ABSTRACT
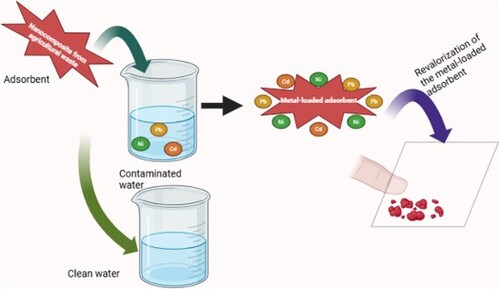
An increasing amount of water pollution is being caused by an increase in industrial activity. Recently, a wide range of methods, including extraction, chemical coagulation, membrane separation, chemical precipitation, adsorption, and ion exchange, have been used to remove heavy metals from aqueous solutions. The adsorption technique is believed to be the most highly effective method for eliminating heavy metals from wastewater among all of them. However, it generates secondary waste that can pose a risk to the environment. Agricultural waste has potential to be collected and converted into carbon nanomaterials, then coated with metal oxides for the removal of Pb2+, Cd2+, and Ni2+ ions and then the reuse of heavy metal spent adsorbents in blood fingerprint detection (BFP) can be studied. This review highlights the eco-friendly nature and abundant availability of these materials while advocating for their integration into mainstream wastewater treatment practices. It explores the prospect of revalorizing spent adsorbents in blood fingerprint applications, demonstrating a dual-purpose utilisation that bridges environmental remediation with advancements forensic sciences. Different method of removal of Pb2+, Cd2+ and Ni2+, removal technique as well as other reuse applications of spent adsorbents are also discussed.
1. Introduction
In recent years, the number of industries manufacturing products containing toxic heavy metals has been increasing rapidly, and their environmental mobilization and transport rates have risen significantly since the 1940s [Citation1]. An unintended consequence of industrialization is environmental pollution due to the emission of materials from industrial processes into the environment. Water resources are a priority recipients of industrial pollutants, predominantly effluents, surface runoff, and incidental spills; this compromises water resources’ quality/health. These growing industries may pollute the water that is essential for our living. South Africa is regarded as a water-scarce country with a high rate of population growth, industrialization, civilization, urbanization, and agriculture. Toxic heavy metals are released from industries such as textile, mining, fertilizer, tanneries, and metal plating into water resources [Citation2]. Other heavy metal releases may also increase from paints, paper, photography, printing, petroleum refining, printing, and dyes into water streams [Citation3]. Such heavy metals include lead (Pb), iron (Fe), copper (Cu), cobalt (Co), cadmium (Cd), nickel (Ni), arsenic (As), hexavalent chromium Cr (VI) [Citation4], manganese (Mn), aluminium (Al), gold (Au), zinc (Zn), and silver (Ag) are used due to their properties.
Contamination of water bodies by heavy metals poses a critical environmental challenge, necessitating effective and sustainable remediation strategies. Among the numerous methods explored, the use of low-cost adsorbents has emerged as a promising avenue for the removal of heavy metals such as Pb2+, Cd2+, and Ni2+ from wastewater. Wastewater may contain components such as inorganic and organic waste, nutrient-suspended solids, and pathogens with biological, physical, and chemical characteristics. Concerns have also been raised regarding adverse side effects on human health. Such effects include reduced haemoglobin, damage to the nervous system and mental retardation [Citation5]. The World Health Organization (WHO) has estimated the maximum permissible limit of Pb2+, Cd2+, and Ni2+ in wastewater to be 0.01, 0.003, and 0.02 mg/L, respectively.
Regarding the removal of pollutants from wastewater during treatment, researchers have developed effective methods for removing various impurities from wastewater. Such pollutants have been considered to lead to secondary pollution. This review paper intends to present a motivation for the use of low-cost adsorbents from agricultural waste (mesoporous carbon, carbon nanorods, carbon nanoparticles) coated with metal oxides (TiO2, ZnO, Al2O3) for the uptake of toxic heavy metals from wastewater and the reuse of the metal-loaded spent adsorbents for fingerprint detection as a viable future research perspective. It is worth mentioning that the reuse of spent adsorbent can enhance latent fingerprint images [Citation6,Citation7]. Besides solving secondary pollution, the reuse of spent adsorbents (carbon nanomaterials, metal oxides, nanoparticles) in latent fingerprint detection has drawn the interest of researchers because they exhibit properties such as good optical stability, excellent affinity, low emission peak, sensitivity, high fluorescent intensity, and selectivity [Citation8,Citation9]. As a result, there is a need to develop cheap, robust, and effective methods of removing heavy metals in wastewater to meet environmental regulations.
This article outlines a comprehensive review of the diverse array of low-cost adsorbents, ranging from agricultural by-products to industrial wastes, to evaluate their efficacy in heavy metal sequestration. and the reuse of spent adsorbents in forensic applications. Specifically, the focus is on Pb2+, Cd2+, and Ni2+ ions, adsorbed on low-cost adsorbent materials. By reviewing current knowledge on low-cost adsorption techniques and their applicability, this research aims to provide insights into sustainable water treatment practices while paving the way for interdisciplinary applications in cutting-edge forensic sciences. Additionally, the forensic applications by reuse of other metal-loaded spent adsorbents are also reviewed.
2.1. Occurrence and environmental release
2.1.1. Lead
Pb2+ is one of the first metal ions that humans discovered [Citation10]. It has been widely employed in numerous industries because of its unique features, such as softness, low melting point, and corrosion resistance. It is generally released from industrial sources such as coal combustion, battery recycling, and lead smelting. Pb is regarded as one of the most highly toxic heavy metals to humans and the environment [Citation11] and occurs at low concentrations in the earth’s crust [Citation12]. The burning and mining of Pb-containing materials release Pb into the atmosphere, and humans may be exposed through inhalation, ingestion, or skin contact [Citation13]. Exposure to Pb2+ poisoning may lead to kidney and liver damage, nervous system difficulties, anaemia, memory loss, and psychological and reproductive issues [Citation14]. Human exposure can also be through the ingestion of Pb-contaminated water, soil, dust, and food.
2.1.2. Cadmium
Cd2+ is categorized as highly toxic and carcinogenic to humans by the International Agency for Research on Cancer [Citation15]. It occurs naturally due to volcanic eruptions and rock and soil erosion/abrasion [Citation16]. Cd2+ is considered among the most toxic heavy metals and is non-degradable and persistent [Citation17]. Untreated wastewater containing Cd2+ may be released from various industrial processes such as phosphate fertilizers, mining, alloy industries, electroplating, sewage sludge, Cd2+ batteries, and pigments [Citation15]. Due to inhalation, oral ingestion, and dermal absorption of Cd2+-containing food, water, or dust, exposure to Cd2+ can last for 10–35 years [Citation18]. The toxicity of Cd2+ arises due to its high mobility and bioaccumulative ability. Excess Cd2+ consumption can result in heart disease, kidney damage, liver, and cancer [Citation19]. According to the World Health Organization, Cd2+ in wastewater should not exceed 0.003 ppm [Citation20]. The global use of Cd2+ is predicted to be approximately 20,00024,000 t/year [Citation21]. Cd2+ in the wastewater should not exceed 2 mg L-1 as the permissible threshold [Citation22].
2.1.3. Nickel
Ni2+ can be released into the environment by volcanic eruptions, anthropogenic activities, and forest fires. Different industries, including rubber, electroplating, alloys, electroforming, plastics, aerospace industry, mining, and batteries, release Ni2+ compounds into the environment [Citation23]. Depending on the exposure route, such as dermal, oral, or inhalation, Ni2+ can have hazardous consequences on human health. Acute and chronic exposure are two types of exposure. Ni2+ does not only cause allergies but is also a risk factor for several illnesses, including dermatitis of the skin, anaemia, hepatitis, renal damage, and diarrhea. WHO has recommended that the maximum permissible of Ni2+ should not exceed 0.02 mg/L [Citation24].
2.2. Remediation techniques for heavy metals in wastewater
To meet the standard of maximum allowable limits industries, different methods have been used to reduce contamination to permissible limits. The advantages and drawbacks of the techniques are provided in .
Table 1. Techniques for eliminating heavy metals in wastewater [Citation25].
2.3. Methods for removal of heavy metals
2.3.1. Chemical precipitation
Chemical precipitation is one of the most common technologies used by most wastewater treatment plants to remove dissolved pollutants in wastewater [Citation26]. It is the process in which chemicals are used to convert substances in wastewater into insoluble precipitates. An impurity in ionic form is transformed into an insoluble form by a chemical reaction, and particulates are removed by settling co-filtration. The effectiveness of chemical precipitation depends on several factors, particularly the pH, solution composition, and pollutant concentration. A detailed schematic diagram of chemical precipitation processes is shown in .
Figure 1. Schematic diagram of the chemical precipitation treatment process [Citation27].
![Figure 1. Schematic diagram of the chemical precipitation treatment process [Citation27].](/cms/asset/7474eb02-6576-421c-b374-11d2563fb49d/tent_a_2358450_f0001_oc.jpg)
2.3.2. Electrochemical treatment
The electrochemical process involves the use of electricity to remove pollutants from water. During the electrolytic method of pollutant removal, an electric current is passed through the anode and cathode immersed in the solution. In between the electrodes, negatively and positively charged substances are caused to attract to each other (). The electrochemical treatment process has proven to remove numerous pollutants that include pharmaceutical residues [Citation28], synthetic dyes [Citation29], inorganic ions [Citation30], humic fulvic acids, and pesticides [Citation31]. Currently, electrochemical treatment processes are utilized in wastewater treatment, including electrochemical oxidation, electro-flotation, and electro-coagulation.
Figure 2. Diagram of the electrochemical reactor (Adapted from: [Citation32]).
![Figure 2. Diagram of the electrochemical reactor (Adapted from: [Citation32]).](/cms/asset/35a36a2f-b582-4ddd-9cc7-7f00cca9d7a5/tent_a_2358450_f0002_oc.jpg)
2.3.3. Membrane separation-based technique
Membrane separation technology is one of the most advanced wastewater treatment technologies for removing dissolved solutes in wastewater. Membranes commonly employ pressure to remove trace levels of contaminants in wastewater. The higher membrane level tehnologies, such as reverse osmosis, remove all types of pollutants that are difficult to remove from any other system. The system is recognized as the most energy-intensive treatment option. The drawbacks of membrane technology include being expensive, not user-friendly, and high rejection efficiency, but the technology is advancing quickly, and performance is improving. Various membrane types, such as liquid, metallic, inorganic, and polymeric membranes, can be used during wastewater treatment. Additionally, various systems, namely, nanofiltration membranes, ultrafiltration, reverse osmosis, and microfiltration, can target pollutants in the membrane system. Amongst them, reverse osmosis, nanofiltration, and ultrafiltration are widely used heavy metal removal techniques. shows a diagram of the membrane separation-based process. In this system, the feed components permeate through the membrane; the other feed components are transported through the membrane [Citation33].
Figure 3. Membrane-based separation process (Adapted from: [Citation33]).
![Figure 3. Membrane-based separation process (Adapted from: [Citation33]).](/cms/asset/dd46024b-7b15-4502-b825-ffe132a7369c/tent_a_2358450_f0003_oc.jpg)
2.3.3.1. Reverse osmosis
Reverse osmosis applies pressure to the membrane, and the permeable membrane permits the water molecules to pass through and flush the dissolved inorganic minerals into the drain. It can reject dissolved inorganic minerals from the water. Thaçi and Gashi, (2018) utilized reverse osmosis for the uptake of heavy metals from synthetic aqueous solution using some agricultural waste such as wheat bran, olive waste, and maize cob. Even though high removal efficiency of ions was achieved, the rejection of heavy metals removed was found to be between 40–90% [Citation34]. In another study, Li et al utilized nanoporous graphenes as reverse osmosis membranes for the uptake of heavy metals (Cu2+, Mn2+, Zn2+) in an aqueous solution, and ion rejection was 100% [Citation35]. Disadvantages of reverse osmosis include higher operating costs, severe fouling, and lower flux [Citation36].
2.3.3.2. Ultrafiltration (UF)
In the UF method, the permeable membrane is used as a separation technique of macromolecules, heavy metals, and suspended solids from the inorganic solution [Citation27]. It has been demonstrated that UF can achieve above 90% removal efficiency in pH above 5.
2.3.3.3. Nanofiltration (NF)
NF is similar to other membrane processes such as ultrafiltration, microfiltration, and reverse osmosis. It has high efficiency and needs less energy to purify inorganic minerals than reverse osmosis. It consists of pore sizes ranging from 0.1–10 nm. Peydayesh et al. 2020 designed nanofiltration self-assembly of Ethylenediamine (ED) grafted multi-walled carbon nanotubes to remove Zn2+, Mg2+, Cd2+, Cu2+, Ca2+, Ni2+ and Pb2+ with a rejection rate ranging from 90.5–96.7% [Citation37].
2.3.4. Ion-exchange
Ion exchange, also known as deionization or demineralization, is the physical process used to remove dissolved ions in wastewater. It is a modern technology used to convert hard water to soft water, which can be used for cleaning, drinking, and washing. Some organic compounds known as resins can exchange ions, and zeolites are among the well-known ion exchangers [Citation38]. It is a reversible chemical reaction that replaces an existing unwanted metal iosn with environmentally friendly ions. Exchange resins can be classified as cationic or anionic, and are either naturally occurring or a synthetic organic material. For water to be free from dissolved ions or minerals, hard water has to pass through a cation exchanger followed by an anion exchanger ().
Figure 4. A typical ion exchange ion treatment process [Citation38].
![Figure 4. A typical ion exchange ion treatment process [Citation38].](/cms/asset/f9a6d186-749f-4358-9bc4-0b6da82972b2/tent_a_2358450_f0004_oc.jpg)
2.3.5. Adsorption
Adsorption is a process that traps dissolved impurities in polluted wastewater onto the surface of the adsorbent. It is a promising technique for removing various contaminants, such as non-degradable organic compounds, heavy metals, and micropollutants [Citation39]. Adsorption can occur in two ways; physisorption or chemisorption. Physisorption involves the van der Waals force for adsorbate to bind to the adsorbent surfaces (). In contrast, chemisorption occurs when chemical bonds intermediate with the adsorbate which is to be attached to the adsorbent. The adsorption mechanism occurs in three ways: mass transfer to the adsorbent surface, intraparticle, and diffusion onto the surface of pores. The advantage of using adsorption processes to remove pollutants in wastewater include ease of operation, low cost, versatility, environmental friendliness, ease of regeneration, and higher removal efficiency of contaminants [Citation39]. Adsorption is an effective, economical, and eco-friendly treatment technique.
Figure 5. Schematic representation of adsorption molecules onto the surface [Citation40]
![Figure 5. Schematic representation of adsorption molecules onto the surface [Citation40]](/cms/asset/8acbb967-a7db-48b9-9222-81983b24c516/tent_a_2358450_f0005_oc.jpg)
2.3.6. Phytoremediation
To treat environmental contamination, a process known as phytoremediation uses plants and the associated soil microbes [Citation41]. It is a brand-new, economical, effective, environmentally friendly, and solar-driven remediation technique that can be used in situ [Citation42]. Heavy metal phytoextraction has a high potential in plants that produce numerous harvests in a single growing cycle, such as Trifolium spp. Because of their high growth rate, greater capacity to adapt to stressful environments, and high biomass, grasses are preferred to shrubs and trees for phytoextraction.
2.4. Adsorbents for removal of heavy metals in wastewater
2.4.1. Nanomaterial-based adsorbents
Several studies reported the use of metal oxides. Some of the conventional adsorbent examples are shown below.
2.4.1.1. Metal-based nanomaterials (MNMs)
MNMs can be produced by either destructive or constructive methods from metals down to nanomaterials diameters. The initial constituents of metal nanostructures are divalent and trivalent metal ions. Using reducing agents, metal ions are transformed into metal nanoparticles. These are good for adsorbing small molecules, have a huge surface area, and are frequently used in numerous applications [Citation43]. summarizes the adsorbents reported for removing Pb2+, Cd2+, and Ni2+ ions from wastewater by MNMs.
Table 2. Pb2+, Cd2+, and Ni2+ ions for removal by MNM.
2.4.1.2. Carbonaceous-based NMs
The detection and treatment of greywater derived from households are accomplished using various carbon-based materials (). Due to their advantage of being effective, inexpensive, and selective, carbon-based compounds are typically seen as excellent absorbents [Citation57]. Graphene, carbon nanotubes (CNTs), graphite, graphite/graphene oxide, and other carbonaceous compounds are among them. More emphasis is placed on nanocarbonaceous materials because it has been found that they are more advantageous than conventionally sized carbonaceous materials [Citation58]. These NMs including nanotubes, nanobeads, nanocomposites, nanofibers, and others, have high surface area-to-mass ratios, porosities, and high permeabilities [Citation59]. Their sorption capacity is increased through surface oxidation, modification, or functionalization [Citation60]. Iijima inadvertently discovered carbon nanotubes in 1991 while researching the graphite electrode surfaces utilized in an electric arc discharge [Citation61]. Based on how their graphene cylinders are arranged, CNTs can be named either single-walled carbon nanotubes (SWCNTs) or multi-walled carbon nanotubes (MWCNTs).
Table 3. Adsorption capacities of carbonaceous-based NM for removing Pb2+, Cd2+, and Ni2+ ions from wastewater.
2.4.2.3. Polymer-based NMs
A natural polymer called alginate is obtained from brown seaweed and contains d-Mannuronate (M) and l-Guluronate (G) linear copolymers joined together by 1,4 – and 1,4-glycosidic linkages. Blocks of homopolymeric M-M, G-G atoms, and heteropolymeric M-G atoms are used to organize M and G units [Citation73]. These blocks are distributed differently depending on where the alginate polymer came from. In fact, this polysaccharide's carboxylate functionalities are negatively charged in neutral and alkaline conditions, which makes them more apt to bind to cations. presents polymer-based as a possible adsorbent for removing selected heavy metals.
Table 4. Summary of polymer-based NMs for adsorption of Pb2+, Cd2+, and Ni2+ ions.
2.4.2. Conventional adsorbents
Activated carbon, agricultural waste, and zeolites are some of the common adsorbents used for the uptake of heavy metal ions in solution. Below is a summary of some examples of convectional adsorbent.
2.4.2.1 Activated carbon
lists forms of activated carbon adsorbents prepared from agricultural wastes. The carbonization of the carbon-based agricultural waste with lower temperatures of 800 °C in an inert environment and the activation of the carbon material are the two primary phases in preparation [Citation88]. Thus, any carbonaceous materials may be converted into activated carbon. However, the characteristics of the end product will vary based on the kind of waste utilized, the type of activating agent employed, and the circumstances surrounding the processes of activation, and carbonization. Using hot gases or steams of water vapour results in the physical activation of carbonized material [Citation89]. This is typically done by using one or a combination of activation/oxidation, which requires high temperatures in the presence of carbon dioxide and steam, and carbonization, which turns this organic precursor into any type of primary carbon like tars, ash, and amorphous carbon. Unfortunately, during the carbonization process, certain tars or decomposition products are trapped in the pores [Citation90]. Contrarily, chemical activation prevents the production of tar, allowing for the one-step production of a carbonized product with a highly developed porosity [Citation91]. Prior to carbonization, chemical activation occurs when agricultural waste is modified with certain chemicals. These chemicals are often an acid like H3PO4, a strong base like NaOH, and KOH or a salt like ZnCl2. Then, at lower temperatures (450–900 °C), the agricultural precursor is carbonized. Therefore, activated carbons are regarded as good adsorbents due to its high surface area and porosity.
Table 5. Remediation of Pb2+, Cd2+, Ni2+ ions by activated carbon.
2.4.2.2 Agricultural waste
Numerous reviews have been published on removing heavy metals using agricultural waste products as adsorbents [Citation105,Citation106]. Due to their ease of availability, low cost in significant quantities as agricultural wastes, agricultural wastes have gained popularity among biosorbents. They typically consist primarily of cellulose and lignin, with several hydroxyl and carboxylic groups that can provide an electron pair to bind metal ions and create complexes [Citation107]. There is evidence that certain vegetable wastes which can filter out heavy metals from water. Heavy metal removal using mushroom residues has been successful [Citation108]. The Cu2+, Zn2+, and Hg2+ removal efficiency ranged from 39.7% to 81.7% based on an analysis of four different kinds of mushroom residues. Potatoes, after rice and wheat, are the most essential foods in developing countries because they include minerals, vitamins, antioxidants, and polysaccharides such as cellulose, hemicellulose, lignin, and starch [Citation109]. lists the maximal adsorption capacities of various agricultural wastes for removing Pb2+, Cd2+, and Ni2+ ions, along with the ideal pH to consider for biosorption.
Table 6. Adsorption of Pb2+, Cd2+, and Ni2+ ions by agricultural wastes.
2.4.2.3 Zeolites
A class of hydrated aluminium-silicates of the alkaline earth metals known as zeolites are distinguished by their availability, cheap cost of mining, high bulk density, and strong resistance to modification [Citation123]. Magnesium, potassium, calcium, and sodium are such classes of aluminium-silicate earth metals. The main component of zeolites is a tetrahedral complex made up of four oxygen ions coordinated tetrahedrally with the Si4+ cation. A negative charge density is produced for the zeolite lattice by the isomorphic substitution of Al3+ for Si4+. Alumosilicates, known as zeolites, are composed of three-dimensional SiO4 and AlO4 tetrahedron networks connected by a common oxygen atom [Citation124]. Zeolites are highly porous crystalline aluminosilicates that have extra-framework charge-balancing cations and tetrahedrally linked three-dimensional frameworks. These frameworks feature pores that can accommodate molecules as small as 1 nm in size [Citation125]. The pore space's dimensions, geometry, and connectivity are frequently used to define zeolite formations. The surface area plays a crucial role in the efficacy of zeolites and zeolite-carbon composites as adsorbents. Zeolites are microporous crystalline materials with a well-defined structure, characterized by a three-dimensional network of channels and cages. Zeolites have been used in various applications, including ammonia removers from urban, chemical sieves, agricultural, and industrial wastewaters, water softeners, toxic gas removers from gaseous emissions, herbicide, odour control filters, animal nutrition, chemical fertilizer, and pesticide carriers. lists the zeolites as adsorbents for removing Pb2+, Cd2+, and Ni2+ ions.
Table 7. Summary of removal of Pb2+, Cd2+, and Ni2+ ions by zeolites.
Despite all the adsorbents reported on the removal of heavy metals and their better adsorption capacity, reports on the heavy metal-loaded adsorbent are still lacking. Hence the reuse of heavy metal loaded spent adsorbent to protect the environment should be investigated.
2.5. Hazards of heavy metal loaded spent adsorbent
Environmental issues have been a significant concern in recent decades, and environmental and health legislation have increased in significance worldwide [Citation132]. One of the most important issues in pollution prevention is integrated and balanced water management. A number of adsorbents are developed from low-cost materials like agricultural and industrial waste, but the concern is the disposal at the end of life. When heavy metals are removed from wastewater, they are generally bound to the surface of the adsorbent, referred to as heavy metals spent adsorbent. The disposal of heavy metal-loaded spent adsorbents is considered a high concern in humans and the aquatic environment. Rufullo et al, investigated the biocidal efficiency of ZnONPs and revealed that ZnONPs hindered the growth of conidiophores and conidia of Penicillium expansum [Citation133]. Kang et al, reported the impact of SWCNTs on microorganisms and their findings were that the size of SWCNT could thereby destroy cells [Citation134]. When carbon NM and metal oxides are used for environmental remediation and are discarded, they interact with pollutants, and the level of toxicity may rise [Citation135]. Carbon NMs can produce responses including lung tumours and cellular inflammation in live tissue due to their small particle size, which allows them to penetrate through cell walls and membranes. These reactions would immediately be harmful to animals and human health [Citation135]. However, recycling the spent adsorbent is necessary to solve secondary pollution. There are various applications (photocatalysis, medical, catalysis) where spent adsorbent can be used [Citation136], but since crime is common in South Africa, there was a necessity to explore the reuse in forensic applications, specifically BFP detection.
Due to inadequate evidence tying the offenders to the crime site during criminal behaviour (which might include physical assault, sexual violence, or murder), the perpetrators may not be prosecuted. Crime scene samples, such as saliva, blood, sperm, and fingerprints, can be collected to acquire evidence. A BFP is an important piece of evidence that has been utilized to potentially link criminals to crime scenes. Three different fingerprint categories can be collected at the crime site; these include latent (non-visible) or patent (visible) or indented [Citation137]. The major features of the fingerprints, like ridge endings, and ridge bifurcations, are called Minutiae [Citation138], shown in . They can be obtained on a porous surface such as clothing items, toilet paper, raw wood, or cardboard, and non-porous surfaces such as tiles, glass, windows, knives, and others.
Figure 6. Images and minuates of latent fingerprint details [Citation139].
![Figure 6. Images and minuates of latent fingerprint details [Citation139].](/cms/asset/de318d8c-8c2a-4450-86b5-2ea44d93c734/tent_a_2358450_f0006_oc.jpg)
Several publications have focused on developing materials for latent fingerprints [Citation140] and carbon-coated metal oxides are one of the materials used as labelling agents to visualize the fingerprints [Citation141]. Other materials enhancing friction ridges include carbon NMs [Citation142] and metal oxides. Criminal investigations frequently heavily rely on fingerprints in blood, whether they are discernible or have been developed. The detection of latent BFP using heavy metals spent adsorbents has superior qualities including higher selection, better background contrast/clear images, and improved sensitivity [Citation143]. A person whose blood-stained fingerprints are discovered at a crime scene may not immediately be the top suspect, but this evidence suggests that they were there when the blood was still fresh or was beginning to dry. Bloodstain pattern analysis and the detection of blood-contaminated fingermarks have been extensively studied over the past 40 years [Citation144].
3. Conclusions
In conclusion, the comprehensive review of heavy metals (Pb2+, Cd2+, Ni2+) removal from wastewater through the utilization of low-cost adsorbents has illuminated a promising avenue for addressing environmental pollution challenges. The exploration of various adsorbents, ranging from agricultural by-products to industrial wastes, underscores the potential of sustainable and cost-effective solutions in mitigating heavy metal contamination. Adsorption has much potential for removing heavy metals from wastewater using inexpensive adsorbents, according to a review of various processes and adsorbents for heavy metal removal. To encourage the widespread use of unconventional adsorbents, more research should be done on low-cost adsorption processes from industrial waste, agricultural waste, etc. Low-cost adsorbents should be developed and used to keep costs down and increase the effectiveness of heavy metal removal. From the preliminary studies mentioned above, most studies conducted did not report the reuse of spent adsorbents. This is a fairly new area of research that has emerged. Hence more current studies focussing on closing this scientific gap are needed. The use of agricultural waste materials for Pb2+, Cd2+, and Ni2+ ions remediation has increased significantly. The main drawbacks, however, have been the high reagent requirements, the production of poisonous waste products, low adsorption capacity, and the inability to remove trace metals. However, in initiating such studies the reuse of spent adsorbents in blood fingerprint detection must also be explored.
Authors contribution
Yvonne Boitumelo Nthwane: Data curation, first draft writing, Conceptualization, editing, Bienvenu Gael Fouda-Mbanga: editing, conceptualization; Melusi Thwala: editing and conceptualization; Kriveshini Pillay: editing, supervision.
Acknowledgements
The authors are grateful to the University of Johannesburg and the Chemical sciences department for their facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5:47–58. doi:10.2478/v10102-012-0009-2
- Abhinaya M, Parthiban R, Kumar PS, et al. A review on cleaner strategies for extraction of chitosan and its application in toxic pollutant removal. Environ Res. 2021;196:110996. doi:10.1016/j.envres.2021.110996
- Kim HC, Jang TW, Chae HJ, et al. Evaluation and management of lead exposure. Ann Occup Environ Med. 2015;27:1–9. doi:10.1186/s40557-014-0044-x
- Majumder AK, Nayeem A Al, Islam M, et al. Critical review of lead pollution In Bangladesh. J Health Pollut. 2021;11:1–21. doi:10.5696/2156-9614-11.31.210902
- Giri DD, Alhazmi A, Mohammad A, et al. Lead removal from synthetic wastewater by biosorbents prepared from seeds of Artocarpus Heterophyllus and Syzygium Cumini. Chemosphere. 2022;287:132016. doi:10.1016/j.chemosphere.2021.132016
- Ayub S, Changani F, Mohammadi AA, et al. Performance evaluation of agro-based adsorbents for the removal of cadmium from wastewater. 2019;142: 293–299.
- Liu T, Lawluvy Y, Shi Y, et al. Adsorption of cadmium and lead from aqueous solution using modified biochar: a review. J Environ Chem Eng. 2022;10:106502. doi:10.1016/j.jece.2021.106502
- Kwikima MM, Mateso S, Chebude Y. Potentials of agricultural wastes as the ultimate alternative adsorbent for cadmium removal from wastewater. A review. Sci Afr. 2021;13:e00934.
- Kinuthia GK, Ngure V, Beti D, et al. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: community health implication. Scientific Reports 2020;10:1–13.
- Haddad MY, Alharbi HF. Enhancement of heavy metal ion adsorption using electrospun polyacrylonitrile nanofibers loaded with ZnO nanoparticles. J Appl Polym Sci. 2019;136:47209. doi:10.1002/app.47209
- Ahmad R, Mirza A. Facile one pot green synthesis of Chitosan-Iron oxide (CS-Fe2O3) nanocomposite: Removal of Pb(II) and Cd(II) from synthetic and industrial wastewater. J Clean Prod. 2018;186:342–352. doi:10.1016/j.jclepro.2018.03.075
- Sadeghi MM, Rad AS, Ardjmand M, et al. Preparation of magnetic nanocomposite based on polyaniline/Fe3O4 towards removal of lead (II) ions from real samples. Synth Met. 2018;245:1–9. doi:10.1016/j.synthmet.2018.08.001
- Razzaz A, Ghorban S, Hosayni L, et al. Chitosan nanofibers functionalized by TiO2 nanoparticles for the removal of heavy metal ions. J Taiwan Inst Chem Eng. 2016;58:333–343. doi:10.1016/j.jtice.2015.06.003
- Khezami L, Taha K, Amami E, et al. Removal of cadmium (II) from aqueous solution by zinc oxide nanoparticles: kinetic and thermodynamic studies. Desalin Water Treat. 2017;62:346–354. doi:10.5004/dwt.2017.0196
- Khan SB, Rahman MM, Marwani HM, et al. An assessment of zinc oxide nanosheets as a selective adsorbent for cadmium. 2013;8:1-8.
- Xiao X, Yang L, Zhou D, et al. Magnetic γ-Fe2O3/Fe-doped hydroxyapatite nanostructures as high-efficiency cadmium adsorbents. Colloids Surf A Physicochem Eng Asp. 2018;555:548–557. doi:10.1016/j.colsurfa.2018.07.036
- Sharma G, Naushad M. Adsorptive removal of noxious cadmium ions from aqueous medium using activated carbon/zirconium oxide composite: isotherm and kinetic modelling. J Mol Liq. 2020;310:113025. doi:10.1016/j.molliq.2020.113025
- Dong L, Zhu Z, Ma H, et al. Simultaneous adsorption of lead and cadmium on MnO2-loaded resin. Journal of Environmental Sciences. 2010;22:225–229. doi:10.1016/S1001-0742(09)60097-8
- Herrera-Barros A, Bitar-Castro N, Villabona-Ortíz Á, et al. Nickel adsorption from aqueous solution using lemon peel biomass chemically modified with TiO2 nanoparticles. Sustain Chem Pharm. 2020;17:100299. doi:10.1016/j.scp.2020.100299
- Shehzad H, Ahmed E, Sharif A, et al. Modified alginate-chitosan-TiO2 composites for adsorptive removal of Ni(II) ions from aqueous medium. Int J Biol Macromol. 2022;194:117–127. doi:10.1016/j.ijbiomac.2021.11.140
- Nassar NN. Kinetics, equilibrium and thermodynamic studies on the adsorptive removal of nickel, cadmium and cobalt from wastewater by superparamagnetic iron oxide nanoadsorbents. Can J Chem Eng. 2012;90:1231–1238. doi:10.1002/cjce.20613
- Zhao J, Liu J, Li N, et al. Highly efficient removal of bivalent heavy metals from aqueous systems by magnetic porous Fe3O4-MnO2: Adsorption behavior and process study. Chem Eng J. 2016;304:737–746. doi:10.1016/j.cej.2016.07.003
- Ren Y, Yan N, Wen Q, et al. Graphene/δ-MnO2 composite as adsorbent for the removal of nickel ions from wastewater. Chem Eng J. 2011;175:1–7. doi:10.1016/j.cej.2010.08.010
- Hemidouche S, Boudriche L, Boudjemaa A, et al. Removal of lead (II) and cadmium (II) cations from water using surface-modified graphene. Can J Chem Eng. 2017;95:508–515. doi:10.1002/cjce.22693
- Agarwal A, Upadhyay U, Sreedhar I, et al. A review on valorization of biomass in heavy metal removal from wastewater. Journal of Water Process Engineering. 2020;38:101602. doi:10.1016/j.jwpe.2020.101602
- Tabish TA, Memon FA, Gomez DE, et al. A facile synthesis of porous graphene for efficient water and wastewater treatment OPEN. 2018;8:1817.
- Ibrahim WAW, Nodeh HR, Sanagi MM. Graphene-based materials as solid phase extraction sorbent for trace metal ions, Organic compounds, and biological Sample Preparation. 2016;46:267–283.
- Narbaitz RM, Karimi-Jashni A. Electrochemical reactivation of granular activated carbon: impact of reactor configuration. Chem Eng J. 2012;197:414–423. doi:10.1016/j.cej.2012.05.049
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58.
- Farghali AA, Abdel Tawab HA, Abdel Moaty SA, et al. Functionalization of acidified multi-walled carbon nanotubes for removal of heavy metals in aqueous solutions. J Nanostructure Chem. 2017;7:101–111. doi:10.1007/s40097-017-0227-4
- Al-Khaldi FA, Abu-Sharkh B, Abulkibash AM, et al. Cadmium removal by activated carbon, carbon nanotubes, carbon nanofibers, and carbon fly ash: a comparative study. New pub: Balaban. 2015;53:1417–1429.
- Ma X, Yang ST, Tang H, et al. Competitive adsorption of heavy metal ions on carbon nanotubes and the desorption in simulated biofluids. J Colloid Interface Sci. 2015;448:347–355. doi:10.1016/j.jcis.2015.02.042
- Liang J, Liu J, Yuan X, et al. Facile synthesis of alumina-decorated multi-walled carbon nanotubes for simultaneous adsorption of cadmium ion and trichloroethylene. Chemical Engineering Journal. 2015;273:101–110. doi:10.1016/j.cej.2015.03.069
- Jun BM, Kim S, Kim Y, et al. Comprehensive evaluation on removal of lead by graphene oxide and metal organic framework. Chemosphere. 2019;231:82–92. doi:10.1016/j.chemosphere.2019.05.076
- Li Y, Xu Z, Liu S, et al. Molecular simulation of reverse osmosis for heavy metal ions using functionalized nanoporous graphenes. Comput Mater Sci. 2017;139:65–74. doi:10.1016/j.commatsci.2017.07.032
- Cho HH, Wepasnick K, Smith BA, et al. Sorption of aqueous Zn[II] and Cd[II] by multiwall carbon nanotubes: The relative roles of oxygen-containing functional groups and graphenic carbon. Langmuir. 2010;26:967–981. doi:10.1021/la902440u
- Alimohammady M, Jahangiri M, Kiani F, et al. A new modified MWCNTs with 3-aminopyrazole as a nanoadsorbent for Cd(II) removal from aqueous solutions. J Environ Chem Eng. 2017;5:3405–3417. doi:10.1016/j.jece.2017.06.045
- Li B, Jin X, Lin J, et al. Green reduction of graphene oxide by sugarcane bagasse extract and its application for the removal of cadmium in aqueous solution. J Clean Prod. 2018;189:128–134. doi:10.1016/j.jclepro.2018.04.018
- Adolph MA, Xavier YM, Kriveshini P, et al. Phosphine functionalised multiwalled carbon nanotubes: a new adsorbent for the removal of nickel from aqueous solution. J Environ Sci (China). 2012;24:1133–1141. doi:10.1016/S1001-0742(11)60880-2
- Sharma R, Saini P. Graphene-based composites and hybrids for water purification applications. Diamond and carbon composites and nanocomposites. 2016;21:59.
- Najafi F, Moradi O, Rajabi M, et al. Thermodynamics of the adsorption of nickel ions from aqueous phase using graphene oxide and glycine functionalized graphene oxide. J Mol Liq. 2015;208:106–113. doi:10.1016/j.molliq.2015.04.033
- Nestle N, Kimmich R. Heavy metal uptake of alginate gels studied by NMR microscopy. Colloids Surf A Physicochem Eng Asp. 1996;115:141–147. doi:10.1016/0927-7757(96)03608-4
- Raval NP, Shah PU, Shah NK. Adsorptive removal of nickel(II) ions from aqueous environment: a review. J Environ Manage. 2016;179:1–20. doi:10.1016/j.jenvman.2016.04.045
- Alakhras F, Al-Shahrani H, Al-Abbad E, et al. Removal of Pb(II) metal ions from aqueous solutions using chitosan-vanillin derivatives of chelating polymers. 2019;28: 1523-1534.
- Hussain MS, Musharraf SG, Bhanger MI, et al. Salicylaldehyde derivative of nano-chitosan as an efficient adsorbent for lead(II), copper(II), and cadmium(II) ions. Int J Biol Macromol. 2020;147:643–652. doi:10.1016/j.ijbiomac.2020.01.091
- Li Y, Guo C, Shi R, et al. Chitosan/nanofibrillated cellulose aerogel with highly oriented microchannel structure for rapid removal of Pb (II) ions from aqueous solution. Elsevier. 2019;
- Mamah SC, Goh PS, Ismail AF, et al. Facile preparation of palygorskite/chitin nanofibers hybrids nanomaterial with remarkable adsorption capacity. Materials Science and Engineering: B. 2020;262:114725. doi:10.1016/j.mseb.2020.114725
- Sabarish R, Unnikrishnan G. Polyvinyl alcohol/carboxymethyl cellulose/ZSM-5 zeolite biocomposite membranes for dye adsorption applications. Carbohydr Polym. 2018;199:129–140. doi:10.1016/j.carbpol.2018.06.123
- Naeimi S, Faghihian H. Application of novel adsorbent prepared by mucor hiemalis biomass impregnated with calcium alginate for removal of sr2+ from aqueous solutions. J Polym Environ. 2019;27:1572–1583. doi:10.1007/s10924-019-01453-8
- Badruddoza AZM, Shawon ZBZ, Tay WJD, et al. Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr Polym. 2013;91:322–332. doi:10.1016/j.carbpol.2012.08.030
- Banivaheb S, Dan S, Hashemipour H, et al. Synthesis of modified chitosan TiO2 and SiO2 hydrogel nanocomposites for cadmium removal. J Saudi Chem Soc. 2021;25:101283. doi:10.1016/j.jscs.2021.101283
- Kucuk AC, Urucu OA. Silsesquioxane-modified chitosan nanocomposite as a nanoadsorbent for the wastewater treatment. React Funct Polym. 2019;140:22–30. doi:10.1016/j.reactfunctpolym.2019.04.011
- Tabatabaeefar A, Keshtkar AR, Talebi M, et al. Polyvinyl alcohol/alginate/zeolite nanohybrid for removal of metals. Chem Eng Technol. 2020;43:343–354. doi:10.1002/ceat.201900231
- Wu J, Cheng X, Yang G. Preparation of nanochitin-contained magnetic chitosan microfibers via continuous injection gelation method for removal of Ni(II) ion from aqueous solution. Int J Biol Macromol. 2019;125:404–413. doi:10.1016/j.ijbiomac.2018.11.212
- Futalan CM, Kan CC, Dalida ML, et al. Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite. Carbohydr Polym. 2011;2:528–536. doi:10.1016/j.carbpol.2010.08.013
- Abu-Saied MA, Wycisk R, Abbassy MM, et al. Sulfated chitosan/PVA absorbent membrane for removal of copper and nickel ions from aqueous solutions-Fabrication and sorption studies. Carbohydr Polym. 2017;165:149–158. doi:10.1016/j.carbpol.2016.12.039
- Lai GS, Lau WJ, Goh PS, et al. Tailor-made thin film nanocomposite membrane incorporated with graphene oxide using novel interfacial polymerization technique for enhanced water separation. Chem Eng J. 2018;344:524–534. doi:10.1016/j.cej.2018.03.116
- Zhang YJ, Xing ZJ, Duan ZK, et al. Effects of steam activation on the pore structure and surface chemistry of activated carbon derived from bamboo waste. Appl Surf Sci. 2014;315:279–286. doi:10.1016/j.apsusc.2014.07.126
- Maciá-Agulló JA, Moore BC, Cazorla-Amorós D, et al. Activation of coal tar pitch carbon fibres: physical activation vs. chemical activation. Carbon N Y. 2004;42:1367–1370. doi:10.1016/j.carbon.2004.01.013
- Kalderis D, Bethanis S, Paraskeva P, et al. Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times. Bioresour Technol. 2008;99:6809–6816. doi:10.1016/j.biortech.2008.01.041
- Sajjadi SA, Meknati A, Lima EC, et al. A novel route for preparation of chemically activated carbon from pistachio wood for highly efficient Pb(II) sorption. J Environ Manage. 2019;236:34–44. doi:10.1016/j.jenvman.2019.01.087
- Al-Onazi WA, Ali MHH, Al-Garni T. Using pomegranate peel and date pit activated carbon for the removal of cadmium and lead ions from aqueous solution. J Chem. 2021;2021:1-13. doi:10.1155/2021/5514118
- Guo J, Song Y, Ji X, et al. Preparation and characterization of nanoporous activated carbon derived from prawn shell and its application for removal of heavy metal ions. Materials (Basel). 2019;12:241. doi:10.3390/ma12020241
- Naeem MA, Imran M, Amjad M, et al. Batch and column scale removal of cadmium from water using raw and acid activated wheat straw biochar. Water. 2019;11:1438. doi:10.3390/w11071438
- Wan Ibrahim WMH, Mohamad Amini MH, Sulaiman NS, et al. Powdered activated carbon prepared from Leucaena leucocephala biomass for cadmium removal in water purification process. 2019;26:30–40.
- Tan IAW, Chan JC, Hameed BH, et al. Adsorption behavior of cadmium ions onto phosphoric acid-impregnated microwave-induced mesoporous activated carbon. Journal of Water Process Engineering. 2016;14:60–70. doi:10.1016/j.jwpe.2016.10.007
- Dandajeh Adamu A, Begianpuye Adie D. Assessment of cadmium adsorption from wastewater onto sugarcane bagasse activated carbon. Bayero Journal of Engineering and Technology. 2020;15:7–14.
- Khedri A, Jafari D, Esfandyari M. Adsorption of Nickel(II) ions from synthetic wastewater using activated carbon prepared from mespilus germanica leaf. Arab J Sci Eng. 2022;47:6155–6166. doi:10.1007/s13369-021-06014-7
- Nnaji CC, Agim AE, Mama CN, et al. Equilibrium and thermodynamic investigation of biosorption of nickel from water by activated carbon made from palm kernel chaff. Sci Rep 2021;11:7808. doi:10.1038/s41598-021-86932-6
- Anitha D, Ramadevi A, Seetharaman R. Biosorptive removal of Nickel(II) from aqueous solution by Mangosteen shell activated carbon. Mater Today Proc. 2021;45:718–722. doi:10.1016/j.matpr.2020.02.748
- Tuomikoski S, Runtti H, Romar H, et al. Multiple heavy metal removal simultaneously by a biomass-based porous carbon. Water Environ Res. 2021;93:1303–1314. doi:10.1002/wer.1514
- Air D, Menggunakan Kurma K, Bahan S, et al. Removal of heavy metals from wastewater using date palm as a biosorbent: a comparative review. Sains Malays. 2018;47:35–49. doi:10.17576/jsm-2018-4701-05
- Tsade H, Murthy A, Muniswamy D. Bio-sorbents from agricultural wastes for eradication of heavy metals: a review. J Mater Environ Sci. 2020;2020:1719–1735.
- Li X, Zhang D, Sheng F, et al. Adsorption characteristics of Copper (Ⅱ), Zinc (Ⅱ) and Mercury (Ⅱ) by four kinds of immobilized fungi residues. Ecotoxicol Environ Saf. 2018;147:357–366. doi:10.1016/j.ecoenv.2017.08.058
- Chohan NA, Aruwajoye GS, Sewsynker-Sukai Y, et al. Valorisation of potato peel wastes for bioethanol production using simultaneous saccharification and fermentation: Process optimization and kinetic assessment. Renew Energy. 2020;146:1031–1040. doi:10.1016/j.renene.2019.07.042
- Tejada-Tovar C, Gonzalez-Delgado AD, Villabona-Ortiz A. Characterization of residual biomasses and its application for the removal of lead ions from aqueous solution. Applied Sciences 2019;9:4486. doi:10.3390/app9214486
- Çelebi H, Gök O. Evaluation of lead adsorption kinetics and isotherms from aqueous solution using natural walnut shell. Int J Environ Res. 2017;11:83–90. doi:10.1007/s41742-017-0009-3
- Basu M, Guha AK, Ray L. Biosorptive removal of lead by lentil husk. J Environ Chem Eng. 2015;3:1088–1095. doi:10.1016/j.jece.2015.04.024
- Gaur N, Kukreja A, Yadav M, et al. Adsorptive removal of lead and arsenic from aqueous solution using soya bean as a novel biosorbent: equilibrium isotherm and thermal stability studies. Appl Water Sci. 2018;8:1–12. doi:10.1007/s13201-017-0639-9
- Afolabi FO, Musonge P, Bakare BF. Bio-sorption of copper and lead ions in single and binary systems onto banana peels. Cogent Engineering. 2021;8. 1886730. doi:10.1080/23311916.2021.1886730
- Basu M, Guha AK, Ray L. Adsorption Behavior of Cadmium on Husk of Lentil. Process Saf Environ Prot. 2017;106:11–22. doi:10.1016/j.psep.2016.11.025
- Abdolali A, Ngo HH, Guo W, et al. A breakthrough biosorbent in removing heavy metals: equilibrium, kinetic, thermodynamic and mechanism analyses in a lab-scale study. Sci Total Environ. 2016;542:603–611. doi:10.1016/j.scitotenv.2015.10.095
- Gupta VK, Nayak A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J. 2012;180:81–90. doi:10.1016/j.cej.2011.11.006
- Akunwa NK, Muhammad MN, Akunna JC. Treatment of metal-contaminated wastewater: a comparison of low-cost biosorbents. J Environ Manage. 2014;146:517–523. doi:10.1016/j.jenvman.2014.08.014
- Kulkarni RM, Shetty KV, Srinikethan G. Biosorption study on Ni(II) and Cd(II) removal in a packed bed column using brewery sludge pellets. Biomass Convers Biorefin. 2022;1:1–12.
- Loiacono S, Crini G, Chanet G, et al. Metals in aqueous solutions and real effluents: biosorption behavior of a hemp-based felt. J Chem Technol Biotechnol. 2018;93:2592–2601. doi:10.1002/jctb.5612
- Chaudhari V, Patkar M. Removal of nickel from aqueous solution by using corncob as adsorbent. Mater Today Proc. 2022;61:307–314. doi:10.1016/j.matpr.2021.09.458
- González-Rodríguez L, García JOP, Rodríguez-López L, et al. Cassava husk powder as an eco-friendly adsorbent for the removal of nickel (II) ions. Springer Proceedings in Earth and Environmental Sciences. 2022;21–38. doi:10.1007/978-3-030-88919-7_3
- Al-Abbad EA, Al Dwairi RA. Removal of nickel (II) ions from water by Jordan natural zeolite as sorbent material. J Saudi Chem Soc. 2021;25:101233. doi:10.1016/j.jscs.2021.101233
- Bao W wei, Zou H feng, Gan S cai, et al. Adsorption of heavy metal ions from aqueous solutions by zeolite based on oil shale ash: kinetic and equilibrium studies. Chem Res Chin Univ. 2013;29:126–131. doi:10.1007/s40242-013-2139-2
- Raval NP, Shah PU, Shah NK. Adsorptive removal of nickel(II) ions from aqueous environment: a review. J Environ Manage. 2016;179:1–20. doi:10.1016/j.jenvman.2016.04.045
- Khan S, Idrees M, Bilal M. Revealing and elucidating chemical speciation mechanisms for lead and nickel adsorption on zeolite in aqueous solutions. Colloids Surf A Physicochem Eng Asp. 2021;623:126711. doi:10.1016/j.colsurfa.2021.126711
- Meneguin JG, Moisés MP, Karchiyappan T, et al. Preparation and characterization of calcium treated bentonite clay and its application for the removal of lead and cadmium ions: Adsorption and thermodynamic modeling. Process Saf Environ Prot. 2017;111:244–252. doi:10.1016/j.psep.2017.07.005
- Alfaro-Cuevas-Villanueva R, Hidalgo-Vázquez AR, Cortés Penagos CDJ, et al. Thermodynamic, kinetic, and equilibrium parameters for the removal of lead and cadmium from aqueous solutions with calcium alginate beads. Scientific World J. 2014;2014: 647512.
- Kragović M, Daković A, Sekulić Ž, et al. Removal of lead from aqueous solutions by using the natural and Fe(III)-modified zeolite. Appl Surf Sci. 2012;258:3667–3673. doi:10.1016/j.apsusc.2011.12.002
- Wang R, Ng DHL, Liu S. Recovery of nickel ions from wastewater by precipitation approach using silica xerogel. J Hazard Mater. 2019;380:120826. doi:10.1016/j.jhazmat.2019.120826
- Jiang D, Yang Y, Huang C, et al. Removal of the heavy metal ion nickel (II) via an adsorption method using flower globular magnesium hydroxide. J Hazard Mater. 2019;373:131–140. doi:10.1016/j.jhazmat.2019.01.096
- Theerthagiri J, Salla S, Senthil RA, et al. A review on ZnO nanostructured materials: energy, environmental and biological applications. Nanotechnology. 2019;30:392001. doi:10.1088/1361-6528/ab268a
- Phothisarattana D, Wongphan P, Promhuad K, et al. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, Vol 13, Page 4192. 2021;13:4192.
- Souza VGL, Fernando AL. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag Shelf Life. 2016;8:63–70. doi:10.1016/j.fpsl.2016.04.001
- Garcia C V., Shin GH, Kim JT. Metal oxide-based nanocomposites in food packaging: applications: migration, and regulations. Trends Food Sci Technol. 2018;82:21–31. doi:10.1016/j.tifs.2018.09.021
- Struller CF, Kelly PJ, Copeland NJ. Aluminum oxide barrier coatings on polymer films for food packaging applications. Surf Coat Technol. 2014;241:130–137. doi:10.1016/j.surfcoat.2013.08.011
- Hirvikorpi T, Vähä-Nissi M, Mustonen T, et al. Atomic layer deposited aluminum oxide barrier coatings for packaging materials. Thin Solid Films. 2010;518:2654–2658. doi:10.1016/j.tsf.2009.08.025
- Lv W, Qiu Q, Wang F, et al. Sonochemical synthesis of cobalt aluminate nanoparticles under various preparation parameters. Ultrason Sonochem. 2010;17:793–801. doi:10.1016/j.ultsonch.2010.01.018
- Thakur AK, Singh R, Teja Pullela R, et al. Green adsorbents for the removal of heavy metals from Wastewater: a review. Mater Today Proc. 2022;57:1468–1472. doi:10.1016/j.matpr.2021.11.373
- Imran-Shaukat M, Wahi R, Ngaini Z. The application of agricultural wastes for heavy metals adsorption: a meta-analysis of recent studies. Bioresour Technol Rep. 2022;17:100902. doi:10.1016/j.biteb.2021.100902
- Thakur V, Sharma E, Guleria A, et al. Modification and management of lignocellulosic waste as an ecofriendly biosorbent for the application of heavy metal ions sorption. Mater Today Proc. 2020;32:608–619. doi:10.1016/j.matpr.2020.02.756
- Wang X, Li X, Liu G, et al. Mixed heavy metal removal from wastewater by using discarded mushroom-stick biochar: adsorption properties and mechanisms. Environ Sci Process Impacts. 2019;21:584–592. doi:10.1039/C8EM00457A
- Sun J, Hui K, Guo Z, et al. Cellulose and lignin contents are negatively correlated with starch accumulation, and their correlation characteristics vary across cassava varieties. J Plant Growth Regul. 2023;42:658–669. doi:10.1007/s00344-022-10573-w
- Cao D, Wang X, Pan L, et al. Nonmetal sulfur-doped coral-like cobalt ferrite nanoparticles with enhanced magnetic properties. J Mater Chem C Mater. 2016;4:951–957. doi:10.1039/C5TC02931G
- Majedi A, Davar F, Abbasi A. Sucrose-mediated sol–gel synthesis of nanosized pure and S-doped zirconia and its catalytic activity for the synthesis of acetyl salicylic acid. J Ind Eng Chem. 2014;20:4215–4223. doi:10.1016/j.jiec.2014.01.023
- Zang Z, Temmyo J, Nakamura A. Single cuprous oxide films synthesized by radical oxidation at low temperature for PV application. Optics Express. 2013;21:11448–11456. doi:10.1364/OE.21.011448
- Wang X, Ahmad M, Sun H. Three-dimensional ZnO hierarchical nanostructures: solution phase synthesis and applications. Materials. 2017;10:1304.
- Gupta SK, Mao Y. A review on molten salt synthesis of metal oxide nanomaterials: status, opportunity, and challenge. Prog Mater Sci. 2021;117:100734. doi:10.1016/j.pmatsci.2020.100734
- Kolahalam LA, Kasi Viswanath IV., Diwakar BS, et al. Review on nanomaterials: synthesis and applications. Mater Today Proc. 2019;18:2182–2190.
- Hyeon T, Su Seong Lee, Park J, et al. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J Am Chem Soc. 2001;123:12798–12801. doi:10.1021/ja016812s
- Kelly CHW, Lein M. Choosing the right precursor for thermal decomposition solution-phase synthesis of iron nanoparticles: tunable dissociation energies of ferrocene derivatives. Phys Chem Chem Phys. 2016;18:32448–32457. doi:10.1039/C6CP06921E
- Kolodziejczak-Radzimska A, Jesionowski T. Zinc oxide—from synthesis to application: a review. Materials. 2014;7:2833–2881. doi:10.3390/ma7042833
- Srivastava R. Synthesis and Characterization Techniques of Nanomaterials. Sage. 2012;4:17–27.
- Naiya TK, Chowdhury P, Bhattacharya AK, et al. Saw dust and neem bark as low-cost natural biosorbent for adsorptive removal of Zn(II) and Cd(II) ions from aqueous solutions. Chem Eng J. 2009;148:68–79. doi:10.1016/j.cej.2008.08.002
- Ahmaruzzaman M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv Colloid Interface Sci. 2011;166:36–59. doi:10.1016/j.cis.2011.04.005
- Oliveira LC, Botero WG, Farias TS, et al. Application of natural organic residues as adsorbents to remove lead from waters. Water Air Soil Pollut. 2019;230:1–11. doi:10.1007/s11270-018-4051-3
- Afroze S, Sen TK. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 2018;229:1–50. doi:10.1007/s11270-018-3869-z
- Kumar J, Balomajumder C, Mondal P. Application of agro-based biomasses for zinc removal from wastewater – a review. Clean (Weinh). 2011;39:641–652.
- Fernández-López JA, Angosto JM, Roca MJ, et al. Taguchi design-based enhancement of heavy metals bioremoval by agroindustrial waste biomass from artichoke. Sci Total Environ. 2019;653:55–63. doi:10.1016/j.scitotenv.2018.10.343
- Anantha RK, Kota S. An evaluation of the major factors influencing the removal of copper ions using the egg shell (Dromaius novaehollandiae): chitosan (Agaricus bisporus) composite. 3 Biotech. 2016;6:1–16.
- SenthilKumar P, Ramalingam S, Sathyaselvabala V, et al. Removal of copper(II) ions from aqueous solution by adsorption using cashew nut shell. Desalination. 2011;266:63–71. doi:10.1016/j.desal.2010.08.003
- Taşar Ş,: Kaya F, Özer A. Biosorption of lead(II) ions from aqueous solution by peanut shells: equilibrium, thermodynamic and kinetic studies. J Environ Chem Eng. 2014;2:1018–1026. doi:10.1016/j.jece.2014.03.015
- Witek-Krowiak A, Szafran RG, Modelski S. Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination. 2011;265:126–134. doi:10.1016/j.desal.2010.07.042
- Al-Anber ZA, Matouq MAD. Batch adsorption of cadmium ions from aqueous solution by means of olive cake. J Hazard Mater. 2008;151:194–201. doi:10.1016/j.jhazmat.2007.05.069
- Joseph L, Jun BM, Flora JRV, et al. Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere. 2019;229:142–159. doi:10.1016/j.chemosphere.2019.04.198
- Rial JB, Ferreira ML. Potential applications of spent adsorbents and catalysts: re-valorization of waste. Science of The Total Environment. 2022;823:153370. doi:10.1016/j.scitotenv.2022.153370
- Ruffolo SA, La Russa MF, Malagodi M, et al. ZnO and ZnTiO3 nanopowders for antimicrobial stone coating. Appl Phys A Mater Sci Process. 2010;100:829–834. doi:10.1007/s00339-010-5658-4
- Azizi-Lalabadi M, Hashemi H, Feng J, et al. Carbon nanomaterials against pathogens: the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv Colloid Interface Sci. 2020;284:102250. doi:10.1016/j.cis.2020.102250
- Peng Z, Liu X, Zhang W, et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: a review. Environ Int. 2020;134:105298. doi:10.1016/j.envint.2019.105298
- Ali NS, Kalash KR, Ahmed AN, et al. Performance of a solar photocatalysis reactor as pretreatment for wastewater via UV, UV/TiO2, and UV/H2O2 to control membrane fouling. Sci Rep. 2022; 12:1.
- Mattson P, Bilous P. Coomassie brilliant blue: an excellent reagent for the enhancement of faint bloody fingerprints. Canadian Society of Forensic Science Journal. 2014;47:20–36. doi:10.1080/00085030.2014.885728
- Patil V, Ingle DR. An association between fingerprint patterns with blood group and lifestyle based diseases: a review. Artif Intell Rev. 2021;54:1803–1839. doi:10.1007/s10462-020-09891-w
- Patil V, Ingle DR. An association between fingerprint patterns with blood group and lifestyle based diseases: a review. Artif Intell Rev. 2021;54:1803–1839. doi:10.1007/s10462-020-09891-w
- Qiu Z, Hao B, Gu X, et al. A general powder dusting method for latent fingerprint development based on AIEgens. Sci China Chem. 2018;61:966–970. doi:10.1007/s11426-018-9280-1
- Zhao Y Bin, Ma YJ, Song D, et al. New luminescent nanoparticles based on carbon dots/SiO2 for the detection of latent fingermarks. Analytical Methods. 2017;9:4770–4775. doi:10.1039/C7AY01316G
- Becue A, Moret S, Champod C, et al. Use of quantum dots in aqueous solution to detect blood fingermarks on non-porous surfaces. Forensic Sci Int. 2009;191:36–41. doi:10.1016/j.forsciint.2009.06.005
- Wang M, Li M, Yu A, et al. Fluorescent Nanomaterials for the Development of Latent Fingerprints in Forensic Sciences. Adv Funct Mater. 2017;27:1606243. doi:10.1002/adfm.201606243
- James SH, Kish PE, Sutton TP. Principles of Bloodstain Pattern Analysis : Theory and Practice. Principles of Bloodstain Pattern Analysis. 2005: 576. doi:10.1201/9781420039467