ABSTRACT
Systematic exposure to odours (olfactory training, OT) is a method of smell loss treatment. Due to olfactory system projections to prefrontal brain areas, OT has been hypothesized to enhance cognitive functions, but its effects have been studied predominantly in adults. This study tested OT effects on working memory (WM), i.e., the ability to store and manipulate information for a short time, in healthy children aged 6-9 years. We expected OT to improve olfactory WM and establish cross-modal transfer to visual and auditory WM. Participants performed 12 weeks of bi-daily OT with either 4 odours (lemon, eucalyptus, rose, cloves; OT group) or odourless propylene glycol (placebo group). Pre- and post-training, participants’ WM was measured utilizing odours (olfactory WM) or pictures (visual WM) and a word-span task (auditory WM). 84 children (40 girls) completed the study. The analyses revealed no changes in the WM performance following OT. The olfactory WM task was the most difficult for children, highlighting the need to include olfactory-related tasks in educational programmes to improve children’s odour knowledge and memory, just as they learn about sounds and pictures. Further neuroimaging research is needed to fully understand the impact of OT on cognitive functions in children.
Introduction
Executive functions (EF) are top-down cognitive processes that are involved in solving problems and pursuing goals (Salehinejad et al., Citation2021). Compared to other cognitive functions, EF’s development is prolonged and maturation expands to late adolescence (Casey et al., Citation2005; Zelazo & Carlson, Citation2012). Enhanced EF facilitate children’s academic performance (Cortés Pascual et al., Citation2019; Spiegel et al., Citation2021) and adults’ psychological resilience (Wu et al., Citation2021), occupational performance (Bailey, Citation2007), and athletic success (Vestberg et al., Citation2012). Three major domains constitute EF – cognitive flexibility, inhibitory control, and working memory (Diamond, Citation2013). The focus of the current study is on working memory which is responsible for temporary storage and active manipulation of information (Diamond, Citation2013).
Working memory (WM) has been described as a system where an executive unit manages information stored in the sensory units (Baddeley, Citation2012). The functioning of WM is based on synchronized neural oscillations between several brain regions (Fell & Axmacher, Citation2011), with a substantial role played by the prefrontal cortex (especially dorsolateral prefrontal cortex; dlPFC), described as the executive control area (D’Esposito & Postle, Citation2015; Kwon et al., Citation2002; Uytun, Citation2018). Although the original views on WM focused on visuospatial and auditory storage units (Baddeley, Citation1992), more recent models account for potential inputs from other sensory modalities (e.g., olfaction) (Baddeley, Citation2012; Jönsson et al., Citation2011; White, Citation2009).
Since the maturing nervous system is plastic and susceptible to changes (Bryck & Fisher, Citation2012; Zelazo & Carlson, Citation2012), multiple interventions have been aimed to improve WM (Rowe et al., Citation2019; Sala & Gobet, Citation2020). Many of these interventions involve direct training of WM (Rowe et al., Citation2019) where participants are asked either (1) to repeat and practice WM tasks without receiving any further instructions (e.g., word recall, remembering spatial patterns) or (2) to follow instructions and implement a specific cognitive strategy (e.g., rehearsal of auditory information, metacognitive monitoring, chunking information) (Jones et al., Citation2020; Passolunghi & Costa, Citation2016; St Clair-Thompson et al., Citation2010). Both types of WM training – with and without instructions – lead to increased performance in the trained skill whereas the findings on transfer effects to other cognitive tasks are mixed (review: Rowe et al., Citation2019).
Indirect interventions have also been shown to improve WM. These programmes involve, for example, regular physical activity (Alesi et al., Citation2016; Kamijo et al., Citation2011), transcranial direct current brain stimulation (Nejati et al., Citation2020), and sensory stimulation (Worthen, Citation2010). For instance, transcranial stimulation of the dorsolateral prefrontal cortex (dlPFC) increased WM abilities in children with attention disorder (Nejati et al., Citation2020). Additionally, healthy children participating in physical activity programmes showed increased and more widespread brain activation during cognitive tasks and better performance on cognitive tasks compared to controls (Erickson et al., Citation2015). The efficacy of such interventions is based on the premise that stimulating brain regions relevant to WM or increasing overall physical fitness might lead to improved performance in WM tasks (Nejati et al., Citation2020; Rowe et al., Citation2019).
Although the studies on that topic are still in their infancy, olfaction appears to be another modality where training may indirectly improve WM. The olfactory system is plastic and susceptible to training (Hummel et al., Citation2018; Kim et al., Citation2020; Watt et al., Citation2004). Odours activate the olfactory receptor neurones in the olfactory epithelium from where the neural activation is conveyed to the olfactory bulb and cortical areas including the piriform cortex, entorhinal cortex, anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC) (Freiherr, Citation2017; Han et al., Citation2019). Importantly, the neural pathway from the olfactory bulb to the higher cortical areas partially bypasses the thalamus (Gottfried, Citation2010; Herz & Engen, Citation1996) resulting in more direct activations evoked by odours compared to other sensory stimuli (Costafreda et al., Citation2008; Freiherr, Citation2017).
Repeated, systematic, bi-daily exposure to odours (i.e., olfactory training; OT) has been introduced to the otorhinolaryngological practice as a tool to rehabilitate the sense of smell in people with olfactory dysfunction (Hummel et al., Citation2009; Pieniak et al., Citation2022). OT has improved the sense of smell in patients with various olfactory dysfunction aetiologies (Konstantinidis et al., Citation2013; Oleszkiewicz et al., Citation2018a; Poletti et al., Citation2017). Moreover, multiple meta-analyses have demonstrated that OT is efficient in restoring both peripheral olfactory sensitivity and higher-order olfactory functions (odour identification and discrimination) (Huang et al., Citation2021; Kattar et al., Citation2021; Sorokowska et al., Citation2017).
Neuroimaging findings indicate that OT alters brain structure and function. In adults with olfactory dysfunction, OT increases the volume of OFC and hippocampus (Gellrich et al., Citation2018), olfactory bulb (Mahmut et al., Citation2020a), the thalamus and cerebellum (Han et al., Citation2021). Additionally, enhanced functional connectivity in brain networks relevant to olfaction and cognitive functions has been reported as a result of OT in adults with olfactory dysfunction (Hosseini et al., Citation2020; Kollndorfer et al., Citation2015; Pellegrino et al., Citation2019). Kollndorfer et al. (Citation2015) reported an increased number of functional connections between the insula, dlPFC, and orbitofrontal gyrus, whereas Hosseini et al. (Citation2020) reported increased connectivity between the insula and cingulate cortex.
As the neurological effects of OT are present in the frontal brain areas relevant for cognition, it has been hypothesized that OT might have an impact on cognitive functions. This assumption has laid the foundations for a new avenue of research investigating the cognitive effects of OT. To date, OT has been mostly used in adult – especially aging – populations, and it has been shown to inhibit cognitive decline (Oleszkiewicz et al., Citation2021) and improve verbal abilities (Oleszkiewicz et al., Citation2022; Wegener et al., Citation2018).
The majority of OT research has focused on minimizing olfactory or cognitive deficits (Pieniak et al., Citation2022). However, OT might successfully stimulate brain regions relevant to cognitive functioning in healthy developing populations, such as children. The effects of OT in children remain understudied as only three studies have been published within this area. OT has been shown to increase olfactory sensitivity (Mori et al., Citation2015) and odour identification ability (Mahmut et al., Citation2021). The third study reported an increase in olfactory sensitivity in children after mild traumatic brain injury who performed OT with low-concentration odours (Pieniak et al., Citation2023).
Although OT increases functional activation in WM-relevant brain regions in adults with olfactory dysfunction (Kollndorfer et al., Citation2015) and has been successfully incorporated in olfactory-focused interventions in children (Mahmut et al., Citation2021; Mori et al., Citation2015; Pieniak et al., Citation2023), the cognitive effects of OT in paediatric population have not been extensively investigated, constituting a substantial gap in the research literature. For instance, if olfactory stimulation benefited cognitive functions in children, OT could be adapted to educational settings and supplement the available psychological interventions. Although a recent study reported that OT did not increase scores in the Tower of Hanoi and London tasks (measures of EF; Pieniak et al., Citation2023) performed by children after mild traumatic brain injury, it is likely that the Tower of Hanoi and London reflect different EF simultaneously (Bull et al., Citation2004) and more specific test is needed to determine if OT indeed benefits EF in children. It is possible that OT affects only specific aspects of EF, particularly WM. Therefore, it remains unclear whether OT may improve WM which formed the aim of the current study.
Interventions targeting WM often investigate whether the skill trained in a particular task transfer to an equivalent task (Zelinski, Citation2009). The transfer might occur either in the same sensory domain (e.g., training visual WM with a visual recognition task improves visual WM measured with the n-back task; Jennings et al., Citation2005) or cross-modal (e.g., training olfactory WM leads to improvement in visual WM; Olofsson et al., Citation2020). Although olfactory WM training involving repeated practice with a smell-based memory game has already demonstrated cross-modal transfer to visual WM measured with a parallel task (Olofsson et al., Citation2020), this training comprised both WM training and regular olfactory stimulation. Therefore, in our study, we used the original procedure of OT that relies on olfactory stimulation only, to verify whether systematic exposure to odours (a task not directly engaging WM) increases WM in olfactory, visual and auditory domains. The choice of OT was also motivated by its previous successful administration in the paediatric population (Mahmut et al., Citation2021; Mori et al., Citation2015), for which more complex training procedures might be too demanding.
We focused on the paediatric population because olfactory development remains understudied in children, and its relationships with cognitive development are yet to be explored. Cognitive interventions aiming to enhance WM were shown to be more effective when performed in children as compared to adults (Wass et al., Citation2012) which is a promising premise for OT to be applied in children as a method to enhance WM. To this end, we investigated changes in olfactory, visual, and auditory WM across time between participants who performed OT for 12 weeks in comparison to a placebo condition where children completed the training with an odourless substance. We hypothesized that OT would improve WM whereas the performance of the placebo group would remain unchanged compared to baseline.
Materials and methods
Ethical statement
This study was conducted in line with the principles of the Declaration of Helsinki. Ethical approval was received from the institutional review board at the Institute of Psychology, University of Wroclaw (decision: 2021/YBKOC). Informed consent was obtained orally from the child and in writing from their parent or legal guardian.
Participants
Based on power analysis conducted with G*Power (Faul et al., Citation2007) we estimated that to detect a moderate effect of f = .25 (Mahmut et al., Citation2021) with α set to .05 and power of .95, the final sample should be at least 54 participants.
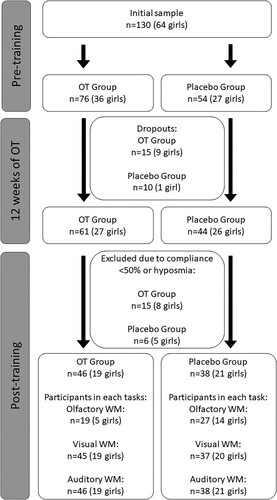
In the initial phase of the study, we recruited 130 participants (64 girls) aged 6–10 years (M = 7.65 years, SD = .66 years). Of these, 25 participants dropped out of the study before the post-training assessment. Based on low (<50%) compliance to OT declared by the parent or legal guardian or on smell impairment (details on the impairment diagnosis below), an additional 21 participants were excluded from the analyses. Of the remaining 84 children (40 girls) aged 6–9 years (M = 7.67 years, SD = .62 years) who returned for the post-training assessment, all were classified as normosmic, and had high (>50%) compliance with OT. Distribution of the compliance with OT is presented in . Some participants did not complete all three WM tests and the final sample size for statistical analyses differs across WM tests (see ). Participants were randomly allocated to the olfactory training (OT) group or placebo group (details on the training procedure below).
Figure 1. Distribution of the parent-reported compliance with OT across each group. The dashed line depicts the inclusion cut-off of 50%. OT = olfactory training.
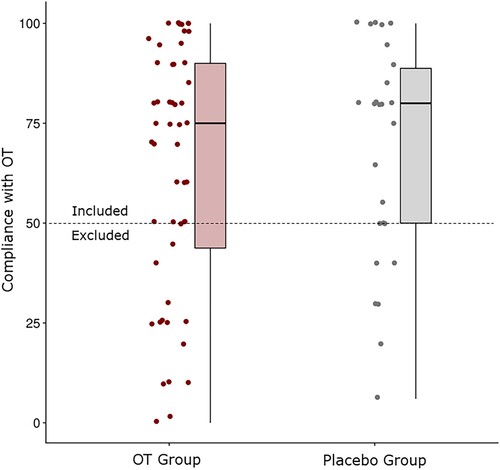
Figure 2. Flowchart presenting the number of participants who completed each part of the study. OT = olfactory training, WM = working memory.
Methods
Visual working memory test
Visual WM was measured with a popular memory game named “Concentration” (Schumann-Hengsteler, Citation1996). In the game used in the study, each participant was presented with a set of 30 cards with 15 pairs of cards depicting various animals. All cards were presented with pictures facing down. The child was asked to uncover two cards at a time and check if a matching pair was found. If the uncovered cards were matching, the pair was removed from the set. If the uncovered cards did not match, they were laid face down again and another pair was uncovered. The task continued until all matching pairs had been found. The number of trials taken to find all pairs was used as the score for this measure, with a greater number of trials indicating the poorer performance of visual WM.
Two parallel versions were used – one depicting animals and plants encountered in the forest and one depicting wild animals from different world regions (sets “In the forest” and “Wild animals” from CzuCzu, Krakow, Poland). The participant was tested with different sets during the pre- and post-training assessments to prevent the learning effect. The distribution of sets was counterbalanced across the participants.
Olfactory working memory test
Olfactory WM was measured with the olfaction-based version of the “Concentration” memory game for children. In the odour-based version, participants were presented with six pairs of odours instead of picture pairs. The child was asked to pick up two jars and smell the contents, one jar after another (they were instructed not to open two at the same time to avoid mixing odours in the air). If the child correctly decided the two jars had the same odour, the pair was removed from the set. If the odours were different, both jars were returned to the pool and the participant continued the task until all the pairs were found. The score for this test equalled the number of trials taken to find all pairs, with a greater number of trials indicating the poorer performance of olfactory WM. A similar olfactory memory task has been previously described by Olofsson et al. (Citation2020).
Odours were presented in 40 ml brown-glass jars with an opening of 3.5 cm in diameter. Each of the jars contained a cotton pad soaked with 4 ml of an odorant. Two equivalent sets of odours were used () and each participant was tested with a different set during the pre- and post-training assessment to avoid the learning effect. Odours sets were counterbalanced across the participants. The set comprised 6 pairs of jars, each pair containing the same odour. Jars were presented on a 3 × 4 rectangular grid. Each jar was labelled with a number on the bottom which enabled the researcher to verify the correctness of each match. Labels were not visible to the participants.
Table 1. Odours used in the olfactory working memory task.
Auditory working memory test
Immediate and delayed recall auditory WM was assessed with a word-span task based on the Rey Auditory-Verbal Learning Test (RAVLT; McMinn et al., Citation1988). The task used in the study employed immediate and delayed recall tasks for a list of 10 words but compared to the original RAVLT procedure we did not introduce an interference list of novel words before the delayed recall, to minimize the difficulty of the task for children. In the immediate recall task, the researcher read aloud a list of ten words, and the participant was asked to repeat as many of the words as possible. Immediate recall was tested twice. The sum of correct recalls varies from 0 to 20 and is used as a measure of immediate auditory working memory. Delayed recall auditory WM was tested approximately 10 min after immediate recall WM was tested. Each participant was asked to recall the ten words again (without the experimenter reading them aloud) and the number of correctly recalled words was used as a measure of delayed auditory WM.
Three parallel word lists were prepared by an experienced Polish linguist. Words in each list were balanced in terms of length, number of syllables, and type of consonants. The participant was presented with one of the three sets during each appointment, and no set was presented twice to the same participant pre- and post-training to prevent the learning effect. The list of original words and their English translation is presented in .
Table 2. Three sets of Polish words used in the auditory working memory task (English word in brackets).
Odour identification test
Participant’s ability to identify odours was measured to screen for potential olfactory dysfunction. We used the U-Sniff odour identification test that is specifically designed for the paediatric population (Schriever et al., Citation2018). The test comprises 12 trials. During each trial, the participant is presented with a felt-tip pen containing 4ml of an odour (e.g., apple, rose). The experimenter uncaps the pen and presents it approximately 2cm underneath the participant’s nostrils. To guide odour naming, a set of four labelled visual descriptors (one target and three distractors) is shown to the participant. Each correct identification is 1 point, resulting in a possible range of 0–12 points. Scores below 6 points indicate olfactory dysfunction in the Polish population (Schriever et al., Citation2018).
Olfactory training task
OT was performed with a set of four odours presented in felt-tip pens (Sniffin’ Sticks, Burghart Messtechnik GmbH, Holm, Germany). We used the original set of odours suggested by Hummel et al. (Citation2009) – lemon (citronellal), eucalyptus (eucalyptol), rose (phenyl ethyl alcohol), and cloves (eugenol). The placebo group received an analogous set of pens filled with odourless propylene glycol, but the participants were informed that the odours are presented in subliminal concentrations. Participants were instructed to engage in the training for 12 weeks, twice per day. Participants were asked to smell each pen for 30 s while moving the pen between both nostrils. They were asked to perform the training in the morning and in the evening and to avoid doing the training immediately after a meal or after brushing their teeth. To enhance children’s motivation to complete the training, each participant received a set of 180 stickers that were placed on a puzzle poster. Parents were asked to administer stickers in a way that the child was given a sticker for completion of a single training session. Completing all training sessions would uncover the poster picture (an art collage of smelling items designed for the project).
During the post-training appointment, the child’s parents or legal guardians were asked to provide a rating on the adherence to the training using a scale ranging from 0 to 100%. Participants with adherence lower than 50% were excluded from the analyses.
Procedure
Participants were assessed twice – pre- and post-training. At the pre-training session, the participants were screened for potential olfactory deficits with the odour identification test. The assessment sessions were separated by 12 weeks during which participants completed OT at home. Each participant was tested individually in a ventilated room. During each session olfactory, visual, and auditory WM tests were performed in a randomized order but with the maintenance of the 10 min break after the first part of the auditory memory task in each case. Due to fatigue, some participants did not complete all WM tests, with the olfactory WM test most often abandoned (see: for details). The olfactory WM test appeared most challenging for the children, likely because in contrast to visual and auditory WM tasks, odour knowledge and memory do not form part of the education programme. Participants were semi-randomly assigned to either the OT group or the placebo group with an allocation ratio of 1.5:1, to minimize the number of participants receiving the placebo treatment while maintaining satisfactory statistical power (Dumville et al., Citation2006).
Statistical analyses
The data were analysed with Jamovi 2.3.18 (The Jamovi Project, Citation2023) with a significance level set to α = .05. Data from participants who finalized the OT were used in the analyses. To verify the effects of OT on working memory performance and odour identification ability, a repeated-measures ANOVA was conducted. Time of assessment (Pre-training and Post-training) was the within-subject factor, and Group (OT and Placebo) was the between-subjects factor. The interaction effect (Time of assessment x Group) was the main effect of interest because it would indicate changes in WM over time related to OT. Due to the continuous and interdependent development of olfactory and cognitive abilities in childhood (Gellrich et al., Citation2019, Citation2021), age (in months) was included as a covariate in the model. Consequently, instead of descriptive statistics, estimated marginal means and standard errors (SE) for the repeated-measures ANOVA models with control of age were reported. Plots were generated with R (Allen et al., Citation2019).
As OT compliance was measured with subjective reports, all analyses were run with and without the participants whose parents declared OT compliance below 50%.
Results
Children from the placebo group were older than children from the OT group (M = 7.95, SD = .61; M = 7.43, SD = .53 years; respectively), t(82) = 4.10; p < .001. Odour identification ability measured with the U-Sniff test did not change between pre- and post-OT assessment in any of the groups (OT group: M = 9.93, SE = .23 vs M = 10.18, SE = .20, p = .347; Placebo group: M = 10.35, SE = .26 vs M = 10.13, SE = .22, p = .452).
At baseline, children from the OT and placebo groups did not differ in any of the WM test scores (Olfactory WM: p = .728; Visual WM: p = .462, Auditory WM Immediate: p = .271, Auditory WM Delayed: p = .292). Estimated marginal means for all the working memory measures are presented in .
Table 3. Estimated marginal means of the working memory scores across the sensory domains presented by group, while controlling for age.
Did olfactory training improve olfactory working memory?
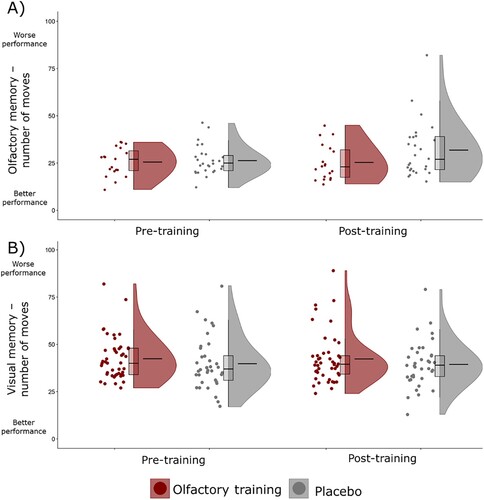
The analyses revealed there was no significant main effect for Group (F1,43 = 2.49, p = .122, η2p = .06) or Time (F1,43<.01, p = .935, η2p < .01). The Group x Time interaction was not significant (F1,43 = 1.7, p = .199, η2p = .04). Therefore, OT did not increase olfactory WM. Age was not a significant covariate (F1,80<.01, p = .931, η2p < .01). Distribution of the scores is presented in (A).
Figure 3. Scores on the olfactory (panel A) and visual (panel B) working memory tests during pre- and post-training by olfactory training and placebo groups. The scores indicate the number of moves required to finish the task where lower scores indicate better performance. Mean scores are represented by the horizontal line in the violin plots.
Did olfactory training improve visual working memory?
The analyses revealed there was no significant main effect for Group (F1,79 = 1.02, p = .316, η2p = .01) or Time (F1,79<.01, p = .967, η2p < .01). The Group x Time interaction was not significant (F1,79 = .02, p = .878, η2p < .01) demonstrating no effects of OT on visual WM. Age was not a significant covariate (F1,79 = .66, p = .419, η2p = .01). Distribution of the scores is presented in (B).
Did olfactory training improve auditory working memory?
The analysis revealed no significant main effect for Group (F1,81 = 1.92, p = .170, η2p = .02) or Time (F1,81 = .31, p = .578, η2p < .01) on auditory immediate memory. The Group x Time interaction was not significant (F1,81 = .01, p = .920, η2p < .01). Therefore, OT did not improve immediate auditory WM. Age was insignificant covariate (F1,81<.01, p = .965, η2p < .01). (A) presents the distribution of the scores.
Figure 4. Scores in auditory working memory task for immediate (panel A) and delayed (panel B) recall of a list of words during pre- and post-training assessment in the olfactory training and placebo groups. Mean scores are represented by the longer line in the middle of violin plots.
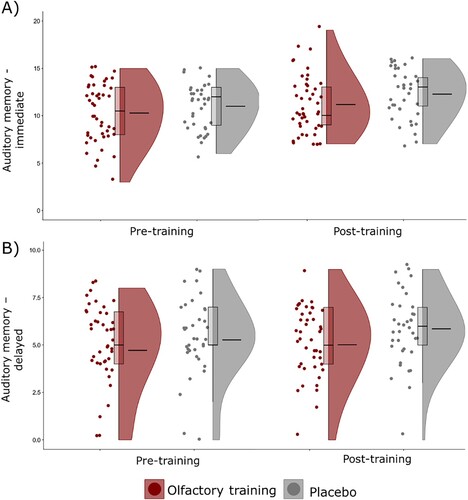
The last model focusing on delayed auditory memory revealed no significant main effect for Group (F1,81 = 3.54, p = .063, η2p = .04) or Time (F1,81<.01, p = .954, η2p < .01). There was no significant Group x Time interaction (F1,81 = .25, p = .620, η2p < .01) demonstrating no effect of OT on delayed auditory WM. Age was not significant covariate (F1,81 = .01, p = .914, η2p < .01). Scores distribution is presented in (B).
Did compliance with OT impact the findings?
Additional analyses were conducted to determine whether including participants with low compliance in all the above analyses changed the results. However, this was not the case – the same pattern of null results was found. Specifically, there was no effect of OT on olfactory (F1,53 = 3.2, p = .080, η2p = .06) or visual (F1,97 = .02, p = .881, η2p = .01) WM as indicated by Group x Time not-significant interaction. There was also no effect on auditory WM for neither immediate (F1,99 = .40, p = .531, η2p < .01) nor delayed (F1,99 = .13, p = .722, η2p < .01) measures.
Discussion
The present study aimed to determine if regular stimulation of the olfactory system using OT improves children’s WM in three sensory domains – olfaction, vision, and audition. Although OT is an intervention developed in the otorhinolaryngological practice, we hypothesized it would benefit cognitive functions, as previous studies in adults with olfactory dysfunction have shown OT enhances functional connectivity and activation in prefrontal brain regions involved in WM (Hosseini et al., Citation2020; Kollndorfer et al., Citation2015; Pellegrino et al., Citation2019). Moreover, research with healthy adults suggests that OT increases verbal abilities (Oleszkiewicz et al., Citation2022; Wegener et al., Citation2018) and inhibits age-related cognitive decline (Oleszkiewicz et al., Citation2021), suggesting cross-domain transfer between olfactory and cognitive abilities.
Contrary to our predictions, we did not observe an improvement in WM performance in children aged 6–9 years. There are a few plausible explanations for this. First, the available research demonstrating OT effects on brain structure and function comes from adult populations, often from clinical cohorts of patients with olfactory dysfunction. Although many olfaction-related brain structures (piriform cortex, entorhinal cortex) develop early, other cortical areas relevant for WM (e.g., dlPFC, OFC) undergo prolonged maturation exceeding childhood (Gogtay et al., Citation2004). Therefore, the OT effects on cognitive functions may depend on these regions, resulting in observable effects in the adult population whose neural development has been completed. Further neuroimaging studies on the OT effects on WM in various age cohorts would shed light on how brain development moderates OT effectiveness in the WM domain.
Studies utilizing transcranial stimulation assume that stimulation of relevant brain regions affects the corresponding cognitive functions (Nejati et al., Citation2020, Citation2022). We based our hypothesis on analogous findings from OT research in adults with olfactory dysfunction (Kollndorfer et al., Citation2015). However, it seems that either mere olfactory stimulation is not enough to cause substantial improvement in WM in children or the OT duration was too short to trigger any significant changes. In adult patients with post-infectious olfactory loss, prolonged duration of OT lead to a more pronounced recovery of olfactory function (Konstantinidis et al., Citation2016) and the effects of OT on children’s fluid intelligence were observed after six months of OT (Pieniak et al., Citation2023). Therefore, extending OT duration may be one way to enhance its effectiveness. However, ensuring high compliance throughout the prolonged training period would be of paramount importance.
Alternatively, observing the impact of olfactory stimulation on cognitive abilities may require more complex olfactory tasks, similar to those proposed by Olofsson et al. (Citation2020), that engage cognitive and olfactory processing simultaneously. However, our investigation revealed that the olfactory WM task was the most difficult WM task, highlighting the need to include olfactory-related education for children, just as children learn about sounds and pictures. Standard OT procedure involves mindful sniffing of four odours but it does not engage any higher-order cognitive processes. It has been previously demonstrated that more complex training tasks lead to transfer effects. For example, an intervention involving three olfactory tasks (odour intensity classification, odour quality classification, target odour detection) leads to improvement in other non-trained olfactory abilities in healthy adults (especially in odour identification) (Al Aïn et al., Citation2019). Additionally, engaging the sense of smell in WM training in healthy adults leads to cross-modal transfer to the visual WM (Olofsson et al., Citation2020). However, in both these studies, participants underwent more cognitively engaging training. In our study, in both assessment sessions, children performed a very similar olfactory WM task to the one described by Olofsson et al. (Citation2020), yet for some children the task was too demanding so they resigned before completing it. Therefore, not every modification of the standard olfactory training regimen will be suitable for children aged 6–9 years due to their limited attention span or proneness to boredom, likely undermining compliance. Nevertheless, a more engaging yet cognitively complex form of olfactory training for children should be sought for future research to address the relationship between OT complexity and its potential cross-modal benefits.
An alternative approach to boost the effects of OT in children is to change the odour exposure method. Instead of a bi-daily presentation of four odours, odours might be presented passively using a nasal clip or immersive exposure which will expand the duration of olfactory stimulation without greater cognitive engagement. Such approaches have already been successfully applied in adults with smell loss and lead to olfactory recovery (Mahmut et al., Citation2022; Mahmut et al., Citation2020b). Another method that may increase compliance with OT is to present the odours in a more child-engaging way, for example, presenting the odours inside figurines of animals or cartoon characters (Metatla et al., Citation2020) or embedding odour presentation in digital cognitive games (Olofsson et al., Citation2017).
Further research on the cognitive effects of OT could analyse the impact of OT on other memory systems that are likely to have overlapping neural systems with olfaction. The promising candidate for improvement following OT is spatial memory that is phylogenetically related to the olfactory function, possibly to facilitate spatial navigation (Dahmani et al., Citation2018; Jacobs, Citation2019, Citation2022). Importantly, both olfaction and spatial memory rely on the hippocampus (Bird & Burgess, Citation2008) which has been demonstrated to increase volume after OT in adult patients with post-infectious olfactory loss (Gellrich et al., Citation2018).
Although it was not the main focus of the presented study, we did not find the effects of OT on children’s ability to identify odours. However, this is in line with previous OT research in the paediatric population (Pieniak et al., Citation2023) and most likely is a result of a ceiling effect caused by high scores of healthy children in a U-Sniff test that was primarily designed as a screening tool for olfactory dysfunction in children.
The present study has several limitations requiring discussion. Firstly, due to the absence of neuroimaging methods verifying structural or functional brain changes, we cannot determine if the lack of OT effect on WM arose from the lack of brain-level changes following OT or the lack of correspondence between brain-level changes and behavioural performance. The latter possibility has been previously described in studies on OT focusing on the adult population, where changes in brain structure or functions did not correlate with changes in the scores of psychophysical tests (Al Aïn et al., Citation2019; Han et al., Citation2021; Negoias et al., Citation2017). Secondly, as the WM tests were prepared in a game-like manner to maximize participants’ engagement, we have no normative data to reference performance. Additionally, participants in the OT and placebo groups differed inadvertently in terms of age, which introduced an additional source of bias in the data. To minimalize the probability of such problems in future studies, block randomization may be considered (Broglio, Citation2018). Finally, we measured OT compliance with a subjective measure that might be prone to recall bias and self-presentation motives. The decision to exclude participants with compliance below 50% may seem liberal, but this compliance threshold is based on the findings from studies involving adult patients (Oleszkiewicz et al., Citation2018b; Saatci et al., Citation2020) and seems reasonable (and less liberal) in healthy children who in the absence of smell abnormalities may not be motivated to engage in OT.
There are also several strengths of the used methodology that overcame the most common challenges in OT research (Pieniak et al., Citation2022). Despite the sample size exceeding our initial benchmark and allowing sensitivity of f = .20 (thus achieving detectability of an even smaller effect size than we initially assumed) our analyses consistently yielded null results. Based on these findings we can say that despite the justified hypothesis, we failed to demonstrate that OT benefits the olfactory, visual, or auditory WM in children aged 6-9 years. In other words, this study provides consistent evidence in favour of the null hypothesis, leaving little room for type II error. Additionally, many cognitive interventions lack a proper placebo group and experimental groups receive different types of treatment (Simons et al., Citation2016). In our study, we investigated the effects of OT and compared it with the impact of an equivalent but odourless task. Finally, we designed WM tasks with parallel versions to prevent the learning effect that is known to be one of the challenges in evaluating psychological intervention effects (Lezak, Citation2012).
In conclusion, based on research demonstrating changes in brain structure and function following OT in adults, we verified its behavioural effects on WM in children. We did not observe any changes in children’s WM in olfactory, visual, and auditory domains at the behavioural level. However, further neuroimaging research is necessary to better understand the potential for OT to trigger structural and functional changes in the nervous system in childhood.
Consent to participate
Informed consent was obtained orally from children and in writing from their parents or legal guardians.
Ethical approval
Ethical approval was received from the institutional review board at the Institute of Psychology, University of Wroclaw (decision: 2021/YBKOC).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Al Aïn, S., Poupon, D., Hétu, S., Mercier, N., Steffener, J., & Frasnelli, J. (2019). Smell training improves olfactory function and alters brain structure. NeuroImage, 189, 45–54. https://doi.org/10.1016/j.neuroimage.2019.01.008
- Alesi, M., Bianco, A., Luppina, G., Palma, A., & Pepi, A. (2016). Improving children’s coordinative skills and executive functions. Perceptual and Motor Skills, 122(1), 27–46. https://doi.org/10.1177/0031512515627527
- Allen, M., Poggiali, D., Whitaker, K., Marshall, T. R., & Kievit, R. A. (2019). Raincloud plots: A multi-platform tool for robust data visualization. Wellcome Open Research, 4, 63. https://doi.org/10.12688/wellcomeopenres.15191.1
- Baddeley, A. (1992). Working memory. Science, 255(5044), 556–559. https://doi.org/10.1126/science.1736359
- Baddeley, A. (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63(1), 1–29. https://doi.org/10.1146/annurev-psych-120710-100422
- Bailey, C. E. (2007). Cognitive accuracy and intelligent executive function in the brain and in business. Annals of the New York Academy of Sciences, 1118(1), 122–141. https://doi.org/10.1196/annals.1412.011
- Bird, C. M., & Burgess, N. (2008). The hippocampus and memory: Insights from spatial processing. Nature Reviews Neuroscience, 9(3), 182–194. https://doi.org/10.1038/nrn2335
- Broglio, K. (2018). Randomization in clinical trials. JAMA, 319(21), 2223–2224. https://doi.org/10.1001/jama.2018.6360
- Bryck, R. L., & Fisher, P. A. (2012). Training the brain: Practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. American Psychologist, 67(2), 87–100. https://doi.org/10.1037/a0024657
- Bull, R., Espy, K. A., & Senn, T. E. (2004). A comparison of performance on the towers of London and Hanoi in young children. Journal of Child Psychology and Psychiatry, 45(4), 743–754. https://doi.org/10.1111/j.1469-7610.2004.00268.x
- Casey, B. J., Tottenhan, N., Liston, C., & Durston, S. (2005). Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Sciences, 9(3), 104–110. https://doi.org/10.1016/j.tics.2005.01.011
- Cortés Pascual, A., Moyano Muñoz, N., & Quílez Robres, A. (2019). The relationship between executive functions and academic performance in primary education: Review and meta-analysis. Frontiers in Psychology, 10, Article 1582. https://doi.org/10.3389/fpsyg.2019.01582
- Costafreda, S. G., Brammer, M. J., David, A. S., & Fu, C. H. Y. (2008). Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews, 58(1), 57–70. https://doi.org/10.1016/J.brainresrev.2007.10.012
- Dahmani, L., Patel, R. M., Yang, Y., Chakravarty, M. M., Fellows, L. K., & Bohbot, V. D. (2018). An intrinsic association between olfactory identification and spatial memory in humans. Nature Communications, 9(1), 4162. https://doi.org/10.1038/s41467-018-06569-4
- D’Esposito, M., & Postle, B. R. (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66(1), 115–142. https://doi.org/10.1146/annurev-psych-010814-015031
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64(1), 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
- Dumville, J. C., Hahn, S., Miles, J. N. V., & Torgerson, D. J. (2006). The use of unequal randomisation ratios in clinical trials: A review. Contemporary Clinical Trials, 27(1), 1–12. https://doi.org/10.1016/j.cct.2005.08.003
- Erickson, K. I., Hillman, C. H., & Kramer, A. F. (2015). Physical activity, brain, and cognition. Current Opinion in Behavioral Sciences, 4, 27–32. https://doi.org/10.1016/j.cobeha.2015.01.005
- Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behaviour Research Methods, 39(2), 175–191. https://doi.org/10.3758/bf03193146
- Fell, J., & Axmacher, N. (2011). The role of phase synchronization in memory processes. Nature Reviews Neuroscience, 12(2), 105–118. https://doi.org/10.1038/nrn2979
- Freiherr, J. (2017). Cortical olfactory processing. In A. Buttner (Ed.), Handbook Of odor (pp. 759–768). Springer Nature.
- Gellrich, J., Han, P., Manesse, C., Betz, A., Junghanns, A., Raue, C., Schriever, V. A., & Hummel, T. (2018). Brain volume changes in hyposmic patients before and after olfactory training. The Laryngoscope, 128(7), 1531–1536. https://doi.org/10.1002/lary.27045
- Gellrich, J., Sparing-Paschke, L.-M., Hummel, T., & Schriever, V. A. (2021). The influence of cognitive parameters on olfactory assessment in healthy children and adolescents. Chemical Senses, 46, bjaa072. https://doi.org/10.1093/chemse/bjaa072
- Gellrich, J., Sparing-Paschke, L. M., Thieme, T., Schwabe, K., Dworschak, A., Hummel, T., & Schriever, V. A. (2019). Normative data for olfactory threshold and odor identification in children and adolescents. International Journal of Pediatric Otorhinolaryngology, 123, 5–9. https://doi.org/10.1016/J.ijporl.2019.01.009
- Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., Nugent, T. F., Herman, D. H., Clasen, L. S., Toga, A. W., Rapoport, J. L., & Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. https://doi.org/10.1073/pnas.0402680101
- Gottfried, J. A. (2010). Central mechanisms of odour object perception. Nature Reviews Neuroscience, 11(9), 628–641. https://doi.org/10.1038/nrn2883
- Han, P., Musch, M., Abolmaali, N., & Hummel, T. (2021). Improved odor identification ability and increased regional gray matter volume after olfactory training in patients with idiopathic olfactory loss. I-Perception, 12(2), 1–11. https://doi.org/10.1177/20416695211005811
- Han, P., Zang, Y., Akshita, J., & Hummel, T. (2019). Magnetic resonance imaging of human olfactory dysfunction. Brain Topography, 32(6), 987–997. https://doi.org/10.1007/s10548-019-00729-5
- Herz, R. S., & Engen, T. (1996). Odor memory: Review and analysis. Psychonomic Bulletin & Review, 3(3), 300–313. https://doi.org/10.3758/bf03210754
- Hosseini, K., Zare-Sadeghi, A., Sadigh-Eteghad, S., Mirsalehi, M., & Khezerloo, D. (2020). Effects of olfactory training on restingstate effective connectivity in patients with posttraumatic olfactory dysfunction. Acta Neurobiologiae Experimentalis, 80(4), 381–388. https://doi.org/10.21307/ane2020035
- Huang, T., Wei, Y., & Wu, D. (2021). Effects of olfactory training on posttraumatic olfactory dysfunction: A systematic review and meta-analysis. International Forum of Allergy & Rhinology, 11(7), 1102–1112. https://doi.org/10.1002/alr.22758
- Hummel, T., Rissom, K., Reden, J., Hähner, A., Weidenbecher, M., & Hüttenbrink, K. B. (2009). Effects of olfactory training in patients with olfactory loss. The Laryngoscope, 119(3), 496–499. https://doi.org/10.1002/lary.20101
- Hummel, T., Stupka, G., Haehner, A., & Poletti, S. C. (2018). Olfactory training changes electrophysiological responses at the level of the olfactory epithelium. Rhinology Journal, 56(4), 330–335. https://doi.org/10.4193/Rhin17.163
- Jacobs, L. F. (2019). The navigational nose: A new hypothesis for the function of the human external pyramid. Journal of Experimental Biology, 222(Suppl_1), jeb186924. https://doi.org/10.1242/jeb.186924
- Jacobs, L. F. (2022). How the evolution of air breathing shaped hippocampal function. Philosophical Transactions of the Royal Society B: Biological Sciences, 377, 20200532. https://doi.org/10.1098/rstb.2020.0532
- Jennings, J. M., Webster, L. M., Kleykamp, B. A., & Dagenbach, D. (2005). Recollection training and transfer effects in older adults: Successful Use of a repetition-Lag procedure. Aging, Neuropsychology, and Cognition, 12(3), 278–298. https://doi.org/10.1080/138255890968312
- Jones, J. S., Milton, F., Mostazir, M., & Adlam, A. R. (2020). The academic outcomes of working memory and metacognitive strategy training in children: A double-blind randomized controlled trial. Developmental Science, 23(4), e12870. https://doi.org/10.1111/desc.12870
- Jönsson, F. U., Møller, P., & Olsson, M. J. (2011). Olfactory working memory: Effects of verbalization on the 2-back task. Memory & Cognition, 39(6), 1023–1032. https://doi.org/10.3758/s13421-011-0080-5
- Kamijo, K., Pontifex, M. B., O’Leary, K. C., Scudder, M. R., Wu, C.-T., Castelli, D. M., & Hillman, C. H. (2011). The effects of an afterschool physical activity program on working memory in preadolescent children. Developmental Science, 14(5), 1046–1058. https://doi.org/10.1111/j.1467-7687.2011.01054.x
- Kattar, N., Do, T., Unis, G., Migneron, M., Thomas, A., & McCoul, E. (2021). Olfactory training for postviral olfactory dysfunction: Systematic review and meta-analysis. Otolaryngology–Head and Neck Surgery : Official Journal of American Academy of Otolaryngology-Head and Neck Surgery, 164(2), 244–254. https://doi.org/10.1177/0194599820943550
- Kim, B. Y., Park, J. Y., Kim, E. J., & Kim, B. G. (2020). Olfactory ensheathing cells mediate neuroplastic mechanisms after olfactory training in mouse model. American Journal of Rhinology and Allergy, 34(2), 217–229. https://doi.org/10.1177/1945892419885036
- Kollndorfer, K., Fischmeister, F. P. S., Kowalczyk, K., Hoche, E., Mueller, C. A., Trattnig, S., & Schöpf, V. (2015). Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. NeuroImage: Clinical, 9, 401–410. https://doi.org/10.1016/j.nicl.2015.09.004
- Konstantinidis, I., Tsakiropoulou, E., Bekiaridou, P., Kazantzidou, C., & Constantinidis, J. (2013). Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. The Laryngoscope, 123(12), E85–E90. https://doi.org/10.1002/lary.24390
- Konstantinidis, I., Tsakiropoulou, E., & Constantinidis, J. (2016). Long term effects of olfactory training in patients with post-infectious olfactory loss. Rhinology Journal, 54(2), 170–175. https://doi.org/10.4193/Rhino15.264
- Kwon, H., Reiss, A. L., & Menon, V. (2002). Neural basis of protracted developmental changes in visuo-spatial working memory. Proceedings of the National Academy of Sciences, 99(20), 13336–13341. https://doi.org/10.1073/pnas.162486399
- Lezak, M. D. (2012). Neuropsychological assessment (5th ed.). Oxford University Press.
- Mahmut, M. K., Musch, M., Han, P., Abolmaali, N., & Hummel, T. (2020a). The effect of olfactory training on olfactory bulb volumes in patients with idiopathic olfactory loss. Rhinology, 58(4), 412–415. https://doi.org/10.4193/Rhin20.223
- Mahmut, M. K., Oelschlägel, A., Haehner, A., & Hummel, T. (2022). The impact of olfactory training using a nasal clip and extended periods of odor exposure. Journal of Sensory Studies, 37(2), e12721. https://doi.org/10.1111/joss.12721
- Mahmut, M. K., Pieniak, M., Resler, K., Schriever, V. A., Haehner, A., & Oleszkiewicz, A. (2021). Olfactory training in 8-year-old increases odour identification ability: A preliminary study. European Journal of Pediatrics, 180(7), 2049–2053. https://doi.org/10.1007/s00431-021-03970-y
- Mahmut, M. K., Uecker, F. C., Göktas, Ö, Georgsdorf, W., Oleszkiewicz, A., & Hummel, T. (2020b). Changes in olfactory function after immersive exposure to odorants. Journal of Sensory Studies, 35(2), e12559. https://doi.org/10.1111/joss.12559
- McMinn, M. R., Wiens, A. N., & Crossen, J. R. (1988). Rey auditory-verbal learning test: Development of norms for healthy young adults. Clinical Neuropsychologist, 2(1), 67–87. https://doi.org/10.1080/13854048808520087
- Metatla, O., Bardot, S., Cullen, C., Serrano, M., & Jouffrais, C. (2020). Robots for inclusive play: Co-designing an educational game with visually impaired and sighted children. Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems, 1–13. https://doi.org/10.1145/3313831.3376270
- Mori, E., Petters, W., Schriever, V. A., Valder, C., & Hummel, T. (2015). Exposure to odours improves olfactory function in healthy children. Rhinology journal, 53(3), 221–226. https://doi.org/10.4193/Rhino14.192
- Negoias, S., Pietsch, K., & Hummel, T. (2017). Changes in olfactory bulb volume following lateralized olfactory training. Brain Imaging and Behaviour, 11(4), 998–1005. https://doi.org/10.1007/s11682-016-9567-9
- Nejati, V., Khorrami, A. S., & Fonoudi, M. (2022). Neuromodulation of facial emotion recognition in health and disease: A systematic review. Neurophysiologie Clinique, 52(3), 183–201. https://doi.org/10.1016/j.neucli.2022.03.005
- Nejati, V., Salehinejad, M. A., Nitsche, M. A., Najian, A., & Javadi, A.-H. (2020). Transcranial direct current stimulation improves executive dysfunctions in ADHD: Implications for inhibitory control, interference control, working memory, and cognitive flexibility. Journal of Attention Disorders, 24(13), 1928–1943. https://doi.org/10.1177/1087054717730611
- Oleszkiewicz, A., Abriat, A., Doelz, G., Azema, E., & Hummel, T. (2021). Beyond olfaction: Beneficial effects of olfactory training extend to aging-related cognitive decline. Behavioral Neuroscience, 135(6), 732–740. https://doi.org/10.1037/bne0000478
- Oleszkiewicz, A., Bottesi, L., Pieniak, M., Fujita, S., Krasteva, N., Nelles, G., & Hummel, T. (2022). Olfactory training with aromastics: Olfactory and cognitive effects. European Archives of Oto-Rhino-Laryngology, 279(1), 225–232. https://doi.org/10.1007/s00405-021-06810-9
- Oleszkiewicz, A., Hanf, S., Whitcroft, K. L., Haehner, A., & Hummel, T. (2018a). Examination of olfactory training effectiveness in relation to its complexity and the cause of olfactory loss. The Laryngoscope, 128(7), 1518–1522. https://doi.org/10.1002/lary.26985
- Oleszkiewicz, A., Schultheiss, T., Schriever, V. A., Linke, J., Cuevas, M., Hahner, A., & Hummel, T. (2018b). Effects of “trigeminal training” on trigeminal sensitivity and self-rated nasal patency. European Archives of Oto-Rhino-Laryngology, 275(7), 1783–1788. https://doi.org/10.1007/s00405-018-4993-5
- Olofsson, J. K., Ekström, I., Lindström, J., Syrjänen, E., Stigsdotter-Neely, A., Nyberg, L., Jonsson, S., & Larsson, M. (2020). Smell-Based memory training: Evidence of olfactory learning and transfer to the visual domain. Chemical Senses, 45(7), 593–600. https://doi.org/10.1093/chemse/bjaa049
- Olofsson, J. K., Niedenthal, S., Ehrndal, M., Zakrzewska, M., Wartel, A., & Larsson, M. (2017). Beyond smell-O-vision: Possibilities for smell-based digital media. Simulation and Gaming, 48(4), 455–479. https://doi.org/10.1177/1046878117702184
- Passolunghi, M. C., & Costa, H. M. (2016). Working memory and early numeracy training in preschool children. Child Neuropsychology, 22(1), 81–98. https://doi.org/10.1080/09297049.2014.971726
- Pellegrino, R., Han, P., Reither, N., & Hummel, T. (2019). Effectiveness of olfactory training on different severities of posttraumatic loss of smell. The Laryngoscope, 129(8), 1737–1743. https://doi.org/10.1002/lary.27832
- Pieniak, M., Oleszkiewicz, A., Avaro, V., Calegari, F., & Hummel, T. (2022). Olfactory training – thirteen years of research reviewed. Neuroscience & Biobehavioral Reviews, 141, 104853. https://doi.org/10.1016/j.neubiorev.2022.104853
- Pieniak, M., Seidel, K., Oleszkiewicz, A., Gellrich, J., Karpinski, C., Fitze, G., & Schriever, V. A. (2023). Olfactory training effects in children after mild traumatic brain injury. Brain Injury, 37(11), 1272–1284. https://doi.org/10.1080/02699052.2023.2237889
- Poletti, S. C., Michel, E., & Hummel, T. (2017). Olfactory training using heavy and light weight molecule odors. Perception, 46(3–4), 343–351. https://doi.org/10.1177/0301006616672881
- Rowe, A., Titterington, J., Holmes, J., Henry, L., & Taggart, L. (2019). Interventions targeting working memory in 4–11 year olds within their everyday contexts: A systematic review. Developmental Review, 52, 1–23. https://doi.org/10.1016/j.dr.2019.02.001
- Saatci, O., Altundag, A., Duz, O. A., & Hummel, T. (2020). Olfactory training ball improves adherence and olfactory outcomes in post-infectious olfactory dysfunction. European Archives of Oto-Rhino-Laryngology, 277(7), 2125–2132. https://doi.org/10.1007/s00405-020-05939-3
- Sala, G., & Gobet, F. (2020). Working memory training in typically developing children: A multilevel meta-analysis. Psychonomic Bulletin & Review, 27(3), 423–434. https://doi.org/10.3758/s13423-019-01681-y
- Salehinejad, M. A., Ghanavati, E., Rashid, M. H. A., & Nitsche, M. A. (2021). Hot and cold executive functions in the brain: A prefrontal-cingular network. Brain and Neuroscience Advances, 5, 1–19. https://doi.org/10.1177/23982128211007769
- Schriever, V. A., Agosin, E., Altundag, A., Avni, H., Cao Van, H., Cornejo, C., de los Santos, G., Fishman, G., Fragola, C., Guarneros, M., Gupta, N., Hudson, R., Kamel, R., Knaapila, A., Konstantinidis, I., Landis, B. N., Larsson, M., Lundström, J. N., Macchi, A., … Hummel, T. (2018). Development of an international odor identification test for children: The universal sniff test. The Journal of Pediatrics, 198, 265–272.e3. https://doi.org/10.1016/j.jpeds.2018.03.011
- Schumann-Hengsteler, R. (1996). Children’s and adults’ visuospatial memory: The game concentration. The Journal of Genetic Psychology, 157(1), 77–92. https://doi.org/10.1080/00221325.1996.9914847
- Simons, D. J., Boot, W. R., Charness, N., Gathercole, S. E., Chabris, C. F., Hambrick, D. Z., & Stine-Morrow, E. A. L. (2016). Do “brain-training” programs work? Psychological Science in the Public Interest, 17(3), 103–186. https://doi.org/10.1177/1529100616661983
- Sorokowska, A., Drechsler, E., Karwowski, M., & Hummel, T. (2017). Effects of olfactory training: A meta-analysis. Rhinology Journal, 55(1), 17–26. https://doi.org/10.4193/Rhino16.195
- Spiegel, J. A., Goodrich, J. M., Morris, B. M., Osborne, C. M., & Lonigan, C. J. (2021). Relations between executive functions and academic outcomes in elementary school children: A meta-analysis. Psychological Bulletin, 147(4), 329–351. https://doi.org/10.1037/bul0000322
- St Clair-Thompson, H., Stevens, R., Hunt, A., & & Bolder, E. (2010). Improving children’s working memory and classroom performance. An International Journal of Experimental Educational Psychology, 30(2), 203–219. https://doi.org/10.1080/01443410903509259
- The Jamovi Project. (2023). jamovi (Version 2.3). https://www.jamovi.org
- Uytun, M. C. (2018). Development period of prefrontal cortex. In Starcevic Ana (Ed.), Prefrontal cortex. InTech. https://doi.org/10.5772/intechopen.78697
- Vestberg, T., Gustafson, R., Maurex, L., Ingvar, M., & Petrovic, P. (2012). Executive functions predict the success of Top-soccer players. PLoS One, 7(4), e34731. https://doi.org/10.1371/journal.pone.0034731
- Wass, S. V., Scerif, G., & Johnson, M. H. (2012). Training attentional control and working memory – Is younger, better? Developmental Review, 32(4), 360–387. https://doi.org/10.1016/j.dr.2012.07.001
- Watt, W. C., Sakano, H., Lee, Z. Y., Reusch, J. E., Trinh, K., & Storm, D. R. (2004). Odorant stimulation enhances survival of olfactory sensory neurons via MAPK and CREB. Neuron, 41(6), 955–967. https://doi.org/10.1016/S0896-6273(04)00075-3
- Wegener, B.-A., Croy, I., Hähner, A., & Hummel, T. (2018). Olfactory training with older people. International Journal of Geriatric Psychiatry, 33(1), 212–220. https://doi.org/10.1002/gps.4725
- White, T. L. (2009). A second look at the structure of human olfactory memory. Annals of the New York Academy of Sciences, 1170(1), 338–342. https://doi.org/10.1111/j.1749-6632.2009.03878.x
- Worthen, E. (2010). Sensory-Based interventions in the general education classroom: A critical appraisal of the topic. Journal of Occupational Therapy, Schools, & Early Intervention, 3(1), 76–94. https://doi.org/10.1080/19411241003684217
- Wu, L., Zhang, X., Wang, J., Sun, J., Mao, F., Han, J., & Cao, F. (2021). The associations of executive functions with resilience in early adulthood: A prospective longitudinal study. Journal of Affective Disorders, 282, 1048–1054. https://doi.org/10.1016/j.jad.2021.01.031
- Zelazo, P. D., & Carlson, S. M. (2012). Hot and cool executive function in childhood and adolescence: Development and plasticity. Child Development Perspectives 6(4), 354–360.
- Zelinski, E. M. (2009). Far transfer in cognitive training of older adults. Restorative Neurology and Neuroscience, 27(5), 455–471. https://doi.org/10.3233/rnn-2009-0495
