neuronal function, even at low doses. Previous studies have reported protein modification alterations in the hippocampus following chronic exposure to LDR (0.3 Gy) (Kempf et al. Citation2016). In addition, high-charged particle radiation (0.1 or 1 Gy) can increase Aβ plaque pathology in animal models of AD (Cherry et al. Citation2012), suggesting a detrimental effect of radiation on AD. Recently, however, a case study reported increases in cognition and speech after 40 mGy detected using CT scans of the AD patients brain (Cuttler et al. Citation2016). In addition, several preclinical studies have attempted to apply ionizing radiation to the treatment of AD (Yang et al. Citation2021). Thus, there is some controversy regarding the effects of radiation on the progression of AD and its associated neuronal functions, necessitating further investigation of specific dose rates and radiation dose analyses.
The aim of this study was to elucidate the molecular alterations induced by LDR in the murine brain. 5xFAD mice were total-body-exposed to cumulative doses of 0.1 Gy or 0.3 Gy given at low-dose-rate of 0.038 mGy/day or 0.113 mGy/day, respectively, over a 112 days. As both amyloid precursor protein (APP) processing and neuroinflammation-related signals can influence the progression of AD, we aimed to demonstrate these modifications in this study. In addition, we examined whether LDR is capable of modulating the innate immunity protein IFITM3, which is related to AD neuropathology.
2. Materials and methods
2.1. Animals
We used female 5xFAD mouse (MMRRC_034848-JAX), which express five familial Alzheimer’s disease (FAD) mutations, including three point mutations (Swedish, Florida, and London) in the human APP gene and two mutations (M146L and L286V) in the human presenilin 1 (PS1) gene. The 5xFAD mice were generated by crossing male 5xFAD (Jackson Laboratory, Bar Harbor, ME, USA) and female SJL/B6 mice.
At 2 months of age, the mice were assigned to the sham-irradiated control group (TG: n = 7), 0.1 Gy LDR group (TG + 0.1 Gy: n = 7), or 0.3 Gy LDR group (TG + 0.3 Gy: n = 7). Age-matched female B6/SJL mice were assigned to the WT group (n = 7). Specific pathogen free (SPF) facility, which under controlled for 12-h light dark cycle (light 7 a.m. to 7 p.m.), temperature (23 ± 2 °C) and humidity (50 ± 10%) conditions, were used for housing the mice and all animals had ad libitum access to autoclaved water and rodent feed.
2.2. Low-dose-rate irradiation
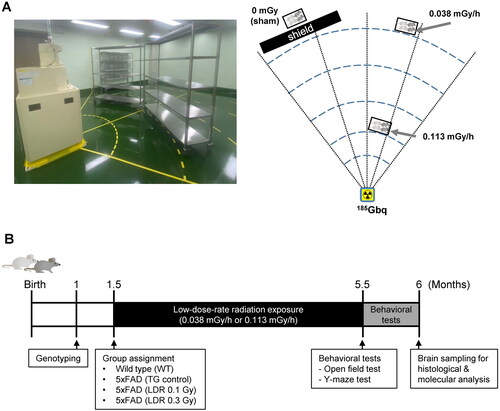
LDR was administered in the animal facility at the DIRAMS and was conducted in a SPF conditioned irradiation room equipped with a 137Cs source (185 GBq). The mouse cages were placed on shelves located at dose rates of 0.038 mGy/h and 0.113 mGy/h for 112 days; the total accumulative radiation dose of the assigned positions were 0.1 and 0.3 Gy, respectively. The animals were continuously exposed to the radiation for 24 h per day, with the exception of 2 h per week during which the room was cleaned, bedding was changed, and food and water were replaced. Sham-irradiated mice were placed on shelves in the same facility and were shielded from radiation ().
2.3. Behavioral tests
2.3.1. Open-field test
We performed the open field test to examine locomotor activity in a novel environment following low-dose-rate LDR exposure. The mice were habituated to the testing room before behavioral experiments. After habituation, the mice were placed in an open acrylic chamber (45 × 45 × 30 cm) without restrictions for 10 min. The camera was placed above the arena, and the movements of the mice were recorded using video tracking. Various parameters, including velocity, percentage of activity, total distance, and time in center were analyzed by using a video recording system (Viewer3 program; Biobserve, St. Augustin, Germany).
2.3.2. Y-maze test
The Y-maze test was used to assess working and reference memory, which can be assessed by monitoring spontaneous alternation behavior. Descriptions of the Y-maze test have been previously described (Jeong et al. Citation2020). Briefly, each mouse was placed into the center of the Y-maze (35 × 5 × 15 cm, 120° apart), and activity was recorded for 8 min with a computer program (Viewer3). Alternation was defined as successive entries into the three different arms on overlapping triplet sets and was calculated as the ratio of actual to possible alternations (defined as the total number of arm entries minus two) multiplied by 100, as follows: % alternation = (number of alternations)/(total arm entries − 2) × 100.
2.4. Immunohistochemistry (IHC) and image analysis
The brain sections were deparaffinized and rehydrated in a graded series of ethanol. After deparaffinization and hydration, each section was incubated with citrate buffer for antigen retrieval. Subsequently, the slides were then incubated with 1.5% normal horse serum (Vector Laboratories Inc., Burlingame, CA, USA) to block nonspecific staining, followed by overnight incubation with mouse anti-amyloid (6E10, 1:2000, sig-39320, Covance, Princeton, NJ, USA) and anti-IFITM3 (1:1000, ab15592, Abcam, Cambridge, MA, USA) antibodies. Subsequently, the slides were washed with PBS containing 0.1% Tween 20 (PBS-T) and incubated with biotinylated anti-mouse IgG and anti-rabbit IgG secondary antibodies (Vector Laboratories, Inc.) for 30 min at room temperature. Following incubation, the sections were then incubated with an avidin–biotin–peroxidase complex (Vector Laboratories Inc.) for 30 min at room temperature. After incubation with diaminobenzidine (DAB; Vector Laboratories Inc.), the images were obtained using a Zeiss microscope (Carl Zeiss, Heidelberg, Germany).
Images of the cortex and DG area of the hippocampus were captured and acquired with a BX-53 microscope (Olympus, Tokyo, Japan) at ×10 magnification. For quantification, images were converted to grayscale and the threshold of each image was adjusted via background subtraction. The mean gray value (256 Gy levels) for each selected area was determined using ImageJ software (version 1.51, NIH, Bethesda, MD, USA). All measurements were performed by the same individual who was blinded to the experimental conditions.
2.5. Western blotting
The cortex and hippocampus were sonicated in lysis buffer (PRO-PREPTM, iNtRon, Gyeonggi-do, Korea) after physical mincing. Protein concentration was quantified using Bradford reagent (Bio-Rad, Hercules, CA, USA), and then denatured by boiling for 10 min. The proteins of each sample were separated by electrophoresis on 10–15% SDS-acrylamide gels and then transferred onto nitrocellulose membranes in Tris/Glycine buffer (Bio-rad), which were subsequently blocked with a 5% bovine serum albumin (BSA) solution for nonspecific blocking. The membranes were incubated at 4 °C with anti-6E10 (1:1000, Sig-39320, Covance), anti-GFAP (1:1000, z0334, Dako, Carpinteria, CA, USA), anti- Iba1 (1:1000, 019-19741, Wako, Osaka, Japan), anti-IFITM3 (1:1000, ab15592, Abcam, Cambridge, MA, USA), or anti-β-actin (1:10,000, A5441, Sigma, St. Louis, MO, USA). After extensive washing in PBS-T buffer, the membranes were incubated for 1 hour with the HRP-conjugated goat anti-mouse or rabbit antibody (1:10,000 dilution; 31462 for mouse and 31432 for rabbit, Invitrogen, Carlsbad, CA, USA). The membranes were then washed, and immunoreactivity was visualized using a chemiluminescence kit (Perkin Elmer, MA, USA). Protein bands were quantified using ImageJ software (National Institutes of Health, MD).
2.6. RNA extraction and quantitative real-time RT-PCR (qRT-PCR)
Total RNA was extracted from the cortex using an RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA, USA), as described by the manufacturer. A NanoDrop ND-One (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the concentration of RNA samples.
cDNA synthesis was performed using random primers (Toyobo Inc., Tokyo, Japan) according to the manufacturer’s instructions and stored at −20 °C. qRT-PCR amplification reactions were performed in 96 well plates using PowerUP 2X SYBR Green mix (Thermo Fisher Scientific) on a StepOne real-time PCR system (Applied Biosystems, CA, USA) according to the manufacturer’s instructions. Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was used as a housekeeping gene. The sequences of the forward and reverse primers are shown in .
Table 1. Primer used in real-time RT-PCR analysis.
2.7. Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM). The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test using GraphPad Prism 9.0 (GraphPad software, San Diego, CA, USA). In all analyses, p < 0.05 was used to indicate statistical significance.
3. Results
3.1. Locomotor activity and memory function in 5xFAD mice following low-dose-rate LDR
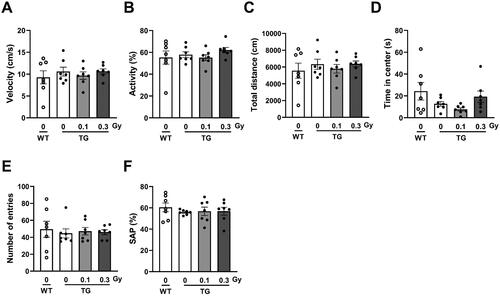
To investigate whether low-dose-rate LDR exposure affected locomotor activity in a mouse model of AD, we first explored basic locomotor activity in a novel environment using an open field test. In the open field test, we did not observe any low-dose-rate LDR-induced differences in the TG mice. There was no difference in velocity, activity (%), or total distance traveled between WT and TG mice with either 0.1 Gy or 0.3 Gy radiation (). In the TG 0.1 Gy group, mice tended to spend less time in the center, and there was no significant difference when compared with the WT group (). Next, we examined whether chronic low-dose-rate LDR affected spatial memory in 5xFAD mice using the Y-maze test. The TG mice that had been exposed to LDR (0.1 or 0.3 Gy) showed no differences in the number of entries or spontaneous alternations in the Y-maze test (). These findings indicate that locomotor and memory function, as examined by the open field and Y-maze tests, did not change after exposure to low-dose-rate LDR in a mouse model of AD.
Figure 2. Locomotor activity and non-spatial memory in 5xFAD mice following low-dose-rate low dose radiation (LDR). (A) Velocity, (B) activity, (C) total distance, and (D) time in center on open field apparatus were assessed in wild-type (WT) and 5xFAD (TG) mice with or without LDR. (E) Number of entries, (F) % of spontaneous alternation (SAP) in Y-maze test were assessed in WT and TG groups. Data are expressed as means ± SE (n = 7 per group).
3.2. Aβ deposition and processing in cortex and hippocampus of 5xFAD mice after low-dose-rate LDR
To determine whether the low-dose-rate LDR affected the levels of Aβ deposition in 5xFAD mice, immunostaining was performed in the cortex and hippocampus with the 6E10 antibody. We focused on the hippocampus and cortex regions of the mouse brain because these regions are known to be the primary site of AD lesions and pathology. In TG mice, there was a prominent accumulation of Aβ (). However, low-dose-rate LDR did not change amyloid plaque deposition in either the hippocampus or cortex (). Next, we investigated whether LDR affected APP processing using Western blotting. The protein levels of APP and carboxyl terminal fragments (CTFs), which are involved in Aβ production, were increased in the brains of 5xFAD mice compared with those of WT mice (). However, there were no significant differences in the expression of APP and CTFs between the sham-exposed 5xFAD and low-dose-rate LDR-exposed groups, in either the cortex or hippocampus ().
Figure 3. Effect of low-dose-rate low dose radiation (LDR) on amyloid beta (Aβ) deposition and APP processing in 5xFAD mice. (A) Representative immunostaining images of Aβ deposits (in brown) in the brain of 5xFAD (TG) mice with or without LDR. (B) Quantification of Aβ burden in the hippocampus and cortex of TG + 0 Gy (sham), TG + 0.1 Gy, and TG + 0.3 Gy (n = 5/group). (C) Representative Western blots of 6E10 in the cortex and hippocampus of 5xFAD mice. (D) Quantification of APP and CTFβ expression in lysates for the indicated groups. (E) The bar graphs show the relative expression levels of insulin-degrading enzyme (IDE) and neprilysin (NEP) in cortex of mice brain. Data are expressed as means ± SEM [n = 4 for (D), n = 6 for (E)]. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT group. ns: not significant.
![Figure 3. Effect of low-dose-rate low dose radiation (LDR) on amyloid beta (Aβ) deposition and APP processing in 5xFAD mice. (A) Representative immunostaining images of Aβ deposits (in brown) in the brain of 5xFAD (TG) mice with or without LDR. (B) Quantification of Aβ burden in the hippocampus and cortex of TG + 0 Gy (sham), TG + 0.1 Gy, and TG + 0.3 Gy (n = 5/group). (C) Representative Western blots of 6E10 in the cortex and hippocampus of 5xFAD mice. (D) Quantification of APP and CTFβ expression in lysates for the indicated groups. (E) The bar graphs show the relative expression levels of insulin-degrading enzyme (IDE) and neprilysin (NEP) in cortex of mice brain. Data are expressed as means ± SEM [n = 4 for (D), n = 6 for (E)]. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT group. ns: not significant.](/cms/asset/fbfbb0c5-fb2d-454d-bb1d-d5a1d5631f8a/irab_a_2211142_f0003_c.jpg)
To demonstrate whether low-dose-rate LDR alters Aβ degradation enzymes, including insulin-degrading enzyme (IDE) and neprilysin (NEP), the mRNA expression levels of these enzymes were observed in a mouse model of AD. While there were no significant differences in IDE expression between the WT and TG groups, the expression of NEP tended to decrease in the TG group compared to the WT group; however, this difference was not statistically significant (). Although LDR tended to increase cortical NEP levels, there was no significant effect on IDE levels (). These data suggested that low-dose-rate LDR did not change Aβ processing or Aβ degradation enzymes in the 5xFAD mouse model of AD.
3.3. Effects of low-dose-rate LDR on the expression levels of inflammatory responses in the brain of 5xFAD mice
To determine whether microglia and astrocytes, markers of neuroinflammation, were altered by low-dose-rate LDR, we observed the levels of Iba1 and GFAP, microglial and astrocyte markers, respectively, in the cortex and hippocampus regions of 5xFAD mice. In the TG group, the protein expression of Iba1 was significantly increased in the hippocampus but not in the cortex compared to that in the WT group (). However, the protein expression of Iba1 was not changed following LDR. The protein levels of GFAP were significantly increased in both the hippocampus and cortex in the TG group compared with those in the WT group (). GFAP protein levels did not differ between the TG group and TG-0.1 Gy or 0.3 Gy group.
Figure 4. Analysis of neuroinflammation-related signaling in 5xFAD mice following low-dose-rate low dose radiation (LDR). (A) Representative Western blots of GFAP and Iba1 in the cortex and hippocampus of 5xFAD mice. (B, C) Quantification of GFAP and Iba1 expression in lysates for the indicated groups. (D) The bar graphs show the mRNA expression levels of Iba1 and Gfap. (E) The bar graphs show the inflammatory cytokines (IL-1β, IL-6, TNF-α, and IFN-γ). Data are expressed as means ± SEM [n = 4 for (B,C), n = 6 for (D,E)]. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT group or TG (0 Gy) group. ns: not significant.
![Figure 4. Analysis of neuroinflammation-related signaling in 5xFAD mice following low-dose-rate low dose radiation (LDR). (A) Representative Western blots of GFAP and Iba1 in the cortex and hippocampus of 5xFAD mice. (B, C) Quantification of GFAP and Iba1 expression in lysates for the indicated groups. (D) The bar graphs show the mRNA expression levels of Iba1 and Gfap. (E) The bar graphs show the inflammatory cytokines (IL-1β, IL-6, TNF-α, and IFN-γ). Data are expressed as means ± SEM [n = 4 for (B,C), n = 6 for (D,E)]. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT group or TG (0 Gy) group. ns: not significant.](/cms/asset/56c773f5-4c06-4e53-9fc3-9efccf1283db/irab_a_2211142_f0004_b.jpg)
Next, we compared the RNA levels of neuroinflammatory markers in WT and TG mice following low-dose-rate LDR exposure. A significant upregulation in the mRNA expression of Iba1 and GFAP was observed in the TG group compared with the WT group, whereas there were no significant differences in Iba1 and Gfap expression between the sham-irradiated and LDR-exposed TG groups (). In addition, to determine whether low-dose-rate LDR affected the levels of inflammatory cytokines, we examined the levels of IL-1β, IL-6, TNF-α, and IFN-γ, markers of pro-inflammatory cytokines, in 5xFAD mice. The mRNA levels of IL-1β, IL-6, TNF-α, and IFN-γ were elevated in TG mice compared to those in age-matched WT mice (). However, there was no change in the levels of IL-1β, IL-6, and TNF-α after exposure to LDR, and the levels of IFN-γ were significantly downregulated following LDR exposure compared with the TG group (). These data suggest that low-dose-rate LDR selectively regulates inflammatory cytokines, which is not correlated with the accumulation of Aβ in the brains of 5xFAD mice.
3.4. Changes in IFN signaling-related genes in the 5xFAD mice following low-dose-rate LDR
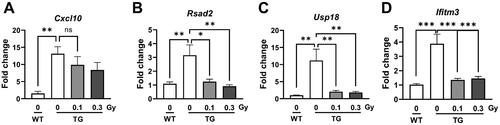
To determine whether low-dose-rate LDR alters IFN-related signaling, including Cxcl10, Rsad2, Usp18, and Ifitm3, the mRNA expression levels of these genes were observed in a mouse model of AD. The expression levels of Cxcl10, a chemokine, as well as Rsad2, Usp18, two interferon-stimulated genes were significantly higher in the TG group than those in the WT group (). In addition, the levels of Ifitm3 were significantly increased in the TG group compared with that in the WT group. In the TG group, there was no change in Cxcl10 levels after exposure to low-dose-rate LDR. LDR significantly decreased the expression of Rsad2, Usp18, and Ifitm3 (). These data suggest that low-dose-rate LDR downregulates the levels of genes related to IFN signaling in the brains of 5xFAD mice.
Figure 5. Analysis of genes related with IFN signaling in 5xFAD mice following low-dose-rate low dose radiation (LDR). The bar graphs show the relative expression levels of (A) Cxcl10, (B) Rsad2, (C) Usp18, and (D) Ifitm3 in cortex of mice brain. Data are expressed as means ± SEM (n = 6 per group). *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT group or TG (0 Gy) group. ns: not significant.
3.5. Changes in IFITM3 in the 5xFAD mice following low-dose-rate LDR
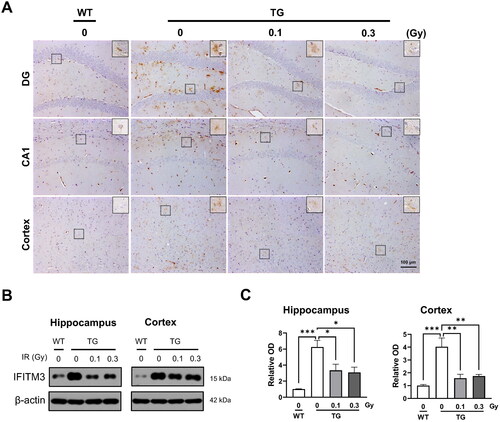
To evaluate the effects of low-dose-rate LDR on amyloid processing, protein levels of IFITM3, an innate immunity protein, were examined in the brains of 5xFAD mice. Representative images show the expression of IFITM3-positive cells in the hippocampus and cortex of the mouse brain (). In the TG group, the protein expression of IFITM3 was significantly increased in both the hippocampus and cortex compared with that in the WT group (). IFITM3 downregulation was observed following low-dose-rate LDR with 0.1 or 0.3 Gy (). This trend was consistent with the results for the mRNA expression of Ifitm3 in the cortex. These data demonstrate a marked decrease in the levels of IFITM3 in the brain following low-dose-rate LDR.
Figure 6. Effect of low-dose-rate low dose radiation (LDR) exposure on IFITM3 in 5xFAD mice. (A) Representative immunostaining images of IFITM3 (in brown) in the brain of wild-type (WT) and 5xFAD (TG) mice with or without LDR. The square shows the merged images at higher magnification (Inserted panel). (B) Representative Western blots of IFITM3 in the cortex and hippocampus of 5xFAD mice. (C) Quantification of IFITM3 expression in lysates for the indicated groups. (D) The bar graphs show the relative expression levels of IFITM3. Data are expressed as means ± SEM (n = 4 per group). *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT group or TG (0 Gy) group.
4. Discussion
AD, the most common neurodegenerative disorder, is a progressive disease that is most commonly associated with initial memory deficits and cognitive impairment. Many researchers have attempted to identify the exact etiology of AD; deposition of senile plaques in the brain, particularly in the cortex and hippocampus, is one of it’s the possible underlying mechanisms (McKhann et al. Citation1984; Findeis Citation2000). However, a successful treatment strategy is yet to be developed. Since a pilot clinical study reported improvement in cognition following repeated CT scans in 2015 (Cuttler et al. Citation2016), many preclinical studies have attempted to examine the effect of LDR on AD. For example, a recent preclinical study demonstrated that low-dose radiotherapy (0.6 Gy × 5 doses) attenuated cognitive impairment examined by the Y-maze test, which persisted for 4–5 weeks after radiation exposure (Yang et al. Citation2021). However, with the increased possibility of LDR in daily life due to medical examination and/or treatment, public concern has been raised about the possible side effects of LDR on human health, especially brain function. In addition, a previous study suggested a possible risk of chronic low-dose-rate LDR (cumulative doses of 0.3 Gy) on AD progression by analyzing the phosphoproteome in the hippocampus of ApoE-deficient mice (Kempf et al. Citation2016). Thus, to demonstrate whether exposure to low-dose-rate LDR affects behavior in a mouse model of AD, 5xFAD mice were subjected to sequential exploration tasks (open field and Y-maze test). LDR in 5xFAD mice began when the mice were 1.5 months old, when Aβ was deposited, to examine whether exposure to LDR is a potential risk factor for AD progression. In the present study, we did not observe a detrimental or beneficial effect of low-dose-rate LDR on locomotor activity or non-spatial memory performance, as determined by OF and Y-maze tests, in 5xFAD mice. Therefore, more comprehensive studies are needed to investigate the effects of different durations of LDR on neurocognitive functions in various AD animal models.
APP processing could contribute to AD pathogenesis, and alterations in these processes may have a significant effect on Aβ production. A previous study showed that amyloid plaques were significantly increased in the dorsal cortex of APP/PS1 mice following proton irradiation at 1 Gy (Rudobeck et al. Citation2017). Another study showed that fractionated LDR (1.8 Gy × 5 doses) did not affect Aβ accumulation in the hippocampus or brain cortex, but significantly ameliorated synaptic degeneration and neuronal loss (Kim et al. Citation2020). It has been reported that 100 mGy of X-ray (dose rate ∼160 mGy/min) induced transcriptional alterations in only three AD-related genes (IL-1α, Apbb1 at 4 h, and Lrp1 at 1 year after irradiation), but did not significantly affect spatial learning and memory or AD-like pathological changes in mice (Wang et al. Citation2014b). It was also reported that low doses of carbon-ion irradiation (50 or 100 mGy) did not induce AD-like pathogenesis in the brain 4 months and 2 years after irradiation (Wang et al. Citation2014a). However, little is known about the effects of low-dose-rate LDR on APP processing. In the present study, exposure to LDR did not result in any differences in the levels of Aβ deposition, APP, or CTFβ, assessed using immunohistochemistry or immunoblotting. Thus, these data suggest that low-dose-rate LDR exposure, initiated at 1.5 months of age, did not affect APP processing or Aβ accumulation in the brains of 5xFAD mice.
The inflammatory response of neuronal structures is complicated in AD; Aβ-induced neuroinflammation involves neuronal loss and formation of Aβ plaques (Baik et al. Citation2016), and activated microglia produce pro-inflammatory cytokines including IL-1β, IL-6, TNF-α, and IFN-γ, which are related to AD pathology (Fairley et al. Citation2021). A previous study demonstrated that the expression of Iba1 and TNF-α was downregulated in the hippocampus of ApoE -/- mice after chronic LDR at 0.3 Gy, but not 6 Gy (Kempf et al. Citation2016). In addition, fractionated LDR decreases neuroinflammation (Iba1 and GFAP) and pro-inflammatory cytokines (IL-1β, IL-6, and CCL6) (Yang et al. Citation2021). However, pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, were not affected by proton irradiation (0.1 ∼ 1 Gy) in the cortex of APP/PS1 mice (Rudobeck et al. Citation2017). Thus, there is some controversy regarding the effects of ionizing radiation on neuroinflammation and associated pro-inflammatory cytokine production, and further mechanistic investigations and analyses are required. IFN-γ is a potent molecule and has numerous immunoregulatory functions within in the CNS, such as activating macrophages/microglia and inducing stimulating them to release toxic oxygen radicals (Blasko et al. Citation2001). In AD, IFN-γ is mainly produced by infiltrating immune cells such as T cells and natural killer (NK) cells, rather than by resident brain cells such as microglia or astrocytes (Blasko et al. Citation2001; Kebir et al. Citation2007; Laurent et al. Citation2017). Whether IFN signaling evokes disease-promoting or disease-ameliorating functions in AD progression remains controversial. For example, while Macchi et al has been reported that treatment of APP/PS1 mice with Aβ reactive T cells accelerated memory impairment and systemic inflammation, increased amyloid burden, elevated microglia activation, and exacerbated neuroinflammation (Machhi et al. Citation2021), other report have found that the expression of hyperphosphorylated tau was decreased by 10 months of IFN-γ expression within the hippocampus of 3xTg-AD mice, suggesting a neuroprotective activity of IFN-γ in AD (Mastrangelo et al. Citation2009). In our study, we observed upregulated gene expression of IFN-γ and activated microglia in the brain of 5xFAD and LDR significantly suppressed the expression of IFN-γ but did not affect those of Iba1. Similar with our study, Gridley et al reported that low-dose, low-dose-rate proton radiation (0. 0.1, 0.05 and 0.1 Gy) modulates CD4(+) T cell gene expression and it was highly dependent upon total dose (Gridley et al. Citation2009). Although LDR down-regulated the expression of IFN-γ, this result was not connected with spatial memory of AD model mice in the present study. Therefore, further investigation is needed to demonstrate how these reductions are mechanistically regulated for degenerative conditions afflicting the AD brain with longer observational duration or higher total radiation dose.
A recent study demonstrated that LDR influences IFN signals; 0.1 Gy induces changes in the phenotypes of endothelial cells (ECs) and alters key molecules involved in the IFN signaling pathway that are associated with cardiovascular disease in cultured primary ECs under both normal and diabetic conditions (Lee, Jeong et al. Citation2022). It was also reported that IFN signaling contributes to neuropathology in AD and increased IFN is found in postmortem brains of AD patients (Roy et al. Citation2020). Previous studies have reported that IFN-stimulated genes (ISGs) are up-regulated in the brains of murine AD models and IFN pathway activation is observed in plaque-associated microglia, suggesting a role of IFN in the neuropathogenic processes of AD (Roy et al. Citation2020). In addition, intracerebroventricular injection of IFN induces microglia activation and reduction in spine density (Roy et al. Citation2020), indicating the negative effect of IFN on memory and brain function (Hayley et al. Citation2013). IFN signaling has been found to modulate the expression of ISGs, including Cxcl10, Rsad2 (also known as viperin), Usp18, and Ifitm3, which are involved in innate immune responses (Schneider et al. Citation2014). Cxcl10, a chemokine, is upregulated in response to IFN signaling and promotes recruitment of inflammatory cells to the site of injury or infection. Increased Cxcl10 levels in the cerebrospinal fluid have been observed in patients with mild cognitive impairment (Liu et al. Citation2014). Rsad2 and Usp18 are two ISGs induced by IFN signaling and have antiviral and anti-inflammatory effects (Ritchie et al. Citation2004). Rsad2 encodes a protein that inhibits viral replication, while Usp18 regulates the activity of the JAK-STAT signaling pathway activated by IFN (Schneider et al. Citation2014). Upregulation of Rsad2 and Usp18 has been reported in the brain tissue of AD patients, suggesting potential driver genes for AD etiology and pathology (Xiang et al. Citation2018). Overall, modulation of IFN signaling-related gene expression in AD patients highlights the complexity of immune responses in the brain and the potential therapeutic targets for AD treatment. In the present study, low-dose-rate LDR significantly downregulated the mRNA levels of Rsad2, Usp18, and Ifitm3, which are involved in IFN signaling. These data suggest that LDR selectively downregulates inflammatory-related genes, which are associated with IFN signaling in the brains of 5xFAD mice.
Since Aβ peptides are generated by the sequential cleavage of APP by β-secretase, BACE1, followed by γ-secretase, many clinical trials have attempted to reduce Aβ production by modulating γ-secretase activity (De Strooper et al. Citation2010). IFITM3, known as the γ-secretase modulatory protein, is involved in innate immunity to defend against many viruses (Zhao et al. Citation2018). In addition, neuroinflammation may cause upregulation of IFITM3, which in turn increases γ-secretase activity and the resultant production of Aβ (Hur et al. Citation2020). Nevertheless, the precise mechanisms associated with γ-secretase modulatory proteins following low-dose-rate LDR remain unknown. In the present study, the expression levels of IFITM3 were significantly increased in the TG group, which is consistent with previous studies on the alterations of IFITM3 in aged and AD mice. The levels of IFITM3 significantly decreased after LDR with 0.1 or 0.3 Gy. The downregulated levels of IFITM3 also support the notion that neuroinflammation is not aggravated after low-dose-rate LDR treatment. However, the expression patterns of IFITM3 did not correlate with unchanged behavioral function and Aβ pathology following chronic low-dose-rate LDR. Consistent with the results of suppression of IFN-γ and IFN-γ signaling-related genes (Rsad2, Cxcl10, Usp18), suppression of IFITM3 expression in the hippocampus and cortex of AD mice suggests that LDR can modulate the immune response and alter cellular proliferation (Friedlová et al. Citation2022), potentially contributing to neuroprotection. However, the lack of significant changes in Aβ deposition following LDR exposure suggests that the regulation of Aβ metabolism may not be directly affected by LDR-mediated changes in IFITM3 expression. Conversely, this contradiction may be due to the experimental conditions of radiation exposure, including the timing, duration, or intensity of the LDR exposure. Therefore, further investigation is needed to fully understand the complex interactions between LDR, IFITM3, and Aβ metabolism in the context of Alzheimer’s disease.
In conclusion, although we did not identify any beneficial effect on neurocognitive function following low-dose-rate LDR on AD progression, it was also demonstrated that LDR did not aggravate Aβ production. Low-dose-rate LDR downregulated IFITM3 expression in the cortex and hippocampus, suggesting that LDR selectively regulates immune response, especially IFN-γ signaling. Even though LDR induced suppression of IFN signaling did not correlate with changes in behavioral function, the present study is the first to examine the expression of IFITM3 in AD mice following chronic low-dose-rate LDR. Consequently, our finding suggests that the LDR exposure could affect immune modulation via IFN signaling in mice model of AD.
Ethical approval
All animal experimental protocols were approved by the Korea Institute of Radiological and Medical Sciences (KIRAMS) and Dongnam Institute of Radiological and Medical Sciences (DIRAMS) Institutional Animal Care and Use Committee (IACUC permit number: KIRAMS-2021-0064, DI-2022-018).
Author contributions
C.G.L. and J.S.K. designed the experiments and analyzed the data. Y.S. and H.J.L. performed the behavioral studies and molecular analyses, and conceived the experiments, analyzed the data, and wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Yeonghoon Son
Yeonghoon Son, D.V.M., Ph.D., is a senior researcher at the Division of Radiation Biomedical Research, Korea Institute of Radiological and Medical Sciences, Republic of Korea.
Chang Geun Lee
Chang Geun Lee, Ph.D., is a senior researcher at the Research Center, Dongnam Institute of Radiological and Medical Sciences, Republic of Korea.
Joong Sun Kim
Joong Sun Kim, D.V.M., Ph.D., is an associate professor at the College of Veterinary Medicine, Chonnam National University, Republic of Korea.
Hae-June Lee
Hae-June Lee, D.V.M., Ph.D., is a principal researcher at the Division of Radiation Biomedical Research, Korea Institute of Radiological and Medical Sciences, Republic of Korea.
References
- Baik SH, Kang S, Son SM, Mook-Jung I. 2016. Microglia contributes to plaque growth by cell death due to uptake of amyloid beta in the brain of Alzheimer’s disease mouse model. Glia. 64(12):2274–2290.
- Blasko I, Ransmayr G, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. 2001. Does IFNgamma play a role in neurodegeneration? J Neuroimmunol. 116(1):1–4.
- Cherry JD, Liu B, Frost JL, Lemere CA, Williams JP, Olschowka JA, O'Banion MK. 2012. Galactic cosmic radiation leads to cognitive impairment and increased abeta plaque accumulation in a mouse model of Alzheimer’s disease. PLoS One. 7(12):e53275.
- Cuttler JM, Moore ER, Hosfeld VD, Nadolski DL. 2016. Treatment of Alzheimer Disease With CT Scans: A Case Report. Dose Response. 14(2):1559325816640073.
- De Strooper B, Vassar R, Golde T. 2010. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 6(2):99–107.
- d‘Errico P, Meyer-Luehmann M. 2020. Mechanisms of Pathogenic Tau and Abeta Protein Spreading in Alzheimer’s Disease. Front Aging Neurosci. 12:265.
- Fairley LH, Wong JH, Barron AM. 2021. Mitochondrial Regulation of Microglial Immunometabolism in Alzheimer’s Disease. Front Immunol. 12:624538.
- Findeis MA. 2000. Approaches to discovery and characterization of inhibitors of amyloid beta-peptide polymerization. Biochim Biophys Acta. 1502(1):76–84.
- Friedlová N, Zavadil Kokáš F, Hupp TR, Vojtěšek B, Nekulová M. 2022. IFITM protein regulation and functions: Far beyond the fight against viruses. Front Immunol. 13:1042368.
- Gridley DS, Rizvi A, Luo-Owen X, Makinde AY, Pecaut MJ. 2009. Low dose, low dose rate photon radiation modifies leukocyte distribution and gene expression in CD4(+) T cells. J Radiat Res. 50(2):139–150.
- Hayley S, Scharf J, Anisman H. 2013. Central administration of murine interferon-alpha induces depressive-like behavioral, brain cytokine and neurochemical alterations in mice: a mini-review and original experiments. Brain Behav Immun. 31:115–127.
- Hur J-Y, Frost GR, Wu X, Crump C, Pan SJ, Wong E, Barros M, Li T, Nie P, Zhai Y, et al. 2020. The innate immunity protein IFITM3 modulates gamma-secretase in Alzheimer’s disease. Nature. 586(7831):735–740.,.
- Jeong YJ, Son Y, Choi HD, Kim N, Lee YS, Ko YG, Lee HJ. 2020. Behavioral changes and gene profile alterations after chronic 1,950-MHz radiofrequency exposure: An observation in C57BL/6 mice. Brain Behav. 10(11):e01815.
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. 2007. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 13(10):1173–1175.
- Kempf SJ, Janik D, Barjaktarovic Z, Braga-Tanaka I, 3rd, Tanaka S, Neff F, Saran A, Larsen MR, Tapio S. 2016. Chronic low-dose-rate ionising radiation affects the hippocampal phosphoproteome in the ApoE-/- Alzheimer’s mouse model. Oncotarget. 7(44):71817–71832.
- Kim S, Nam Y, Kim C, Lee H, Hong S, Kim HS, Shin SJ, Park YH, Mai HN, Oh SM, et al. 2020. Neuroprotective and anti-inflammatory effects of low-moderate dose ionizing radiation in models of alzheimer’s disease. Int J Mol Sci. 21:3678.
- Laurent C, Dorothee G, Hunot S, Martin E, Monnet Y, Duchamp M, Dong Y, Legeron FP, Leboucher A, Burnouf S, et al. 2017. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain. 140(1):184–200.
- Lee SH, Jeong YJ, Park J, Kim HY, Son Y, Kim KS, Lee HJ. 2022. Low-dose radiation affects cardiovascular disease risk in human aortic endothelial cells by altering gene expression under normal and diabetic conditions. Int J Mol Sci. 23:8577.
- Lee HJ, Kim JS, Moon C, Son Y. 2022. Profiling of gene expression in the brain associated with anxiety-related behaviors in the chronic phase following cranial irradiation. Sci Rep. 12(1):13162.
- Liu C, Cui G, Zhu M, Kang X, Guo H. 2014. Neuroinflammation in Alzheimer’s disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Pathol. 7(12):8342–8355.
- Long JM, Holtzman DM. 2019. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 179(2):312–339.
- Machhi J, Yeapuri P, Lu Y, Foster E, Chikhale R, Herskovitz J, Namminga KL, Olson KE, Abdelmoaty MM, Gao J, et al. 2021. CD4+ effector T cells accelerate Alzheimer’s disease in mice. J Neuroinflammation. 18(1):272.
- Mastrangelo MA, Sudol KL, Narrow WC, Bowers WJ. 2009. Interferon-gamma differentially affects Alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am J Pathol. 175(5):2076–2088.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. 1984. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 34(7):939–944.
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. 2004. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 162(1):39–47.
- Ritchie KJ, Hahn CS, Kim KI, Yan M, Rosario D, Li L, de la Torre JC, Zhang DE. 2004. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat Med. 10(12):1374–1378.
- Roy ER, Wang B, Wan YW, Chiu G, Cole A, Yin Z, Propson NE, Xu Y, Jankowsky JL, Liu Z, et al. 2020. Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J Clin Invest. 130(4):1912–1930.
- Rudobeck E, Bellone JA, Szucs A, Bonnick K, Mehrotra-Carter S, Badaut J, Nelson GA, Hartman RE, Vlkolinsky R. 2017. Low-dose proton radiation effects in a transgenic mouse model of Alzheimer’s disease - Implications for space travel. PLoS One. 12(11):e0186168.
- Schneider WM, Chevillotte MD, Rice CM. 2014. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 32:513–545.
- Son Y, Kang S, Kim J, Lee S, Kim JC, Kim SH, Kim JS, Jo SK, Jung U, Youn B, et al. 2016. Possible involvement of hippocampal immediate-early genes in contextual fear memory deficit induced by cranial irradiation. Neurobiol Learn Mem. 133:19–29.
- Son Y, Yang M, Kim JS, Kim J, Kim SH, Kim JC, Shin T, Wang H, Jo SK, Jung U, et al. 2014. Hippocampal dysfunction during the chronic phase following a single exposure to cranial irradiation. Exp Neurol. 254:134–144.
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, et al. 2023. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 388(1):9–21.
- Wang B, Tanaka K, Ji B, Ono M, Fang Y, Ninomiya Y, Maruyama K, Izumi-Nakajima N, Begum N, Higuchi M, et al. 2014a. Low-dose total-body carbon-ion irradiations induce early transcriptional alteration without late Alzheimer’s disease-like pathogenesis and memory impairment in mice. J Neurosci Res. 92(7):915–926.
- Wang B, Tanaka K, Ji B, Ono M, Fang Y, Ninomiya Y, Maruyama K, Izumi-Nakajima N, Begum N, Higuchi M, et al. 2014b. Total body 100-mGy X-irradiation does not induce Alzheimer’s disease-like pathogenesis or memory impairment in mice. J Radiat Res. 55(1):84–96.
- Xiang S, Huang Z, Wang T, Han Z, Yu CY, Ni D, Huang K, Zhang J. 2018. Condition-specific gene co-expression network mining identifies key pathways and regulators in the brain tissue of Alzheimer’s disease patients. BMC Med Genomics. 11(Suppl 6):115.
- Yang EJ, Kim H, Choi Y, Kim HJ, Kim JH, Yoon J, Seo YS, Kim HS. 2021. Modulation of Neuroinflammation by Low-Dose Radiation Therapy in an Animal Model of Alzheimer’s Disease. Int J Radiat Oncol Biol Phys. 111(3):658–670.
- Yin E, Nelson DO, Coleman MA, Peterson LE, Wyrobek AJ. 2003. Gene expression changes in mouse brain after exposure to low-dose ionizing radiation. Int J Radiat Biol. 79(10):759–775.
- Zhao X, Li J, Winkler CA, An P, Guo JT. 2018. IFITM Genes, Variants, and Their Roles in the Control and Pathogenesis of Viral Infections. Front Microbiol. 9:3228.