Abstract
The effects of soaking and cooking on soluble sugars, alpha-galacto-oligosaccharides (α-GOS) and soluble dietary fibres (SDF) and insoluble dietary fibres (IDF) were assessed in five legumes (lentil, chickpea, fenugreek, faba bean and Egyptian faba bean). In raw seeds, total α-GOS content ranged from 2500 mg/100 g (chickpea) to over 4000 mg/100 g (fenugreek). Stachyose was predominant in fenugreek, lentil and chickpea, whereas verbascose was the main α-GOS in faba bean and Egyptian faba bean. IDF represented 69–87% of the total dietary fibres in all studied legumes, while SDF content varied noticeably. During soaking, total α-GOS content decreased between 10% (lentil and faba bean) and 40% (chickpea). In fenugreek, soaking reduced IDF and increased SDF concentration, possibly due to partial IDF solubilisation from the cell wall. Cooking further decreased α-GOS and increased total dietary fibre content. The different behaviours of these five legumes during processing illustrate the high biodiversity within legume species.
Introduction
Legumes are key foods in the Mediterranean diet and excellent candidates to help ensuring the sustainability of food systems. Legume cultures improve the soil nitrogen level and the net primary productivity, and may deliver additional ecosystem benefits. Legumes play a central role in food systems as a source of plant proteins and thanks to their various beneficial health effects (Viguiliouk et al. Citation2017). Indeed, besides their protein content, legumes are an excellent source of nutrients, including carbohydrates (soluble sugars and dietary fibres) (Berrios et al. Citation2010), B-group vitamins and minerals. However, they also contain several compounds that display anti-nutritional activity (for instance, polyphenols and phytic acid) and inhibit mineral absorption, or that are non-digestible, such as alpha-galacto-oligosaccharides (α-GOS) (Aguilera et al. Citation2009; Wang et al. Citation2009; Vasishtha and Srivastava Citation2013). α-GOS contain 1–3 units of galactose linked to sucrose by α-1,6 linkages that are not hydrolysed in the upper part of the human gastrointestinal tract, due to the absence of the enzyme α-galactosidase. In the colon, they are fermented together with soluble dietary fibres by the colon microbiota, generating significant amounts of short-chain fatty acids (SCFA). These fermentation substrates stimulate the growth of lactobacilli and bifidobacteria and the decrease of enterobacteria in the intestinal microflora. This prebiotic action (Martínez-Villaluenga et al. Citation2008; Slavin Citation2013) is beneficial for the host’s well-being and health. However, fermentation also produces gases (carbon dioxide, hydrogen and methane) that generate bloating and flatulence. Flatus production is considered to be the most important deterrent to the consumption of grain legumes (Aguilera et al. Citation2009; Berrios et al. Citation2010).
Legumes are recognised as good sources of dietary fibres and their consumption is promoted for their positive effects on the treatment and prevention of constipation, the control of serum cholesterol levels, the reduction of the risk of diabetes and intestinal cancer, and the stimulation of the growth of beneficial microorganisms. Soluble dietary fibres (SDF) have been linked to cholesterol lowering in blood, while insoluble dietary fibres (IDF) have been associated with water absorption and regulatory intestinal effects (Perry and Ying Citation2016). IDF has a mechanical and irritating effect on the mucosa of the large bowel, thus stimulating the secretion of water and mucous as a defence mechanism against abrasion. IDF are poorly fermented, and remain relatively intact throughout the large intestine (McRorie and McKeown Citation2017).
As all dietary poorly absorbed short-chain carbohydrates have similar and additive effects in the intestine, they have been collectively designated as fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) (Shepherd et al. Citation2013). FODMAPs, among which α-GOS, trigger gastrointestinal symptoms in patients with irritable bowel syndrome, while SDF could have a protecting effect. Therefore, FODMAP reduction in the diet, possibly associated with other dietary strategies, could be an efficient approach for managing irritable bowel syndrome.
Legumes are widely used in many traditional Mediterranean dishes. Several traditional household food-processing methods, including soaking and cooking (Hotz and Gibson Citation2007), can reduce their anti-nutritional factors, particularly dietary fibres and α-GOS (Lestienne et al. Citation2005). Bahthoula is a Tunisian dish prepared with various soaked legume species (lentil, faba bean, Egyptian faba bean, fenugreek and chickpea). Using this dish as a model, we studied the changes in dietary fibres and α-GOS content during soaking and cooking. This study was undertaken (i) to determine the content in soluble sugars, especially α-GOS (raffinose, stachyose and verbascose), and in SDF and IDF in the five Mediterranean legumes used for Bahthoula preparation; (ii) to investigate and elaborate hypotheses on the mechanisms of their changes during soaking in traditional conditions; and finally (iii) to study the effect of cooking on the whole Bahthoula dish, as eaten. This study was performed in the framework of the “Medina” project (http://www6.inra.fr/medina) with the aim of preserving the cultural heritage of the Mediterranean diet, as an outstanding resource for sustainable development because it contributes to promote the consumption of locally produced traditional foods.
Materials and methods
Legumes
Whole lentil (Lens culinaris), faba bean (Vicia faba L. var. major), fenugreek (Trigonella foenum-graecum), chickpea (Cicer arietinum) and Egyptian faba bean (Vicia faba L. var. minor; hereinafter, Egyptian FB) seeds were purchased from a local market in Tunis (Tunisia). As usual, Egyptian FB seeds were decorticated and split. Samples of the five raw seed species were ground using a laboratory mill (IKA M20, Labortechnik, Staufen, Germany) before soaking and cooking.
Soaking
Seeds (24 g) and mineral water (Volvic™) were mixed at a ratio of 1:4 (w/v), except for fenugreek seeds where the ratio was 1:5 (w/v) due to their high swelling capacity, and left to soak at 25 °C in an oven incubator for different times (1, 3, 6, 16 and 24 h). Before soaking, the containers and water were kept at 25 °C for few hours to reach the soaking temperature. At each time point during soaking, the water was drained from the container and stored at −18 °C for analysis. The soaked seeds were washed and blotted dry (by patting with paper towel) and then water absorption by the seeds was monitored by weighing. The dry matter (DM) content of the soaked seeds and pH of the soaking waters were also measured. Soaked seeds were then frozen, lyophilised, ground using a laboratory mill (IKA M20) and stored at −18 °C for chemical analysis. Soaking waters were also kept at −18 °C before analysis.
Experimental preparation of Bahthoula based on the traditional Tunisian recipe
An average recipe and a standard preparation procedure were derived from preparations of Bahthoula observed in four Tunisian households. The cooks were also asked to indicate a mean portion size at the end of the preparation. Each legume species (100 g) used for the preparation of the dish was separately soaked for 16 h in mineral water to mimic the overnight soaking performed in the households. Shortly before cooking, dried salted anchovies were bleached for 5 min to reduce saltiness. Bahthoula was prepared by mixing the five soaked legumes (lentil, faba bean, Egyptian FB, fenugreek and chickpea) with 70 g of anchovies, 150 g of Mhamsa (couscous of coarse grain size obtained by rolling wheat semolina), 50 g of olive oil, 100 g of tomato paste, 90 g of onions, 15 g of garlic and spices (14 g of a caraway-coriander mixture, 10 g of red pepper powder, 2 g of black pepper, 3 g of cloves, 12 g of curcuma and 10 g of salt). Then, 3 L of boiling demineralised water was added, leading to a hot and heterogeneous mixture.
The preparation was simmered on a hot plate for 1 h at boiling temperature. Analyses were carried out on raw and cooked samples to determine the effect of the traditional cooking practice on mono, di and oligosaccharide content. Samples were freeze-dried, grinded to obtain a homogeneous sample and kept at −18 °C until analysis.
Physico-chemical analyses
The thousand seed weight (TSW) was measured for the five legume species (three replicates) as described in Ribeiro et al. (Citation2012). The soaking water pH was monitored at different time points (1, 3, 6, 16 and 24 h). The DM content was measured gravimetrically in triplicate, by drying in an oven at 105 °C until constant weight (AOAC Citation1995).
Insoluble and soluble dietary fibres
A standardised enzymatic-gravimetric method (Megazyme K-TDFR Kit) was used for determination of SDF and IDF, the sum of the two fractions corresponding to the TDF, also designed as the high molecular weight dietary fibres. The method is based on the procedure of the methods AOAC 985.29 and the Prosky method (Prosky et al. Citation1992). Each determination was made on duplicate samples. Shortly, MES-TRIS blend buffer solution (pH 8.2) was added to 1 g of dried sample and stirred until complete dispersion. Samples were incubated with pancreatic α-amylase at 96 °C in a shaking water bath for 30 min. Proteins in the sample were denatured and digested with protease at 60 °C for 30 min. Then, after adjusting the pH to 4.1–4.8, samples were incubated with amyloglucosidase at 60 °C for 30 min. IDF was recovered in a crucible after vacuum filtration of the aqueous reaction mixture; the residue R1 was washed twice with distilled water preheated to 70 °C. The filtrate and water washings were saved for determination of SDF. The residue was then washed twice again with 10 ml of 95% ethanol and acetone, and dried at 105 °C overnight before weighing to determine R1 weight. For the determination of SDF, the mixture of filtrate and water washings was precipitated by adding four volumes of 95% ethanol. The precipitated mixture was vacuum-filtered and washed successively with ethanol 78%, ethanol 95% and acetone. The residue R2 was dried at 105 °C overnight and weighed. For both R1 and R2 residues, one duplicate was analysed for protein using the method of Kjeldahl (ISO 1871:2009) and the other was mineralised at 525 °C to determine ash. R1 and R2 weights were corrected for protein and ash contents for the final calculation of the IDF and SDF contents. The whole procedure was done in duplicate for all samples, and results were averaged.
Analysis of mono, disaccharides and α-GOS
Mono and disaccharides (glucose, fructose, arabinose, galactose, melibiose and sucrose) and α-GOS (raffinose, stachyose and verbascose) were extracted by mixing 80 mg of each sample with ethanol (3 ml, 78%). Tubes were placed in a water bath at 80 °C for 20 min. After centrifugation at 4500 rpm at 4 °C for 15 min, supernatants were transferred to special Speedvac tubes and centrifuged again. Pellets were then rinsed with 2 ml of ethanol (without heating), centrifuged and transferred to new tubes. The supernatants of each sample from the three centrifugation steps were pooled (8 ml) and evaporated under vacuum (Speedvac RC 10). The dry extract was resuspended in 1–2 ml of H2O before high-performance anion-exchange chromatography (HPAEC) analysis (Dionex), as previously described (Baye et al. Citation2013). HPAEC analyses were performed with a CarboPac PA1 Guard pre-column (4 × 50 mm) and a CarboPac PA1 Analytical Column (4 × 250 mm). The mobile phase was 60 mM sodium hydroxide (NaOH), the flow rate was set at 1 ml/min, and the injection volume was 30 μL.
Statistical analysis
Data were analysed using one-way ANOVA and the SPSS software version 24.0 (SPSS Inc., Chicago, IL). The Tukey’s multiple comparison test was used for pair-wise comparisons. A p-value <.05 was the chosen level of significance.
Results and discussion
Characterisation of raw legume seeds
The TSW ranged from 24.6 ± 1.3 to 2098.8 ± 31.6 g depending on the species and variety (), highlighting the great differences in seed size (fenugreek seeds were the smallest and faba bean seeds the largest in this study). Moreover, seeds had very different morphologies: lens-shaped, spherical, or kidney-shaped (or elongated cardioid). The main sugars detected in the five legume species were monosaccharides (fructose, glucose, arabinose and galactose), disaccharides (sucrose and melibiose) and α-GOS (raffinose, stachyose and verbascose) (). The qualitative sugar and fibre profiles of the studied legumes were comparable, but with quantitative differences illustrating the diversity within this food group. Monosaccharide content was low in all seeds, mostly below 30 mg/100 g DM, except for glucose and fructose in faba bean (80 mg/100 g DM). Arabinose was present in minor concentration in fenugreek and lentil, and only in traces in faba bean, Egyptian FB and chickpea. Egyptian FB had the lowest contents in monosaccharides. Sucrose was the main disaccharide in raw seeds. The highest quantities were found in faba bean and chickpea, where it represented more than 2.5% of the seed weight. Melibiose was present in minor concentration in lentil and faba bean and in trace amounts in fenugreek, chickpea and Egyptian FB. Raw legume seeds contained large amounts of total α-GOS (), from about 2500 mg/100 g DM in chickpea to more than 4000 mg/100 g DM in fenugreek. The main α-GOS were raffinose, stachyose and verbascose. Huge differences in α-GOS content were observed among the five legume seeds. Raffinose was present in moderate to low amounts in most legumes, as reported by Reddy et al. (Citation1984), and the highest quantities were detected in fenugreek and chickpea. Stachyose was clearly the predominant α-GOS in fenugreek, lentil and chickpea, while verbascose was the main α-GOS in faba bean and Egyptian FB. Although Egyptian FB seeds were decorticated, their sugar profile was similar to that of the faba bean (both are of the same species) because these sugars are mainly located inside the seeds, and not in the external coat. Overall, the sugar profiles of the five types of legume were consistent with the literature data (Rupérez Citation1998; Sanchez-Mata et al. Citation1998; Martin-Cabrejas et al. Citation2006).
Table 1. Mono, di, oligosaccharide and dietary fibre contents in five raw legumes.
IDF represented between 69 and 87% of all dietary fibres in all five legumes (). SDF contents varied noticeably among legume species. They represented about 30% of all dietary fibres in fenugreek and 13–27% in the other legume seeds. Fenugreek seeds were the richest in both IDF and SDF (almost two thirds of the total DM). This could be partly explained by the small seed size, as indicated by their low TSW, making the proportion of seed coat larger. In agreement, Zdunczyk et al. (Citation1997) reported that smaller-sized pea seeds have significantly higher fibre content than larger-sized seeds. However, in fenugreek seeds, the seed coat is separated from the embryo by a whitish translucent endosperm that is mainly composed of soluble galactomannan gum (Sakhare et al. Citation2015). These authors found that fenugreek seeds contain about 30% of gel-forming soluble fibres, like guar and psyllium seeds. In the other four legume seeds, fibre content was still high, although not as high as in fenugreek. Their SDF fractions were comparable, whereas the IDF fraction was higher in faba bean due to its tough seed coat (Rowland Citation1977), and much lower in decorticated Egyptian FB because IDF is mainly located in the seed coat (Rowland Citation1977).
Effect of soaking
Water absorption
The legume seed samples displayed typical water absorption behaviours, characterised by an initial phase of rapid water imbibition (water gain increased sharply), followed by an equilibrium phase, during which the legumes approached their full soaking capacity (the water absorption rate steadily decreased) (). Similar curves were previously reported for other grains and seeds (Sayar et al. Citation2001). The rapid initial water uptake is attributed to the filling of capillaries on the surface of the seed coat. Then, the water absorption rate decreases sharply when water fills open capillary and inter-micellar spaces. The decrease in water absorption rate could also be explained by the fact that water filling reduces the driving force (i.e. the concentration difference between the soaking medium and the legumes) (Sayar et al. Citation2001).
Figure 1. Effects of soaking on (a) water absorption of legume seeds, (b) pH of the soaking water and (c) leached DM.
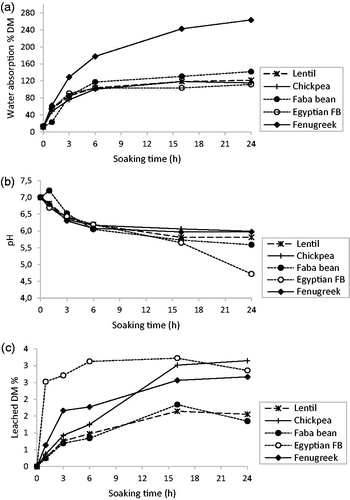
Again, fenugreek exhibited a different behaviour compared with the other seeds. The other four seeds reached the saturation moisture content (around 100–110% DM) after 6 h of soaking. Conversely, fenugreek absorbed higher quantity of water and faster, and continued to absorb water, reaching around 250% DM after 24 h and without attaining a clear equilibrium. This huge water absorption capacity is due to the soft and mucilaginous layer of the seed coat, rich in galactomannans, as reported by Meghwal and Goswami (Citation2012).
Dry matter and pH of soaking water
For all seeds, the pH of the soaking water decreased from approximatively 7.0 to 6.0–4.7 (lowest value for Egyptian FB) after 24 h of soaking (). This acidification suggests leaching of acidic compounds, such as malic or citric acids, during soaking (Sarmento et al. Citation2015) and/or spontaneous lactic acid fermentation. Indeed, it was reported that pH decreases during lentil fermentation (Vidal-Valverde et al. Citation1994; Frias et al. Citation1996).
After 24 h of soaking, the DM leached in soaking water was 1.35, 1.55, 3.15, 2.86 and 2.67% in faba bean, lentil, chickpea, Egyptian FB and fenugreek seeds, respectively (). The amount of DM leached in the soaking water was lower than the DM amount lost by the seeds during soaking (results not shown), and this could be explained by losses in gaseous form, by respiration or fermentation (Dung et al. Citation2010).
Changes in soluble sugar and α-GOS content during soaking
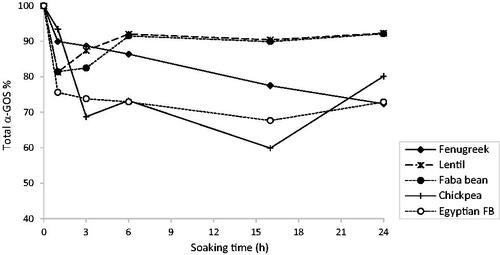
For all legumes, soaking led to a variable reduction in the total α-GOS content (), depending on the seed type and soaking time point. This decrease could result from leaching i.e. diffusion in the soaking water, or from enzymatic degradation that can take place in the seeds or in the soaking water (Coffigniez et al. Citation2018). After 16 h of soaking (the traditional soaking time in Tunisian households), the total α-GOS loss ranged from 10% (lentil and faba bean) to 40% (chickpea seeds). Longer soaking time further reduced the α-GOS content only in fenugreek that exhibited an almost linear decrease of α-GOS content. In the other seed types, a slight increase in total α-GOS was observed between 16 and 24 h. This could be a passive increase caused by the leaching of other soluble compounds from the seeds in the soaking water.
Analysis of the changes in the content of each α-GOS (expressed as percentage of the initial content; ) showed that verbascose displayed the fastest and largest decrease during the first 3 h of soaking, except in fenugreek. Previous works reported that α-GOS spatial distribution in legume seeds can be heterogeneous (Sreerama et al. Citation2010). In chickpea and horse grain, higher concentrations of α-GOS are present in cotyledons than in seed coat, and verbascose is the major α-GOS in the seed coat (Sreerama et al. Citation2010). Stachyose is present in higher amounts in both cotyledon and embryonic axe fractions, although substantial amounts of raffinose and verbascose can also be found in these fractions. This could explain why verbascose content decreased early during soaking. However, verbascose content strongly decreased also in decorticated Egyptian FB seeds. The decreases in stachyose and raffinose, the two lower forms of α-GOS, was slower and moderate, particularly in faba bean and Egyptian FB. Their leaching could be offset by partial verbascose hydrolysis by α-galactosidase, as indicated by the increase (147% after 1 h of soaking) of galactose content in both seed varieties (). Significant decreases in stachyose and raffinose contents were observed in chickpea (40% for both α-GOS) and fenugreek (26 and 49%, respectively) (). As a consequence of this more pronounced α-GOS hydrolysis, galactose production was more important (above 200% after 1 h and around 1000% after 24 h of soaking).
Figure 3. Effect of soaking at 25 °C on the α-GOS content of legume seeds. Error bars are for average deviation.
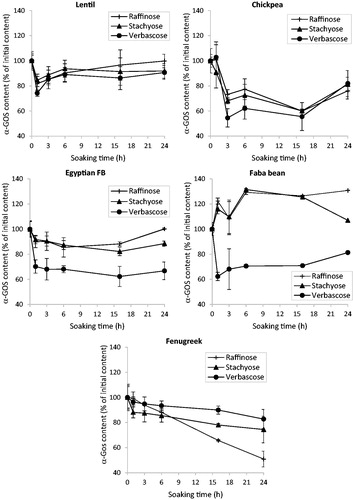
Figure 4. Effect of soaking at 25 °C on the content of some mono and disaccharides in the five legume seeds. Error bars are for average deviation.
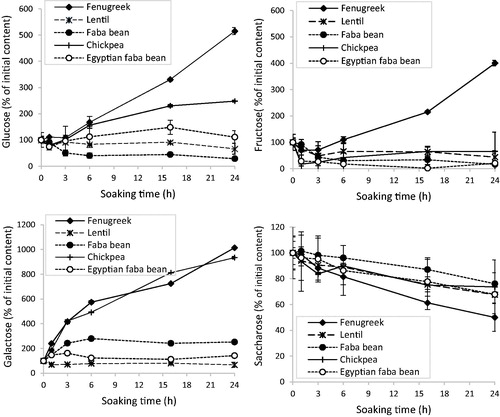
A significant sucrose hydrolysis occurred in all five legumes during soaking. This resulted in an increase in the glucose and fructose content particularly in fenugreek (). Conversely, in the other legumes, glucose and fructose increased, but then mostly diffused in the soaking water. At the end of soaking period, their concentration decreased probably because they were used for respiration (in the seed) and/or fermentation (in the soaking water).
Significant α-GOS reduction by soaking has been previously reported in soybean (Mulimani et al. Citation1997) and various legumes, including lentil and faba bean (Abdel-Gawad Citation1993). During soaking, seeds absorb water, while raffinose, stachyose and verbascose, which are all water-soluble, may leach out of the seeds into the soaking water (Han and Baik Citation2006; Coffigniez et al. Citation2018), or are enzymatically degraded to lower molecular weight sugars (Vidal-Valverde et al. Citation2002; Martínez-Villaluenga et al. Citation2008; Berrios et al. Citation2010). Indeed, very low amounts of α-GOS were found in soaking water (Supplementary Figure 1). And large amounts of hydrolysis products, such as galactose, glucose and fructose, were found in the soaked seeds and also in the soaking water (Supplementary Figure 1). This indicated that enzymatic degradation occurred in the seeds and in the soaking water, after leaching. Endogenous alpha-galactosidase is present in seeds (Coffigniez et al. Citation2018) and produces raffinose from stachyose, and stachyose from verbascose, at low soaking temperatures. Alpha-galactosidase hydrolyses α-GOS by cutting the terminal galactose, and this could explain the increase in galactose content observed in fenugreek, faba bean, Egyptian FB and chickpea during soaking.
Overall, similar changes were observed for the five legumes. Nevertheless, the more detailed analysis revealed specific behaviours that could be due to several factors, such as the presence and thickness of the seed coat, the shape and size of the seeds and also the metabolic specificity of each legume species, related for example to the presence of mucilage or α-galactosidase activity.
Changes in insoluble and soluble dietary fibre content during soaking
The impact of soaking on the dietary fibres was variable. In some legumes, IDF content decreased significantly during the first hours of soaking (fenugreek showed the highest reduction (−28% at 6 h) while in other legumes, the reduction was low (lentil, −17% at 6 h) or even not significant (). This reduction could be due to partial IDF solubilisation from cell wall material (Rehman et al. Citation2004; Aguilera et al. Citation2009), which is not closely linked to the fibre matrix. In chickpea, faba bean and Egyptian FB, a small IDF reduction could have been masked by its passive increase due to leaching of other soluble compounds after several hours of soaking. On the other hand, the SDF fraction showed a quantitative increase of +41% in fenugreek and of +3% in lentil seeds at 1 h. In the other legumes, SDF content tended to increase during the first-hour soaking, but this was significant only for Egyptian FB (+27% at 1 h).
Table 2. Changes in soluble and insoluble fibre contents during seed soaking.
SDF increase can be of health interest because fibres have recognised positive effects on health and are better tolerated when soluble (Fernandes et al. Citation2010). Similar results (significant increase in SDF with a concomitant decrease in IDF contents) were previously reported for chickpea, pink mottled cream bean, and white bean (Aguilera et al. Citation2009).
Legumes display different behaviours due to the specific structure and composition of their cell wall network (Martin-Cabrejas et al. Citation2006). The composition of the dietary fibre fraction depends on its localisation in the seed coat (outer fibres) or in the cotyledons (inner fibres). The relative content of cellulosic and non-cellulosic polysaccharides is significantly different between inner and outer dietary fibres. The cotyledon cell walls contain mainly a range of polysaccharides with various degrees of solubility (e.g. hemicellulose and pectin and cellulose). The seed coat contains mainly water-insoluble, non-digestible carbohydrates (primarily cellulose) and lower amounts of hemicellulose and pectin (Guillon and Champ Citation2002).
Effect of cooking
Changes in mono, di, oligosaccharide and soluble and insoluble fibre content in the Bahthoula dish at the end of cooking
Cooking after soaking led to a further decrease in raffinose (−32%), stachyose (−25%) and verbascose (−35%) and to a significant increase in galactose content (+54%) in the whole dish (Supplementary Table 1). This should be attributed to further enzymatic degradation, due to better conditions for the expression of α-galactosidase activity. Alpha-galactosidase from lentils are active in the temperature range 20–50 °C and up to 65 °C, and have optimal pH of 4.7, 5.5 or 6.1, depending on their isoforms (Dey et al. Citation1983; Celem et al. Citation2009). Indeed the pH of all soaked legumes after 16 h-soaking was around 6.0 as shown in . During heating, the temperature increased progressively and conditions were met for higher α-galactosidase action.
Cooking also increased the IDF content of the Bahthoula dish (+23%), while the SDF fraction remained almost constant (Supplementary Table 1). Similarly, a previous study showed that cooking markedly increases IDF content in chickpea (Perez-Hidalgo et al. Citation1997; Vasishtha and Srivastava Citation2013). This IDF increase can be due to the formation of resistant starch that is partially measured by the method used in our study for fibre determination. Authors suggested that such IDF increase during cooking could be due to Maillard’s reaction (Vasishtha and Srivastava Citation2013). However, Maillard’s reaction is usually limited during hydrothermal processing.
Contribution of the Bahthoula dish to the fibre and α-GOS intake
The minimum total dietary fibre intake recommended by WHO and FAO (Citation2003) is at least 25 g/d, with an optimal intake of 30 g/d for adults. The consumption of a portion of 296 g of Bahthoula (the mean usual portion indicated by Tunisian cooks) would provide 18.7 g of total dietary fibres, thus covering 62% of the average recommended intake for adults. The Bahthoula dish could be considered as an outstanding source of total dietary fibres. α-GOS act as beneficial compounds due to their prebiotic action, but could also cause digestive troubles and act as anti-nutritional factors. The balance between the beneficial and adverse effects mainly depends on the dose at which the α-GOS are consumed. It has been suggested that a daily amount of 3 g of α-GOS is enough to obtain the beneficial prebiotic action, while higher doses could cause too much flatulence and digestive troubles (Martínez-Villaluenga et al. Citation2008). A portion of 296 g of Bahthoula provides 0.7 g of total α-GOS, thus contributing to 22% of the effective daily dose. However, for people suffering from irritable bowel disease, further decrease in α-GOS content would be desirable. This could be obtained by determining the optimal temperature and pH conditions to allow the maximum alpha-galactosidase activity. Characterizing and modelling alpha-galactosidase activity as a function of the processing conditions has been proposed in order to identify optimised pathways to enhance enzymatic degradation and hence reduce α-GOS content more efficiently (Coffigniez et al. Citation2018). This would require the development of models specific for each seed species.
The results of this study highlight that traditional dishes, which prominently include legumes, represent an important component of healthy diets and contribute to human nutrition and food security. This may, in turn, foster strategies of sustainable gastronomy, promoting awareness for the need of healthy and balanced diets (Marinangeli et al. Citation2017).
Conclusion
The consumption of legumes is generally recommended to improve dietary profiles, but is hindered by gastrointestinal discomfort associated with their richness in α-GOS and fibre. This study shows that traditional soaking in water (i) reduces total α-GOS content due to partial leaching and enzymatic degradation occurring both inside and outside the seeds and (ii) induces partial solubilisation of IDF with a concomitant increase of SDF. Cooking further decreased the levels of total α-GOS but increased IDF. Further studies will be carried out to explore the effects of germination/fermentation that are usual in Mediterranean countries, and could enhance α-GOS and fibre degradation. Traditional culinary practices offer healthy legume-based dishes that contribute to the adequate amounts of dietary fibres and α-GOS required for a well-balanced diet.
Supplementary Figure 1 and Table 1
Download PDF (480.1 KB)Disclosure statement
All authors have no conflicts of interest.
Additional information
Funding
References
- Abdel-Gawad AS. 1993. Effect of domestic processing on oligosaccharide content of some dry legume seeds. Food Chem. 46:25–31.
- Aguilera Y, Martın-Cabrejas MA, Benıtez V, Molla E, Lopez-Andreu FJ, Esteban RM. 2009. Changes in carbohydrate fraction during dehydration process of common legumes. J Food Compos Anal. 22:678–683.
- AOAC. 1995. Official methods of analysis. Washington (DC): Association of Official Analytical Chemists.
- Baye K, Mouquet-Rivier C, Icard-Vernière C, Rochette I, Guyot JP. 2013. Influence of flour blend composition on fermentation kinetics and phytate hydrolysis of sourdough used to make injera. Food Chem. 138:430–436.
- Berrios JJ, Morales P, Cámara M, Sánchez-Mata MC. 2010. Carbohydrate composition of raw and extruded pulse flours. Food Res Int. 43:531–536.
- Celem EB, Bolle SS, Onal S. 2009. Efficient and rapid purification of lentil alpha-galactosidase by affinity precipitation with alginate. Indian J Biochem Biophys. 46:366–370.
- Coffigniez F, Briffaz A, Mestres C, Alter P, Durand N, Bohuon P. 2018. Multi-response modeling of reaction-diffusion to explain alpha-galactoside behavior during the soaking-cooking process in cowpea. Food Chem. 242:279–287.
- Dey PM, Del-Campillo EM, Lezica RP. 1983. Characterization of a glycoprotein alpha-galactosidase from lentil seeds (Lens culinaris). J Biol Chem. 258:923–929.
- Dung DD, Godwin IR, Nolan JV. 2010. Nutrient content and in sacco degradation of hydroponic barley sprouts using nutrient solution or tap water. J Anim Vet Adv. 9:2432–2436.
- Fernandes AC, Nishida W, Costa-Proença RP. 2010. Influence of soaking on the nutritional quality of common beans (Phaseolus vulgaris L.) cooked with or without the soaking water: a review. Int J Food Sci Technol. 45:2209–2218.
- Frias J, Vidal-Valverde C, Kozlowska H, Tabera J, Honke J, Hedley CL. 1996. Natural fermentation of lentils. Influence of time, concentration and temperature on the kinetics of monosaccharide, disaccharide and a-galactosides. J Agric Food Chem. 44:579–584.
- Guillon F, Champ MMJ. 2002. Carbohydrate fractions of legumes: uses in human nutrition and potential for health. Br J Nutr. 88:293–306.
- Han IH, Baik BK. 2006. Oligosaccharide content and composition of legumes and their reduction by soaking, cooking, ultrasound, and high hydrostatic pressure. Cereal Chem. 83:428–433.
- Hotz C, Gibson RS. 2007. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J Nutr. 137:1097–1100.
- Lestienne I, Icard-Vernière C, Mouquet C, Picq C, Trèche S. 2005. Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chem. 89:421–425.
- Marinangeli CPF, Curran J, Barr SI, Slavin J, Puri S, Swaminathan S, Tapsell L, Patterson CA. 2017. Enhancing nutrition with pulses: defining a recommended serving size for adults. Nutr Rev. 75:990–1006.
- Martin-Cabrejas MA, Aguilera Y, Benitez V, Mollà E, Lopez-Andréu FJ, Esteban RM. 2006. Effect of industrial dehydration on the soluble carbohydrates and dietary fiber fractions in legumes. J Agric Food Chem. 54:7652–7657.
- Martínez-Villaluenga C, Frias J, Vidal-Valverde C. 2008. Alpha-galactosides: antinutritional factors or functional ingredients? Critic Rev Food Sci Nutr. 48:301–316.
- McRorie JW, McKeown NM. 2017. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J Acad Nutr Diet. 117:251–264.
- Meghwal M, Goswami TK. 2012. A review on the functional properties, nutritional content, medicinal utilization and potential application of fenugreek. J Food Process Technol. 3:1–10.
- Mulimani VH, Thippeswamy S, Ramalingam S. 1997. Effect of soaking, cooking and crude α-galactosidase treatment on the oligosaccharide content of soybean flour. Food Chem. 59:279–282.
- Perez-Hidalgo MA, Guerra-Hernández E, Garcı́a-Villanova B. 1997. Dietary fiber in three raw legumes and processing effect on chickpeas by an enzymatic-gravimetric method. J Food Compos Anal. 10:66–72.
- Perry JR, Ying W. 2016. A review of physiological effects of soluble and insoluble dietary fibers. J Nutr Food Sci. 6:476.
- Prosky L, Asp NG, Schweizer TF, DeVries JW, Furda I. 1992. Determination of insoluble and soluble dietary fiber in foods and food products: collaborative study. J AOAC. 75:360–367.
- Reddy NR, Pierson MD, Sathe SK, Salunkhe DK. 1984. Chemical, nutritional and physiological aspects of dry bean carbohydrates-a review. Food Chem. 13:25–68.
- Rehman ZU, Rashid M, Shah WH. 2004. Insoluble dietary fibre components of food legumes as affected by soaking and cooking processes. Food Chem. 85:245–249.
- Ribeiro ND, Maziero SM, Prigol M, Nogueira CW, Rosa DP, Possobom MTDF. 2012. Mineral concentrations in the embryo and seed coat of common bean cultivars. J Food Compos Anal. 26:89–95.
- Rowland GC. 1977. Seed coat thickness and seed crude fibre in faba beans (Vicia faba). Can J Plant Sci. 57:951–953.
- Rupérez P. 1998. Oligosaccharides in raw and processed legumes. Z Lebensm Unters Forsch 206:130–133.
- Sakhare SD, Inamdar AA, Prabhasankar P. 2015. Roller milling process for fractionation of fenugreek seeds (Trigonella foenumgraecum) and characterization of milled fractions. J Food Sci Technol. 52:2211–2219.
- Sanchez-Mata MC, Penuela-Teruel MJ, Camara-Hurtado M, Diez-Marques C, Torija-Isasa ME. 1998. Determination of mono-, di-, and oligosaccharides in legumes by high-performance liquid chromatography using an amino-bonded silica column. J Agric Food Chem. 46:3648–3652.
- Sarmento A, Barros L, Fernandes Â, Carvalho AM, Ferreira IC. 2015. Valorization of traditional foods: nutritional and bioactive properties of Cicer arietinum L. and Lathyrus sativus L. pulses. J Sci Food Agric. 95:179–185.
- Sayar S, Turhan M, Gunasekaran S. 2001. Analysis of chickpea soaking by simultaneous water transfer and water-starch reaction. J Food Eng. 50:91–98.
- Shepherd SJ, Lomer MCE, Gibson PR. 2013. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 108:707–717.
- Slavin J. 2013. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 5:1417–1435.
- Sreerama YN, Neelam DA, Sashikala VB, Pratape VM. 2010. Distribution of nutrients and antinutrients in milled fractions of chickpea and horse gram: seed coat phenolics and their distinct modes of enzyme inhibition. J Agric Food Chem. 58:4322–4330.
- Vasishtha H, Srivastava RP. 2013. Effect of soaking and cooking on dietary fibre components of different type of chickpea genotypes. J Food Sci Technol. 50:579–584.
- Vidal-Valverde C, Frias J, Estrella I, Gorospe MJ, Ruiz R, Bacon J. 1994. Effect of processing on some antinutritional factors of lentils. J Agric Food Chem. 42:2291–2295.
- Vidal-Valverde C, Sierra I, Frias J, Prodanov M, Sotomayor C, Hedley C, Urbano G. 2002. Nutritional evaluation of lentil flours obtained after short-time soaking process. Eur Food Res Technol. 215:138–144.
- Viguiliouk E, Mejia SB, Kendall CWC, Sievenpiper JL. 2017. Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Annal N Y Acad Sci. 1392:43–57.
- Wang N, Hatcher DW, Toews R, Gawalko EJ. 2009. Influence of cooking and dehulling on nutritional composition of several varieties of lentils (Lens culinaris). LWT Food Sci Technol. 42:842–848.
- WHO, FAO. 2003. Diet, nutrition and the prevention of chronic diseases. Geneva (Switzerland): WHO Technical Report.
- Zdunczyk Z, Godycka I, Amarowicz R. 1997. Chemical composition and content of antinutritional factors in Polish cultivars of peas. Plant Foods Hum Nutr. 50:37–45.