?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
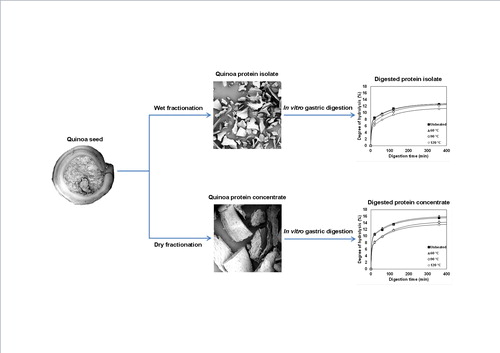
Quinoa protein was isolated from quinoa seeds using wet fractionation that resulted in a protein isolate (QPI) with a high protein purity of 87.1% (w/dw) and a protein yield of around 54%, and a dry fractionation method delivered a quinoa protein concentrate (QPC) with a purity of 27.8% (w/dw) and yield of around 47%. The dry fractionation process only involves milling and sieving and keeps the protein in its natural, native state. The aim was to study the in vitro gastric digestibility of both protein. Attention was paid to thermal pre-treatment of QPI and QPC. QPC showed significantly higher (p < .05) digestibility than QPI samples. The results were interpreted with a simple double exponential model. The fraction of easily digested protein in QPC is higher than for QPI. The better digestibility of the QPC was explained by the prevention of the formation of large aggregates during pre-heating of the protein.
Graphical Abstract
Introduction
Quinoa (Chenopodium quinoa Willd) has been cultivated in the Andean region of Latin America for thousands of years (Tang et al. Citation2015). Its production was largely replaced by European crops after the Spanish conquest (Martínez et al. Citation2009). Nowadays, there is a renewed interest worldwide in quinoa due to its high nutritional value, especially the essential amino acid balance. Quinoa proteins are therefore considered promising food ingredients as they can supplement other plant proteins to increase their nutritional value (Abugoch et al. Citation2008). In addition, quinoa, being a gluten-free pseudo-cereal, has attracted the attention of gluten-free manufacturers.
Traditionally, wet fractionation has been used to obtain protein-rich fractions. During this process, the starting material is reduced in size and subsequently diluted to achieve complete disentanglement of the tissue structures to allow extraction of individual or classes of components as proteins, starch and lipids (Schutyser & van der Goot Citation2011). This process is not only energy intensive, but also affects the functionality of the protein (Pelgrom et al. Citation2014). Dry fractionation is a more sustainable alternative to wet fractionation for quinoa seeds. During dry fractionation, a protein-enriched fraction can be obtained by milling and dry separation by for example, sieving or air classification. This delivers a protein fraction that is still in its natural state. A disadvantage of this technique is the lower protein purity that can be obtained in the concentrate.
While the amino acid profile including the essential amino acids is important for the nutritive quality of a protein source, its digestibility is another important factor in determining the quality of a protein source (FAO/WHO/UNU Citation2007). Generally, the potential use of plant proteins and thus also quinoa protein as a food ingredient is limited by their relatively lower digestibility as compared with animal proteins (Guillaume et al. Citation2001).
Protein digestion in the human stomach is facilitated by the presence of acids and pepsin and subsequently by the pancreatic and intestinal enzymes in the small intestine (Whitney et al. Citation1998). Heating often leads to an increase in digestibility. For example, heat treatment of sweet potato protein isolate (PPI) at 100 °C (20 and 60 min), 110 and 127 °C for 20 min resulted in a significant increase in the gastrointestinal digestibility compared to that of native protein (Sun et al. Citation2012). Whey protein isolate (WPI) heat treated at 80 °C for 30 min significantly enhanced its gastric digestibility compared with native WPI (He et al. Citation2013). However, heating can also result in a decrease of the digestibility. Heating soy protein isolate (SPI) at 100 °C for 60 min decreased its gastric digestibility compared with native SPI (Sun et al. Citation2012).
In vitro assays simulating digestion processes have been used to study the effect of temperature on quinoa protein isolate (Avila et al. Citation2016a) and quinoa seeds (Ruales & Nair Citation1992). However, the in vitro gastric digestion of quinoa protein concentrate in solution has not been studied before. Since the fractionation processes to obtain quinoa protein isolate (QPI) and quinoa protein concentrate (QPC) are different, there may be differences in the digestibility of the protein fractions. The aim of this paper is to study in vitro gastric digestion of the untreated protein fractions as well as heat-treated protein fractions in solution. Both QPI and QPC will be studied. We hypothesise that QPC, where the protein is in its natural, native state, is more digestible as compared to QPI, where the protein properties may have changed due to the harsh conditions during the wet fractionation process.
Materials and methods
Materials
Quinoa (Chenopodium quinoa Willd) with a protein content of 11.6% (w/dw) purchased from Notenstore (The Netherlands). Pepsin from porcine gastric mucosa (400–800 units/mg, P7125), mucin from porcine stomach (Type III, M2378-100 G) and all other chemicals were purchased from Sigma-Aldrich Inc. (St. Louis, MO). Milli-Q water (18.2 MΩ cm at 25 °C, Millipore Corporation, Molsheim, France) was used for all experiments.
Wet fractionation method
The wet fractionation method was carried out according to Avila et al. (Citation2016a) with minor modifications. Quinoa seeds were pre-milled with a laboratory scale mill (Fritsch Mill Pulverisette 14, Indar-Oberstein, Germany) at 7000 rpm and sieved through a 200 μm sieve. Oil extraction was performed in a Soxhlet for 24 h using petroleum ether as solvent. The defatted flour was suspended in deionised water (10% w/w) and the pH was adjusted to 8 by addition of 2 N NaOH. The extraction was performed at room temperature for 4 h. The suspensions were centrifuged for 30 min at 6000 g and 10 °C. The supernatants were then acidified to pH 4.5 by addition of 2 N HCl and incubated at room temperature for 1 h. The suspensions were centrifuged for 30 min at 13,000g and 10 °C. The precipitated pellets were re-suspended in deionised water (5% w/w). To rinse remaining salts the suspensions were centrifuged twice for 30 min at 13000 g and 10 °C, re-suspended in deionised water (5% w/w) and neutralised by addition of 2 N NaOH. The suspensions were frozen overnight and subsequently freeze-dried for 72 h (Chris Epsilon 2–6 D Freeze Dryer, Osterode am Harz, Germany). The dried protein isolates were mixed and ground with an IKA A11 basic grinder (IKA-Werke GmbH and Co., Staufen, Germany) for a few seconds to obtain powders.
Dry fractionation method
Quinoa seeds were pre-milled to separate the cotyledons from the seed with a laboratory scale mill (Fritsch Mill Pulverisette 14, Idar-Oberstein, Germany) with a 1.5 and 2.0 mm screen at room temperature. The rotor speed was 6000 rpm with a feed rate of ∼20 g/min. The milling experiments were performed in triplicate.
The pre-milled quinoa seeds were sieved by air jet sieving (Alpine200 LS-N, Hosokawa-Alpine, Augsburg, Germany) with different sieves (1, 0.85, 0.63, 0.5 and 0.315 mm) at 1500 Pa for 2.5 min. During these sieving experiments, each time a sample of 25 g of pre-milled seeds was sieved. The protein separation efficiency was measured as the percentage of protein in each fraction. All experiments were performed in triplicate. The fraction with the highest protein content was chosen for gastric digestion analysis.
Determination of protein content
The protein content was measured by Dumas analysis (Nitrogen analyser, FlashEA 1112 series, Thermo Scientific, Interscience, Breda, The Netherlands). A conversion factor of N × 6.25 for quinoa protein was used (Ruales & Nair Citation1994; Nascimento et al. Citation2014). Protein purity was defined as mass protein/mass dry matter (w/dw). The measurements were carried out in triplicate. All protein contents reported are based on dry matter basis.
Heat treatment of quinoa protein solutions
All solutions were prepared by dissolving 0.1 g of pure quinoa protein in 2 mL of solution with Milli-Q water, prepared at room temperature into an Eppendorf tube of 2 mL. The solutions were stirred vigorously using a stirrer for 30 min. Subsequently, the solutions were subjected to heat treatment at 60 and 90 °C, 30 min and 1400 rpm of shaking in a pre-heated Eppendorf thermomixer (Eppendorf AG, Hamburg, Germany). Heat treatment at 120 °C during 30 min was carried out without shaking in a heating block (Grant QBT4, Cambridge, UK). After heating, the samples were immediately cooled and kept at room temperature until measurement the same day.
In vitro gastric digestion of quinoa protein
The suspensions of 5% protein (w/v, in Milli-Q water) were incubated in the simulated gastric juice at 37 °C for 6 h. The simulated gastric juice was prepared according to Luo et al. (Citation2015). For this, pepsin (1 g/L), mucin (1.5 g/L) and NaCl (8.775 g/L) were dissolved in Milli-Q water and the pH was adjusted to 2.0. The enzyme:substrate ratio during all experiment was constant at 1:2 (weight/weight).
The quinoa suspension was added to 50 mL of simulated gastric juice in a jacketed glass vessel connected to a water bath of 37 °C (Julabo GmbH, Seelbach, Germany). The solution was stirred at 100 rpm and the vessel was sealed with Parafilm (Pechiney Plastic Packaging Inc., IL) to avoid evaporation. Samples were taken at 5, 10, 20, 30, 60, 90, 120, 180, 240 and 360 min for further analyses. Immediately after sampling, the samples were heated in a pre-heated Eppendorf thermomixer (Eppendorf AG, Hamburg, Germany) at 90 °C and 1400 rpm for 5 min to inactivate the pepsin, which is rapidly inactivated at a temperature above 62 °C (Casey & Laidler Citation1951). All digestion experiments were performed in triplicate.
Effect of starch concentration on digestibility
Because starch is the main component in the dry fractionated protein concentrate, the effect of starch was evaluated. The effect of starch on protein digestibility was measured using two different ratios of protein and starch. Starch is obtained by the dry fractionation method (fraction >1 mm). The suspensions with 5% of protein (w/v, in Milli-Q water) were used and starch was added. Solutions of QPI with 20% and 50% of starch added were used. These solutions were heated at 90 and 120 °C for 30 min and the protein digestibility was measured. All measurements were carried out in triplicate.
Size exclusion chromatography (SEC)
In vitro digested samples were analysed via high-performance size-exclusion chromatography using an Ultimate 3000 UHPLC system (Thermo Scientific, MA) equipped with a TSKgel G2000SWXl column (7.8 mm × 300 mm) (Tosoh Bioscience LLC, PA). For analysis 0.5 mL of undiluted sample was filtered using a 0.22 μm filter. A 10 μL sample was injected each time. The mobile phase was acetonitrile (30%) in Milli-Q water (70%) containing trifluoroacetic acid (0.1%). The flow rate was 1 mL/min and the UV detector was set at 214 nm. Calibration was carried out with: carbonic anhydrase (29 kDa), α-lactalbumin (14.1 kDa), aprotinin (6.51 kDa), insulin (5.7 kDa), bacitracin (1.42 kDa) and phenylalanine (165 Da) (Sigma-Aldrich Inc., St. Louis, MO). The molecular mass was estimated based on the elution time of molecular weights markers. All measurements were carried out in triplicate.
Degree of hydrolysis (DH)
The degree of hydrolysis was measured using the OPA method (Nielsen et al. Citation2001) in order to determine the degree of hydrolysis attained. The OPA reagent (100 mL) was prepared by dissolving 3.81 g sodium tetraborate decahydrate (Borax) and 0.1 g of sodium dodecyl sulphate (SDS) in 80 mL milli-Q water. o-Phthaldialdehyde (OPA), 80 mg dissolved in 2 mL ethanol, was added to the Borax-SDS solution together with 88 mg of dithiothreitol (DTT). The solution was filled up to 100 mL with milli-Q water and filtered over a 0.45 μm filter. The solution was stored in a bottle covered with aluminium foil because OPA reagent is sensitive to light.
A standard curve was prepared using l-serine in a concentration range of 50–200 mg/L (Nielsen et al. Citation2001). The OPA assay was carried out by the addition of 200 μL of sample (or standard) to 1.5 mL of OPA reagent. The samples were pipetted into the Amicon Ultra-0.5 10 K Centrifugal Filter Units (Millipore) and centrifuged for 20 min at 14,000 g. The absorbance of these solutions was measured after 3 minutes at 340 nm with a spectrophotometer DU 720 (Beckman Coulter Inc., Pasadena, CA). Free amino groups in quinoa protein digest were expressed as serine amino equivalents (Serine NH2). The DH was calculated using the following equations:
(1)
(1)
(2)
(2)
Where, the value of constants α and β used here are the values reported by Adler-Nielsen (Citation1986), α equal 1 and β equal 0.4. While htot was estimated according to the concentration of each amino acid present in the protein (Lindeboom Citation2005) and found to be 7.4 mequv/g for quinoa protein. All measurements were carried out in triplicate.
Scanning Electron Microscope
The images of quinoa fractions were obtained by scanning electron microscopy (Phenom G2 Pure, Phenom-World BV, Eindhoven, The Netherlands). Carbon tabs (SPI Supplies/Structure Probe Inc., West Chester, PA) were used to fix the samples on aluminium pin mounts (SPI Supplies/Structure Probe Inc., West Chester, PA). Pre-treatment of the samples was not necessary.
Particle size distribution
Particle size distribution of QPI samples unheated and heated at 60, 90 and 120 °C for 30 min was measured using a Mastersizer 2000 (Malvern Instruments Ltd., Worcestershire, UK). Before the measurements were taken, the samples were diluted to 2% at pH 2. A refractive index of 1.45 was used for the dispersed phase and 1.33 for the continuous phase (water). Samples were diluted in milli-Q water in the measurement cell of the equipment until the obscuration reached 15% for the digested samples. The mean particle sizes and distribution were determined as the average of three repeated measurements.
Optical microscopy
The quinoa protein isolates (QPI) unheated and heated (60, 90 and 120 °C for 30 min) were studied using optical light microscopy (Axio Scope A1, Carl Zeiss Microscopy GmbH, Göttingen, Germany). The images were captured by the connected video camera (Axio Cam MRc5, Carl Zeiss Meditec) and acquisition software Zeiss AxioVision Rel 4.8. Images were obtained with a 40× objective.
Statistical analysis
Significance testing was performed using Fisher’s least significant difference (LSD) test and the differences were taken to be statistically significant when the p value was < .05. The multiple range test (MRT) included in the statistical programme was used to prove the existence of homogeneous groups within each of the parameters analysed. All analyses were performed using Statgraphics Centurion XVI Statistical Software (Statistical Graphics Corp., Herdon, VA).
Results and discussion
Dry fractionation process
Dry fractionation by milling and subsequent sieving appears to be a good alternative to wet fractionation for most grains and especially for quinoa. The milling process must be controlled to obtain the parts of interest. Of these, the embryo that consists of the radicle and two cotyledons () is the part of the seed which is richest in protein. The embryo contains 23.5% protein, while the bran and the perisperm contain only 6.1 and 7.2%, respectively (Ando et al. Citation2002).
Figure 1. SEM image of medial longitudinal section of quinoa seed. Perisperm (P), hypocotyl-radical axis (H), shoot appendix (SA), cotyledons (C), radicle (R), funicle (F) and pericarp (PE).
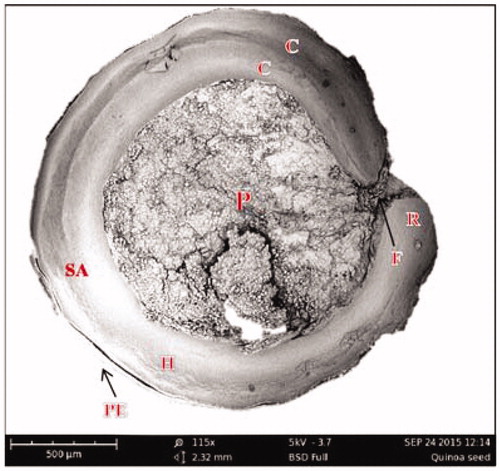
The results of the air jet sieving experiments are presented in . The coarse material (>1 mm) has a low protein content. The protein content of the coarse fraction is slightly dependent on the sieve used during the milling (1.5 or 2.0 mm). The richer protein fraction was obtained between the sieves 0.315–0.5 mm and reached 27.8% (w/dw), which is almost three times higher than the protein content of the whole quinoa seed (11.5% w/dw). The protein yield of this fraction was around 45%. This is higher compared to the literature values for wet fractionation (Avila et al. Citation2016b) and can be explained with the help of SEM images (), where the different quinoa fractions are shown. The coarse fraction mainly consists of the body of the quinoa seed (perisperm), which contains mostly starch (around 82%) and only low amounts of protein (Lindeboom Citation2005). The 0.315–0.5 mm fraction contains high amounts of the radicle/cotyledons, which is in agreement with the high protein content.
Table 1. Experimental characterisation of quinoa fractions after sieving.
The protein content obtained is higher than was reported by previous studies (Becker & Hanners Citation1990; Ando et al. Citation2002; Avila et al. Citation2016c). Differences in protein content are related to the yield, where increased protein purity is usually reflected in lower yield. Föste et al. (Citation2015) obtained similar results after sieving and subsequent purification of quinoa bran (31.3%), however, they were purified using water and chemicals. There is no evidence in our fractions of damage to the perisperm after pre-milling and subsequent sieving; thus, the high-starch fraction will also remain a high value.
Hydrolysis of quinoa protein solutions
In vitro gastric digestion of quinoa protein isolate (QPI)
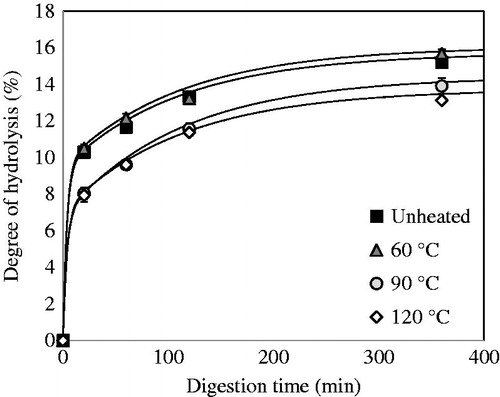
QPI with a protein content of 87.1% (w/dw) was used during digestion experiments. The yield of this method was around 54%. The in vitro gastric digestion of QPI that was obtained via wet fractionation was measured on time and is shown in . Before digestion, QPI was pre-treated at various temperatures. The degree of hydrolysis (DH) increased rapidly in the first 20 min of digestion by pepsin, and then increased steadily from 20 to 360 min. The unheated samples and samples heated at 60 °C yielded significantly higher DH values (p < .05) at 20 min of digestion as compared to samples pre-treated at 90 and 120 °C, while above 20 min of digestion, the rates of digestion are basically similar. After 360 min, only the samples heated at 120 °C exhibited significantly lower digestibility (p < .05). These DH values are slightly lower compared to the previous study by Avila et al. (Citation2016a) using the same conditions. The reason may be the variety used in the previous study. In fact, a sweet variety (saponins free) was used, while in our study a bitter variety was used (with saponins). Avila et al. (Citation2016b) indicated that the absence of saponins increases the solubility of proteins, so this factor could increase protein digestibility.
Figure 3. Degree of hydrolysis (DH) of QPI obtained by the wet fractionation process unheated and pre-heated at 60, 90 and 120 °C.
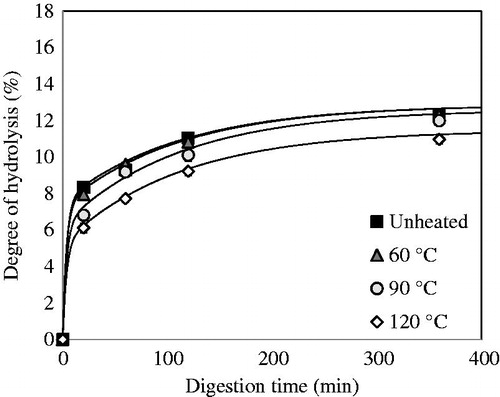
The final amount of hydrolysed peptide bonds produced during in vitro gastric digestion is higher for a native protein solution compared to denatured protein solutions. These results are in line with the hypothesis that heating protein above the denaturation temperature (98.1 °C) (Abugoch et al. Citation2008) leads to the formation of protein aggregates which become less accessible for pepsin to hydrolyse (). The fact that heating to 120 °C results in slower overall digestion suggests that the aggregation here leads to poorer accessibility due to stronger aggregation. This may imply a different localisation of amino acid residues that are specific to pepsin action in the quinoa protein after heating.
Figure 4. Light microscopy images of QPI solutions obtained by wet fractionation unheated and pre-heated at 60, 90 and 120 °C.
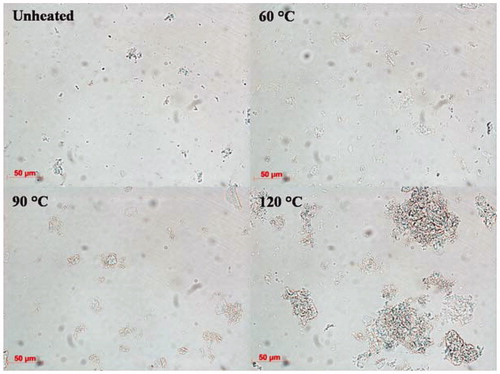
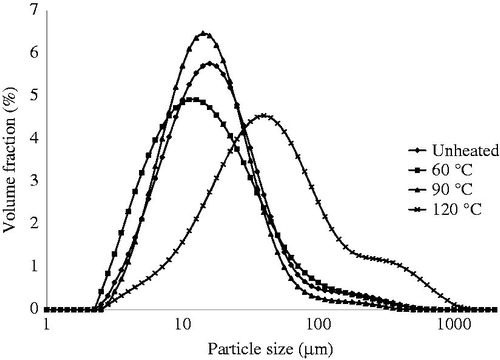
To evaluate the protein aggregation, particle size distribution of the suspensions before digestion was carried out (). The particle size distributions showed that the QPI heated at 60 and 90 °C did not show any difference with the unheated protein, the QPI heated at 120 °C showed much larger aggregates, which was supported by microscopy. The samples heated at 120 °C after 30 min also had a gel-like substance. A similar effect was observed for spaghetti made from durum wheat, where protein aggregation due to intensive heat treatment reduced protein digestibility (Stuknyte et al. Citation2014).
Figure 5. Particle size distribution of QPI unheated and pre-heated at 60, 90 and 120 °C and dissolved in Milli-Q water at pH2. Curves represent the average of three independent measurements.
The differences in protein digestion between heated and unheated samples are similar to previous observations for casein digestion. In pre-heated casein, the formation of protein aggregates and thus curd-like structure in simulated gastric juice slows down the protein digestibility (Lambers et al. Citation2013). Some authors ascribe the decreased digestibility to the reduced solubility of the native protein after denaturation. Carbonaro et al. (Citation1997) indicated that heating is responsible for protein denaturation, possibly followed by aggregation of the unfolded molecules, which results in reduced solubility. This effect was also observed for meat proteins, where protein aggregation during heating was linked to the increase in surface hydrophobicity which resulted in protein insolubility (Bax et al. Citation2012).
The effect of temperature on plant proteins has not been studied extensively. Lupine protein concentrate unheated and heated at 60 °C showed a higher amount of peptides formed after 30 min of gastric digestion than samples heated at 90 °C (Pelgrom et al. Citation2014). Soy protein isolate (SPI) heated at 100 °C for 20 min showed a lower digestibility than native SPI, while autoclaving at 110 and 127 °C for 20 min significantly enhanced its digestibility (Sun et al. Citation2012).
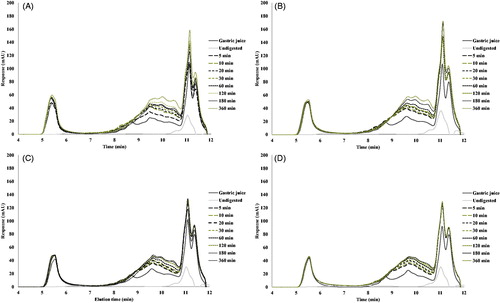
From the different digestion stages of each sample, the size exclusion chromatograms (SEC) of the digested samples are presented in . For all samples (heated and unheated), QPI showed a significant increase in the molecular range of 0.5–5 kDa. This confirmed the fact that when digestion progresses, pepsin cleaves more and more peptide sites, resulting in an increase of oligopeptides of widely varying sizes (Kaur & Boland Citation2013). As the digestion time increased, larger molecules are gradually converted into smaller peptides. After 2 h of pepsin proteolysis, the increase of peptides between 0.5 and 5 kDa slows down.
Figure 6. SEC-HPLC profiles of gastric digestion of QPI digested by pepsin for 6 h at 37 °C. (A) Unheated samples, (B) pre-heated at 60 °C, (C) pre-heated at 90 °C and (D) pre-heated at 120 °C.
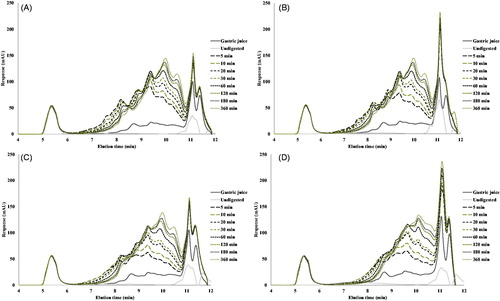
The size exclusion chromatograms show only minor differences between the proteins heated to different temperatures. The main difference is that from the QPI heated to 120 °C, less larger peptides (elution time between 6 and 9 min) are produced, and more smaller peptides (elution time around 11 min); this is indicative of the poorer accessibility of the aggregated protein for hydrolysis.
In vitro gastric digestion of quinoa protein concentrate (QPC)
While QPI obtained by means of wet fractionation is relatively pure, it has been dissolved and dehydrated by freeze drying, and its properties may have been changed by this. Dry fractionation leaves the protein in its original state, however the concentrate obtained is less pure. To determine the digestibility of quinoa protein obtained by dry fractionation, the protein fraction with a particle size of 0.315–0.5 mm obtained via air jet sieving was used. This quinoa protein concentrate (QPC) has a protein content of 27.8% (w/dw) (compare with the 87.1% for the wet fractionated QPI). The in vitro gastric digestion of this protein-rich concentrate, dispersed in water and pre-treated at various temperatures, was followed in time (). This QPC, whether heated or unheated, is digested more quickly than the QPI. This may be explained by the fact that the protein is more available for pepsin after dry fractionation. The samples heated at 60 °C do not present significant differences (p < .05) in their digestibility during 6 h of gastric digestion process as compared to the unheated samples. However, the samples heated at 90 and 120 °C presented a significantly lower digestibility (p < .05) compared with the others during 6 h of digestion by pepsin.
Figure 7. Degree of hydrolysis (DH) of quinoa protein obtained by the dry fractionation process unheated and pre-heated at 60, 90 and 120 °C.
These results are opposite to those obtained with unfractionated quinoa flour heated at 91 °C for 30 min and autoclaved for 10 and 30 min, because the protein digestibility increased significantly as compared with unheated quinoa flour (Ruales & Nair Citation1994). Likewise, Rathod and Annapure (Citation2016) found that lentil protein was digested more quickly after heat treatment at 140 °C. However, sorghum heated for 20 min in boiling water increased the amount of molecular aggregates, and reduced protein digestibility (Nunes et al. Citation2004).
The chromatograms of the digested QPC are presented in . For all samples (heated and unheated), the chromatograms show an increase in the molecular range of 0.5–5 kDa. A comparison of the chromatograms (obtained by dry and wet fractionation) shows that in general QPI releases more small peptides than QPC. This, in combination with the faster hydrolysis of the QPC, increases the number of very small aggregates with a large specific surface area, but which do not allow access to cleave off big peptides.
Double exponential model
We can interpret the results in and with a simple model, in which we assume that the protein consists of a part that is easily hydrolysed (e.g. the relatively exposed residues), one part that is hydrolysed with more difficulty and one part that is not hydrolysed at all. This is represented in a simple double-exponential model according to:
(3)
(3)
In which α1 is the fraction that is most easily digested, α2 the fraction that is hydrolysed with more difficulty, and k1 and k2 are the hydrolysis rate constants. Fitting the results with this model, assuming that k1 and k2 are the same for all temperatures, we obtain . While a pre-treatment below 60 °C does not have much effect on the digestion, with a pre-treatment above this temperature the quickly digestible fraction is reduced, and the slowly digestible and the undigested fraction increases above 60 °C.
Figure 9. Digested fractions (left hand figure) and undigested fraction (right hand figure), as function of the pre-treatment temperature. The rate constants were assumed to be the same for QPI and QPC, and were fitted at k1=0.280 min−1 and k2=0.00895 min−1. (A) Wet fractionated QPI and (B) dry fractionated QPC.
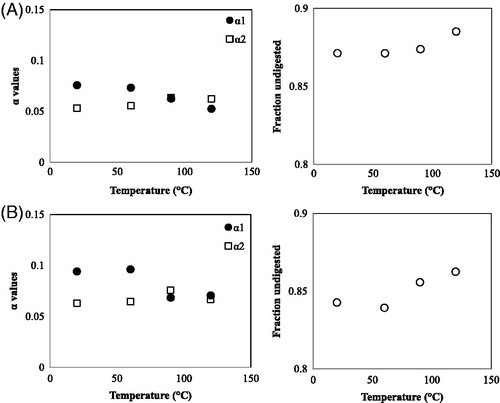
We see that the wet fractionated QPI () and the dry fractionated QPC () both show similar behaviour: both show an increase of the indigestible fraction, and a decrease of the rapidly digestible fraction, when the protein is pre-heated above 60 °C. The non-digested fraction of the QPC is lower than that of the QPI, while the slowly digested fraction is only slightly higher. Overall, QPC is better digestible. In the fits, the values for the rate constants were assumed to be the same for QPI and QPC. This can, of course, be disputed; the precise value of at least k1 was found to barely influence the quality of the fit; the values for k2 are more important, however, fitting separate values for QPI and QPC gives almost the same value.
One can observe that pre-heating the QPC to temperatures higher than 60 °C, leads to a sudden loss in rapidly digestible protein. Even though the digestibility of the QPI also decreases at higher temperatures, this drop is more gradual. We hypothesise that this is because of the presence of starch in the QPC, which will reduce the accessibility of the protein for pepsin.
Effect of starch on digestibility of quinoa protein
To asses this hypothesis, we added starch to QPI, and heated solutions to 90 and 120 °C. shows the digestibility, compared to QPI, that was pre-heated at 90 and 120 °C (compare ). Indeed, an increase in the starch concentration results in a significantly lower (p < .05) protein digestibility. In fact, with QPI heated at 90 °C the lower digestibility does not seem to depend on the starch concentration, while at 120 °C a dependency on the starch concentration is shown. The DH after 6 h of digestion by pepsin was 11.5 ± 0.2% for heated samples at 120 °C, while these were 9.4 ± 0.1% and 8.4 ± 0.2% for the samples with 20 and 50% of starch, respectively. These values are significantly lower (p < .05) than those obtained by digestion of dry fractionated samples subjected to the same treatment (). This result shows that starch has an effect on protein digestibility. Wong et al. (Citation2009) found that when starch was removed by α-amylase from sorghum flour, the protein digestibility by pepsin became considerably higher. When starch is removed, the quinoa proteins are more exposed and thus more accessible to pepsin digestion. Furthermore, the increase in viscosity reduces the diffusivity of both the enzyme and the protein. The quinoa starch yields a high final viscosity 5.67 Pa s (measured in this work) in comparison with rice (4.47 Pa s), potato (3.89 Pa s), cassava (2.91 Pa s), wheat (2.99 Pa s) and corn (2.99 Pa s) (Araujo-Farro et al. Citation2005). This final viscosity is associated with retrogradation between starch molecules (particularly amylose component) and in sufficient concentration causes the formation of a gel.
Figure 10. Degree of hydrolysis (DH) of the mixture of 5% of QPI and starch added different concentrations (0, 20 and 50% of starch) and pre-heated at (A) 90 °C and (B) 120 °C for 30 min.
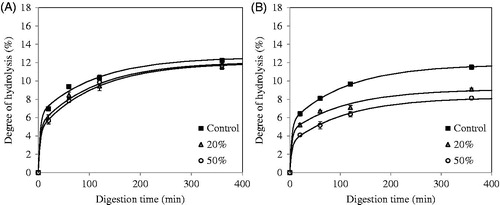
Our finding that QPC is better digestible than the QPI can therefore not be ascribed to the presence of starch and possibly other components, but must be attributed to the condition of the protein itself. In our experiments, starch was added only after isolation of the quinoa protein. It may be then that the protein is already aggregated into larger aggregates, making the protein relatively inaccessible to pepsin. In the case of QPC, the protein was kept in its natural state, i.e. in the form of protein bodies surrounded by some matrix components, such as carbohydrates and starch. These matrix components may inhibit the formation of larger aggregates when the protein is denatured and lead to smaller aggregates that are better accessible for pepsin.
Conclusions
In conclusion, the method proposed in the present study can provide a protein concentrate with a protein purity of 28% (w/dw) and a protein yield of 45%. QPI showed slower digestibility than QPC with all pre-heating temperatures, even though all fractions showed reduced digestibility when preheated to higher temperatures. QPC showed reduced digestibility above 60 °C. This could be explained by the presence of starch, which after being heated above its gelatinisation temperature (64.5 °C) increases the viscosity and reduces the accessibility of the protein for pepsin. The better digestibility of the dry fractionated QPC was found not to be linked to the carbohydrates present in this fraction, but may be due to the prevention of the formation of large aggregates during pre-heating of the protein.
Acknowledgements
Mauricio Opazo Navarrete contributed to this research. Thanks to a PhD scholarship from CONICYT (Formation of Advanced Human Capital Program), Chile.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Abugoch LE, Romero N, Tapia CA, Silva J, Rivera M. 2008. Study of some physicochemical and functional properties of quinoa (Chenopodium quinoa Willd) protein isolates. J Agric Food Chem. 56:4745–4750.
- Adler-Nielsen J. 1986. Enzymic hydrolysis of food proteins. New York: Elsevier Applied Science Publishers. p. 110–169.
- Ando H, Chen Y-C, Tang H, Shimizu M, Watanabe K, Mitsunaga T. 2002. Food components in fractions of quinoa seed. Food Sci Technol Res. 8:80–84.
- Araujo-Farro PC, Amaral JP, Menegalli FC. 2005. Comparison of starch pasting and retrogradation properties of Quinoa (Chenopodium quinoa Willd), rice, potato, cassava, wheat and corn starches. Presented at 2nd Mercosur Congress on Chemical Engineering; Rio de Janeiro, Brazil.
- Avila RG, Opazo-Navarrete M, Meurs M, Minor M, Sala G, van Boekel M, Janssen AEM. 2016a. Denaturation and in vitro gastric digestion of heat-treated quinoa protein isolates obtained at various extraction pH. Food Biophys. 11:184–197.
- Avila RG, Xiao W, van Boekel M, Minor M, Stieger M. 2016b. Effect of extraction pH on heat induced, gelation and microstructure of protein isolate from quinoa (Chenopodium quinoa Willd). Food Chem. 209:203–210.
- Avila RG, Arts A, Minor M, Schutyser MAI. 2016c. A hybrid dry and aqueous fractionation method to obtain protein-rich fractions from Quinoa (Chenopodium quinoa Willd). Food Bioprocess Techn. 9:1502–1510.
- Bax ML, Aubry L, Ferreira C, Daudin JD, Gatellier P, Rémond D, Santé-Lhoutellier V. 2012. Cooking temperature is a key determinant of in vitro meat protein digestion rate: investigation of underlying mechanisms. J Agric Food Chem. 60:2569–2576.
- Becker R, Hanners GD. 1990. Compositional and nutritional evaluation of quinoa whole grain flour and mill fractions. LWT-Food Sci. Technol. 23:441–444.
- Carbonaro M, Cappelloni M, Nicoli S, Lucarini M, Carnovale E. 1997. Solubility − digestibility relationship of legume proteins. J Agric Food Chem. 45:3387–3394.
- Casey BE, Laidler KJ. 1951. The kinetics and mechanism of the heat inactivation of pepsin. J Am Chem Soc. 73:1455–1457.
- FAO/WHO/UNU. 2007. Report of a Joint FAO/WHO/UNU Expert Consultation; Protein and Amino Acid Requirements in Human Nutrition. WHO Technical Report Series; Geneva, Switzerland.
- Föste M, Elgeti D, Brunner A-K, Jekle M, Becker T. 2015. Isolation of quinoa protein by milling fractionation and solvent extraction. Food Bioprod Process. 96:20–26.
- Guillaume J, Kaushik S, Bergot P, Metailler R. 2001. Digestive physiology and nutrient digestibility in fishes. In Nutrition and Feeding of Fish and Crustaceans. Chichester, U.K: Praxis Publishing.
- He J-S, Mu T-H, Wang J. 2013. A comparative in vitro study of the digestibility of heat- and high pressure-induced gels prepared from industrial milk whey proteins. High Press Res. 33:328–335.
- Kaur L, Boland M. 2013. Influence of kiwifruit on protein digestion. Adv Food Nutr Res. 68:149–167.
- Lambers TT, Van Den Bosch WG, De Jong S. 2013. Fast and slow proteins: modulation of the gastric behavior of whey and casein in vitro. Food Dig. 4:1–6.
- Lindeboom N. 2005. Studies on the characterization, biosynthesis and isolation of starch and protein from quinoa (Chenopodium quinoa Willd). Department of Applied Microbiology and Food Science University of Saskatchewan; Saskatoon, Canada (PhD Thesis).
- Luo Q, Boom RM, Janssen AEM. 2015. Digestion of protein and protein gels in simulated gastric environment. LWT-Food Sci Technol. 63:161–168.
- Martínez EA, Veas E, Jorquera C, San Martín R, Jara P. 2009. Re-introduction of Quínoa into arid Chile: cultivation of two lowland races under extremely low irrigation. J Agron Crop Sci. 195:1–10.
- Nascimento AC, Mota C, Coelho I, Gueifão S, Santos M, Matos AS, Gimenez A, Lobo M, Samman N, Castanheira I. 2014. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: proximates, minerals and trace elements. Food Chem. 148:420–426.
- Nielsen PM, Petersen D, Dambmann C. 2001. An improved method for determining food protein degree of hydrolysis. J Food Sci. 66:642–646.
- Nunes A, Correia I, Barros A, Delgadillo I. 2004. Sequential in vitro pepsin digestion of uncooked and cooked sorghum and maize samples. J Agric Food Chem. 52:2052–2058.
- Pelgrom PJM, Berghout JAM, van der Goot AJ, Boom RM, Schutyser MAI. 2014. Preparation of functional lupine protein fractions by dry separation. LWT-Food Sci Technol. 59:680–688.
- Rathod RP, Annapure US. 2016. Effect of extrusion process on antinutritional factors and protein and starch digestibility of lentil splits. LWT-Food Sci Technol. 66:114–123.
- Ruales J, Nair BM. 1994. Effect of processing on in vitro digestibility of protein and starch in quinoa seeds. Int J Food Sci Technol. 29:449–456.
- Ruales J, Nair BM. 1992. Nutritional quality of the protein in quinoa (Chenopodium quinoa Willd) seeds. Plant Foods Hum Nutr. 42:1–11.
- Schutyser MAI, van der Goot AJ. 2011. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci Technol. 22:154–164.
- Sun M, Mu T, Zhang M, Arogundade LA. 2012. Nutritional assessment and effects of heat processing on digestibility of Chinese sweet potato protein. J Food Comp Anal. 26:104–110.
- Stuknyte M, Cattaneo S, Pagani MA, Marti A, Micard V, Hogenboom J, De Noni I. 2014. Spaghetti from durum wheat: effect of drying conditions on heat damage, ultrastructure and in vitro digestibility. Food Chem. 149:40–46.
- Tang Y, Li X, Zhang B, Chen PX, Liu R, Tsao R. 2015. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 166:380–388.
- Whitney EN, Cataldo BC, Sharon RR. 1998. Understanding normal and clinical nutrition. 5th ed. Belmont, USA: Wadsworth Publishing Company Inc.
- Wong JH, Lau T, Cai N, Singh J, Pedersen JF, Vensel WH, Hurkman WL, Wilson JD, Lemaux PG, Buchanan B. 2009. Digestibility of protein and starch from sorghum (Sorghum bicolor) is linked to biochemical and structural features of grain endosperm. J Cereal Sci. 49:73–82.